Abstract
We investigated the development of the HPA axis in group-living rhesus monkeys. Forty-three infants were studied from birth through their third year of life; 22 infants were physically abused by their mothers, while 21 infants were not abused. Plasma cortisol levels in basal conditions and in response to a novel environment test were assessed at 6-month intervals. Both basal and stress cortisol increased steadily from 6 to 24 months of age and then dropped. Across all ages, stress cortisol levels were significantly higher than the basal levels. The cortisol responses to stress at 30 and 36 months of age were significantly lower than the responses at all other ages. At most ages there was an inverse relationship between basal and stress cortisol levels. Individual differences in basal cortisol levels were generally stable in the first 2 years and more variable in the third year while the opposite for true for cortisol responses to stress. At the end of the first year, but not later in life, abused infants had higher cortisol levels than controls across the basal and stress conditions. Rates of social interactions with the mother and other group members were positively correlated with basal cortisol levels early in life, and with cortisol responses to stress later in life. Altogether, these results indicate that there are strong individual differences in HPA function, that there is a relationship between basal activity and stress reactivity, and that early abuse has the short-term effect of increasing both basal activity and stress reactivity.
Keywords: stress, HPA axis, development, early experience, individual differences, rhesus monkeys
Introduction
Nonhuman primates are excellent animal models for investigating the effects of early experience on interindividual variation in reactivity to stress and its relation to behavior, emotion, and cognition (e.g., Del Giudice, Ellis, & Shirtcliff, 2011; Parker & Maestripieri, 2011; Sanchez, 2006). In particular, monkey species such as rhesus macaques, which have a shorter lifespan and are characterized by faster maturation than humans (e.g., they reach puberty at approximately 3 years of age), provide an opportunity to investigate interindividual variation in the development of stress reactivity from birth to adulthood in a short period of time, relative to humans.
Previous studies of rhesus macaques have investigated the effects of different rearing environments (e.g., isolation-rearing, peer-rearing, and mother-rearing) on the development the hypothalamic–pituitary–adrenal (HPA) axis and other stress-sensitive physiological systems (e.g., Champoux, Coe, Schanberg, Kuhn, & Suomi, 1989; Clarke, 1993; Higley, Linnoila, & Suomi, 1992; Meyer & Bowman, 1972; Sackett, Bowman, Meyer, Tripp, & Grady, 1973; Shannon, Champoux, & Suomi, 1998). In some of these studies, peer-reared individuals have been reported to have higher basal cortisol levels than mother-reared individuals (first month of life; Champoux et al., 1989; first 2 years of life: Higley et al., 1992), lower basal cortisol levels (14–30 days of age: Shannon et al., 1998), or lower basal ACTH but similar cortisol levels (1–6 months of age: Clarke, 1993). Studies comparing peer-reared and isolation-reared animals have reported higher basal cortisol levels in peer-reared animals (19 months of age: Sackett et al., 1973) or no differences in cortisol levels (4 years of age: Meyer & Bowman, 1972). When physiological responses to stressful challenges were assessed, some studies found no differences in cortisol levels between peer-reared, mother-reared, and isolation-reared monkeys (Champoux et al., 1989; Meyer & Bowman, 1972; Sackett et al., 1973), one study found that peer-reared monkeys had lower increases in ACTH and cortisol than mother-reared monkeys (Clarke, 1993), and another study reported that the cortisol levels of peer-reared monkeys were higher than those of isolation-reared monkeys but similar to those of mother-reared monkeys (Shannon et al., 1998). Shannon et al. (1998) suggested that these discrepancies in the activity of the HPA axis in relation to early rearing may have resulted from differences in the housing environment, type of stressful experiences, feeding regimen, or diurnal variation in HPA axis activity across studies.
Previous research in our laboratory has investigated the effects of early exposure to variable parenting style (along its dimensions of maternal protectiveness and rejection) and of maternal physical abuse on the development of offspring's behavior, brain monoamine systems, and the HPA axis in group-living rhesus macaques (e.g., Maestripieri et al., 2006; McCormack, Newman, Higley, Maestripieri, & Sanchez, 2009; Sanchez et al., 2010). Among other findings, we reported that male and female infants that were exposed to maternal physical abuse in the first 2– 3 months of life exhibited significantly higher plasma cortisol responses to a corticotropin-releasing hormone (CRH) challenge at 6, 12, 18, 24, 30, and 36 months of life than nonabused infants (Sanchez et al., 2010; see also Tarullo & Gunnar, 2006).
Although previous studies have suggested that early psychosocial stress has the potential to result in long-term alterations in HPA responsiveness to challenges (see Parker & Maestripieri, 2011, for review), it remains to be ascertained whether these alterations may also include HPA basal activity or HPA responses to different kinds of environmental and psychosocial stress. Since few previous longitudinal studies have assessed the development of the HPA axis in group-living rhesus monkeys (e.g., Higley, Suomi, & Linnoila, 1992), little is known about age-related changes in both basal HPA activity and HPA reactivity to stress, and about the relationship between these two parameters from a developmental perspective. This lack of information is problematic because previous research has shown that variation in HPA basal activity is associated with variation in HPA reactivity to stress (e.g., in humans, there is a negative relationship between basal cortisol levels and cortisol responses to stress; e.g., Dickerson & Kemeny, 2004; Lewis & Thomas, 1990; Maestripieri, Baran, Sapienza, & Zingales, 2010) and it is possible that the effects of early stress on later HPA reactivity to challenges may be more apparent at some ages than at others depending on differential HPA basal activity at these ages.
In this study, we investigated plasma cortisol levels in baseline condition and in response to mild psychosocial stress (a novel environment test) in male and female rhesus monkeys during their first 3 years of life. The subjects were the same as those in our previous study in which we assessed cortisol responses to a CRH challenge (Sanchez et al., 2010). Half of these subjects were physically abused by their mothers and half were reared by nonabusive control mothers.
The rationale for this study is that investigating the development of HPA axis activity in a relatively large number of mother-reared rhesus monkey infants living in naturalistic social groups can provide useful information for understanding basic aspects of neuroendocrine development in this species and in other primates, including humans. A repeated, longitudinal assessment of plasma cortisol levels under both basal and stressful conditions can also enhance our understanding of basic aspects of physiological reactivity to the environment, including documenting the occurrence and temporal stability of individual differences in HPA function.
In this study, we tested the following predictions: (1) that exposure to stress resulted in a significant elevation in plasma cortisol levels at all ages; (2) that both basal and stress cortisol levels varied significantly in relation to age, reflecting maturational changes in the HPA axis, and/or developmental changes in its sensitivity to stress; (3) that there was a negative correlation between basal and stress cortisol levels at each age; (4) that individual differences in basal cortisol levels and/or cortisol responses to stress were stable across different ages; (5) that abused infants exhibited higher basal and/or stress cortisol levels relative to controls; (6) that sex was not a significant variable affecting basal or stress cortisol levels; and finally, (7) that there were significant associations between basal or stress cortisol levels and selected aspects of infant behavior.
Stable individual differences in basal and stress cortisol levels have been reported by many previous studies of rhesus monkeys, including some involving free-ranging individuals (e.g., Hoffman, Ayala, Mas-Rivera, & Maestripieri, 2010; Suomi, 1991). Our prediction concerning the absence of significant sex differences in basal or stress cortisol levels is justified by previous human and nonhuman primate studies indicating that such sex differences, if they occur at all, tend to be relatively small (e.g., Dickerson & Kemeny, 2004; Higley et al., 1992). Our prediction that abused infants may exhibit higher basal and/or stress cortisol levels relative to controls is based on both human and nonhuman primate studies investigating the developmental effects of early traumatic experiences, including parental maltreatment (e.g., Parker & Maestripieri, 2011; Tarullo & Gunnar, 2006). The relatively infrequent parental abuse experienced by our study subjects, however, probably has less dramatic consequences for neuroendocrine function and behavior than more severe instances of child maltreatment in humans or experimentally induced traumatic experiences in nonhuman primates such as maternal separation and social deprivation. Finally, although variation in early experience is expected to significantly affect socio-behavioral development, investigating such relationship is beyond the scope of this study; in our analysis of behavioral variables, our goal was simply to assess whether differences in HPA function under basal or stressful conditions are associated with differences in rates of social interactions in order to determine whether subjects that appear to be more or less socially active or inhibited have different physiological activity or reactivity profiles.
Methods
Subjects and Housing
Subjects were 43 rhesus monkey infants living in large social groups at the Field Station of the Yerkes National Primate Research Center in Lawrenceville, GA (USA). The groups were housed in 38 m × 38 m outdoor compounds with indoor housing areas. They consisted of 20–50 adult females with their immature offspring and two to five unrelated adult males. All groups had a stable matrilineal structure and a linear dominance hierarchy. Female dominance ranks were calculated using data on unidirectional aggression and submission collected during previous studies. The monkeys were fed Purina brand monkey chow twice daily and water was always available. All research followed the guidelines in the NIH Guide for the Care and Use of Laboratory Animals and was approved by the Emory University Institutional Animal Care and Use Committee.
Twenty-one infants (9 males, 12 females) were reared by multiparous mothers with a history of abusive parenting, whereas 22 of them (9 males, 13 females) were reared by nonabusive controls. The abusive mothers used in this study had been observed in previous years and their abusive behavior had been documented (Maestripieri, 1998; Maestripieri, Tomaszycki, & Carroll, 1999). Only mothers whose frequency and severity of abuse did not jeopardize their infant's life were used. These abusive mothers were typically consistent in the frequency and severity with which they abused offspring born in successive years (Maestripieri et al., 1999). Control mothers were multiparous females without a history of abusive parenting who had similar characteristics (e.g., age, parity, dominance rank, and infant sex) as the abusive mothers, and who gave birth in the same time period and in the same social groups as the abusive mothers.
Behavioral Data Collection
All 43 infants were studied longitudinally, in their own social groups, from birth to 36 months of age. Infants and their mothers were focally observed in 30-min sessions, 5 times per week during the first month of life, 2 times per week during the second month of life, and 1 time per week from the third month through the end of the study. All behavioral data were converted into mean hourly rates of behavior per month for the purposes of data analysis. For some analyses the monthly averages were combined across 6-month time spans. The three observers who collected the data were tested for reliability prior to the beginning of data collection (they simultaneously observed the same subjects and coded their behavior: this procedure was repeated until there was at least 90% agreement among the observers and Cohen's kappa was >.8).
Analysis of the behavioral data focused on hourly rates of maternal abuse. Infant abuse was operationally defined as (see Maestripieri, 1998): dragging: the mother drags her infant by its tail or leg while walking or running; crushing: the mother pushes her infant on the ground with both hands; rough grooming: the mother forces her infant onto the ground, and pulls out the infant's hair with force causing distress calls; throwing: the mother throws her infant a short distance with one hand while standing or walking; hitting: the mother violently slaps her infant with one hand or arm; biting: common definition; stepping or sitting on: the mother steps on her infant with one foot or both feet, or sits on her infant; abusive carrying: the mother carries the infant with one arm away from her body, preventing the infant from clinging. Abuse events did not last more than a few seconds, and therefore only their frequency was recorded.
In addition to the abusive behavior, behavioral data analyzed here include: frequencies of contacts made and broken by infants; frequencies of social play done and received by infants; frequencies of aggression done and received by infants; and frequencies of avoidant behavior by infants.
Training and Capture Procedures
To speed the capture process and minimize arousal, study subjects were trained to move on command from the outdoor corral into an indoor capture unit, from this to a transfer box, and from the box to a squeeze cage, where blood samples could be collected. Training was done using positive reinforcement and following guidelines and protocols approved by the Emory University IACUC. Infants <12 months of age were typically carried into the cage by their mothers and then separated from them. However, they learned the training procedure during the first year of life so that, in subsequent years, they could be captured without their mothers and with the same procedure used for the adults. Previous work has shown that infants who have experienced these procedures exhibit no alterations in normal development (Wilson, Gordon, & Collins, 1986). Once in the cage, one blood sample was collected, without anesthesia, within <10 min from the time the monkeys saw the experimenters approach their outdoor compound. Under these conditions, elevations in plasma cortisol concentrations are minimal or nonexistent (e.g., Blank, Gordon, & Wilson, 1983). All blood samples were collected at approximately the same time, between 9 and 11 am.
Novel Environment Test
At 6-month intervals, when the subjects were 6-, 12-, 18-, 24-, 30-, and 36-month old, they were captured, removed from the social group, and placed alone in a cage, in a novel room, for 30 min. In the first year, and in some cases also in the second year, the subjects were captured along with their mothers and were separated from them before the novel environment test. When subjects were older they were captured without their mothers. At each age, the test took place in a different room to minimize habituation to the procedure. A blood sample was collected by femoral venipuncture from each subject at the beginning (pretest: 0 min) and another at the end (posttest: 30 min) of the test. Blood samples were collected without anesthesia. The subject was then quickly returned to the social group. Throughout the Results and Discussion Section, the novel environment test is referred to as the stress test or the stress condition. The cortisol response to stress was assessed by calculating the difference between the stress and the basal cortisol levels.
Hormonal Assays
Blood samples were collected in pre-chilled polypropylene tubes containing EDTA and immediately placed on ice. Plasma was separated by centrifugation at 1,000g for 10 min at 4°C and then aliquoted and stored at −80°C until assayed. Plasma concentrations of cortisol were assayed in duplicated 10-μl aliquots by radioimmunoassay using commercially available kits (Diagnostic Systems Lab, Webster, TX). The sensitivity of this assay was 1.25 μ/dl and intra- and inter-assay coefficients of variation were <10%.
Data Analyses
The comparison between basal and stress cortisol levels across ages was performed using a repeated measures ANOVA. Possible differences between male and female infants, and between abused and control infants in basal cortisol, stress cortisol, or in the difference score were analyzed with mixed-design ANOVAs, with age as a repeated measure. Possible interactions between sex and early experience (abuse vs. control) in the cortisol measures were assessed with 2 × 2 ANOVAs calculated separately at each age. t-Tests, regression, and correlation (Pearson's coefficient) analyses were also used. All tests were two-tailed and probabilities <.05 were considered statistically significant.
Results
Age, Sex, and Stress Effects on Cortisol Levels
The comparison of cortisol levels in the basal and stress condition over the six time points revealed a main effect of age, F(5, 200) = 29.94, p ≤ .001, a main effect of stress, F(1, 40) = 322.21, p ≤ .001, and a significant interaction between age and stress, F(5, 200) = 7.46, p ≤ .001. There were no significant differences in cortisol levels between males and females in the basal condition, F(1, 5) = .62, p = .68, in the stress condition, F(1, 5) = .36, p = .55, or in the difference scores, F(1, 39) = .13, p = .71. There were no significant interactions between sex and age or sex and stress in any cortisol measures.
Both basal and stress cortisol increased steadily from 6 to 24 months of age and then dropped at 30 and 36 months such that cortisol levels in the third year of life were the lowest across the entire time span (Fig. 1). Across all ages cortisol levels after the stress test were higher than the basal levels. The difference between stress and basal cortisol levels was significant at all six ages (all p-values were <.05). Post hoc pairwise comparisons at each time point using the Bonferroni correction revealed that the increases in cortisol concentrations at 30 and 36 months of age were significantly lower than the increases in cortisol at all other ages (p < .05; mean increase year 1: M = 12.15 ± 6.87; mean increase in year 2: M = 12.99 ± 9.79; mean increase in year 3: M = 7.64 ± 3.33; see also Fig. 1). Therefore, the stress test had a weaker effect on cortisol levels in the third year of life than in the first 2 years.
Figure 1.
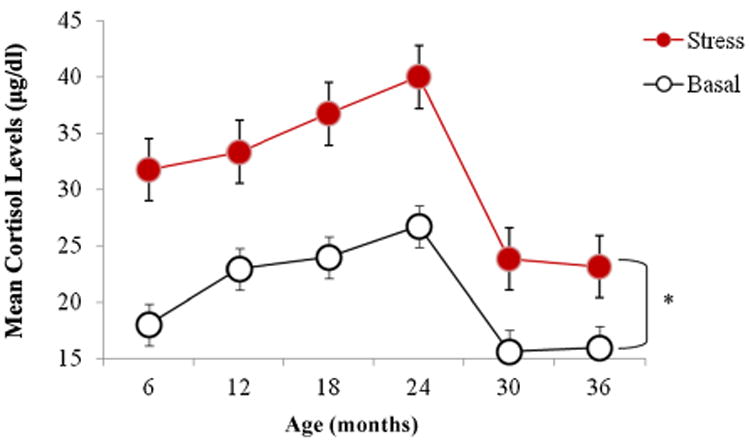
Mean (±SEM) basal and stress cortisol levels at 6, 12, 18, 24, 30, and 36 months of age. The difference between stress and basal levels is statistically significant at all ages. Both basal and stress cortisol levels (as well as the difference between them) are significantly lower at 30 and 36 months than at previous months (*p < .05).
Relationship Between Basal and Stress Cortisol and Stability of Individual Differences in Cortisol Levels Over Time
At each of the six ages there was an inverse relationship between basal cortisol levels and the magnitude of the cortisol increase following the stress test such that lower baseline values were associated with larger increases, whereas higher baseline values were associated with smaller increases. Regression analysis revealed that these relationships were significant at ages 6 months (R2 = .21, p = .002), 12 months (R2 = .60, p < .001), and 36 months (R2 = .46, p < .001) and approached statistical significance at the other ages.
To investigate whether individual differences in cortisol were consistent over time we calculated a correlation matrix for basal cortisol levels and cortisol responses to stress (difference between stress and basal levels) at all ages (see Tabs. 1 and 2). The basal levels at 6 months were positively correlated with the basal levels at 12 months, and the values at 12 months were positively correlated with those at every other age. Basal levels at 18 months were correlated with levels at 24 and 30 months, and levels at 24 months were correlated with levels at 30 months. Overall, basal cortisol levels were fairly stable across the first 2 years, especially at adjacent 6-month intervals, while there was more variability in the third year (see Tab. 1).
Table 1. Correlations Between Basal Cortisol Levels at Each Age and Basal Cortisol Levels at Every Other Age.
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|
| 1 | 6 months | — | |||||
| 2 | 12 months | .41* (p < .01) | — | ||||
| 3 | 18 months | .21 (p = .18) | .53* (p < .01) | — | |||
| 4 | 24 months | .17 (p = .28) | .69* (p < .01) | .66 (p < .01) | — | ||
| 5 | 30months | .05 (p = .73) | .376* (p = .01) | .370* (p = .01) | .334* (p = .03) | — | |
| 6 | 36 months | .12 (p = .48) | .36* (p = .01) | .19 (p = .21) | .25 (p = .10) | .14 (p = .36) | — |
Note: Statistically significant.
Table 2. Correlations Between Cortisol Responses to Stress at Each Age and Stress Cortisol Levels at Every Other Age.
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|
| 1 | 6 months | — | |||||
| 2 | 12 months | .21 (p = .17) | — | ||||
| 3 | 18 months | .15 (p = .33) | .10 (p = .51) | — | |||
| 4 | 24 months | .17 (p = .28) | .11 (p = .47) | .39* (p = .01) | — | ||
| 5 | 30 months | .24 (p = .12) | .10 (p = .52) | .34* (p = .02) | .46* (p < .01) | — | |
| 6 | 36 months | −.05 (p = .72) | −.05 (p = .74) | −.01 (p = .95) | .11 (p = .49) | .05 (p = .71) | — |
Note: Statistically significant.
Table 2 shows that cortisol stress responses at 18 months were correlated with stress responses at 24 and 30 months, and stress responses at 24 months were correlated with responses at 30 months. All other correlations were not significant. Overall, individual differences in cortisol stress response were more variable than basal levels of cortisol, and the highest variability was in the first 2 years, in which no correlation was significant.
Occurrence of Infant Abuse and Effects of Abuse on Cortisol Levels Across the 3 Years
All infants reared by abusive mothers experienced abuse, while none of the infants of control mothers were abused. The abuse occurred most frequently in the first month of life and virtually disappeared after the third month. Abuse resulted in infant distress behavior and superficial bruises and scratches. No abused subject in this study suffered injuries that required the intervention of a veterinarian. Figure 2 illustrates the mean rates of abuse per hour experienced by male and female infants in the first 3 months of life. There was a significant effect of age F(2, 38) = 4.49, p = .01, but no significant sex differences in abuse, F(1, 38) = 1.38, p = .25, or a significant interaction between age and sex, F(2, 38) = .02, p = .97.
Figure 2.
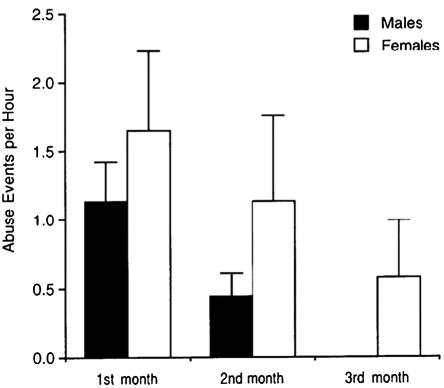
Mean (±SEM) hourly rates of maternal abuse in the first 3 months of life. Data refer to the 22 subjects reared by abusive mothers. Differences between males and females are not statistically significant.
The comparison of basal cortisol levels in abused infants and controls across all ages revealed a significant effect of age, F(5, 195) = 25.13, p < .001, but no main effect of experience, F(1, 39) = .63, p = .43 or a significant interaction between age and experience F(5, 195) = .64, p = .66. Similarly, there was no significant difference between abused and control infants in the cortisol response to stress across the six ages, F(1, 39) = .01, p = .91, and no significant interaction between age and experience, F(5, 195) = .11, p = .98. Holding basal levels as a covariate, there were no significant differences in stress cortisol levels relative to the baseline between the abused and the control infants over time, F(1, 33) = 1.04, p = .31.
A correlational analysis of cortisol concentrations across the six ages revealed that abused and control infants were generally similar in the extent to which basal cortisol and stress cortisol were consistent or inconsistent over time (data not shown). In other words, we found no evidence that the abused infants had less stable cortisol levels over time than controls. Finally, there was no significant relationship between the frequency of abuse experienced by the subjects in the first 3 months of life and any cortisol measure at any point in time.
Relationship Between Abuse, Stress, Sex, and Cortisol in the First Year of Life
Since infant abuse was concentrated in the first 3 months of life, it is possible that the effects of abuse on cortisol levels, if any, are stronger in the first year of life than in the second or third year. Therefore, we examined the possible relationship between abuse, stress, and cortisol levels in greater detail for this time period. At both 6 and 12 months of age, the cortisol responses to stress were predicted by the basal levels for both abused (6 months: R2 = .12, p = .06; 12 months: R2 = .23, p < .001) and control infants (6 months: R2 = .16, p = .02; 12 months: R2 = .19, p = .01). At 12 months of age, the regression between basal and stress cortisol levels for abused infants was significantly different from the regression between basal and stress cortisol levels for controls (in other words, the y-intercepts of the two functions were significantly different, t(35,3) = 2.32, p < .05 (see Fig. 3), indicating that abused infants had higher cortisol levels than controls across the basal and stress conditions. This relationship was not significant at 6 months of age. Furthermore, at 12 months of age, but not at 6 months, if basal cortisol level was held as covariate, there was a significant interactive effect of experience and sex on the cortisol stress response, F(1, 36) = 5.92, p = .02, such that abused males had a lower stress response than control males, whereas abused females had a higher stress response than control females (see Fig. 4).
Figure 3.
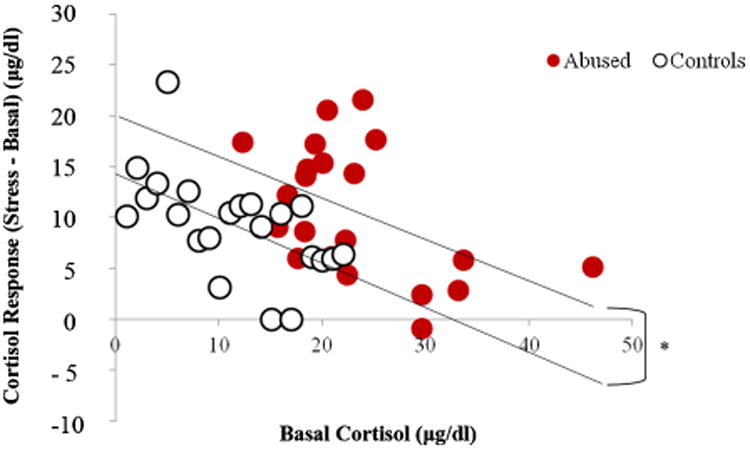
Regression of cortisol responses to stress (differences between stress and basal cortisol) and basal cortisol levels for abused and control subjects at 12 months of age. Abusedsubjects had significantly (*p < .05) values than controls (i.e., the value of the y-intercept line for the abused subjects is significantly higher than the value for the controls).
Figure 4.
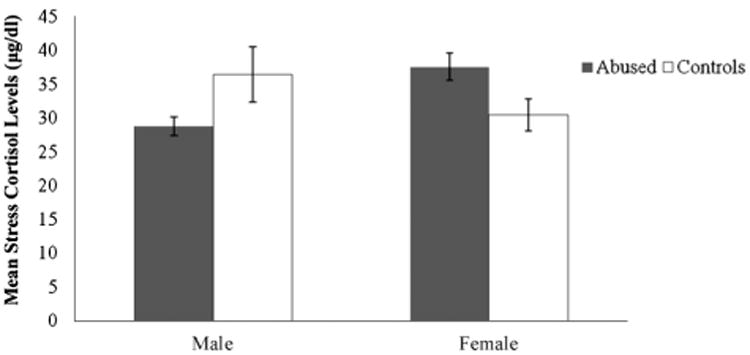
Mean (±SEM) cortisol levels (differences between stress and basal cortisol) in male and female abused subjects and controls at 12 months of age. The interaction between sex and experience group is statistically significant (*p = .02).
Relationships Between Behavior, Age, Abuse, Sex, Stress, and Cortisol Levels
Six of the eight behavioral measures considered in this study showed significant changes in relation to age. Specifically, the frequency with which subjects made or broke contact with their mothers and the frequency with which they initiated or received social play decreased significantly across the 3 years of life, while the frequency with which subjects initiated or received aggression increased with age (Tab. 3). The rates of scratching and avoidance did not vary significantly in relation to age.
Table 3. Mean Hourly Rates of Behavioral Measures at Each Age.
| Age | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 6 | 12 | 18 | 24 | 30 | 36 | |
| Makes contact | 10.01 | 7.64 | 3.65 | 2.52 | 1.81 | 1.54 |
| Breaks contact | 7.91 | 4.26 | 2.22 | 1.53 | 1.06 | .96 |
| Initiates play | 3.41 | 3.08 | 3.14 | 2.54 | 2.48 | 1.47 |
| Receives play | 2.25 | 1.66 | 1.57 | 1.10 | .76 | .32 |
| Scratches | 4.04 | 3.13 | 5.01 | 3.84 | 5.28 | 3.29 |
| Initiates aggression | .01 | .11 | .18 | .20 | .32 | .32 |
| Receives aggression | .12 | .20 | .38 | .53 | .38 | .29 |
| Avoids | .68 | 1.77 | 2.18 | 1.94 | 1.99 | 1.65 |
With the exception of the first 6 months, there were significant sex differences in the rates of social play done and received such that males had significantly higher rates than females at all ages (all ps < .05). The only significant difference in behavior between abused and control infants was observed at 36 months of age, t(34) = −2.30, p = .02, when control infants had a higher avoidance rate (M = 1.96, SD = .95) than abused infants (M = 1.361, SD = .67).
When the eight behavioral measures were analyzed in relation to basal cortisol and cortisol responses to stress, we found that the basal cortisol levels predicted the behavioral measures at the end of the first year: basal cortisol levels were positively correlated with the rates of making and breaking contact, social play done and received, and avoidance rates. The stress responses predicted the behavioral measures at the end of the second year and the beginning of the third year: stress responses were positively correlated with the rates of making and breaking contact, social play done and received, and aggression done and received. Therefore, individual differences in rates of social interactions (both with mothers and other individuals) were correlated with basal cortisol levels in the first year of life and with cortisol responses to stress in the second and third year.
Discussion
This study of rhesus monkeys provided evidence for significant developmental changes in both basal activity of the HPA axis and its reactivity to stress in the first 3 years of life. Similar to what has been reported by some previous studies (e.g., Clarke, 1993; Higley et al., 1992; Shannon et al., 1998), basal cortisol levels increased steadily during the first 2 years of life; however, in the third year, they dropped to levels similar to those measured at 6 months of age. Subjects showed significant increases in cortisol levels following a novel environment test at all ages, regardless of their gender and early experience (see also Champoux et al., 1989; Meyer & Bowman, 1972). However, the cortisol response to stress was lower in the third year than in the first 2 years. This result may be due, in part to habituation to the procedure and in part to the fact that younger subjects had to be separated from their mothers before the novel environment test, while in the third year subjects were more socially independent and separation from the mother was not necessary.
The baseline cortisol levels significantly predicted the responses to stress such that subjects with a low baseline had a large cortisol increase while subjects with a high baseline had a small cortisol increase. This finding has been reported by other studies of cortisol responses to psychological stress (e.g., in humans, Lewis & Thomas, 1990; Maestripieri et al., 2010). In part, it reflects the statistical phenomenon of regression to the mean, and in part biological constraints on hormonal activity, that is, the fact that individuals who have high baseline cortisol levels may not be able to increase their cortisol secretion as much as individuals with lower cortisol baseline. Individual differences in basal cortisol levels were fairly stable across the first 2 years, especially at adjacent 6-month intervals, while there was more variability in the third year. In contrast, individual differences in cortisol stress responses were more variable than those in basal cortisol levels; the highest variability was in the first 2 years, with more stability in the third year.
Infants reared by mothers with a history of abusive parenting were abused by them with a frequency of approximately 1.5 events per hour in their first month of life. Abuse was less frequent in the second month, and in most cases ended in the third month. Although the frequency of abuse appeared to be higher for female than for male infants, this difference was not statistically significant. Across the 3 years, there were no overall significant effects of abuse on the cortisol response to the novel environment test. There was also no evidence that the abused infants had less stable cortisol levels over time than controls. However, in the first year, abused infants had significantly higher cortisol levels than controls across the basal and stress conditions. At 1 year, there was also a significant interaction between early experience and infant sex on the cortisol stress response such that abused males had a lower stress response than control males, whereas abused females had a higher stress response than control females. These results suggest that early abuse was associated with an alteration in HPA axis function under both basal conditions and in response to stress, that the altered response to stress was different in male and female infants, and that this was primarily a short-term effect that was detectable in the first year or life but not in subsequent years (see also McCormack et al., 2009). The novel environment test used in this study, however, must be considered a relatively mild stressful challenge. The subjects of this study were also tested for cortisol responses to a CRH challenge (a much more powerful manipulation of the HPA axis than mild psy-chosocial stress) at 6-month intervals and, in this test, abused infants exhibited greater cortisol responses than controls at all ages at which they were tested (Sanchez et al., 2010).
The subjects of this study lived in large social groups and we recorded many aspects of their behavioral development over the first 3 years of life. Presenting a detailed analysis of interindividual variation in behavior in relation to cortisol measures, infant sex, or early experience is beyond the scope of this article.
Our interest here is in documenting broad developmental changes in the rates at which some behaviors are expressed and exploring whether differences in HPA axis function under basal or stressful conditions are associated with differences in rates of social interactions in order to determine whether subjects that appear to be more or less socially active or inhibited have different physiological activity or reactivity profiles.
Infant-initiated interactions with the mother, such as making or breaking contact with her, decreased steadily over the 3 years. Social play with peers was frequent in the first and second year of life but decreased significantly in the third year. In contrast, the frequency with which subjects initiated or received aggression increased with age. As expected, there was a significant sex difference in social play, as males initiated and received social play at higher frequencies than females at all ages. The only significant difference in behavior between abused and control infants was observed at 36 months of age, when control infants had a higher avoidance rate than abused infants.
Rates of social interactions (both with mothers and other individuals) were correlated with basal cortisol levels in the first year of life and with cortisol responses to stress in the second and third year. In both cases, the higher the cortisol levels the higher the rates of social interaction. Therefore, individuals that were more socially active had higher basal HPA activity or greater HPA reactivity to stress, while individuals who were more socially inhibited had physiological profiles characterized by lower HPA activity or reactivity. The correlations between behavior and basal cortisol level earlier in life and between behavior and stress cortisol later in life might reflect the finding that individual differences in basal cortisol level were most stable in the first year while those in stress cortisol were most stable in the third year. One possible explanation for these different correlations is that infants spend most of their time with their mothers in the first year of life, and most of their time alone in the third year. Thus, it is possible that individual differences in both basal cortisol and social behavior in the first year are influenced by variation in the mother's activity level or her behavior toward the infant, which is consistent with the observed effects of maternal abuse on basal cortisol levels at the end of the first year, while individual differences in stress cortisol and social behavior in the third year reflect variation in reactivity to the environment that is genetically based or affected by social experience with individuals other than the mother, or both.
Taken together, these results suggest that age has significant effects on the development of HPA axis activity and reactivity during the first 3 years of life and that variation in HPA function is associated with variation in behavior. With a few exceptions, infant sex is not a strong determinant of variation in HPA function or behavior. The early experience of abuse affected HPA function, but the effect was detectable only in the first year of life. It is worth emphasizing that the abuse experienced by the infant monkeys in this study was relatively mild (mothers with much higher rates of abuse were not included in this study because their infants' lives would have been at risk) and the experimental stressful manipulation was brief and mainly psychosocial in nature. It is possible that the effects of abuse on physiology and behavior might have been much stronger if infants who experienced higher rates of abuse had been included in the study and if more dramatic stressful perturbations had been performed. These limitations of this study, however, are compensated by the fact that the study was conducted in semi-naturalistic conditions, while the subjects lived in their large and complex social groups. The results of this study, therefore, have considerable ecological validity and can be potentially extrapolated to other naturally occurring situations and other species, including humans. Moreover, since extremely severe cases of child maltreatment represent only a small minority of all reported occurrences of child maltreatment (e.g., in the U.S., approximately 1,500 children per year die because of parental abuse or neglect, while the annual total number of reported cases of child maltreatment is close to 1 million; National Child Abuse and Neglect Data System), the occurrence of relatively mild infant abuse in rhesus monkeys provides a good animal model for many cases of human maltreatment, in which children do not necessarily experience serious physical trauma but may exhibit long-term physiological and psychological alterations as a result of this early experience.
Acknowledgments
Notes:The data analyses presented in this article were part of Hannah Koch's undergraduate honor's thesis at the University of Chicago. She thanks Anne Henly and Bob Martin for advice on her thesis. The authors also thank Richelle Scales and Anne Graff for technical assistance with the research procedures. This research was supported by NIH grants R01-MH62577 and R01-HD06717 to D.M., R21-MH01005 to M.M.S., and RR-00165 to the Yerkes Center. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Contract grant sponsor: NIH
Contract grant numbers: R01-MH62577, R01-HD06717, R21-MH01005, RR-00165
Footnotes
Conflicts of interest: nothing to declare.
References
- Blank MS, Gordon TP, Wilson ME. Effects of venipuncture on serum levels of prolactin, growth hormone, and cortisol in outdoor compound-housed female rhesus monkeys. Acta Endocrinologica. 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Champoux M, Coe CL, Schanberg SM, Kuhn CM, Suomi SJ. Hormonal effects of early rearing conditions in the infant rhesus monkey. American Journal of Primatology. 1989;19:111–117. doi: 10.1002/ajp.1350190204. [DOI] [PubMed] [Google Scholar]
- Clarke AS. Social rearing effects on HPA activity over early development and in response to stress in rhesus monkeys. Developmental Psychobiology. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson S, Kemeny M. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal study of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys: Effects of early experience, age, sex and stress on continuity of interindividual differences. Biological Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hoffman CL, Ayala JE, Mas-Rivera A, Maestripieri D. Effects of reproductive condition and dominance rank on cortisol responsiveness to stress in free-ranging female rhesus macaques. American Journal of Primatology. 2010;72:559–565. doi: 10.1002/ajp.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Thomas D. Cortisol release in infants in response to inoculation. Child Development. 1990;61:50–59. [PubMed] [Google Scholar]
- Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Animal Behavior. 1998;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Baran NM, Sapienza P, Zingales L. Between- and within-sex variation in hormonal responses to psychological stress in a large sample of college students. Stress. 2010;13:413–424. doi: 10.3109/10253891003681137. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack K, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques. Behavioral Neuroscience. 2006;120:1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Developmental Psychobiology. 1999;34:29–35. [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Bowman RE. Rearing experience, stress, and adrenocorticosteroids in the rhesus monkey. Physiology and Behavior. 1972;8:339–343. doi: 10.1016/0031-9384(72)90382-4. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Maestripieri D. Identifying features of early stressful experiences that produce stress vulnerability and resilience in primates. Neuroscience & Biobehavioral Reviews. 2011;35:1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett GP, Bowman RE, Meyer JS, Tripp RL, Grady SA. Adrenocortical and behavioral responses by differentially raised rhesus monkeys. Physiological Psychology. 1973;1:209–212. [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack K, Grand A, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and corti-sol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Development and Psychopathology. 2010;22:45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. American Journal of Primatology. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Up-tight and laid-back monkeys: Individual differences in the response to social challenges. In: Brauth S, Hall W, Dooling R, editors. Plasticity of development. Cambridge, MA: MIT Press; 1991. pp. 27–56. [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Gordon TP, Collins DC. Ontogeny of luteinizing hormone secretion and first ovulation in seasonally breeding rhesus monkeys. Endocrinology. 1986;118:293–301. doi: 10.1210/endo-118-1-293. [DOI] [PubMed] [Google Scholar]


