Abstract
In this cross-sectional study, mycobacteria specimens from 189 tuberculosis (TB) patients living in an urban area in Brazil were characterised from 2008-2010 using phenotypic and molecular speciation methods (pncA gene and oxyR pseudogene analysis). Of these samples, 174 isolates simultaneously grew on Löwenstein-Jensen (LJ) and Stonebrink (SB)-containing media and presented phenotypic and molecular profiles of Mycobacterium tuberculosis, whereas 12 had molecular profiles of M. tuberculosis based on the DNA analysis of formalin-fixed paraffin wax-embedded tissue samples (paraffin blocks). One patient produced two sputum isolates, the first of which simultaneously grew on LJ and SB media and presented phenotypic and molecular profiles of M. tuberculosis, and the second of which only grew on SB media and presented phenotypic profiles of Mycobacterium bovis. One patient provided a bronchial lavage isolate, which simultaneously grew on LJ and SB media and presented phenotypic and molecular profiles of M. tuberculosis, but had molecular profiles of M. bovis from paraffin block DNA analysis, and one sample had molecular profiles of M. tuberculosis and M. bovis identified from two distinct paraffin blocks. Moreover, we found a low prevalence (1.6%) of M. bovis among these isolates, which suggests that local health service procedures likely underestimate its real frequency and that it deserves more attention from public health officials.
Keywords: Mycobacterium bovis, Mycobacterium tuberculosis, zoonotic tuberculosis
Although most cases of human tuberculosis (TB) are caused by Mycobacterium tuberculosis, there are rising concerns over infection with Mycobacterium bovis. First, there have been outbreaks of multidrug-resistant (MDR) M. bovis strains among hospitalised patients with human immunodeficiency virus (HIV) (Samper et al. 1997) and these outbreaks highlight the risk associated with MDR M. bovis, especially in countries where animals with M. bovis and humans with HIV co-exist. Second, evidence for person-to-person transmission of M. bovis has been demonstrated (Evans et al. 2007) and, third, zoonotic TB has re-emerged at the United States of America-Mexico border among immigrants from regions where bovine TB is endemic (Dankner et al. 1993, Dankner & Davis 2000, LoBue et al. 2003, Rodwell et al. 2010).
A Mexican study found that a high proportion of human TB cases were due to M. bovis in an endemic area of bovine TB (Pérez-Guerrero et al. 2008) and another Mexican study identified, through microbiological methods, the presence of mycobacteria in sputum samples of human TB patients with chronic respiratory problems. In this previous study, each sample was cultured in both Löwenstein-Jensen (LJ) broth with glycerol and Stonebrink (SB) medium containing pyruvate and 19 of 50 samples tested positive by culture; of these, 13 (68.4%) were identified as M. tuberculosis, three (15.8%) as M. bovis and three (15.8%) tested positive for both M. tuberculosis and M. bovis (Ordoñez et al. 1999).
In Latin America, the estimated proportion of zoonotic TB that is caused by M. bovis accounts for 2% and 8% of pulmonary (PTB) and extrapulmonary (EPTB) TB cases, respectively (Cosivi et al. 1998, de Kantor et al. 2008). In Brazil, a more representative study found that the proportion of zoonotic cases due to M. bovis was estimated to be 3.5% of all TB cases (Corrêa & Corrêa 1974). Moreover, de Kantor et al. (2008) conducted a historical report of M. bovis diagnoses made at several reference laboratories in Brazil and showed that the Professor Hélio Fraga National Referral Centre, located in the city of Rio de Janeiro (RJ), identified only one case of TB due to M. bovis during a 20-year period (1987-2006). In addition, the Adolfo Lutz Institute, located in the city of São Paulo, tested approximately 355,000 EPTB cultures using pyruvate-containing medium and isolated only two M. bovis strains from 2001-2005. Furthermore, Rocha et al. (2011) and Silva et al. (2011) failed to identify any contribution of M. bovis to PTB cases in the Brazilian cities of Rio de Janeiro and Juiz de Fora.
The best estimate of the prevalence of zoonotic TB due to M. bovis in humans in Argentina, Brazil, Chile, Colombia, Costa Rica, Dominican Republic, Ecuador, Peru, Uruguay and Venezuela was published in 2008 (de Kantor et al. 2008). In Chile, Colombia, Costa Rica, the Dominican Republic, Peru and Uruguay, M. bovis was most likely never isolated from humans and only four of these countries reported bacteriologically confirmed cases of M. bovis infection in humans. Most of these cases were diagnosed in Argentina, where the mean prevalence of M. bovis cases in relation to those due to M. tuberculosis (2000-2006) ranged from 0.34-1%, according to region. In most of these 10 countries, the low coverage of M. bovis-appropriate culture methods, including pyruvate-containing media, likely contributes to the underestimation of this infection (de Kantor et al. 2008).
The differentiation between M. bovis and the M. tuberculosis complex (MTC) impacts TB treatment because M. bovis is intrinsically resistant to pyrazinamide (Konno et al. 1967). Moreover, M. bovis displays a dysgonic colony shape on conventional LJ medium and the inclusion of pyruvate-containing medium is useful for the detection of M. bovis in humans. Molecular biology constitutes another more recent tool for the detection of M. bovis in humans (Gutiérrez et al. 1999).
Sputum acid-fast bacilli (AFB) microscopy and histopathology are the standard criteria for TB diagnosis in Brazil, which may potentially overlook zoonotic TB cases. This potential has been demonstrated, as 5% of the herds and 0.85% of the cattle in the state of Minas Gerais (MG), Brazil (1999) were considered to represent tuberculin reactors according to the Brazilian Ministry of Agriculture, Livestock and Supply (MAPA 2006). Although no zoonotic TB cases have been reported by the Municipal Health Department of Juiz de Fora, these previous authors suspected that cases were overlooked because sputum AFB microscopy and histopathology served as the major criteria for TB diagnosis.
The main objective of this cross-sectional study was to determine the prevalence of M. bovis among patients at two referral centres in Juiz de Fora, a predominantly urban area of MG.
PATIENTS, MATERIALS AND METHODS
Study site and design - The patients were recruited from two TB referral centres between March 2008-February 2010. The first centre was located in a TB regional hospital, where direct microscopy for AFB and mycobacteria cultures for hospitalised patients was performed. The second centre was a local referral centre that performed direct microscopy for AFB and cultures in response to outpatient demands. These two centres treated 75% (n = 357) of all the TB cases (n = 476) reported in Juiz de Fora during the study period. The majority (98%) of the study population lived in urban settlements.
TB patients were selected using specific inclusion criteria. Subjects who did not provide clinical specimens, such as sputum, bronchoalveolar lavage fluid, pleural fluid, biopsies for histopathological or microbiological diagnosis or culture samples for mycobacteria characterisation were excluded from the study. Most of the subjects (TB patients) who were excluded consisted of children and adolescents (< 17 years old) and EPTB cases, as local physicians generally did not request the complementary TB diagnosis tests for these patients (direct microscopy for AFB or culture for mycobacteria). Finally, all TB patients with at least one MTC strain characterised by phenotypic and molecular speciation methods, including culture (n = 176) or molecular methods from DNA extracted from tissue samples (n = 13), were considered study subjects.
Ethical approval - Written informed consent was obtained and the study was approved by the Ethical Research Review Board of the Federal University of Juiz de Fora (protocol 819.125.2006) and the Hospital Foundation of Minas Gerais (FHEMIG) (protocol 52/08). No personal information was disclosed and the samples were coded for analysis.
Primary isolation of mycobacteria - The Brazilian Ministry of Health (MS 2005) currently considers culture methods imperative for the diagnosis of paucibacillary TB, EPTB, nontuberculous mycobacteria (NTM) and suspected TB in HIV/acquired immune deficiency syndrome patients.
In Juiz de Fora, clinical examinations, X-rays and sputum bacilloscopy were performed in all cases where PTB was suspected and histopathology was performed in all cases where EPTB was suspected. However, cultures for AFB were not performed for all paucibacillary, EPTB and sputum AFB-positive PTB cases. Additionally, bacterial isolation was performed using only LJ glycerol-containing medium without simultaneously using SB pyruvate-containing medium and the lack of pyruvate in conventional LJ medium can make primary isolation of M. bovis more difficult.
Changes in the procedures for TB diagnosis were implemented to detect zoonotic TB due to M. bovis. The samples were collected by clinical officers who were responsible for TB cases in the referral centres. All biological specimens (negative and positive for AFB) underwent decontamination using Darzins' method and the sediments were inoculated simultaneously in LJ and SB-containing media. Readings were taken at seven, 30, 45 and 60 days of incubation at 36.5ºC (Kent & Kubica 1985). Media samples that remained incubated for at least 60 days without any colonisation were considered negative for mycobacteria growth (Drobniewski et al. 2003). The primary isolation of mycobacteria was carried out at the João Penido Regional Hospital of the FHEMIG.
Phenotype-based speciation methods - Isolates that grew on both the conventional LJ and pyruvate-containing SB media were suggestive of M. tuberculosis, while those that grew exclusively on pyruvate-containing SB media were suggestive of M. bovis. All positive AFB cultures were subjected to additional phenotypic speciation methods. Standard biochemical tests, such as tests for catalase at both room temperature (RT) and at 68ºC; niacin, nitrate, pyrazinamidase and urease, as well as drug susceptibility testing to thiophen-2-carboxylic acid hydrazide (TCH) were performed to distinguish M. bovis strains from other MTC strains (Kent & Kubica 1985). These analyses were carried out at the National Agricultural Laboratory of the MAPA. M. tuberculosis usually presents positive results while M. bovis is negative to niacin, nitrate, pyrazinamidase and urease tests. Both strains present negative results to the catalase test at RT, as well as at 68ºC tests. M. tuberculosis strains are resistant to TCH, whereas the M. bovis strains are susceptible to TCH.
Molecular speciation methods - A replicate of all positive AFB cultures that grew on LJ or SB-containing media was separately heat-inactivated (Van Embden et al. 1993) and DNA extraction (Van Embden et al. 1993) was performed. A two-step approach was used to distinguish between M. bovis and other types in the MTC from inactivated cultures. The first step consisted of amplification of the pncA gene (Scorpio & Zhang 1996) and polymorphism detection by DNA restriction enzyme analysis (Eco065 I) (Barouni et al. 2004). The second step consisted of partial amplification (150 bp) of the oxyR pseudogene (Taylor et al. 1999) and polymorphism detection by DNA restriction enzyme analysis (Alu I) (Sreevatsan et al. 1996). This analysis was carried out at the Laboratory of Molecular Biology Applied to Mycobacteria at the Oswaldo Cruz Institute, Oswaldo Cruz Foundation (IOC-Fiocruz).
Local physicians did not request mycobacteria cultures on a routine basis. Rather, histopathology was performed on all suspected EPTB specimens. For this reason, an alternative to culture was needed to include this subgroup in the present study. Formalin-fixed and paraffin wax-embedded biopsy tissue samples from subjects who presented lesions suggestive of EPTB (chronic granulomatous inflammatory lesions with caseous necrosis) were subjected to DNA extraction and partial amplification (150 bp) of the oxyR pseudogene (Zink & Nerlich 2004) and restriction enzyme (Alu I) analysis (Sreevatsan et al. 1996) or DNA sequencing (Taylor et al. 1999) to identify MTC carriers. The sequence data were assembled and edited electronically with the SeqScape 2.6V program and were compared to published sequences of the oxyR pseudogene of M. bovis subsp. bovis AF2122/97 (GenBank accession BX248342.1) and M. tuberculosis H37Rv (GenBank accession BX842579.1). This analyses was carried out at the Laboratory of Molecular Biology Applied to Mycobacteria at the IOC-Fiocruz.
Epidemiological data collection - Verbal consent was obtained from each TB patient upon explanation of the study purpose and counselling for the laboratory tests. On presentation at the hospital, the patients were interviewed and the clinical forms were completed. The interview was conducted to collect information on a range of variables relating to clinical data, behavioural data, patterns of milk product consumption and occupation. Milk and cheese consumption was assessed according to the frequency of consumption and the type of food preparation (boiled, pasteurised or raw). BCG status was also recorded. Zoonotic exposure to M. bovis in humans was defined as unpasteurised milk and cheese consumption or occupations related to livestock and agro-food industries of animal origin.
Additional patient data were obtained from notification records at the Department of Epidemiology of the Municipal Health Department of Juiz de Fora.
Data processing and analysis - The data entry and database management were performed using Epi Info software. The potential differences between included and excluded subjects were assessed using chi-squared tests and the level of significance was set to 0.05. The proportions of each type of mycobacterial infection in the population were determined (Dean et al. 1994) and an overall description of all M. bovis infections was compiled.
RESULTS
A total of 476 TB and mycobacteriosis cases were reported in Juiz de Fora during the study period. Of these 476 cases, 119 (25%) were referred to health centres that were not included in this study and 357 (75%) were referred to the two health centres that were included in this study. Of the 357 cases of TB and mycobacteriosis, 168 were excluded because (i) the cultures were not performed (n = 2), (ii) the specimens could not be cultivated due to either technical problems (n = 46) or the absence of specimens (n = 61), (iii) biopsies were not available (n = 15), (iv) the culture was lost (n = 1) or the mycobacteria characterisation was inconclusive (n = 41) or (v) only Mycobacterium avium complex profiles (n = 2) were identified. After these cases were excluded, 189 cases remained for analysis.
Table I shows the descriptive characteristics of the included subjects and the comparison to TB cases that were reported in the county, but not included in the study. There were no significant differences (p > 0.05) in regards to the municipality of residence, current residence in a rural area or diagnosis of HIV between these two subpopulations of TB patients. However, gender, age, clinical TB form and educational level were significantly different (p < 0.05). Of the 189 cases, 168 (88.9%), 17 (9%) and four (2.1%) presented PTB, EPTB and PTB/EPTB clinical TB forms, respectively.
TABLE I. Descriptive characteristics of included subjects (n = 189) and their comparison (chi-square test) with tuberculosis (TB) cases non-included (n = 285), city of Juiz de Fora, state of Minas Gerais, Brazil, 2008-2010.
| Characteristics | Included n = 189 n (%) | Non-included n = 285 n (%) | p |
| Sex | |||
| Male | 140 (74) | 180 (63.2) | 0.01 |
| Female | 49 (26) | 105 (36.8) | |
| Age | |||
| < 17 | 0 (0) | 23 (8) | < 0.0001 |
| 17-38 | 89 (47.1) | 148 (52) | |
| > 38 | 100 (52.8) | 114 (40) | |
| Educational level | |||
| None-fourth step of elementary school | 62 (32.8) | 37 (13) | < 0.001 |
| Fifth-eighth step of elementary school | 53 (28) | 24 (8.5) | |
| Complete or incomplete high school | 25 (13.2) | 27 (9.4) | |
| Complete or incomplete undergraduate | 6 (3.2) | 19 (6.6) | |
| Unknown a | 43 (22.8) | 178 (62.5) | |
| Residence | |||
| Juiz de Fora | 167 (88.4) | 259 (90.9) | 0.23 |
| Other cities of Brazil | 22 (11.6) | 26 (9.1) | |
| Live in rural area at this moment | |||
| Yes | 4 (2.1) | 2 (0.7) | 0.17 b |
| No | 185 (97.9) | 283 (99.3) | |
| Clinical TB forms | |||
| Pulmonary | 168 (88.9) | 199 (69.8) | < 0.0001 |
| Extrapulmonary | 17 (9) | 81 (28.4) | |
| Pulmonary and extrapulmonary | 4 (2.1) | 5 (1.8) | |
| Serological diagnosis of HIV | |||
| Positive | 24 (12.7) | 26 (9.1) | 0.43 |
| Negative | 86 (45.5) | 103 (36.2) | |
| Unknown a | 79 (41.8) | 156 (54.7) |
a: not included in χ2 calculation; b: Fisher exact test; HIV: human immunodeficiency virus.
Table II presents the potential zoonotic exposures to M. bovis. Most of the included subjects (97.4%) lived in an urban area of Juiz de Fora, some were current consumers of unpasteurised milk, many were current (31.2%) or former (27%) consumers of unpasteurised cheeses and many of these subjects (34.4%) had a history of occupations related to livestock or agro-food industries of animal origin.
TABLE II. Possible zoonotic exposures to Mycobacterium bovis infections in study population (n = 189), city of Juiz de Fora, state of Minas Gerais, Brazil, March 2008-February 2010.
| Characteristics | n (%) |
| Reside in rural areas | |
| At this moment | 4 (2.6) |
| In the past | 54 (28.6) |
| Never lived in rural areas | 131 (69.3) |
| History of livestock or agro-food industry of animal origin | |
| Yes | 65 (34.4) |
| No | 67 (35.5) |
| Unknown | 57 (30.1) |
| History of unpasteurised cheese consumption | |
| Current consumers | 59 (31.2) |
| Ex-consumers | 51 (27) |
| Never consumed | 23 (12.1) |
| Unknown | 56 (29.7) |
| History of unpasteurised milk consumption | |
| Current consumers | 15 (7.9) |
| Ex-consumers | 72 (38.1) |
| Never consumed | 46 (24.3) |
| Unknown | 56 (29.7) |
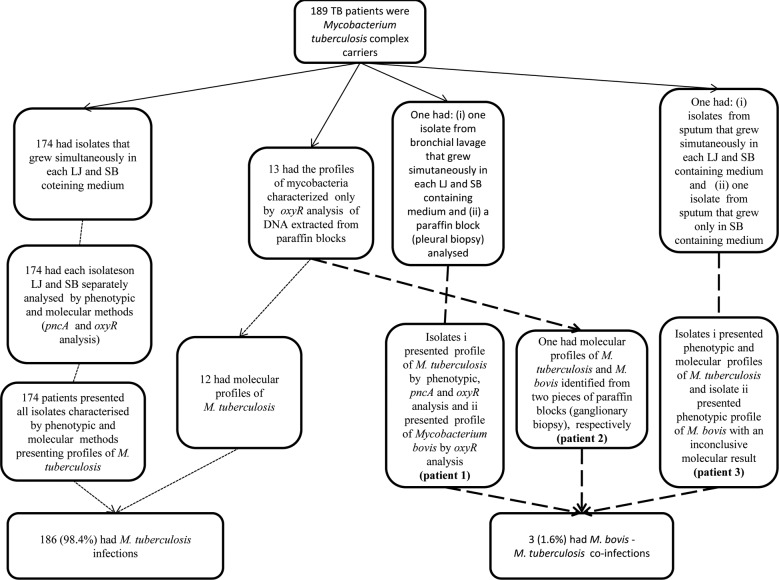
Results of mycobacteria characterization of specimens from study population (n = 189), city of Juiz de Fora, state of Minas Gerais, Brazil, 2008-2010. LJ: conventional Löwenstein-Jensen containing medium; oxyR, oxyR: pseudogene; pncA, pncA: gene; SB: Stonebrink containing medium; TB: tuberculosis.
The Figure shows the conventional and molecular results of the mycobacteria characterisation from specimens of TB patients (n = 189). Of the 189 TB patients, 186 exclusively presented M. tuberculosis profiles in all the clinical specimens analysed. Three patients presented profiles of both M. tuberculosis and M. bovis in different specimens (co-infections). Of these co-infected patients, the amplification and sequencing results of the oxyR pseudogene from DNA extracted from a pleural biopsy of patient 1 (PTB and EPTB) showed a profile of M. bovis, whereas M. tuberculosis was cultured and confirmed by phenotypic and molecular speciation methods from a bronchial lavage specimen. For patient 2 (EPTB), no isolation by culture was obtained, but the amplification and sequencing of the oxyR pseudogene of DNA extracted from two biopsy fragments revealed profiles of M. bovis and M. tuberculosis. For patient 3 (PTB), two isolates from two different sputum samples showed profiles of M. bovis and M. tuberculosis, which were confirmed by phenotypic and phenotypic/molecular methods, respectively.
Results of mycobacteria characterization of specimens from study population (n = 189), city of Juiz de Fora, state of Minas Gerais, Brazil, 2008-2010. LJ: conventional Löwenstein-Jensen containing medium; oxyR, oxyR: pseudogene; pncA, pncA: gene; SB: Stonebrink containing medium; TB: tuberculosis.
Among the three patients who had M. bovis co-infections, two cases were confirmed by molecular techniques involving the amplification and sequencing of the oxyR pseudogene from DNA extracted from biopsies. The re-sultant partial sequences of the oxyR pseudogene were more concordant with M. bovis subsp. bovis AF2122/97 than with M. tuberculosis H37Rv. Furthermore, these sequences displayed the molecular profiles of M. bovis (adenine instead of guanine at position 285 of the pseudogene). In addition, one PTB patient had an isolate that scantily grew only in SB medium and presented the phenotypic profile of M. bovis (Figure).
All the co-infected patients were HIV-negative, but they had received potential zoonotic exposure to M. bovis (unpasteurised cheese consumption or occupations concerning livestock and agro-food industries of animal origin) throughout their lifetimes (Table III). In addition, one (5.8%) of the 17 EPTB cases, one (25%) of the four EPTB/PTB cases and one (0.6%) of the 168 PTB cases presented profiles of M. bovis infection (see also clinical forms, Table I). With regard to the TB treatment outcomes, patient 1 had been cured, but developed recurrent TB one year after the end of treatment, patient 2 was cured and patient 3 died.
TABLE III. Comparative characteristics of three patients who were Mycobacterium bovis carriers, city of Juiz de Fora, state of Minas Gerais, Brazil, 2008-2010.
| Patients | Clinical tuberculosis forms | Age (years) | HIV | Occupation with livestock and agro industry | Unpasteurised cheese consumption | Unpasteurised milk consumption |
| 1 | Pulmonary and pleural | 20 | N | Never | Current | Never |
| 2 | Ganglionary | 50 | N | Never | Current | Never |
| 3 | Pulmonary | 33 | N | Goats and slaughterhouse | Ex-consumer | Never |
HIV: human immunodeficiency virus; N: negativ
DISCUSSION
We found evidence of M. bovis co-infection in three (1.6%) out of 189 patients and two of these cases were confirmed using molecular speciation methods.
The potential source of M. bovis infection for two of these TB patients was unpasteurised cheese, where zoonotic agents can remain viable and cause EPTB. For the PTB patient, the potential source of M. bovis infection was related to work with goats or slaughterhouses; goats can be carriers of M. bovis and slaughterhouses may contain contaminated aerosols that could cause PTB.
There are several potential explanations for the absence of pncA gene and oxyR pseudogene amplification from the isolate of the PTB patient with an M. bovis profile as detected using phenotypic methods. For instance, there may have been incomplete cell lysis and DNA release due to incorrect timing or temperatures during the mycobacteria DNA extraction or this procedure may have affected the integrity of the genetic material. In general, a variety of methods can be used for DNA isolation from different biological materials. However, the isolation of nucleic acids from mycobacteria is more difficult than from other microorganisms due to the thick peptidoglycan layer in the cell wall (Wards et al. 1995, Cornejo et al. 1998). In the present study, we were unable to amplify DNA on the first attempt for 13% of the isolates that had M. tuberculosis molecular profiles and the DNA extractions therefore had to be repeated. However, the sample diagnosed as M. bovis using phenotypic methods was of insufficient quantity for a new DNA extraction.
All of the subjects in this study with M. bovis infections were co-infected with M. tuberculosis. We classified these as co-infections because both species are human obliged pathogens. In Mexico, a previous study found a high proportion (15.7%) of M. bovis-M. tuberculosis co-infections and exclusive M. bovis infections (15.7%) in chronic cases of TB (Ordoñez et al. 1999) and these authors reinforced the importance of including mycobacteria cultures on both LJ and pyruvate-containing media and identifying species during routine TB diagnosis where bovine TB is endemic.
The proportion of M. bovis in this study (1.6%) was slightly lower than the 3.5% reported in a previous Brazilian study (Corrêa & Corrêa 1974), but higher than those reported in recent Brazilian studies that either could not find any profiles of M. bovis (Rocha et al. 2011, Silva et al. 2011) or found a much lower prevalence (de Kantor et al. 2008). These recent findings (Rocha et al. 2011, Silva et al. 2011), including our own data, suggests that the importance of M. bovis in the global burden of PTB in Brazil is marginal (Rocha et al. 2011).
However, the molecular profile of M. bovis, as revealed by pleural biopsy examination, was useful for the local physicians to redefine anti-TB therapy for the patient who experienced recurrent TB one year after the end of the first treatment. Moreover, the patient with PTB due to M. bovis developed chronic TB and died and these clinical and epidemiological findings strengthen the involvement of M. bovis, which is intrinsically resistant to pyrazinamide and therefore may be more involved in TB relapses or chronic infections (Konno et al. 1967, Ordoñez et al. 1999).
There are potential limitations to this study. For instance, some potential participants were excluded from the study because many sputum samples with positive bacilloscopy did not have culture performed due to a lack of compliance to request mycobacteria cultures on a routine basis or a failure to cultivate mycobacteria due to technical problems, whereas other excluded subjects (most EPTB patients) lacked either cultures or paraffin blocks. Of the remaining TB patients reported in Juiz de Fora who were not included in this study, a large proportion of them were either younger than 17 years of age (χ2 = 19.9; p < 0.0001) or had EPTB (χ2 = 26.1; p < 0.0001). This may have been due to a lack of compliance to request mycobacteria cultures on a routine basis. Moreover, the non-inclusion of children and the lower proportion of EPTB cases among the included subjects could have underestimated M. bovis infection because both groups are historically more susceptible to this zoonotic agent (Corrêa & Corrêa 1974). Finally, the present study only involved urban health centres in a predominantly urban population. These limitations are present in the majority of Brazilian studies (de Kantor et al. 2008, Rocha et al. 2011, Silva et al. 2011) and may result in the underestimation of the prevalence of M. bovis.
Perhaps because of similar limitations, only four of 10 Latin American countries (1970-2007) reported the occurrence of human TB due to M. bovis. In the same study, Argentina had the most reported M. bovis cases (there were 2 cases in Ecuador, 10 cases in Brazil and 1 case in Venezuela), which may be because this country conducts more representative studies and traditionally uses either egg-based pyruvate-containing SB or semisynthetic media to preferentially grow M. bovis. Taking into account the limitations cited above, these previous authors concluded that the negative data from other countries was likely the result of underestimation of the relative prevalence of M. bovis (de Kantor et al. 2008).
In conclusion, this study presented evidence of M. bovis infections in the urban setting in Brazil. However, further work to assess the prevalence of M. bovis infections in small cities and rural populations in Brazil is needed. A national-based survey of human TB due to M. bovis is also needed and this could be nested into the current national survey of MDR TB. Moreover, widespread use of pyruvate-containing media for the detection of mycobacteria should also be considered as a potential strategy to improve our current knowledge of the contribution of M. bovis to the Brazilian TB burden. Finally, the potential health hazards of unpasteurised milk consumption should be emphasised for regions in which bovine TB is still prevalent.
Acknowledgments
ACKNOWLEDGEMENTS
To the CITO, EBLEN, Cytopathology, Macro-regional and Ezequiel Dias Foundation, by kindly provide us the clinical specimens, and to Marcos Thadeu Rangel L'Hotellier, Mayumi Seito and Mércia Guadalupe Ramos, for assistance in the project.
Funding Statement
CNPq (410595/2006-3)/MCT/CNPq/MS-SCTIEDECIT 25/2006 MRS received a fellowship from the EMBRAPA
REFERENCES
- Barouni AS, Augusto CJ, Lopes MT, Zanini MS, Salas CE. A pncA polymorphism to differentiate between Mycobacterium bovis and Mycobacterium tuberculosis . Mol Cell Probes. 2004;18:167–170. doi: 10.1016/j.mcp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Cornejo BJ, Sahagun-Ruiz A, Suarez-Guemes F, Thornton CG, Ficht TA, Adams LG. Comparison of C-18-carboxypropylbetaine and glass bead DNA extraction methods for detection of Mycobacterium bovis in bovine milk samples and analysis of samples by PCR. Appl Environ Microbiol. 1998;64:3099–3101. doi: 10.1128/aem.64.8.3099-3101.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa CNM, Corrêa WM. Tuberculose humana por bacilo bovino em São Paulo, Brasil. Arq Inst Biol. 1974;41:131–134. [PubMed] [Google Scholar]
- Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, Cousins D, Robinson RA, Huchzermeyer HF, de Kantor I, Meslin FX. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis. 1998;4:59–70. doi: 10.3201/eid0401.980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankner WM, Davis CE. Mycobacterium bovis as a significant cause of tuberculosis in children residing along the United States-Mexico border in the Baja California region. Pediatrics. 2000;105: doi: 10.1542/peds.105.6.e79. [DOI] [PubMed] [Google Scholar]
- Dankner WM, Waecker NJ, Essey MA, Moser K, Thompson M, Davis CE. Medicine. Vol. 72. Baltimore: 1993. Mycobacterium bovis infections in San Diego: a clinicoepidemiologic study of 73 patients and a historical review of a forgotten pathogen; pp. 11–37. [PubMed] [Google Scholar]
- de Kantor IN, Ambroggi M, Poggi S, Morcillo N, da Silva Telles MA, Osório Ribeiro M, Garzón Torres MC, Llerena Polo C, Ribón W, García V, Kuffo D, Asencios L, Vásquez Campos LM, Rivas C, de Waard JH. Tuberculosis. Vol. 88. Edinb: 2008. Human Mycobacterium bovis infection in ten Latin American countries; pp. 358–365. [DOI] [PubMed] [Google Scholar]
- Dean AG, Dean JA, Coulombier D, Brendel KA, Smith DC, Burton AH, Dicker RC, Sullivan K, Fagan RF, Arner TG. Epi Info Version 6.01. A word processing, database and statistics program for epidemiology on microcomputers. Centers for Disease Control and Prevention; Atlanta, GA: 1994. [Google Scholar]
- Drobniewski F, Strutt M, Smith G, Magee J, Flanagan P. Audit of scope and culture techniques applied to samples for the diagnosis of Mycobacterium bovis by hospital laboratories in England and Wales. Epidemiol Infect. 2003;130:235–237. doi: 10.1017/s0950268802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JT, Smith EG, Banerjee A, Smith RMM, Dale J, Innes JA, Hunt D, Tweddell A, Wood A, Anderson C, Hewinson RG, Simth NH, Hawkey PM, Sonnenberg P. Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to-person transmission in the UK. Lancet. 2007;369:1270–1276. doi: 10.1016/S0140-6736(07)60598-4. [DOI] [PubMed] [Google Scholar]
- Gutiérrez MC, Galán JC, Blázquez J, Bouvet E, Vincent V. Molecular markers demonstrate that the first described multidrug-resistant Mycobacterium bovis outbreak was due to Mycobacterium tuberculosis . J Clin Microbiol. 1999;37:971–975. doi: 10.1128/jcm.37.4.971-975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent PT, Kubica GP 1985. Public health mycobacteriology: a guide for the level III laboratory. US Department of Health and Human Services/Public Health Service/Centers for Disease Control; Atlanta: 207 pp [Google Scholar]
- Konno K, Feldman FM, McDermott W. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis. 1967;95:461–469. doi: 10.1164/arrd.1967.95.3.461. [DOI] [PubMed] [Google Scholar]
- LoBue PA, Betacourt W, Peter C, Moser KS. Epidemiology of Mycobacterium bovis disease in San Diego county, 1994-2000. Int J Tuberc Lung Dis. 2003;7:180–185. [PubMed] [Google Scholar]
- MAPA - Ministério da Agricultura, Pecuária e Abastecimento Brasil . Programa Nacional de Controle e Erradicação da Brucelose e da Tuberculose Animal (PNCEBT) MAPA/SDA/DSA; Brasília: 2006. 188 pp [Google Scholar]
- MS - Ministério da Saúde Brasil . Manual de bacteriologia da tuberculose. 3rd ed. . Centro de Referência Professor Hélio Fraga/Secretaria de Vigilância em Saúde; Rio de Janeiro: 2005. 240 pp [Google Scholar]
- Ordoñez PT, Sauzo FM, Flores MAS, Casillas ICR. Aislamiento e identificación de Mycobacterium bovis a partir de muestras de expectoración de pacientes humanos com problemas respiratórios crônicos. Vet Mex. 1999;30:227–229. [Google Scholar]
- Pérez-Guerrero L, Milián-Suazo F, Arriga-Díaz C, Romero-Torres C, Escartín-Chávez M. Epidemiología molecular de las tuberculosis bovina y humana em uma zona endêmica de Querétaro, México. Salud Publica Mex. 2008;50:286–291. doi: 10.1590/s0036-36342008000400006. [DOI] [PubMed] [Google Scholar]
- Rocha A, Elias AR, Sobral LF, Soares DF, Santos AC, Marsico AG, Hacker MA, Caldas PC, Parente LC, Silva MR, Fonseca L, Suffys P, Boéchat N. Tuberculosis. Vol. 91. Edinb: 2011. Genotyping did not evidence any contribution of Mycobacterium bovis to human tuberculosis in Brazil; pp. 14–21. [DOI] [PubMed] [Google Scholar]
- Rodwell TC, Kapasi AJ, Moore M, Milian-Suazo F, Harris B, Guerrero LP, Moser K, Strathdee SA, Garfein RS. Tracing the origins of Mycobacterium bovis tuberculosis in humans in the USA to cattle in Mexico using spoligotyping. Int J Infect Dis. 2010;14:29–35. doi: 10.1016/j.ijid.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samper S, Martín C, Pinedo A, Rivero A, Blázquez J, Baquero F, van Soolingen D, van Embden J. Transmission between HIV-infected patients of multidrug-resistant tuberculosis caused by Mycobacterium bovis . AIDS. 1997;11:1237–1242. doi: 10.1097/00002030-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Scorpio A, Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med. 1996;2:635–636. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- Silva MR, Guimarães MDC, de Oliveira VM, Moreira AS, da Costa RR, Abi-Zaid KCF, Rocha AS, Suffys PN. Identification of Mycobacterium tuberculosis complex based on amplification and sequencing of the oxyR pseudogene from stored Ziehl-Neelsen-stained sputum smears in Brazil. Mem Inst Oswaldo Cruz. 2011;106:9–15. doi: 10.1590/s0074-02762011000100002. [DOI] [PubMed] [Google Scholar]
- Sreevatsan S, Escalante P, Pan X, Gillies DA 2nd, Siddiqui S, Khalaf CN, Kreiswirth BN, Bifani P, Adams LG, Ficht T, Perumaalla VS, Cave MD, van Embden JD, Musser JM. Identification of a polymorphic nucleotide in oxyR specific for Mycobacterium bovis . J Clin Microbiol. 1996;34:2007–2010. doi: 10.1128/jcm.34.8.2007-2010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GM, Goyal M, Legge AJ, Shaw RJ, Young D. Genotypic analysis of Mycobacterium tuberculosis from medieval human remains. Microbiology. 1999;145:899–904. doi: 10.1099/13500872-145-4-899. [DOI] [PubMed] [Google Scholar]
- Van Embden JDA, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendation for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wards BJ, Collins DM, de Lisle GW. Detection of Mycobacterium bovis in tissues by polymerase chain reaction. Vet Microbiol. 1995;43:227–240. doi: 10.1016/0378-1135(94)00096-f. [DOI] [PubMed] [Google Scholar]
- Zink AR, Nerlich AG. Molecular strain identification of the Mycobacterium tuberculosis complex in archival tissue samples. J Clin Pathol. 2004;57:1185–1192. doi: 10.1136/jcp.2003.015719. [DOI] [PMC free article] [PubMed] [Google Scholar]