Abstract
The growing number of antibiotic-resistant bacteria necessitates the search for new antimicrobial agents and the principles by which they work. We report that cell membrane-permeant rhodamine B (RhB)-conjugated peptides based on the phosphatidylinositol-4,5-bisphosphate binding site of gelsolin can kill the gram-negative organisms Escherichia coli and Pseudomonas aeruginosa and the gram-positive organism Streptococcus pneumoniae. RhB linkage to the QRLFQVKGRR sequence in gelsolin was essential for the antibacterial function, since the unconjugated peptide had no effect on the bacteria tested. Because RhB-QRLFQVKGRR (also termed PBP10), its scrambled sequence (RhB-FRVKLKQGQR), and PBP10 synthesized from d-isomer amino acids show similar antibacterial properties, the physical and chemical properties of these derivatives appear to be more important than specific peptide folding for their antibacterial functions. The similar activities of PBP10 and all-d-amino-acid PBP10 also indicate that a specific interaction between RhB derivatives and bacterial proteins is unlikely to be involved in the bacterial killing function of PBP10. By using a phospholipid monolayer system, we found a positive correlation between the antibacterial function of PBP10, as well as some naturally occurring antibacterial peptides, and the intrinsic surface pressure activity at the hydrophobic-hydrophilic interface. Surprisingly, we observed little or no dependence of the insertion of these peptides into lipid monolayers on the phospholipid composition. These studies show that an effective antimicrobial agent can be produced from a peptide sequence with specificity to a phospholipid not found in bacteria, and comparisons with other antimicrobial agents suggest that the surface activities of these peptides are more important than specific binding to bacterial proteins or lipids for their antimicrobial functions.
Antimicrobial peptides are nonadaptive host defense molecules that provide a first line of defense against a wide spectrum of pathogens. They are found in all species ranging from protozoa to vertebrates. In mammals, these peptides are stored in granules of leukocytes and are present on mucosal surfaces and skin (31). In the last decade, they have become an important focus of study due to their possible applications as a new source of antibiotics, anticancer drugs, food preservatives, and antiseptic agents (32). It has been proposed that a variety of antimicrobial peptides kill bacteria by interacting with the anionic phospholipid of the bacterial inner membrane or with the hydrophilic lipopolysaccharide (LPS) of the cell wall of gram-negative bacteria. As a result of this relatively nonspecific interaction, insertion of antibacterial peptides in the bacterial membrane occurs, resulting in either its permeabilization or disruption. This leads to changes in secondary messenger systems that further augment the abnormal electrical activity and that disrupt signal transduction, causing bacterial death (14). However, understanding of the peptide-mediated bacterial killing mechanism(s) is still incomplete. The proline- and arginine-rich bactenecin (Bac) peptides (Bac5 and Bac7, respectively) from bovine neutrophil granules inhibit incorporation of precursor molecules into Escherichia coli protein and RNA (27), and cecropin (PR-39) from pig small intestine has been shown to prevent protein and DNA synthesis in gram-negative bacteria (3). Antimicrobial peptides might also act through mechanisms other than bacterial membrane destabilization or nucleic acid and protein synthesis inhibition. Recently, receptor-mediated stimulation of host defense mechanisms by some antibacterial peptides has been established (7). The antimicrobial peptides magainin II, indolicidin, and temporins (B and L) were found to modulate the hydrolytic activity of secretory phospholipase A2 from bee venom and human lacrimal fluid (36).
Coupling between the innate and adaptive immune systems is likely, and antimicrobial peptides appear to influence both the quality and the effectiveness of the immune responses (31, 36). Rabbit cathelicidin-derived CAP18 and human cathelicidin-derived CAP18 (peptide LL37) inhibit the biological activity of LPS and reduce the lethality of LPS in murine models of endotoxemia (15, 16), whereas PR-39 can induce expression of cell surface heparan sulfate proteoglycans (syndecan-1 and -4) as part of the wound repair process (11, 33).
The relationship between the bactericidal activities and the physicochemical properties of antibacterial peptides has been intensively studied. Peptide activities correlated positively with the gradient of hydrophobicity along the peptide backbone, with a net positive charge at neutral pH and with α-helical structures (13, 34). There is, however, no sequence homology among families of antimicrobial peptides.
The PBP10 peptide, whose sequence is derived from the phosphoinositide (PPI)-binding region in gelsolin, bears some resemblance to the antibiotic peptides, such as a short sequence with a net positive charge and membrane permeation. Additionally, circular dichroism and nuclear magnetic resonance imaging studies show that peptides based on gelsolin residues 150 to 169 undergo a coil-to-helix transition in the presence of phosphatidylinositol-4,5-bisphosphate (PIP2) and other acidic surfactants (30). This helical structure produces an amphipathic molecule with positive charges arranged on one face and hydrophobic residues arranged on the other. Unique properties of PBP10 include PPI binding due to the cationic amino acid composition and hydrophobicity due in part to the linked fluorophore. These traits allow PBP10 to enter cells passively and to affect many cellular functions dependent on PPI signaling, including cell motility (6), platelet activation (5), and canalicular membrane ATP-dependent vesicle transport (24).
In this study we characterized the bacterial killing properties of peptides based on the PIP2-binding site of gelsolin and compared them with the antibacterial functions of cathelicidin LL37, magainin II, and melittin. We found a positive correlation between the antibacterial functions of those agents and their surface pressure activities.
MATERIALS AND METHODS
Materials.
Magainin II (catalog no. M-7402), synthetic melittin (catalog no. M-4171), l-α-phosphatidylcholine (catalog no. P-6638), l-α-phosphatidyl-l-serine (catalog no. P-6641), cholesterol (catalog no. C-8667), l-α-phosphatidylglycerol dipalmitoyl (catalog no. P-9789), and cardiolipin (catalog no. C-1649) were obtained from Sigma. Brain PIP2, tri-ammonium salt (catalog no. 840046), was from Avanti Polar Lipids. Tryptic soy broth (catalog no. 0370-17-3), Pseudomonas isolation agar (catalog no. 0927-17-1), and blood-based agar (catalog no. 0045-17) were from Difco. Peptide LL37 was synthesized with an automatic peptide synthesizer monitored by mass spectrometry and capillary electrophoresis at the Louisiana State University Medical Center Core Laboratories. The set of gelsolin-related peptides, including QRL, QRLFQV, QRLFQVKGRR (GS 160-169), all-d-amino-acid QRLFQVKGRR (all-d-QRLFQVKGRR), FRVKLKQGQR, and KHVVPNEVVVQRLFQVKGRR (GS 150-169), were prepared in the Peptide Synthesis Laboratory (Riga, Republic of Latvia) either as free peptides or as peptides conjugated to rhodamine B (RhB) with an amide link at the N terminus of the peptide (denoted with RhB as a prefix to the peptide sequence), as described previously (6).
Antimicrobial activities.
The bactericidal activities of the different antibacterial peptides were measured as described previously (26); kanamycin-resistant E. coli (SG 13009), kanamycin-resistant Pseudomonas aeruginosa (PAO1), and Streptococcus pneumoniae (STG) were grown to mid-log phase at 37°C, resuspended in phosphate-buffered saline (PBS; 140 mM NaCl, 7.5 mM Na2HPO4 [pH 7.4]), and brought to 108 CFU/ml (with the assumption that an optical density at 620 nm of 0.35 corresponds to 108 CFU/ml). They were then diluted 10 times in 100 μl of PBS containing different antibacterial and control peptides. After 1 h of incubation at 37°C, the suspensions were placed on ice and diluted 10- to 10,000-fold in PBS, and 10-μl aliquots were spotted on Lura-Bertani agar plates, Pseudomonas isolation agar plates, or blood-based agar plates (for E. coli, PAO1, and STG, respectively) for overnight culture at 37°C. Manual counting of the numbers of CFU was performed.
Examination of bacterial morphological changes by electron microscopy.
Samples containing E. coli (108 CFU/ml) in 140 mM NaCl-10 mM Na2HPO4 (pH 7.4) were incubated for 45 min with 5 to 10 μM PBP10, 10 μM RhB-QRL, or 5 μM peptide LL37. After centrifugation at 3,000 × g for 5 min, a drop of the bacterial suspension was deposited onto a grid and negatively stained with 0.25% phosphotungstic acid (PTA). The grids were examined with a JEOL JEM-1010 electron microscope.
Surface pressure and incorporation of the peptides into lipid monolayers.
The surface activities of the peptides were measured with a μTrough S instrument (Kibron Inc., Helsinki, Finland), as described elsewhere (20). PBS (1 ml) was placed in each of the 15 wells in a multiple-well trough, and the surface pressure (π) was measured for 30 min after injection of the desired volume of the peptide stock solution below the monolayer-covered surface. Kinetic measurements of the π values showed stable readings after 5 min, and the π values remained constant (within 5%) for at least 45 min for all pressures reported. The lipid monolayers residing on the air-water interface provided a convenient means to assess the lipophilicities of the peptides by monitoring the increase in π caused by peptide insertion into the lipid film (4). To investigate the interaction of peptide LL37 and the RhB-QRLFQVKGRR peptide with lipid monolayers, various amounts of different phospholipids from a chloroform stock solution were spread at the liquid-air interface to give an initial π (π0). Thirty minutes after monolayer formation (π0), the desired volume of peptide stock solution was injected below the monolayer-covered surface through an injection septum, and the subphase was stirred for 30 s. The change in π (Δπ) from π0 after the addition of peptide was complete in approximately 30 min, and the difference between π0 and the value observed after equilibration of the surface with the peptide solution was taken as Δπ. The data are represented as Δπ versus π0 (4). All of the measurements were performed at room temperature.
Lipid extractions and analysis.
Extraction of lipids from the bacteria was performed by the methods described by Rozgonyi et al. (23). The bacterial culture was washed with ice-cold buffer A (140 mM NaCl, 10 mM Tris [pH 5]) and centrifuged; the pellet was extracted in 5% trichloroacetic acid for 15 min to remove nonlipid materials that can be dissolved in lipid solvent. The extraction of lipids from the bacterial mass was performed in a mixture of chloroform-methanol-HCl at relative concentrations of 20:40:1 (by volume) for 2 h at room temperature. Separation of the organic phase was performed by adding water to this system. After the lipids were extracted, they were separated into different fractions by thin-layer chromatography with silica plates (0.25 mm; Merck) developed with chloroform-methanol-acetic acid-H2O (77:49:10:6, by volume) for phospholipid analysis (35).
RESULTS
Bactericidal activity.
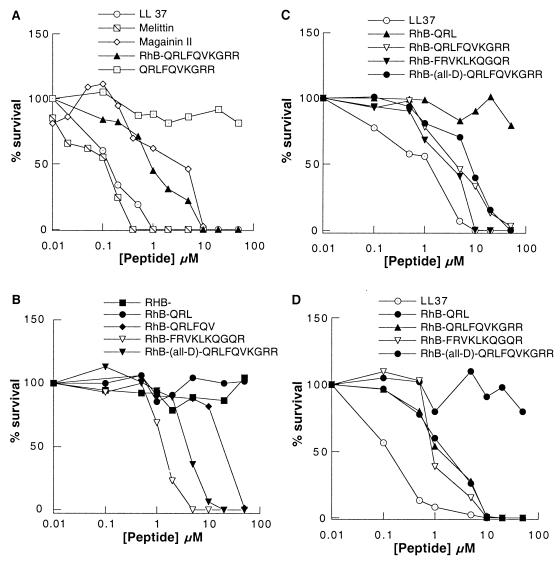
By using a standard killing assay for E. coli, we compared the antimicrobial activities of the naturally occurring antibacterial agents mellitin, magainin II, and peptide LL37 (Table 1) with those of synthetic RhB-labeled derivatives of the PIP2-binding site of gelsolin (Fig. 1A and B). Additionally, we tested the ability of RhB-labeled peptides to kill other bacterial strains, including the gram-negative species P. aeruginosa and the gram-positive species S. pneumoniae (Fig. 1C and D). As determined by in vitro assays, the antibacterial concentrations of naturally occurring antimicrobial peptides varied from 1 to 100 μM. PBP10 and its derivatives, RhB-all-d-QRLFQVKGRR and RhB-FRVKLKQGQR, quickly and efficiently killed nearly all E. coli organisms at concentrations of 5 to 15 μM (Fig. 1). This antibacterial activity is similar to that of magainin II but is 10 times less than those of mellitin and peptide LL37, which at concentrations of 1 μM and 0.5 to 1 μM, respectively, kill approximately 100% of E. coli isolates. Under the same conditions, the number of growing colonies was not affected by RhB, RhB-QRL, GS 150-169, GS 160-169, or full-length gelsolin (data not shown), indicating the importance of the RhB link to the peptide sequences for the antibacterial actions of these agents. The GS 150-169 peptide, which has physical properties similar to those of the active α-helical peptides, had no antibacterial activity, possibly because it lacked the tryptophan residue required for insertion of the peptide into the lipid membrane (10). The RhB-QRLFQV peptide was the shortest peptide with antibacterial activity and killed nearly 100% of the E. coli organisms at a concentration of 50 μM. PBP10 also killed bacteria of the genera Pseudomonas and Streptococcus, but at higher concentrations compared to that necessary to kill E. coli.
TABLE 1.
Sequences of selected antibacterial peptides
| Peptide | Sequence | Source |
|---|---|---|
| LL37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | Human cathelicidin (hCAP18) |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | Honeybee (Apis mellifera) venom |
| Magainin II | GIGLFLHSALLFGLAFVGGIMNS | Frog (Xenopus laevis) skin |
| PBP10 | RhB-QRLFQVKGRR | PIP2-binding site of gelsolin (positions 150-169) |
FIG. 1.
Viabilities of E. coli (A and B), P. aeruginosa (C), and S. pneumoniae (D) after 1 h of incubation with various concentrations of antibacterial peptides. Each datum point represents the mean of three to five experiments. The effects of the peptides were calculated by taking the number of CFU in the sample with no drug treatment (growth control) as 100%. The standard deviation is less than 15% and for the sake of clarity is not shown.
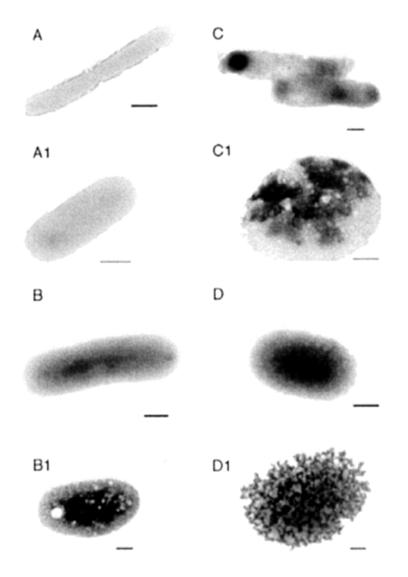
The morphological changes of the bacteria after treatments with PBP10 and peptide LL37 are shown in Fig. 2. PBP10 at a concentration of 5 μM, which killed ∼80% of the E. coli organisms, caused an increase in bacterial membrane permeability on the basis of the intensity of PTA staining. This result suggests that PBP10 mainly targets the bacterial membrane. An increase in the PBP10 concentration (to 10 μM) resulted in the irregular disruption of the bacterial membrane and wall. Round PTA-stained remnants of completely lysed bacteria after PBP10 and LL37 treatments are shown in Fig. 2C1 and D1, respectively.
FIG. 2.
Electron micrographs of negatively stained E. coli untreated (A and A1) and treated with antibacterial peptides: 5 μM PBP10 (B and B1), 10 μM PBP10 (C and C1), and 5 μM LL37 peptide (D and D1). The images shown were obtained in a single experiment. Two other experiments gave similar images. Bars, 500 nm.
Surface activities of antibacterial peptides.
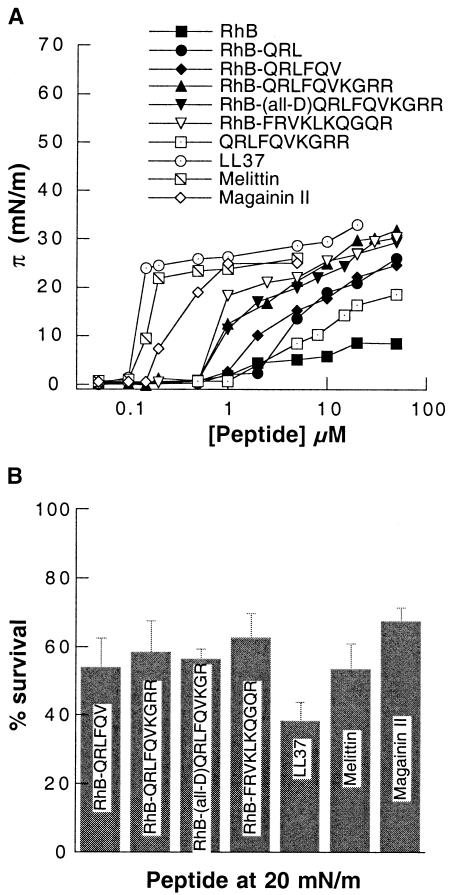
One of the common characteristics of antibacterial peptides is their ability to destabilize the bacterial membrane and increase its permeability. To determine whether the membrane permeation activities of those agents correlate with their amphipathic character, which promotes partitioning to the hydrophobic interface, the π values at the air-water interface were measured in the presence of various concentrations of the RhB-linked peptides mellitin, magainin II, and peptide LL37. Figure 3 shows how π changes when increasing amounts of antibacterial peptides are added to PBS solutions. A significant increase in π occurred at RhB-linked peptide concentrations between 0.5 and 10 μM. The surface activities of the naturally occurring antibacterial peptides were approximately 10 times stronger than those of the RhB derivatives. The surface activities of RhB-labeled and unlabeled peptide QRLFQVKGRR are much weaker than those of the RhB conjugates.
FIG. 3.
(A) π values at the liquid-air interface for PBS with different concentrations of peptides. Each datum point represents the mean of five to six experiments. The standard deviation is less than 1 mN/m and for the sake of clarity is not shown. (B) Viability of E. coli after 1 h of incubation with different antibacterial peptides at concentrations at which π was 20 ± 0.5 mN/m. The data shown are the means ± standard deviations of three experiments.
Bactericidal and π activities of antibacterial peptides.
As shown in Fig. 3, the antibacterial peptides exhibited different π activities, and there appears to be a relationship between π activity and bacterial killing efficiency. To investigate more quantitatively the relation between partitioning to hydrophobic interfaces and the killing activities of antibacterial peptides, we conducted a standard bacterial killing assay with E. coli using concentrations of the various peptides just sufficient to produce π values at the air-water interface of 20 ± 0.5 mN/m (0.11 μM LL37, 0.12 μM melittin, 0.15 μM magainin II, 1.1 μM RhB-FRVKLKQGQR, 1.4 μM RhB-QRLFQVKGRR or RhB-all-d-QRLFQVKGRR, and 12 μM RhB-QRLFQV). Figure 4 shows that these concentrations of the structurally distinct peptides killed 60% ± 15% of the E. coli organisms, indicating a positive correlation between π and the killing activities of these antibacterial agents.
FIG. 4.
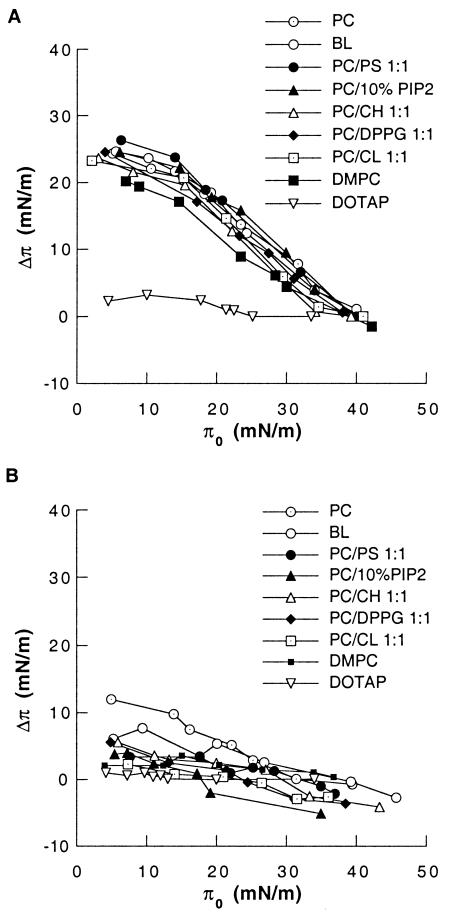
Interaction of LL37 (A) and PBP10 (B) peptides (both at a concentration of 0.1 μM) with lipid monolayers obtained by spreading chloroform solutions of different lipids on the liquid-air interface. The data shown on panels A and B were obtained in a single experiment. Two other experiments gave similar data. DOTAP, positively charged 1,2-dioleoyl-3-trimethylammonium propane.
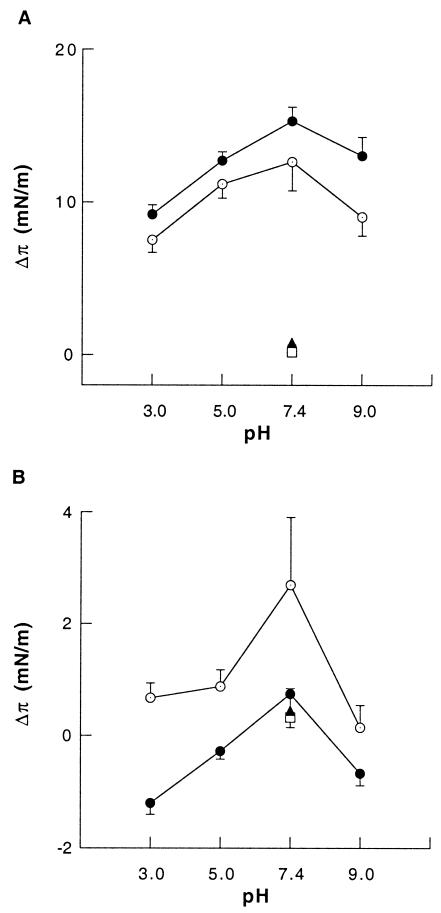
Insertion of LL37 and PBP10 peptides into lipid monolayers.
Partitioning to the air-water interface is expected for any amphipathic solute; but partitioning of amphipathic peptides to the membrane can depend on the lipid packing density, the structure of the peptide, the distribution of surface charges, and other features that mediate the balance between surface adsorption and incorporation into the hydrophobic layer of the membrane. The intercalation of antimicrobial peptides into lipid monolayers compressed at the air-water interface to different π0 values was observed by measuring Δπ following the addition of the PBP10 or the LL37 peptide into the aqueous subphase (37). As shown in Fig. 4A, insertion of LL37 peptides (0.1 μM) into various phospholipid monolayers composed of phosphatidylcholine (PC), 1,2-dimyristoyl-glycero-3-phosphocholine (DMPC), PC-cholesterol (CH), PC-phosphatidylserine (PS), PC-PIP2, PC-l-α-phosphatidylglycerol dipalmitoyl (DPPG), PC-cardiolipin (CL), and total lipid extract from E. coli (bacterial lipids [BLs]) did not result in any significant differences in π values. The phospholipid composition of BL was as follows: PS, 2.94% ± 0.57%; phosphatidylethanolamine, 77% ± 1.3%; CL, 12% ± 1.41%; and other phospholipids (including phosphatidylglycerol), 12.1% ± 1.2%. The observation of a lack of significant differences in π values suggests that the phospholipid composition of the outer leaflet of bacterial membranes with high concentrations of negatively charged phospholipids, which are not found in mammalian cells, is not the only factor determining the high degree of toxicity of the LL37 peptide against E. coli cells but a negligible effect on platelets or red blood cells (data not shown). However, the lack of LL37 insertion into the positively charged 1,2-dioleoyl-3-trimethylammonium propane monolayer (Fig. 4A) shows the importance of charge interactions during insertion of antibacterial peptides into the cell membrane. A similar experiment with 0.1 μM PBP10 (Fig. 4B) shows a smaller increase in π values (less than 10-mN/m increases even at low π0 values). At higher values of π0, the addition of PBP10 causes a small decrease in π, but as expected for a peptide based on a specific lipid-binding domain, there is a significant dependence on the lipid composition.
Since the antibacterial activity of the LL37 peptide is strongly dependent on its α-helicity, which can be modulated by changing the ion composition and the pH of the buffer (13), we designed an experiment in which the same amount of PC or BL was spread on the surfaces (π0 = 20 ± 0.5 mN/m) of hypotonic (pH 7.4) or isotonic (pH 3.0 to 9.0) buffers. As shown in Fig. 5A, insertion of the LL37 peptide (0.1 μM) into the PC and BL monolayer occurred only when the phospholipids were spread on isotonic solutions. Insertion of LL37 peptides into PC as well as BL monolayers was also modulated by the H+ concentration, with a maximal effect at pH 7.4. Insertion of PBP10 into PC monolayers was also stronger in isotonic buffers at physiological pH than in acidic or basic environments (Fig. 5B), suggesting either pH-dependent conformational changes in PBP10 or, possibly, changes in the degree of ionization or conformation in the lipid. This result confirms previous observations that showed a strong dependence of PBP10 fluorescence on the H+ concentration (6). The interactions of PBP10 with BL monolayers spread on isotonic or hypotonic medium at pH 7.4 were not significantly different. Interestingly, a decrease in π was observed during the interaction of the PBP10 peptide with BL phospholipids spread on the buffer when the pH was <5 or >9.
FIG. 5.
Effect of pH and ionic strength on LL37 (A) and PBP10 (RhB-QRLFQVKGRR) (B) interaction with PC monolayers spread on isotonic (circles with points) and hypotonic (empty squares) buffer or BL monolayers spread on isotonic (filled circles) and hypotonic (filled triangles) buffers. The π0 value for all PC and BL monolayers was 20 ± 0.5 mN/m. Data shown represent Δπ (π − π0) and are the means ± standard deviations of four to five experiments.
DISCUSSION
Defining the structure-function relationships for antimicrobial peptides would have many implications for the design of new therapeutic agents that could be used to counter infections and avoid antibiotic resistance. Nearly all antimicrobial peptides are cationic amphiphiles; but a common mechanism that accounts for their selective destruction of bacterial membranes while leaving eukaryotic membranes intact has not been revealed, and different antimicrobial peptides may function by distinct mechanisms. The present work contributes two results that may have implications for the function of antimicrobial peptides. An effective antimicrobial agent was produced from a peptide sequence with no relation to any natural antibiotic function. Antibacterial RhB conjugates are derived from the gelsolin binding site for PPIs, a class of lipids not present in the vast majority of known bacteria, and the PPI-binding sequence bears little homology to the sequences of antimicrobial peptides, aside from its basic and hydrophobic character. When the bactericidal activities of structurally distinct peptides at different concentrations are compared, there appears to be a relationship between their bactericidal efficiencies and their surface activities at the air-water interface.
The structures and amino acid compositions of natural antibacterial peptides have been modified experimentally to produce improved antibacterial and antifungal agents with high levels of antibiotic activity but low levels of hemolytic activity. In part, this goal is attainable by increasing the net positive charge by lysine substitution (25), increasing the net hydrophobicity by tryptophan substitution (17, 18), conjugation of peptides with lipophilic acid (1), incorporation of a carbamate bond(s) (19), synthesis of hybrid peptides (22), and synthesis of truncated sequences that omit hemolytic regions (29). The results presented here show that linkage of the hydrophobic molecule RhB to the QRLFQVKGRR, all-d-QRLFQVKGRR, and FRVKLKQGQR sequences based on the PIP2-binding site of gelsolin (1) also produced new antimicrobial agents.
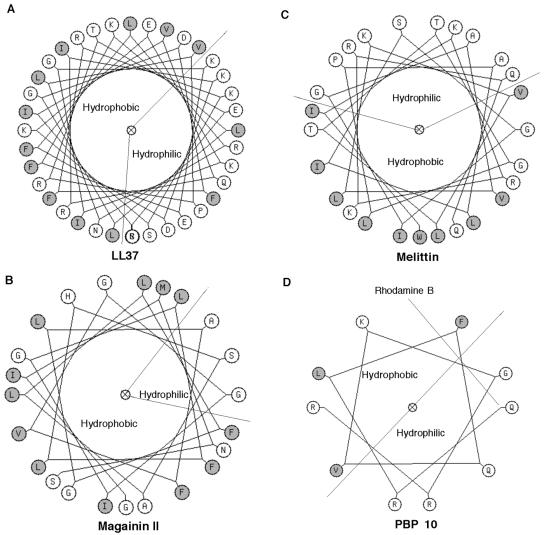
PBP10 has antibacterial activity against gram-negative and gram-positive bacteria, while free RhB, nonlabeled QRLFQVKGRR and KHVVPNEVVVQRLFQVKGRR peptides, and gelsolin lack the ability to kill bacteria. By linking RhB to the GS 160-169 sequence, the net hydrophobicity of this molecule increases, and this effect may increase its membrane permeation function (Fig. 6). Some cellular processes associated with PPI metabolism are effectively modulated by PBP10 (5, 6, 12, 24), but not by the scrambled form of PBP10, in which changes in the amino acid sequence disrupt its PPI-binding ability (6, 12). Because the scrambled form of PBP10 kills bacteria over the same concentration range as the original peptide, the net positive charge of RhB-labeled peptides appears to be more important than their specific structure or their ability to bind to PPI for the antibacterial functions of the peptides. PBP10 synthesized from the d-isomer forms of the amino acids also had antibacterial properties similar to those of PBP10 synthesized from the l-isomer forms. This observation rules out the involvement of a specific PBP10-bacterial protein interaction and agrees with recent observations showing that the natural and synthetic (all-d-isomer-form) forms of cecropin have comparable antibacterial activities (2).
FIG. 6.
Edmundson helical wheel plots for LL37 (A), magainin II (B), mellitin (C), and PBP10 (D) peptides. Lines divide the helix into hydrophobic (shaded residues) and hydrophilic parts. An amphipathic helical structural prediction has been confirmed by circular dichroism spectroscopy for most of these peptides (34). On the basis of the circular dichroism of GS 150-169 (30), we assumed that PBP10 can also adopt a helical conformation.
The membrane permeation function of antibacterial peptides is associated with an amphipathic character that promotes partitioning to the hydrophobic interface. Measurement of the intrinsic surface activities of the PBP10, melittin, magainin II, and LL37 peptides reveals a positive correlation between their π activities and their antibacterial functions. Molecule hydrophobicity does not ensure binding of the molecule to different phospholipids (21), but it favors penetration of the molecule into phospholipid monolayers. In effect, hydrophobic forces seem to govern the interaction of antimicrobials with lipid membranes. We propose that simple measurements of intrinsic π activity can be used to estimate the concentrations of antibacterial agents required for their membrane-destabilizing insertion.
The interaction between the peptides absorbing into the lipid monolayer and the individual molecules composing this surface is determined by a number of factors (8, 28). No significant head-group dependency was observed for the interaction of LL37 with monolayers of PC, DMPC, PC-CH, PC-PS, PC-PIP2, PC-DPPG, PC-CL, and a mixture of BLs, suggesting little or no specificity of the LL37 peptide for the polar head groups of the lipids under the test conditions used in our experiments. This observation suggests that a negative charge, even in the context of a zwitterionic lipid, and its density on the bacterial surface could be more important than the specific type of phospholipid present in the bacterial membrane.
When PBP10 (0.1 μM) was injected under a monolayer containing negatively charged phospholipids, especially PC-PIP2, a decrease in π (below π0) was observed. This result suggests that during the interaction of PBP10 with lipid monolayers, anionic phospholipids attracted by the positively charged PBP10 peptide reorganized their packing to reduce π. Previous microscopy observations have demonstrated that addition of fluorescein-labeled peptide QRLFQVKGRR to the aqueous subphase of a PC-, PIP-, Texas Red-, and phosphatidylethanolamine-containing monolayer caused segregation of highly charged phospholipids into domains (9). A similar formation of lipid domains may precede or accompany the cell membrane reorganization that eventually leads to permeabilization or rupture. Whether the induction of lipid domains is a general property of antimicrobial peptides has not been investigated, but the data presented here suggest that nonspecific surface activity independent of or in addition to the presence of specific binding sites for antimicrobial peptides may be an important determinant of their biological function.
Acknowledgments
We gratefully acknowledge Qian-Chun Yu and Neelima Shah from the Biomedical Imaging Core Laboratory (University of Pennsylvania) for help with preparing electron microscopy images.
This work was supported by USPHS under NIH grants R01 HL67286 and AR38910. R.B. was supported by a postdoctoral fellowship based on CFF grant R881.
REFERENCES
- 1.Avrahami, D., and Y. Shai. 2002. Conjugation of a magainin analogue with lipophilic acids controls hydrophobicity, solution assembly, and cell selectivity. Biochemistry 41:2254-2263. [DOI] [PubMed] [Google Scholar]
- 2.Bland, J. M., A. J. De Lucca, T. J. Jacks, and C. B. Vigo. 2001. All-d-cecropin B: synthesis, conformation, lipopolysaccharide binding, and antibacterial activity. Mol. Cell. Biochem. 218:105-111. [DOI] [PubMed] [Google Scholar]
- 3.Boman, H. G., B. Agerberth, and A. Boman. 1993. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun. 61:2978-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockman, H. 1999. Lipid monolayers: why use half a membrane to characterize protein-membrane interactions? Curr. Opin. Struct. Biol. 9:438-443. [DOI] [PubMed] [Google Scholar]
- 5.Bucki, R., P. A. Janmey, R. Vegners, F. Giraud, and J. C. Sulpice. 2001. Involvement of phosphatidylinositol 4,5-bisphosphate in phosphatidylserine exposure in platelets: use of a permeant phosphoinositide-binding peptide. Biochemistry 40:15752-15761. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham, C. C., R. Vegners, R. Bucki, M. Funaki, N. Korde, J. H. Hartwig, T. P. Stossel, and P. A. Janmey. 2001. Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly. J. Biol. Chem. 276:43390-43399. [DOI] [PubMed] [Google Scholar]
- 7.De, Y., Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demel, R. A., Y. London, W. S. Geurts van Kessel, F. G. Vossenberg, and L. L. van Deenen. 1973. The specific interaction of myelin basic protein with lipids at the air-water interface. Biochim. Biophys. Acta 311:507-519. [DOI] [PubMed] [Google Scholar]
- 9.Foster, W. J., Jr., and P. A. Janmey. 2001. The distribution of polyphosphoinositides in lipid films. Biophys. Chem. 91:211-218. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich, C., M. G. Scott, N. Karunaratne, H. Yan, and R. E. Hancock. 1999. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 43:1542-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallo, R. L., M. Ono, T. Povsic, C. Page, E. Eriksson, M. Klagsbrun, and M. Bernfield. 1994. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Acad. Sci. USA 91:11035-11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttman, J. A., P. Janmey, and A. W. Vogl. 2002. Gelsolin—evidence for a role in turnover of junction-related actin filaments in Sertoli cells. J. Cell Sci. 115:499-505. [DOI] [PubMed] [Google Scholar]
- 13.Johansson, J., G. H. Gudmundsson, M. E. Rottenberg, K. D. Berndt, and B. Agerberth. 1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273:3718-3724. [DOI] [PubMed] [Google Scholar]
- 14.Kourie, J. I., and A. A. Shorthouse. 2000. Properties of cytotoxic peptide-formed ion channels. Am. J. Physiol. Cell Physiol. 278:C1063-C1087. [DOI] [PubMed] [Google Scholar]
- 15.Larrick, J. W., M. Hirata, R. F. Balint, J. Lee, J. Zhong, and S. C. Wright. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larrick, J. W., M. Hirata, H. Zheng, J. Zhong, D. Bolin, J. M. Cavaillon, H. S. Warren, and S. C. Wright. 1994. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J. Immunol. 152:231-240. [PubMed] [Google Scholar]
- 17.Lee, D. G., H. N. Kim, Y. Park, H. K. Kim, B. H. Choi, C. H. Choi, and K. S. Hahm. 2002. Design of novel analogue peptides with potent antibiotic activity based on the antimicrobial peptide, HP (2-20), derived from N-terminus of Helicobacter pylori ribosomal protein L1. Biochim. Biophys. Acta 1598:185-194. [DOI] [PubMed] [Google Scholar]
- 18.Lee, D. G., P. I. Kim, Y. Park, E. R. Woo, J. S. Choi, C. H. Choi, and K. S. Hahm. 2002. Design of novel peptide analogs with potent fungicidal activity, based on PMAP-23 antimicrobial peptide isolated from porcine myeloid. Biochem. Biophys. Res. Commun. 293:231-238. [DOI] [PubMed] [Google Scholar]
- 19.Lee, K. H., and J. E. Oh. 2000. Design and synthesis of novel antimicrobial pseudopeptides with selective membrane-perturbation activity. Bioorg. Med. Chem. 8:833-839. [DOI] [PubMed] [Google Scholar]
- 20.Medina, O. P., T. Soderlund, L. J. Laakkonen, E. K. Tuominen, E. Koivunen, and P. K. Kinnunen. 2001. Binding of novel peptide inhibitors of type IV collagenases to phospholipid membranes and use in liposome targeting to tumor cells in vitro. Cancer Res. 61:3978-3985. [PubMed] [Google Scholar]
- 21.Ogasawara, Y., Y. Kuroki, and T. Akino. 1992. Pulmonary surfactant protein D specifically binds to phosphatidylinositol. J. Biol. Chem. 267:21244-21249. [PubMed] [Google Scholar]
- 22.Oh, D., S. Y. Shin, S. Lee, J. H. Kang, S. D. Kim, P. D. Ryu, K. S. Hahm, and Y. Kim. 2000. Role of the hinge region and the tryptophan residue in the synthetic antimicrobial peptides, cecropin A(1-8)-magainin 2(1-12) and its analogues, on their antibiotic activities and structures. Biochemistry 39:11855-11864. [DOI] [PubMed] [Google Scholar]
- 23.Rozgonyi, F., J. Kiss, and K. Szitha. 1980. Lipid extraction and phospholipid phosphorus determination in gram-positive bacteria. Acta Microbiol. Acad. Sci. Hung. 27:23-29. [PubMed] [Google Scholar]
- 24.Sai, Y., A. T. Nies, and I. M. Arias. 1999. Bile acid secretion and direct targeting of mdr1-green fluorescent protein from Golgi to the canalicular membrane in polarized WIF-B cells. J. Cell Sci. 112(Pt 24):4535-4545. [DOI] [PubMed] [Google Scholar]
- 25.Shin, S. Y., S. W. Kang, D. G. Lee, S. H. Eom, W. K. Song, and J. I. Kim. 2000. CRAMP analogues having potent antibiotic activity against bacterial, fungal, and tumor cells without hemolytic activity. Biochem. Biophys. Res. Commun. 275:904-909. [DOI] [PubMed] [Google Scholar]
- 26.Silvestro, L., J. N. Weiser, and P. H. Axelsen. 2000. Antibacterial and antimembrane activities of cecropin A in Escherichia coli. Antimicrob. Agents Chemother. 44:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skerlavaj, B., D. Romeo, and R. Gennaro. 1990. Rapid membrane permeabilization and inhibition of vital functions of gram-negative bacteria by bactenecins. Infect. Immun. 58:3724-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taneva, S., D. R. Voelker, and K. M. Keough. 1997. Adsorption of pulmonary surfactant protein D to phospholipid monolayers at the air-water interface. Biochemistry 36:8173-8179. [DOI] [PubMed] [Google Scholar]
- 29.Travis, S. M., N. N. Anderson, W. R. Forsyth, C. Espiritu, B. D. Conway, E. P. Greenberg, P. B. McCray, Jr., R. I. Lehrer, M. J. Welsh, and B. F. Tack. 2000. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 68:2748-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xian, W., R. Vegners, P. A. Janmey, and W. H. Braunlin. 1995. Spectroscopic studies of a phosphoinositide-binding peptide from gelsolin: behavior in solutions of mixed solvent and anionic micelles. Biophys. J. 69:2695-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, D., O. Chertov, and J. J. Oppenheim. 2001. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol. Life Sci. 58:978-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanetti, M., R. Gennaro, and D. Romeo. 1997. The cathelicidin family of antimicrobial peptide precursors: a component of the oxygen-independent defense mechanisms of neutrophils. Ann. N. Y. Acad. Sci. 832:147-162. [DOI] [PubMed] [Google Scholar]
- 33.Zanetti, M., R. Gennaro, and D. Romeo. 1995. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1-5. [DOI] [PubMed] [Google Scholar]
- 34.Zanetti, M., R. Gennaro, M. Scocchi, and B. Skerlavaj. 2000. Structure and biology of cathelicidins. Adv. Exp. Med. Biol. 479:203-218. [DOI] [PubMed] [Google Scholar]
- 35.Zendzian-Piotrowska, M., R. Bucki, M. Gorska, and J. Gorski. 2000. Diabetes affects phospholipid content in the nuclei of the rat liver. Horm. Metab. Res. 32:386-389. [DOI] [PubMed] [Google Scholar]
- 36.Zhao, H., and P. K. Kinnunen. 2003. Modulation of the activity of secretory phospholipase A2 by antimicrobial peptides. Antimicrob. Agents Chemother. 47:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao, H., J. P. Mattila, J. M. Holopainen, and P. K. Kinnunen. 2001. Comparison of the membrane association of two antimicrobial peptides, magainin 2 and indolicidin. Biophys. J. 81:2979-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]