Abstract
Background
Pulmonary tuberculosis (PTB) and paragonimiasis remain as health problems in certain areas in the Philippines. Both share similar clinical manifestations, which include chronic productive cough, hemoptysis, dyspnea, fever, weight loss, and night sweats. This study aimed to determine the prevalence of PTB, paragonimiasis, and co-infections in Zamboanga del Norte, Philippines.
Methods
This study was conducted in selected villages in two municipalities in Zamboanga del Norte. Patients with chronic cough were interviewed, examined, and requested to submit two sputum samples which were processed using Ziehl–Neelsen method to detect acid-fast bacilli (AFB), and NaOH concentration technique for the detection of Paragonimus ova.
Results
A total of 836 patients submitted sputum samples for examination. Prevalence was 6.7% (2.5–12.7%) for paragonimiasis and 1.9% (0.9–6.3%) for PTB. Co-infection rate was 0.3%, with two identified cases. Positivity rates for males and females were 9.6 and 5.8% for paragonimiasis and 3.4 and 1.2% for PTB.
Conclusion
Pulmonary tuberculosis and paragonimiasis are co-endemic in Zamboanga del Norte, suggesting the need to integrate surveillance and control efforts. Strengthening local health systems through collaboration between different sectors is recommended for effective disease control. Development of more sensitive diagnostic tests is important for more accurate disease surveillance.
Keywords: Pulmonary tuberculosis, Paragonimiasis, Integrated surveillance and control
Introduction
Pulmonary tuberculosis (PTB) and paragonimiasis remain as health problems in certain areas in the Philippines.1,2 The Philippines is one of the 22 countries heavily burdened by PTB. National health data indicate that PTB ranked as the eight leading cause of morbidity in the country.2 Paragonimiasis on the other hand, is caused by Paragonimus westermani, a foodborne trematode which is transmitted via ingestion of raw or inadequately cooked crabs. Socio-economic status and cultural practices influence its perpetuation. Identified endemic areas in the country include Mindoro, Camarines, Sorsogon, Samar, Leyte, Zamboanga del Norte, Davao, Davao Oriental, Cotabato, and Basilan.3,4 The total population at-risk of PTB and paragonimiasis in these co-endemic areas are approximately 11 million based on the 2010 Philippine Census.
Pulmonary tuberculosis and paragonimiasis share similar clinical manifestations, including chronic productive cough, hemoptysis, dyspnea, fever, weight loss, and night sweats. Often times, chest radiographs prove little or no definitive role in differentiating one disease from the other,5 resulting in misdiagnosis. In addition, PTB and paragonimiasis co-infections, which have not been well described in the past, in some instances may not be uncommon especially in areas where both are endemic.6 The cost of incorrect diagnosis may be significantly high since it results in the use of inappropriate diagnostic modalities and chemotherapy. It has been proposed that routine exclusion of paragonimiasis infection be carried out in areas where both diseases are endemic.3,4
This study aimed to determine the prevalence of PTB, paragonimiasis, and co-infection. Results of which can be used as bases for policy formulation to improve existing control efforts for PTB and paragonimiasis.
Materials and Methods
Study sites
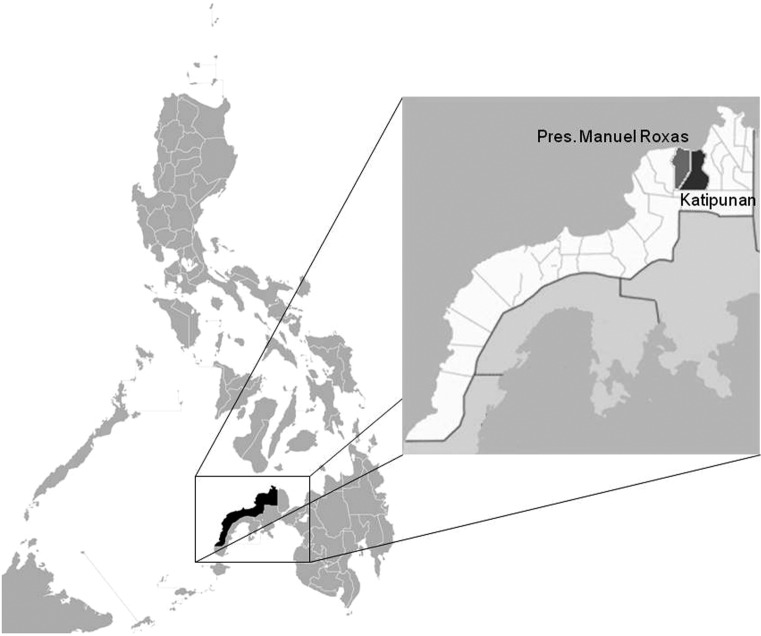
The study was conducted in selected villages from two municipalities in the province of Zamboanga del Norte, namely, Katipunan and Pres. Manuel Roxas (Fig. 1). The rural health unit (RHU) in Pres. Manuel Roxas reported that PTB ranked as the tenth leading cause of mortality in 2003.7 In Katipunan, PTB was reported in 37.9% of patients consulting the RHU in 2010.8 A study conducted by Belizario et al. (2007) reported a prevalence of paragonimiasis of 14.8% in Pres. Manuel Roxas.9
Figure 1.
Map of the Philippines showing the location of the study sites (modified map from http://commons.wikimedia.org/wiki).
Integrated surveillance was done in both municipalities in 2011, with a follow-up surveillance in Pres. Manuel Roxas in 2013.The selected villages in Katipunan included Matam, Miatan, and Nanginan. In Pres. Manuel Roxas, Marupay, Piñalan, Piñamar, and Sibatog were included in the study, with the addition of Pangulogon in the follow-up surveillance. Consultation with the local government units of the two municipalities, together with the Department of Health-Center for Health Development Region IX (DOH-CHD IX), the Provincial Health Team Office (PHTO), and the Provincial Health Office (PHO) of Zamboanga del Norte, was done as part of the selection of the villages. Selection of the study sites was based on the established endemicity of paragonimiasis, accessibility, peace and order situation, and willingness of the local government units to participate.
Study design and population
This study utilized a cross-sectional study design to determine the prevalence of PTB, paragonimiasis, and co-infection. Patients included in the study should have had complaints of chronic productive cough, with or without hemoptysis, for at least 2 weeks before consultation.
Data collection
Before the scheduled data collection, village health workers, who had been oriented on the study, distributed sputum collection containers to patients with chronic cough who were then instructed to bring early morning sputum samples at the designated area (e.g., Village Hall, Village Health Station) on the day of the consultation. Consent to participate in the study was requested by trained local health unit (LHU) staff from all patients before specimen collection, screening, and examination.
As part of data collection, members of the study team and trained staff from the LHU screened and interviewed the patients for the purpose of reviewing their history of illness. Pertinent data were recorded on case record forms (CRF). Patients were examined for abnormal chest findings by trained project physicians.
Diagnosis of PTB and paragonimiasis
Patients were asked to submit two sputum specimens (i.e., early morning and spot sample). The specimens were collected in a clean container and were brought to the designated area in the selected villages for immediate processing and examination by trained microscopists.
For the detection of Mycobacterium tuberculosis, two direct acid-fast bacilli (AFB) smears were prepared from each sputum sample. In accordance with the revised National TB Control Program (NTP) guidelines, the standard Ziehl–Neelsen method was used in staining the smear.10 The smears were then examined following the existing guidelines of the NTP for the diagnosis of tuberculosis by trained microscopists of the Philippine General Hospital-Infectious Disease Section (PGH-IDS) and PHO of Zamboanga del Norte.
For the detection of Paragonimus westermani eggs, the remaining sputum specimen was processed for clearing and concentration purposes using 3% sodium hydroxide (NaOH) and centrifuged at high-speed setting for 5 minutes. The resulting sediment was then examined. The field microscopists who performed the diagnostic procedure were properly trained before the data collection, and cross-checking between the microscopists was done to ensure quality of slide reading.
Quality control of AFB smears was performed by a senior microscopist of the PGH-IDS through blinded slide rechecking based on the NTP guidelines.10
Data processing and analysis
Data from the CRF (providing information on patient demographics, signs and symptoms, and physical examination findings) and laboratory result forms were double encoded using the prepared Microsoft Excel data forms. Verification of data entries with source documents was done to ensure accuracy of recorded data. Prevalence was computed using the following formulas:
Formula for computation of prevalence of PTB
 |
Formula for computation of prevalence of Paragonimus
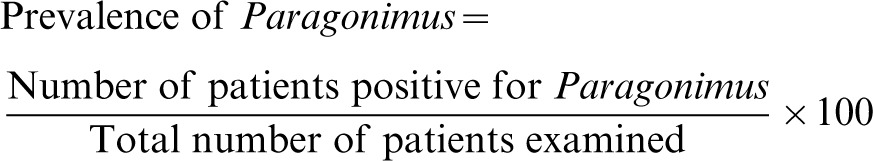 |
Formula for computation of prevalence of co-infection
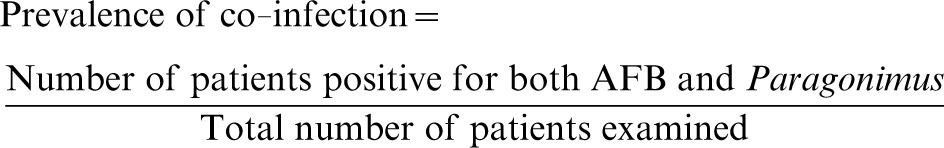 |
Detection rate was computed by dividing the total number of positive cases detected using a specific type sputum sample (i.e., early morning or spot) with the total number of actual positive cases identified based on positivity for either early morning or spot sample.
The chi-square test of association and distribution was used to determine significant difference between detection of Paragonimus eggs using early morning and spot sputum sample, as well as detection using one sample and two samples. Level of significance was set at P < 0.05.
Ethical considerations
The study protocol was reviewed and approved by the University of the Philippines Manila-Research Ethics Board (NIH 2009-023). Following established ethical guidelines, all patients were only included into the study after signing an informed consent. Health information collected were kept confidential. All patients diagnosed with TB were treated with anti-TB medications according to the national guidelines using the directly observed treatment shortcourse (DOTS) strategy. For patients diagnosed with paragonimiasis, praziquantel 25 mg/kg was given three times daily for 2 days. Drugs were dispensed through the RHUs or the PHTO. Adult patients and minor patients with their guardians were also provided with health education.
Results
A total of 836 patients from both municipalities submitted sputum samples for examination for AFB and Paragonimus, of which 231 and 605 were from Katipunan and Pres. Manuel Roxas, respectively. Among these patients, 474 were interviewed and corresponding CRF were completed. Out of the 836 patients, 56 patients were found positive for Paragonimus, giving a prevalence of 6.7%.
Out of the 56 patients found positive for Paragonimus, 18 were identified as positive after examination of both early morning sputum and spot samples. Twenty-seven were positive after examination of early morning sputum sample only, while 11 patients were positive for Paragonimus after examination of spot sputum sample only. These values suggest a significantly higher detection rate for Paragonimus ova using early morning sputum sample (80.4%) compared with spot sputum sample (51.8%) (P = 0.001). Furthermore, detection of Paragonimus was significantly higher with the examination of two samples (8.2%) compared with the examination of just one sample (4.7%) (P = 0.041) (Table 1).
Table 1. Number of patients examined and positivity for Paragonimus infection according to number and type of sputum samples Katipunan and Pres. Manuel Roxas, Zamboanga del Norte August–September 2011 and May 2013.
| Katipunan no. (%) | Pres. Manuel Roxas no. (%) | Total no. (%) | |
| No. of patients with sputum samples examined | 231 | 605 | 836 |
| Examined with case record form | 92 (39.8) | 382 (63.1) | 474 (56.7) |
| Examined for Paragonimus | 231 (100.0) | 605 (100.0) | 836 (100.0) |
| with two samples | 78 (33.8) | 395 (65.3) | 473 (56.6) |
| with single sample | 153 (66.2) | 210 (34.7) | 363 (43.4) |
| with early morning sample only | 54 (23.4) | 157 (26.0) | 211 (25.2) |
| with spot sample only | 99 (42.9) | 53 (8.8) | 152 (18.2) |
| Positive for Paragonimus | 15 (6.5) | 41 (6.8) | 56 (6.7) |
| with two samples | 7 (9.0) | 32 (8.1) | 39 (8.2) |
| positive in both samples | 4 (5.1) | 14 (3.5) | 18 (3.8) |
| positive in early morning sample only | 3 (3.8) | 11 (2.8) | 14 (3.0) |
| positive in spot sample only | 0 (0.0) | 7 (1.8) | 7 (1.5) |
| with single sample | 8 (5.2) | 9 (4.3) | 17 (4.7) |
| positive in early morning sample | 6 (11.1) | 7 (4.5) | 13 (6.2) |
| positive in spot sample | 2 (2.0) | 2 (3.8) | 4 (2.6) |
Examination of AFB smears revealed a prevalence of 1.9% as shown in Table 2. Pulmonary tuberculosis and paragonimiasis co-infection rate was 0.3%, with just two identified cases. The first case was an 82-year-old female in Piñamar, and the second case was a 41-year-old female in Pangulogon. The distribution of cases per barangay in each municipality is shown in Table 3.
Table 2. Number of patients examined and positivity for pulmonary tuberculosis (PTB) according to number and type of sputum samples Katipunan and Pres. Manuel Roxas, Zamboanga del Norte August–September 2011 and May 2013.
| Katipunan no. (%) | Pres. Manuel Roxas no. (%) | Total no. (%) | |
| No. of patients | 231 | 605 | 836 |
| Examined with case record form | 92 (39.8) | 382 (63.1) | 474 (56.7) |
| Examined for AFB | 231 (100.0) | 602(99.5) | 833 (99.6) |
| with two samples | 72 (31.2) | 367 (61.0) | 439 (52.7) |
| with single sample | 159 (68.8) | 235 (39.0) | 394 (47.3) |
| with early morning sample only | 39 (16.9) | 171 (28.4) | 210 (25.2) |
| with spot sample only | 120 (51.9) | 64 (10.6) | 184 (22.1) |
| Positive for AFB | 6 (2.6) | 10 (1.7) | 16 (1.9) |
| with two samples | 0 (0.0) | 6 (1.6) | 6(1.4) |
| positive in both samples | 0 (0.0) | 3 (0.8) | 3 (0.7) |
| positive in early morning sample only | 0 (0.0) | 2 (0.5) | 2 (0.5) |
| positive in spot sample only | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| with single sample | 5 (3.1) | 3 (1.3) | 8 (2.0) |
| positive in early morning sample | 1 (2.6) | 3 (1.8) | 4 (1.9) |
| positive in spot sample | 4 (3.3) | 0 (0.0) | 4 (2.2) |
AFB: acid-fast bacilli.
Table 3. Number of patients examined and positivity rate for Paragonimus and pulmonary tuberculosis (PTB) according to barangays Katipunan and Pres. Manuel Roxas, Zamboanga del Norte August 2011 and May 2013.
| Marupay no. (%) | Piñalan no. (%) | Piñamar* no. (%) | Sibatog no. (%) | Subtotal no. (%) | Matam no. (%) | Miatan no. (%) | Nanginan no. (%) | Others no. (%) | Subtotal no. (%) | Total no. (%) | |
| Number of patients with sputum samples examined | 134 | 130 | 186 | 155 | 605 | 33 | 79 | 112 | 7 | 231 | 836 |
| Examined, with case record form | 88 (65.7) | 83 (63.8) | 113 (60.8) | 98 (63.2) | 382 (63.1) | 29 (87.9) | 48 (60.8) | 15 (13.4) | 0 (0.0) | 92 (39.8) | 474 (56.7) |
| Positive for Paragonimus | 5 (3.7) | 6 (4.6) | 17 (9.1) | 13 (8.4) | 41 (6.8) | 0 (0.0) | 2 (2.5) | 11(9.8) | 2 (28.6) | 15 (6.5) | 56 (6.7) |
| Positive for AFB | 0 (0.0) | 4 (3.1) | 6 (3.2) | 0 (0.0) | 10 (1.7) | 0 (0.0) | 5 (6.3) | 1 (0.9) | 0 (0.0) | 6 (2.6) | 16 (1.9) |
| Positive for Paragonimus and AFB | 0 (0.0) | 0 (0.0) | 2 (1.1) | 0 (0.0) | 2 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.2) |
Includes Pangulogon in the follow-up surveillance
AFB: acid-fast bacilli
The mean ages of patients positive for Paragonimus and AFB were 49.7 years old and 44.1 years old, respectively. The youngest patient positive for paragonimiasis was 6 years old and the oldest was 82 years old. The mean ages of male and female Paragonimus patients were 50.1 years old and 50.6 years old, respectively. Paragonimus positivity rates between males (9.6%) and females (5.8%) were not significantly different (P = 0.05). On the other hand, the mean ages of male and female AFB positive patients were 39.3 years old and 55.5 years old, respectively. The ages of AFB positive patients ranged from 8 years old to 82 years old. On the other hand, AFB positivity rate among males and females were 3.4 and 1.2%, respectively.
Among the study participants who submitted sputum samples, 474 patients were interviewed and underwent physical examination. In terms of occupation, positivity for Paragonimus and PTB were highest among farmers (66.7% for both diseases). The most frequent chief complaint of patients positive for Paragonimus and AFB was productive cough (77.4 and 82.1%, respectively). Back pain was also reported by 12.8% of paragonimiasis patients and 8.3% of AFB positive patients. Based on the symptoms reported on review of systems, all patients positive for paragonimiasis and AFB experienced productive cough, while 82.1% of the patients with paragonimiasis and 66.7% of AFB positive patients had back pain. Chest pain, hemoptysis, and difficulty of breathing were also notably high among Paragonimus and AFB positive patients (Table 4). Patients were also asked regarding past history of eating raw freshwater crab and/or crayfish. Results showed that only 74.4% of the paragonimiasis patients reported eating raw freshwater crabs. Chest findings among the patients examined by physicians showed that 51.3% of the paragonimiasis patients had significant chest findings, of which decreased breath sounds (25.6%) was the most common finding (Table 5).
Table 4. Frequency of symptoms reported by patients examined for Paragonimus infection and pulmonary tuberculosis (PTB) Katipunan and Pres. Manuel Roxas, Zamboanga del Norte August–September 2011 and May 2013.
| Symptoms | Total examined no. (%) n = 474 | Paragonimus positive no. (%) n = 39 | AFB positive no. (%) n = 12 |
| Productive cough | 442 (93.2) | 39 (100.0) | 12 (100.0) |
| Back Pain | 329 (69.4) | 32 (82.1) | 8 (66.7) |
| Chest Pain | 270 (57.2) | 18 (46.2) | 6 (50.0) |
| Hemoptysis | 129 (27.2) | 29 (74.4) | 2 (16.7) |
| Difficulty of breathing | 213 (44.9) | 16 (41.0) | 3 (25.0) |
| Weight loss | 152 (32.1) | 8 (20.5) | 4 (33.3) |
| Anorexia | 119 (25.1) | 9 (23.1) | 4 (33.3) |
| Fever | 105 (22.2) | 6 (15.4) | 2 (16.7) |
AFB: acid-fast bacilli.
Table 5. Chest findings among examined patients for Paragonimus infection and pulmonary tuberculosis (PTB) Katipunan and Pres. Manuel Roxas, Zamboanga del Norte August–September 2011 and May 2013.
| Chest findings | Total examined no. (%) n = 474 | Paragonimus positive no. (%) n = 39 | AFB positive no. (%) n = 12 |
| No significant chest findings | 267 (56.3) | 20 (51.3) | 9 (75.0) |
| Abnormal chest findings | 207 (43.7) | 19 (48.7) | 3 (25.0) |
| Chest Asymmetry | 1 (0.2) | 0 (0.0) | 1 (8.3) |
| Decreased breath sounds | 113 (23.8) | 10 (25.6) | 0 (0.0) |
| Decreased chest expansion | 10 (2.1) | 4 (10.3) | 0 (0.0) |
| Increased vocal fremitus | 22 (4.6) | 3 (7.7) | 0 (0.0) |
| Wheezing | 30 (6.3) | 3 (7.7) | 1 (8.3) |
| Rales | 58 (12.2) | 1 (2.6) | 1 (8.3) |
| Rhonchi | 16 (3.4) | 1 (2.6) | 0 (0.0) |
| Decreased vocal fremitus | 16 (3.4) | 1 (2.6) | 0 (0.0) |
| Dullness | 4 (0.8) | 0 (0.0) | 0 (0.0) |
AFB: acid-fast bacilli.
Discussion
In this study, patients with chronic productive cough, with or without hemoptysis, were examined for both PTB and paragonimiasis using two sputum specimens instead of three specimens in line with the World Health Organization (WHO) recommendation.11 A systematic review on the incremental increase in the sensitivity of direct sputum microscopy with increasing number of sputum samples revealed that a second sample increased the sensitivity of AFB diagnosis by 11.9%, while a third only increased the sensitivity by 3.1%.12 In a multi-country study comparing the sensitivity of the use of two versus three sputum samples in direct sputum microscopy, with AFB culture as the gold standard, two sputum samples collected on the first day showed a sensitivity of 63.6%, which was not markedly different compared with the sensitivity of using three sputum samples at 65.9%.13 According to the policy statement of the WHO on the same-day diagnosis of tuberculosis by microscopy, diagnosis of PTB using two samples submitted by a patient with chronic cough on the same day has been shown to be equally accurate as the conventional scheme, which utilizes three sputum samples collected over a period of 2 days.14 The same-day diagnosis contributes to better patient compliance and reduced cost of diagnosis. In countries like the Philippines where the three-specimen case-finding strategy is still being utilized,10 the WHO recommends ‘a gradual change to same-day diagnosis, once WHO-recommended external microscopy quality assurance systems are in place and good quality microscopy results have been documented’.14
The WHO recommends examination of sputum specimen for Paragonimus ova in patients suspected of tuberculosis in all endemic areas.15 For PTB, although the WHO recommends diagnosis using two spot samples,14 patients in this study were required to submit one early morning sputum sample and one spot sample for greater detection rate in terms of diagnosis for paragonimiasis. Comparing the detection rates of the use of early morning versus spot sample in detecting Paragonimus ova, examination of early morning sample yielded significantly a higher rate (80.4%) in the diagnosis of paragonimiasis compared with the examination of spot sample (51.8%). Thus, it is recommended that in areas endemic for Paragonimus, in line with the integration of diagnosis and surveillance for the two diseases, patients with chronic cough should be examined using one early morning and one spot sample. This will allow greater sensitivity for the diagnosis of lung fluke infection without compromising the accuracy of the TB diagnosis.
The WHO recognizes the need for integration of programs for the control and prevention of foodborne trematodes with other existing public health programs to allow more efficient and cost-effective means of service delivery.16 While programs for the control of tuberculosis are in place, measures for the control and prevention of paragonimiasis are limited, especially in poor endemic countries.17 In the Philippines, the NTP program has been in existence since the 1950s and is constantly being improved and updated ever since. For the proper case finding and treatment of PTB patients, the NTP has provided guidelines that serve as the basis for capacity building activities for local government units.10 On the other hand, a recent administrative order (AO) on paragonimiasis released in 2010 enumerated the clinical presentation of the disease, diagnostic modalities, treatment, and roles and responsibilities of national and local public health workers.18 However, neither the NTP Manual of Procedures, nor the AO on paragonimiasis had clear guidelines that promote integration of the program strategies in co-endemic areas in the country.
Proper integration requires identification of cost-effective approaches, as well as appropriate delivery systems.19 Surveillance for paragonimiasis may well be integrated with case finding for PTB.16,20 Case finding of patients with paragonimiasis can easily be incorporated in the existing scheme of the NTP of the DOH. In this study, the inclusion criteria used for screening patients was chronic productive cough for 2 weeks or more. However, there may also be a need to consider lowering the criterion to at least 1 week since PTB and paragonimiasis can also be detected within this time period as demonstrated in a study by Devi et al.21 Furthermore, case-holding and treatment of patients with paragonimiasis can also be integrated with the treatment schedule and follow-up of PTB cases. National policy to strengthen the integration of the Paragonimus control program into the NTP is highly recommended.
Knowledge on the symptoms of lung fluke infection is important. A case of pleuropulmonary paragonimiasis was reported in a soldier in Eastern India who presented with symptoms similar to PTB, namely: chest pain, hemoptysis, and eosinophilia.22 In addition, a community survey done by community leaders in Lao PDR was conducted through the use of a questionnaire that inquired about patient symptoms (i.e., chronic cough lasting longer than 3 weeks and/or with a sputum with blood/red brownish color, without nocturnal fever) to identify suspected community members with paragonimiasis and PTB. Sputum samples from 129 suspected cases were examined; seven (5.4%) were found positive for Paragonimus, and one (0.8%) was positive for AFB.23
Capacity building of LHUs is one of the main components of the NTP, and is part of the recommendations of the AO on paragonimiasis.10,18 This, in turn, will translate to early and appropriate disease management, as well as early morbidity control. Apart from history of consuming raw or poorly cooked crabs, common symptoms elicited from patients included in this study (Table 4), as well as physical examination findings observed among infected patients (Table 5) may be used by health workers in identifying patients with chronic cough who should be examined for paragonimiasis.
The willingness of different sectors is important in the successful integration of prevention and control measures.16 Advocacy to local government units is important to obtain support for the provision of manpower and facilities for the control program. Optimizing the existing local health infrastructure can contribute toward effective integration of disease control measures. Collaboration between the government, health, education, and agricultural sectors is also recommended for the effective control of foodborne trematodes.24 The WHO also recommends regular monitoring and reporting of cases of paragonimiasis in areas where they are suspected to occur as part of disease surveillance.15 Surveillance may be done through passive case finding, or through active case finding if the health unit has the necessary human resources and logistics. There may also be a need for the development and testing of a more sensitive diagnostic technique that can be used in the field for more accurate disease surveillance. Disease mapping will also be important, especially in resource-poor settings like the Philippines, in order to pinpoint areas that need to be prioritized.
In summary, this study reported the prevalence of PTB and paragonimiasis among patients with chronic cough in the municipalities of Katipunan and Pres. Manuel Roxas in the Province of Zamboanga del Norte. In co-endemic areas, the WHO recommends integration of control programs for foodborne trematode infections with existing public health programs for more cost-effective implementation of control strategies.16 In integrating tuberculosis and paragonimiasis case finding and diagnosis, same-day diagnosis is proposed to be utilized. This follows the scheme recommended by the WHO for diagnosis of PTB, which entails examination of two sputum samples in the same day. Same-day diagnosis for PTB as recommended by the WHO refers to diagnosis using two spot samples collected on the same day. Same-day diagnosis of PTB and paragonimiasis as proposed by this study, on the other hand, refers to diagnosis using one early morning and one spot sputum sample collected on the same day. Collection of both early morning and spot sputum sample on the same day is therefore recommended to ensure greater sensitivity for the diagnosis of Paragonimus infection. Disease mapping is also recommended in order to identify endemic barangays where integrated control and prevention efforts should be focused. An integrated implementation strategy, coupled with active collaboration of local government is recommended for an effective integrated surveillance system for PTB and paragonimiasis.
Acknowledgments
The authors wish to extend their warmest gratitude to the Department of Health-Center for Health Development Region IX, Zamboanga del Norte PHTO, and the Local Government Units of Katipunan and Pres. Manuel Roxas for their assistance and collaboration, and to the Department of Science and Technology – Philippine Council for Health Research and Development for providing financial support for the conduct of this study.
References
- 1.Belizario VY, de Leon WU, Bugayong MPG, de Guzman AD, Valderama MTG. An assessment of re-infection rates and treatment outcomes of patients with pulmonary paragonimiasis. Natl Res Counc Philipp Res J. 2006;8/9:10–9. [Google Scholar]
- 2.Department of Health (DOH) Field health services information system. 2009. Annual Report. Manila, Philippines: Department of Health. [Google Scholar]
- 3.Belizario VY, Guan M, Borja L, Ortega AR, Tiri R. Pulmonary paragonimiasis in non-responding tuberculosis patients in Irosin, Sorsogon. Phil J Microbiol Infect Dis. 1997;26(1):13–5. [Google Scholar]
- 4.Toscano C, Hai YS, Nunn P, Mott KE. Paragonimiasis and tuberculosis – diagnostic confusion: a review of the literature. Trop Dis Bull. 1995;92:R1–27. [Google Scholar]
- 5.Singh TN, Kananbala S, Devi KD. Pleuropulmonary paragonimiasis mimicking pulmonary tuberculosis – a report of three cases. Indian J Med Microbiol. 2005;23:131–4. doi: 10.4103/0255-0857.16056. [DOI] [PubMed] [Google Scholar]
- 6.Belizario VY, Ortega AR, Guan M, Leonardia W. Pulmonary paragonimiasis and tuberculosis in Sorsogon. Proceedings of the 2nd Seminar on Food-borne Parasitic Zoonoses: Current Problems, Epidemiology, Food Safety and Control. Supplement to the Southeast Asian J Trop Med Public Health, Vol. 63. 1997; p. 241–56. [Google Scholar]
- 7.Rural Health Unit of Pres. Manuel Roxas. Municipal Health Office Report 2000-2004. Unpublished report. [Google Scholar]
- 8.Rural Health Unit of Katipunan. 2010. Personal Communication on number of reported PTB cases. [Google Scholar]
- 9.Belizario VY, Mallari AO, de Leon WU, Lucero AC. Assessment of the efficacy, safety, and tolerability of praziquantel and triclabendazole in the treatment of paragonimiasis. Southeast Asian J Trop Med Public Health. 2007;38(Suppl 1):1–9. [Google Scholar]
- 10.Department of Health (DOH) The National Tuberculosis Control Program (NTP) Manual of Procedures (MOP) Manila, Philippines: Department of Health; 2005. [Google Scholar]
- 11.World Health Organization (WHO) Policy on reduction of number of smears for the diagnosis of pulmonary TB. 2007. Geneva: World Health Organization. [Google Scholar]
- 12.Mase SR, Ramsay A, Ng V, Henry M, Hopewell PC, Cunningham J, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2007;11(5):485–95. [PubMed] [Google Scholar]
- 13.Cuevas LE, Yassin MA, Al-Sonboli N, Lawson L, Arbide I, Al-Aghbari N, et al. A multi-country non-inferiority cluster randomized trial of frontloaded smear microscopy for the diagnosis of pulmonary tuberculosis. PLoS Med. 2007;8(7):e1000443. doi: 10.1371/journal.pmed.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) Same-day diagnosis of tuberculosis by microscopy: policy statement. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) Report of the WHO expert consultation on foodborne trematode infections and taeniasis/cysticercosis. Geneva: World Health Organization; 2011. [Google Scholar]
- 16.World Health Organization (WHO) Joint WHO/FAO Workshop on Foodborne Trematode Infections in Asia. World Health Organization Regional Office for the Western Pacific. 2002. Report Series Number. [Google Scholar]
- 17.Furst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet. 2011;12(3):210–21. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- 18.Department of Health (DOH) Administrative order No.2010-0037 ‘diagnosis and treatment guidelines for paragonimiasis’; 2010. [Google Scholar]
- 19.Lammie PJ, Fenwick A, Utzinger J. A blueprint for success: integration of neglected tropical disease control programmes. Trends Parasitol. 2006;22(7):313–21. doi: 10.1016/j.pt.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Belizario VY, de Leon WU, Solon JA, Marquez AI, Galang JM, Valderama MTG. The clinical epidemiology of pulmonary paragonimiasis and tuberculosis in Sorsogon, Philippines. Part II. An integrated tuberculosis-paragonimiasis surveillance program in two municipalities in Sorsogon, Philippines. NRCP Res J. 1998;5:65–85. [Google Scholar]
- 21.Devi KR, Narain K, Mahanta J, Deori R, Lego K, Goswami D, et al. Active detection of tuberculosis and paragonimiasis in the remote areas in North-Eastern India using cough as a simple indicator. Pathog Glob Health. 2013;107(3):153–6. doi: 10.1179/2047773213Y.0000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lall M, Sahni AK, Rajput AK. Pleuropulmonary paragonimiasis: mimicker of tuberculosis. Pathog Glob Health</emph>. 2013;107(1):40–2. doi: 10.1179/2047773212Y.0000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odermatt P, Veasna D, Zhang W, Vannavong N, Phrommala S, Habe S, et al. Rapid identification of paragonimiasis foci by lay informants in Lao People’s Democratic Republic. PLoS Negl Trop Dis. 2009;3(9):e521. doi: 10.1371/journal.pntd.0000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keiser J, Utzinger J. Food-borne trematodiases. Clin Microbiol Rev. 2009;22(3):466–83. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]