Abstract
Background
The primary objective of this study was to find the performance of the 2009 probable case definition of dengue and compare it with the definition given by the WHO-SEAR expert group in 2011.
Methods
A cross-sectional study was conducted in Thiruvananthapuram district of Kerala, which is hyperendemic for dengue. A consecutive series of 851 participants defined by the selection criteria were recruited from the primary, secondary, and tertiary health care settings. Sensitivity, specificity, predictive values, and likelihood ratios of the clinical case definitions were calculated using reverse transcriptase-polymerized chain reaction (RT-PCR) as gold standard in case of fever less than or equal to 5 days and serology (IgM positivity) for fever >5 days. Diagnostic odds ratio (DOR) was also calculated as a single indicator of performance of the case definition.
Results
The 2009 World Health Organization (WHO) case definition had a sensitivity of 76.4% (69.6–82.1) and negative predictive value of 87.5%. The 2011 WHO-SEAR expert group case definition had a higher sensitivity of 87.9% (82.2–91.9) but lower negative predictive value of 86.6%. The three independent criteria which were significantly associated with dengue were thrombocytopenia less than 150 000 (OR 2.80), leukopenia (OR 2.28), and absence of backache (OR 2.68). The performance of 2009 case definition was better (DOR 2.4) than the 2011 WHO-SEAR expert group case definition. This was further enhanced when thrombocytopenia was specified as platelet count less than 150 000 (DOR2.7). When ‘no backahe’ was added as an additional criteria, the performance of both definitions improved.
Conclusions
The 2009 WHO case definition has better discriminatory power than the 2011 WHO-SEAR expert group case definition. The performance of 2009 WHO case definition is enhanced by specifying thrombocytopenia as platelet count less than 150 000. The inclusion of ‘no backache’ further improves the discriminatory power. This may be more useful in primary care settings, to rule out dengue.
Keywords: Dengue, Case definition, WHO, SEAR, Diagnostic odds ratio
Introduction
Dengue is one of the most serious and fast emerging tropical mosquito-borne disease and its burden is 465 000 DALYs across the globe.1 India is heading to transform into a country that is highly endemic for dengue infection.2 In 2003, Kerala reported the maximum number of deaths due to dengue in India. Over the years, the reported cases of dengue have been increasing.3 Kerala is now hyperendemic for dengue with presence of multiple serotypes, high rates of coinfection, and local genomic evolution of viral strains.4 Thiruvananthapuram district reports maximum number of cases in the state.3
Case definitions have been recognized to be important elements of public health surveillance systems.5 They assure comparability and consistency of surveillance data. The World Health Organization (WHO) has been encouraging the use of case definitions to make surveillance data comparable between countries.6
Despite the widespread recognition of the usefulness of the 1997 classification, difficulties in documenting all of the clinical manifestations required to define severe cases of dengue has resulted in alternative designations of certain clinical presentations seen in dengue, such as ‘Dengue with Signs Associated with Shock’ (DSAS)7 and ‘Dengue with Severe Bleeding’ (DFB).8 As a result of this situation, Bandyopadhyay9 proposed the creation of a multicentric prospective study, and from it emerged a revised proposal for dengue classification in 2009.10,11 The validation studies of the 2009 classification system, did not include the testing of performance of probable case definition.12 Hadinegoro13 found difficulties in the application of revised classification in Indonesia and suggested that elements from the revised classification (2009) should be incorporated into the 1997 guidelines.14 It has also been recommended that there should be separate guidelines for dengue case management of children and adults.15 In view of these limitations, the expert group of WHO-SEAR expert group reinforced the dengue classification of Dengue Fever/ Dengue Haemorrhagic Fever/ Dengue Shock Syndrome (DF/DHF/DSS) which was developed in Bangkok in the early 1970s.16 A comparative analysis of the 2009 WHO case definition and 2011 WHO-SEAR expert group case definition is given by Dash,17 but it was not a study.
There is a confusion in the literature about the term ‘dengue case definition’ as distinct from ‘case classification’. Case definition and case classification serve different purposes. Typically, a case definition is used for discovery, epidemiologic, or diagnostic purposes, usually in the absence of confirmatory laboratory tests, but case classification separates patients into different disease categories based on predefined criteria.18 The revised dengue case classification into different levels of severity (WHO 2009) has shown its superiority compared to the DF/DHF/DSS classification in a 18 country study12 and other analyses.19 In contrast this paper focuses at the dengue case definition and the question of the clinical diagnosis of dengue and/or the use of the probable case definition for surveillance without laboratory support. We tried to compare the performance of the 2009 WHO definition of ‘probable dengue cases’ and the WHO-SEAR expert group definition, 2011 for this purpose.
Methods
Setting and study population
A cross-sectional study was conducted in the outpatient (OP) departments and casualty of primary, secondary, and tertiary health care institutions of Thiruvananthapuram. Patients more than 5 years, with acute febrile illness of 2–7 days duration without a definitive diagnosis/definite focus of infection were recruited. Those patients who were not willing to give an informed consent were excluded. Patients with fever, already diagnosed as dengue and presenting to the setting for review were also excluded.
Participant sampling
Sample size was estimated by initially estimating number of cases using the formula 4pq/l2 taking p as sensitivity (taken as 71%),20 ‘q’ as 100−p (29) and ‘l’ as precision (5%). The figure thus obtained as 329 was divided by a prevalence of 0.3921 to estimate dengue among acute febrile illness. The required sample size was estimated to be 843. Then based on the average daily OP in the study settings, the number days of work was estimated. During these days, a consecutive series of 939 participants defined by the eligibility criteria were recruited. In total, 851 such individuals who gave informed consent from the various settings were selected. Of these, 314 cases of fever were taken from the primary setting, 217 from secondary, and 320 from tertiary settings. Primary health care settings were the primary health centers. Secondary settings included the community health centers and taluk hospitals. Tertiary setting was the district hospital.
Data collection
Clearance of the institutional research committee (IRC/SBMR/1/2011/MCT/2) and the institutional ethical committee (IEC No: 06/6/2012/MCT) were obtained. Permission was obtained from heads of institutions concerned. Informed written consent was taken from all study participants. Orientation was given to the heads of institutions included in the study. Training of the personnel involved in data collection was also given. Baseline socio-demographic variables, clinical symptoms and signs included in the WHO case definition, were collected using a questionnaire. The symptoms experienced at the time of enrollment or at any time before enrollment were considered, regardless of whether or not they were present at the time of enrollment. Blood samples for the required investigations were also collected. Blood investigations done were total count (TC), differential count, platelet count, packed cell volume, reverse transcriptase-polymerized chain reaction (RT-PCR), and serology (IgM and IgG). Reverse transcriptase-polymerized chain reaction was used as the gold standard, against which the diagnostic properties of the probable case definition was evaluated in patients with fever of less than or equal to 5 days. IgM ELISA was used as confirmatory test when the patients had fever of more than 5 days. In cases where the duration of fever was not reliably obtained, both tests were performed. The phone numbers of the patients and concerned medical officers were noted in the proforma and the results of the investigations were communicated to them, as early as possible. The procedure for blood collection and the techniques followed for RT-PCR and serology are detailed in a previous publication.20
Statistical methods
Analysis was done in SPSS version 11. Sensitivity, specificity, predictive values, and likelihood ratios positive and negative (LR+ and LR−) of the case definitions were calculated. Diagnostic odds ratio (DOR) was also calculated as a single indicator of performance of the case definition.22 Different combinations of symptoms and negative symptoms were analyzed separately and compared with the existing case definition. Receiver operator characteristic (ROC) curve was plotted using the score calculated by giving a score of one to each of the criteria in the case definition.
Results
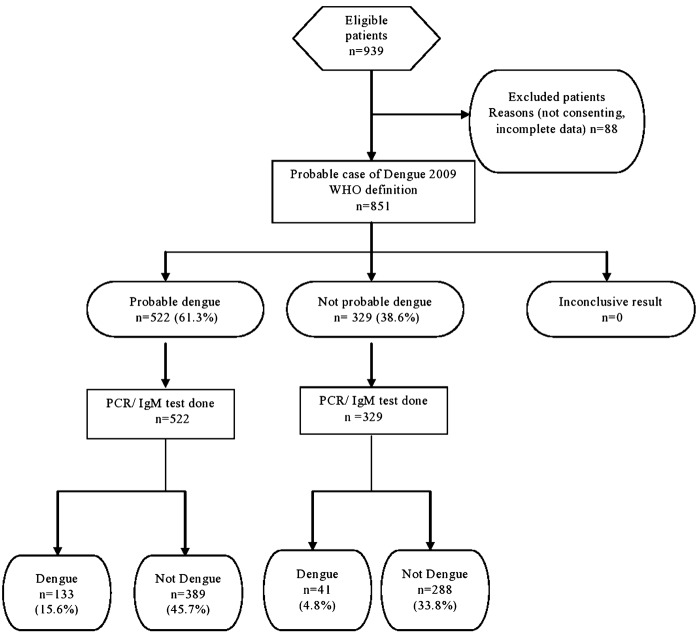
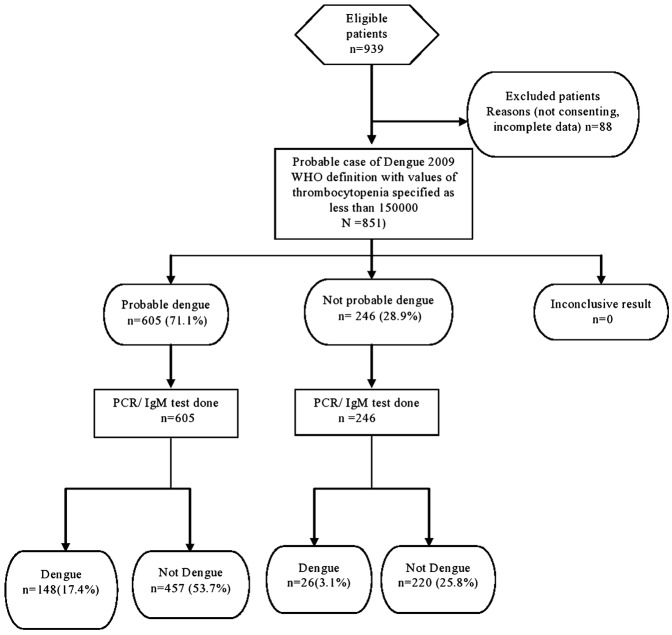
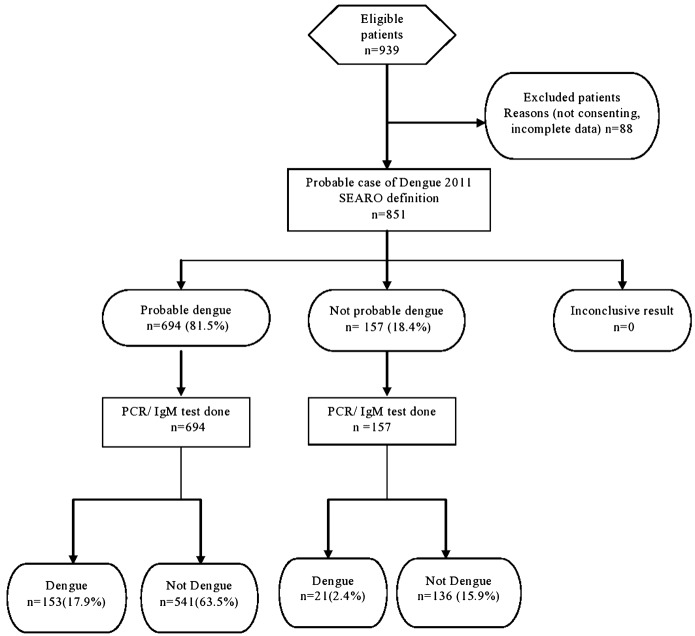
Among the 851 cases of acute febrile illness, 53.1% were males and 46.9% were females (Table 1). Sixty one percentage (518/851) of the study population were educated till secondary/higher secondary school. Those from the below poverty line formed 46.9% (399/851) of the study population. Acute febrile illness in this population was most commonly seen in adolescence 10–19 years (24.6%). The mean age was 30.9 years (SD 16.9). Among the 851 cases 174 cases of acute febrile illness (20.4%) [95% CI 17.8–23.3] were positive for dengue (Fig. 1). Dengue positivity was almost similar in all age groups with the highest prevalence (23.7%) in the age group of 40–49 years. A higher proportion of females (21.05%) with acute febrile illness had dengue compared to males (19.9%). These differences were however not statistically significant. The 2009 probable case definition by the WHO was satisfied by 522/851 (61.3%) of patients whereas 81.5% (694/851) were probable dengue, by the 2011 SEARO case definition. The 2009 WHO case definition had a sensitivity of 76.4% (69.6–82.1), specificity of 42.5% (38.9–46.3), positive predictive value of 25.5%, and negative predictive value of 87.5%. False positivity rate was 57.5% and false negativity was 23.6%. The 2011 WHO-SEAR expert group case definition had a sensitivity of 87.9% (82.2–91.9), specificity of 20.1% (17.2–23.3), positive predictive value was 22.0%, negative predictive value of 86.6%, false positivity rate of 79.9%, and false negativity of 12.1%. The 2009 WHO case definition, with values of thrombocytopenia specified as less than 150 000 performed best, with a sensitivity of 85.1% (79.0–89.6), specificity of 32.5% (29.1–36.1), positive predictive value of 24.5%, and negative predictive value of 89.4%. False positivity rate was 71.1% and false negativity was 14.9% (Fig. 1–3).
Table 1. Age–sex distribution of the study population.
| Age group | sex | Total | |
| Male | Female | ||
| 5–10 | 40 (8.9%) | 26 (6.5%) | 66 (7.8%) |
| 10–19 | 102 (22.4%) | 106 (26.6%) | 208 (24.6%) |
| 20–29 | 104 (23.1%) | 60 (15.0%) | 164 (19.3%) |
| 30–39 | 73 (16.2%) | 80 (20.1%) | 153 (18.0%) |
| 40–49 | 59 (13.1%) | 55 (13.8%) | 114 (13.4%) |
| 50–59 | 47 (10.4%) | 46 (11.5%) | 93 (11.0%) |
| >60 | 27 (6.0%) | 26 (6.5%) | 53 (6.2%) |
| Total | 452 (100%) | 399 (100%) | 851 (100%) |
Figure 1.
STARD flow diagram 2009 WHO case definition.
Figure 3.
STARD flow diagram WHO 2009 case definition (with thrombocytopenia specified as 150 000).
Figure 2.
STARD flow diagram 2011 WHO-SEAR expert group case definition.
The three independent criteria significantly associated with dengue, on multivariable analysis using logistic regression were thrombocytopenia less than 150 000, leukopenia, and absence of backache (Table 2). Among these ‘no backache’ is a feature which is not mentioned in any of these case definitions. Performance was improved after addition of ‘no backache’ to the 2009 and 2011 case definitions (Table 3).
Table 2. Symptoms/signs associated with dengue in the study participants.
| Symptom/sign | Dengue N = 174 | Fevers other than dengue N = 677 | Chi-square | P-value | Odds ratio | Adjusted odds ratios |
| Nausea | 63 (36.2%) | 164 (24.2%) | 10.2 | 0.001 | 1.78 (1.2–2.5) | 0.7 (0.3–1.3) |
| Vomiting | 49 (28.2%) | 123 (18.2%) | 8.6 | 0.003 | 1.77 (1.2–2.6) | 1.2 (0.6–2.6) |
| Abdominal pain | 32 (18.4%) | 95 (14%) | 2.07 | 0.15 | ||
| Melena | 3 (1.7%) | 2 (0.3%) | 4.8 | 0.03 | 5.92 (1.0–35.7) | 3.6 (0.1–89.7) |
| Petechie | 2 (1.1%) | 1 (0.1%) | 3.95 | 0.05 | NS | |
| Any bleed | 7 (4.0%) | 12 (1.8%) | 3.21 | 0.07 | NS | |
| Lethargy | 65 (37.4%) | 203 (30.0%) | 3.49 | 0.06 | NS | |
| Restlessness | 5 (2.9%) | 19 (2.8%) | 0.002 | 0.96 | NS | |
| Rash symptom | 6 (3.4%) | 11 (1.6%) | 2.35 | 0.13 | NS | |
| Rash sign | 3 (1.7%) | 8 (1.2%) | 0.32 | 0.57 | NS | |
| Headache | 134 (77.0%) | 503 (74.3%) | 0.54 | 0.46 | NS | |
| Retroorbital pain | 40 (23.0%) | 148 (21.9%) | 0.10 | 0.75 | NS | |
| Myalgia | 126 (72.4%) | 446 (65.9%) | 2.68 | 0.10 | NS | |
| Arthralgia | 83 (47.7%) | 311 (45.9%) | 0.17 | 0.68 | NS | |
| No Backache | 146 (83.9%) | 515 (76.1%) | 4.9 | 0.03 | 1.6 (1.1–2.6) | 2.7 (1.1–6.7) |
| Any ache | 162 (93.1%) | 598 (88.3%) | 3.3 | 0.07 | NS | |
| Tourniquet test positive | 1 (0.6%) | 0 (0%) | 3.9 | 0.05 | NS | |
| Persistent vomiting | 5 (2.9%) | 12 (1.8%) | 0.86 | 0.36 | NS | |
| Fluid accumulation | 0 (0) | 4 (0.6) | 1.03 | 0.31 | NS | |
| Liver enlargement | 1 (0.6) | 2 (0.3) | 0.31 | 0.58 | NS | |
| Raised hematocrit | 8 (4.6%) | 27 (4%) | 0.13 | 0.72 | NS | |
| Thrombocytopenia(<100 000) | 49 (28.2%) | 84 (12.4%) | 26.05 | 0.000 | 2.8 (1.9–4.1) | 2.9 (1.6–5.1) |
| Thrombocytopenia(<150 000) | 108 (62.1%) | 192 (28.4%) | 68.90 | 0.000 | 4.1 (2.9–5.9) | |
| Leukopenia (<4000) | 65 (39.2%) | 78 (12.8%) | 60.08 | 0.000 | 4.4 (2.9–6.5) | 2.3 (1.2–4.3) |
| Leukopenia (<5000) | 85 (48.9%) | 153 (22.6%) | 47.35 | 0.000 | 3.3 (2.3–4.6) | |
| No Cough | 17 (25.0%) | 35 (11.7%) | 8.05 | 0.005 | 2.5 (1.3–4.4) | 1.8 (0.9–3.6) |
| Chills | 124 (71.7%) | 490 (79.8%) | 0.81 | 0.37 | NS | |
| No Throat pain | 148 (85.5%) | 503 (77.1%) | 5.79 | 0.02 | 1.8 (1.1–2.8) | 1.6 (0.8–3.2) |
NS: not significant.
Bold signifies those variables which were significant in bivariable analysis.
Table 3. Comparison of the different case definitions.
| 2009 | 2011 | 2009 (with values of thrombocytopenia specified as less than 150 000) | With backache included in the criteria of any 2 in the 2011 definition | With backache included in the criteria of any 2 in the 2009 definition | |
| Sensitivity | 76.4 (69.6–82.1) | 87.9 (82.2–91.9) | 85.1 (79.0–89.6) | 97.7 (94.2–99.1) | 96.6 (95.4–97.8) |
| Specificity | 42.5 (38.9–46.3) | 20.1 (17.2–23.3) | 32.5 (29.1–36.1) | 9.3 (7.3–11.7) | 14.3 (11.9–17.1) |
| LR+ | 1.3 (1.2–1.5) | 1.1 (1.0–1.2) | 1.3 (1.2–1.4) | 1.1 (1.0–1.1) | 1.1 (1.0–1.2) |
| LR− | 0.6 (0.4–0.7) | 0.6 (0.4–0.9) | 0.5 (0.3–0.7) | 0.3 (0.1–0.7) | 0.2 (0.1–0.5) |
| DOR | 2.4 (1.6–3.5) | 1.8 (1.1–3) | 2.7 (1.7–4.3) | 4.4 (1.6–12.2) | 4.7 (2.0–10.9) |
DOR: diagnostic odds ratio.
The sensitivities of the case definitions increased from primary to tertiary level. This is expected, since those febrile illnesses that are unlikely to be dengue will be screened out at the lower settings. The specificity was best at the secondary level (Table 4). This could be because the more typical cases of dengue are seen at the secondary level and the acute febrile illness, which are more atypical gets referred to the tertiary settings.
Table 4. Setting wise performance of the 2009 and 2011 case definitions.
| Primary (n = 314) | Secondary (n = 217) | Tertiary (n = 320) | ||
| Sensitivity | 2009 | 75.7 (64.8–84.0) | 67.7 (50.1–81.4) | 81.2 (70.4–88.7) |
| 2011 | 83.7 (73.7–90.5) | 87.1 (71.2–94.8) | 92.7 (84.1–96.9) | |
| Specificity | 2009 | 42.9 (36.8–49.2) | 47.9 (40.8–55.0) | 38.3 (32.5–44.4) |
| 2011 | 21.7 (16.9–27.3) | 27.9 (22.0–34.8) | 16.7 (12.6–21.8) | |
| PPV | 2009 | 29.0 (23.9–34.01) | 17.8 (12.7–22.9) | 26.5 (21.6–31.3) |
| 2011 | 24.8 (20.0–29.6) | 16.8 (11.8–21.8) | 23.4 (18.8–28.0) | |
| NPV | 2009 | 85.1 (81.2–89.0) | 89.9 (85.9–93.9) | 88.1 (84.5–91.6) |
| 2011 | 81.2 (76.9–85.5) | 92.9 (89.5–96.3) | 89.4 (84.6–91.6) | |
| LR+ | 2009 | 1.3 (1.1–1.5) | 1.3 (1.0–1.8) | 1.3 (1.1–1.5) |
| 2011 | 1.1 (0.95–1.21) | 1.2 (1.0–1.4) | 1.1 (1.0–1.2) | |
| LR− | 2009 | 0.56 (0.37–0.87) | 0.67 (0.40–1.1) | 0.49 (0.30–0.82) |
| 2011 | 0.75 (0.42–1.33) | 0.5 (0.18–1.2) | 0.4 (0.18–1.1) | |
| DOR | 2009 | 2.3 (1.3–4.2) | 1.9 (0.86–4.3) | 2.7 (1.4–5.2) |
| 2011 | 1.4 (0.7–2.9) | 2.6 (0.87–7.85) | 2.6 (0.98–6.8) |
DOR: diagnostic odds ratio; PPV: positive predictive value; NPV: negative predictive value.
Discussion
The validity of the probable case definition of dengue given by the WHO-SEAR expert group in 2011 has not been previously studied scientifically, according to our knowledge. However the development, evidence base, and application of the revised dengue case classification has been extensively reviewed.23 The differences in the probable case definition of dengue, given by the WHO-SEAR expert group in 2011 as compared to the 2009 WHO case definition are the following. Retro orbital pain, headache, myalgia, and arthralgia have been specified, whereas in the 2009 WHO case definition it has been generalized as aches. Leukopenia has been included and count has been specified as less than 5000. Thrombocytopenia has been specified as platelet count less than 150 000. In 2009 WHO guidelines, generally the definitions either describe a rapid decline in platelet count or specify a platelet count <100 000 as a criteria for DHF. Other than these, there are not many differences between the probable case definitions in the 2009 and 2011 classifications. The 2009 WHO dengue classification was developed based on a prospective cohort study while the 2011 SEAR and the 1997 case classifications were based on expert consensus.
According to the results of our study, the 2009 WHO probable case definition performs better than the 2011 SEAR expert group definition. The performance of the 2009 definition can be improved by specifying the value of thrombocytopenia as less than 150 000.There are other literature evidences that say that the WHO 2009 criteria lack clarity in quantifying the degree and rate of change in hematocrit and platelet count.24 It has been previously reported that the sensitivity of the probable case definition is 74.1%, but specificity is low at 30.8% and the specificity could be improved by a two-stage screening process using a five criteria along with two.20 Passive surveillance using the WHO case definitions alone lack specificity since many other infectious diseases, such as influenza, chikungunya fever, enterovirus infections, leptospirosis, malaria, and typhoid fever all present with similar symptoms and signs as dengue in the acute phase of illness.25,26
The 2009 WHO case definition is also useful for identifying those with severe dengue,10,27–29 patients at risk of progression to severe disease, and those needing hospitalization.19 This definition has much greater sensitivity and specificity (92.1 and 78.5%) than the 1997 WHO classification system for diagnosing severe dengue.11 The 1997 case definition could capture only 18% of severe dengue.30 The 2009 definition has a high potential for facilitating dengue case management and surveillance. It was shown and perceived to be more sensitive than the DF/DHF/DSS classification for timely recognition of severe disease. Both acceptance and perceived user friendliness of the 2009 system were high, particularly in relation to triage and case management.12
Addition of absence of backache as a criteria for probable diagnosis, improved the performance of both 2009 and 2011 probable case definitions. Backache is generally a non-specific clinical feature seen in many diseases which present as acute febrile illness. The proportion of dengue patients with backache in different studies varied. Although myalgia and arthralgia have been found to be important clinical features of dengue, backache as a specific symptom has not been reported in patients with dengue in several studies.31–37 It could also be because it was not specifically asked for. Contrary to this some studies have mentioned backache as an important symptom.38–40 In Kerala, among the four most important features of dengue along with fever were headache (85%), myalgia (77%), retroorbital pain, and arthralgia (47%).35 In a study from Kollam, a district very close to Thiruvananthapuram, the major clinical features obtained were fever (96.8%), headache (77.2%), abdominal pain (62.4%), diarrhea (15.2%), bleeding (15.2%), skin rash (13.2%), pruritus (10.4%), sore throat (5.2%), and seizures (0.8%).35 Backache was not an important symptom in these studies. The use of absence of backache, for differentiating dengue from other febrile illness needs to be further investigated.
The limitations of the study are the following. 5–10% increase in hematocrit was not evaluated by two consecutive PCV estimations. IgM remains positive for 3 months, therefore the use of a single value might not always reflect that the present episode of fever is due to dengue. Another important limitation of the study is that the study was cross-sectional. The dengue illness evolves different phases and to capture at only one time point means that different patients were captured at different time points in their evolving febrile illness.
Conclusions and Recommendations
Dengue fever accounts for one fifth of cases of acute febrile illness. The 2009 WHO case definition has better discriminatory power than the 2011 WHO-SEAR expert group case definition. It also has higher negative predictive value. The performance of 2009 WHO case definition is further improved if the thrombocytopenia is defined as platelet count less than 150 000. The addition of ‘no backache’ as a criteria into the list of any two criteria required for diagnosing probable Dengue, improves the discriminatory power of the 2009 and 2011 definitions. A further comparative analysis needs to be done with other fevers to find whether the absence of backache could be used to differentiate dengue from other fevers. Studies have to be done in other parts of the world to find out whether this finding could be region specific.
Disclaimer Statements
Contributors Major contributors are the authors and minor contributors have been acknowledged below.
Funding The fund for the study was given by the State Board of Medical Research, Kerala, India (Order No: A2-8557/2010/MCT). Reverse transcriptase-polymerized chain reaction and Serology was done at Rajiv Gandhi Centre for Biotechnology, Kerala, India. ICMR Virology Network Program, Government of India, funded the investigations done at Rajeev Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala, India.
Conflicts of interest The authors have no conflicts of interest to declare.
Ethics approval Clearance of the institutional ethical committee of Medical College, Thiruvananthapuram, Kerala, India was also obtained (IEC No: 06/6/2012/MCT).
Acknowledgments
We place on record our gratitude to the faculty of State Board of Medical Research (SBMR), especially our Principal Dr Ramdas Pisharody, for funding the study and giving the necessary support and guidance (Order No: A2-8557/2010/MCT). We gratefully acknowledge ICMR Virology Network Program, Government of India, as they funded the investigations done at Rajeev Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala, India. We thank the staff of the various institutions under the Directorate of Health Services for the support provided in the conduct of the study. We would like to mention a special word of gratitude to Dr Syam Sunder, general hospital, Thiruvananthapuram in this regard.
The interns posted in our department during the period had participated sincerely in the project and we wish to acknowledge their work as well.
References
- 1.Gubler DJ, Kuno G. Dengue and dengue haemorrhagic fever. New York: CABI Publishing; 1978. [Google Scholar]
- 2.Lall R, Dhanda V. Dengue haemorrahagic fever and the dengue shock syndrome in India. Natl Med J India. 1996;9:20. [PubMed] [Google Scholar]
- 3.State Bulletin, Thiruvananthapuram. 2010. Integrated Disease Surveillance Project, State Surveillance Unit, Directorate of Health Services: Government of Kerala. [Google Scholar]
- 4.Anoop M, Issac A, Mathew T, Philip S, Kareem NA, Unnikrishnan R, et al. Genetic characterization of dengue virus serotypes causing concurrent infection in an outbreak in Ernakulam, Kerala, South India. Indian J Exp Biol. 2010; 488849–57. [PubMed] [Google Scholar]
- 5.World Health Organisation. Norms and standards in epidemiology: case definitions. Epidemiol Bull. 1999201123. [PubMed] [Google Scholar]
- 6.CDC. Update on acquired immune deficiency syndrome (AIDS). MMWR Morb Mortal Wkly Rep. 19823137507–8. [PubMed] [Google Scholar]
- 7.Harris E, Videa E, Perez L, Sandoval E, Téllez Y, Pérez ML, et al. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg. 2000;63:5–11. doi: 10.4269/ajtmh.2000.63.5. [DOI] [PubMed] [Google Scholar]
- 8.Kabra SK, Jain Y, Pandey RM, Madhulika SinghalT, Tripathi P, et al. Dengue haemorrhagic fever in children in the 1996 Delhi epidemic. Trans R Soc Trop Med Hyg. 1999;93:294–8. doi: 10.1016/s0035-9203(99)90027-5. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay S, Lum LC, Kroeger A. Classifying dengue: a review of the difficulties in using the WHO case classification for dengue haemorrhagic fever. Trop Med Int Health. 2006;11:1238–55. doi: 10.1111/j.1365-3156.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 10.Alexander N, Balmaseda A, Coelho IC, Dimaano E, Hien TT, Hung NT, et al. Multicentre prospective study on dengue classification in four South-East Asian and three Latin American countries. Trop Med Int Health. 2011;16(8):936–48. doi: 10.1111/j.1365-3156.2011.02793.x. [DOI] [PubMed] [Google Scholar]
- 11.Federico N, Gamaliel G, Pérez MA, Elizondo D, Nuñez A, Balmaseda A, et al. Evaluation of the traditional and revised WHO classifications of dengue disease severity. PLoS Negl Trop Dis. 2011. Available from: http://www.plosntds.org/article/fetchObject.action?uri = info%3Adoi%2F10.1371%2Fjournal.pntd.0001397& representation = PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barniol J, Gaczkowski R, Barbato EV, da Cunha RV, Salgado D, Martínez E, et al. Usefulness and applicability of the revised dengue case classification by disease: multicentre study in 18 countries. BMC Infect Dis. 2011;11:106. doi: 10.1186/1471-2334-11-106. Available from: http://www.biomedcentral.com/1471-2334/11/106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadinegoro SR. The revised WHO dengue case classification: does the system need to be modified? Paediatr Int Child Health. 2012;32(Suppl 1):33–8. doi: 10.1179/2046904712Z.00000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. Dengue haemorrhagic fever: diagnosis, treatment prevention and control, 2nd edn. Geneva: WHO; 1997. [Google Scholar]
- 15. TDR-WHO Expert Meeting on Effective, Affordable and Evidence-based Dengue Early Warning and Response Systems: Country Case Studies for Sharing Experiences. Freiburg, 2012 June 28–30 (Provisional Report) [Google Scholar]
- 16.WHO Regional Office for South-East Asia. Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever revised and expanded edition. New Delhi: WHO Regional Office for South-East Asia; 2011. [Google Scholar]
- 17.Dash AP, Rajesh B, Kalra NL. Dengue in South-East Asia: an appraisal of case management and vector control. Dengue Bull. 2012;36:1–13. [Google Scholar]
- 18.Farrar JJ, Hien TT, Horstick O, Hung NT, Jaenisch T, Junghanns T, et al. Dogma in classifying dengue disease. Am J Trop Med Hyg. 2013;89:198–201. doi: 10.4269/ajtmh.13-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima FR, Croda MG, Muniz DA, Gomes IT, Soares KR, Cardoso MR, et al. Evaluation of the traditional and revised world health organization classifications of dengue cases in Brazil. Clinics (Sao Paulo). 2013;68(10):1299–304. doi: 10.6061/clinics/2013(10)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinia TN, Vijayakumar K, Pradeep Kumar AS, Anoop M, Sreekumar E, Ramani Bai JT, et al. Performance of WHO probable case definition of dengue in Kerala, India, and its implications for surveillance and referral. Dengue Bull. 2012;36:94–104. [Google Scholar]
- 21.Quader AJ, Gandham P, Nandeshwar AJ. Screening for dengue infection in clinically suspected cases in a rural teaching hospital. J Microbiol Biotech Res. 2013;3(2):26–9. [Google Scholar]
- 22.Glas AS, Lijmerb JG, Prinsc MH, Bonseld GJ, Bossuyta PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–35. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 23.Horstick O, Farrar L, Lum L, Martinez E, San Martin JL, Ehrenberg J, et al. Reviewing the development, evidence base, and application of the revised dengue case classification. Pathog Glob Health. 2012;106(2):94–101. doi: 10.1179/2047773212Y.0000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR) Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: WHO; 2009. [Google Scholar]
- 25.WHO Regional Office for South-East Asia. Guidelines for treatment of dengue fever/dengue hemorrhagic fever in small hospitals. New Delhi: WHO Regional Office for South-East Asia; 1999. [Google Scholar]
- 26.Pan American Health Organization (PAHO) Dengue and dengue hemorrhagic fever in the Americas: guidelines for prevention and control. Washington, DC: Pan American Health Organization; 1994. [Google Scholar]
- 27.Yee Sin L, Tun LT, Fisher DA, Low JG, Oh HM, Narayanan RL, et al. Confirmed adult dengue deaths in Singapore: 5-year multi-center retrospective study. BMC Infect Dis. 2011;11:123. doi: 10.1186/1471-2334-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sam SS, Omar SF, Teoh BT, Abd-Jamil J, AbuBakar S. Review of dengue hemorrhagic fever fatal cases seen among adults: a retrospective study. PLoS Negl Trop Dis. 2013;7(5):e2194. doi: 10.1371/journal.pntd.0002194. Available from www.plosntds.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman MG, Alvarez M, Rodriguez R, Rosario D, Vázquez S, Valdes L, et al. Fatal dengue hemorrhagic fever in Cuba, 1997. Int J Infect Dis. 1999;3(3):130–5. doi: 10.1016/s1201-9712(99)90033-4. [DOI] [PubMed] [Google Scholar]
- 30.Balmaseda A, Hammond SN, Pérez MA, Cuadra R, Solano S, Rocha J, et al. Short report: assessment of the World Health Organization scheme for classification of dengue severity in Nicaragua. Am J Trop Med Hyg. 2005;73:1059–62. [PubMed] [Google Scholar]
- 31.Shahid A, Ali N, Ashraf S, Ilyas M, Tariq WU, Chotani RA. Dengue fever outbreak: a clinical management experience. J Coll Physicians Surg Pak. 2008;18(1):8–12. [PubMed] [Google Scholar]
- 32. Dengue fever. e-book. Chapter 2. Clinical diagnosis. WHO. Available from: http://www.who.int/csr/resources/publications/dengue/012-23.pdf. [Google Scholar]
- 33.Khan NA, Azhar EI, El-Fiky S, Madani HH, Abuljadial MA, Ashshi AM, et al. Clinical profile and outcome of hospitalized patients during first outbreak of dengue in Makkah, Saudi Arabia. Acta Trop. 2008;105(1):39–44. doi: 10.1016/j.actatropica.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Gascón J, Giner V, Vidal J, Jou JM, Mas E, Corachán M. Dengue: a re-emerging disease. A clinical and epidemiological study in 57 Spanish travellers. Med Clin (Barc). 1998;111(15):583–6. [PubMed] [Google Scholar]
- 35.Tyagi BK, Hiriyan J, Philip S, Tewari C, Paramasivan R. Dengue in Kerala: a critical review. ICMR Bull. 2006;36(4–5):13–22. Available from http://icmr.nic.in/bulletin/english/2006/april-may06.pdf. [Google Scholar]
- 36.Tewari KN, Tuli NR, Devgun SC. Clinical profile of dengue fever and use of platelets in four tertiary level hospitals of Delhi in the year 2009. JIACM. 2013;14(1):8–12. [Google Scholar]
- 37.Rachel D, Rajamohanan AbyZP. A study of clinical profile of dengue fever in Kollam, Kerala, India. Dengue Bull. 2005;29:197–202. [Google Scholar]
- 38.Singh NP, Jhamb R, Agarwal SK, Gaiha M, Dewan R, Daga MK, et al. The 2003 outbreak of dengue fever in Delhi, India. Southeast Asian J Trop Med Public Health. 2005;36:1174–8. [PubMed] [Google Scholar]
- 39.Abdallah TM, Ali AA, Karsany MS, Adam I. Epidemiology of dengue infections in Kassala, Eastern Sudan. J Med Virol. 2012;84(3):500–3. doi: 10.1002/jmv.23218. [DOI] [PubMed] [Google Scholar]
- 40.Madani TA, Abuelzein el-TM, Al-Bar HM, Azhar EI, Kao M, Alshoeb HO, et al. Outbreak of viral hemorrhagic fever caused by dengue virus type 3 in Al-Mukalla, Yemen. BMC Infect Dis. 2013;13:136. doi: 10.1186/1471-2334-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]