Abstract
Information provided to patients is thought to influence placebo and drug effects. We investigated the potential relationship between treatment labeling and its outcome in a prospective, within-subjects, repeated measures study of episodic migraine. A cohort of 66 participants documented 7 separate migraine attack: one untreated attack, followed by six attacks that were randomly assigned for either rizatriptan (10 mg Maxalt) or placebo treatments, each of which labeled once as ‘Maxalt’, once as ‘Placebo’, and once as ‘Maxalt or Placebo’ (459 documented attacks). Data were analyzed using generalized linear mixed model statistics. While Maxalt was generally superior to placebo, the placebo effect, and to a lesser extent Maxalt efficacy, increased monotonically with treatment labeling as follows: ‘Placebo’ label < ‘Maxalt or placebo’ label ≤ ‘Maxalt’ label. Efficacy of Maxalt mislabeled as placebo was not significantly different from the efficacy of placebo mislabeled as Maxalt. The placebo effect was significant under each labeling condition relative to no treatment, amounting in magnitude to >50% of Maxalt effect under the corresponding labeling condition. Thus, incremental “positive” information yielded incremental efficacy of placebo and medication during migraine attacks.
INTRODUCTION
It is generally thought that placebo and medication efficacies are influenced by contextual factors such as the expectations embedded in the information clinicians provide (1). Much of the evidence for such beliefs is based on “balanced placebo design” experiments concerning mostly addictive or stimulant substances or their placebo controls that disentangle and reassemble placebo and medication effects by providing subjects with various statements, including true, uncertain and false information. These between-subjects studies have shown that information can significantly modulate the impact of such substances (2–6). To ascertain whether these findings apply in a clinical condition, we used a randomized 2x3 within-subjects expanded “balanced placebo design" to test the hypothesis that, in acute migraine, clinical outcomes with both placebo and medication treatment would increase monotonically as information varied from negative (0% chance of receiving active medication) to uncertain (50% chance of medication) to positive (100% chance). A seventh session provided a no-treatment control baseline. As secondary questions, we planned to examine whether medication with negative information (0% chance) was different from placebo with positive information (100% chance) and whether open-label placebo was superior to no-treatment control. In an exploratory fashion, we also planned to examine whether the difference between medication and placebo changes under varying information conditions. We used migraine headache as a model because it is a naturally-recurring neurological disorder of unilateral throbbing headache associated with variable incidence of aura, nausea, photophobia, allodynia, fatigue, and irritability (7). The recurring nature of migraine allowed us to compare within each subject the efficacies of treatment and placebo over consecutive attacks using varying conditions of information.
RESULTS
Participant Enrollment and Characteristics
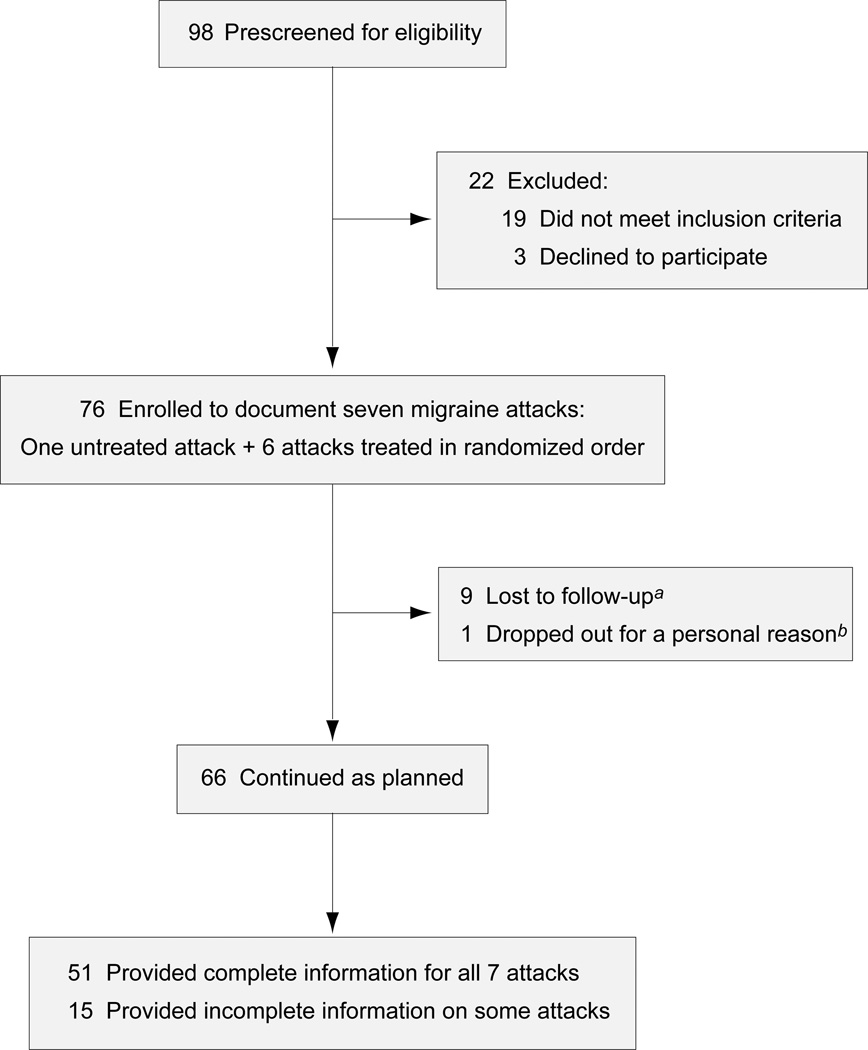
Of 98 persons prescreened for eligibility between December 2008 and March 2010, 19 were excluded for reasons listed in table S1 and 3 declined to participate. The remaining 76 persons signed the consent form, but 10 of them dropped out of the study for various reasons (Fig. 1). The demographic characteristics of the 66 participants and the 10 dropouts were very similar (table S2). Participants had experienced a median of 4 episodic migraine attacks per month (interquartile range [IQR] 2–8), cumulatively lasting a median of 4 (IQR 3–8) migraine days per month (table S3); 25% of them used migraine prophylactic drugs. Of the 66 participants, 51 provided complete data on all 7 attacks, and 15 submitted incomplete data on 1–3 attacks and complete data on the remaining attacks (Fig. 1). None of the subjects reported any unexpected adverse event other than the typical side effects listed in the drug information.
Figure 1. CONSORT flow diagram.
aNine subjects submitted no diary (2 relocated, 3 withdrew from the study, 4 gave no specific reason). bOne withdrew after submitting a diary for the untreated attack.
Study Design and Execution
Participants were required to document one untreated attack at the beginning of the study, and 6 attacks randomly assigned for treatment with a pill of rizatriptan (10 mg Maxalt) or placebo, each labeled once as ‘Maxalt’, once as ‘Placebo’ and once with ‘Maxalt or Placebo’ (Fig. 2 and Table 1). They were asked to record one pain score 30 min after the onset of headache (baseline), take the study pill at the same baseline time, and record a second pain score 2.5 h after the onset of headache. Rescue medications were provide for each attack to be used as needed 2.5 h after the onset of headache. Baseline pain scores were reported in 459 attacks, whereas the 2.5-h pain scores were reported in 435 attacks. Using additional information available in the diaries, we were able to impute 18 of the missing pain scores at 2.5 h, resulting in 453 analyzable attacks (Table 2 and table S4). Generalized linear mixed models were used to analyze the data as described in detail in Materials and Methods and Supplementary Materials.
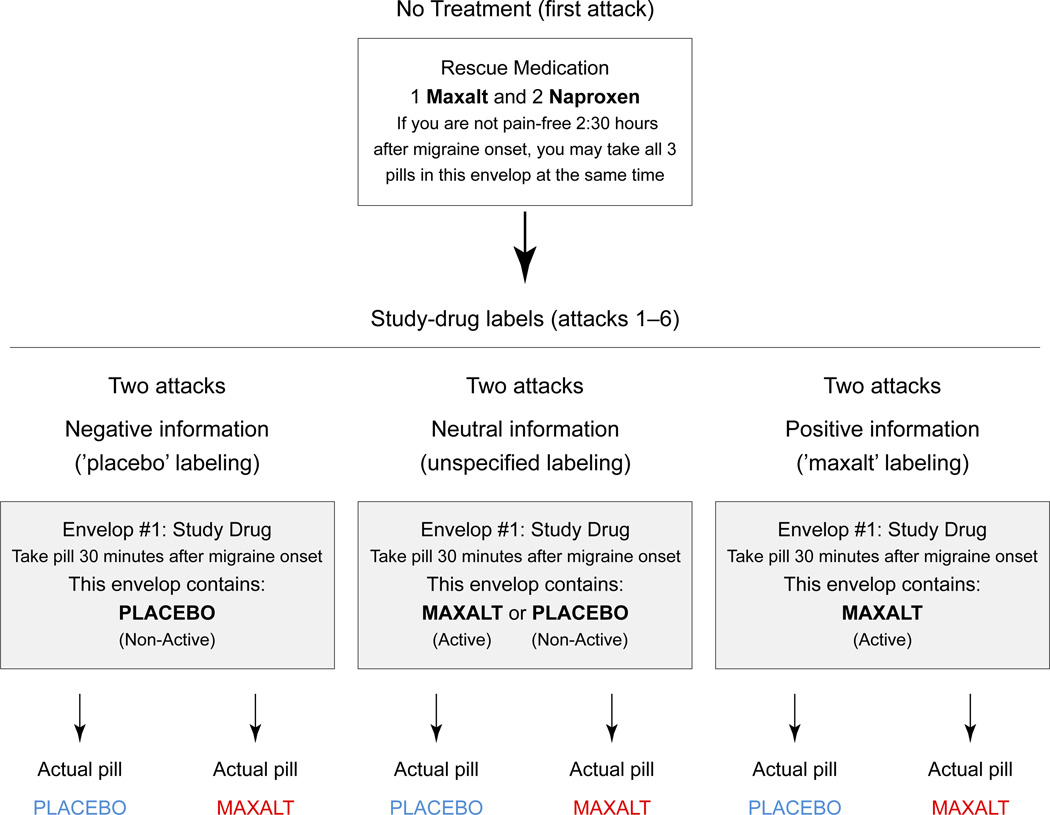
Figure 2. Labeling of the ‘study drug’ and optional ‘rescue medications’ envelopes.
The study drug envelope was labeled ‘placebo’ (2 attacks), ‘Maxalt or placebo’ (2 attacks) or ‘Maxalt’ (2 attacks), in order to provide negative, neutral or positive information, respectively. Subjects were instructed to open the envelope and swallow the pill 30 min after onset of headache. They were asked to refrain from taking rescue medications during the first 2.5 h of each attack, including untreated attack (control).
Table 1.
Study design combining 3 types of labeling and 2 types of treatment
| Accuracy of treatment labeling |
||
|---|---|---|
| Labeling of treatment pill | Given placebo pill | Given Maxalt pill |
| Placebo (negative information) | Correct | Incorrect |
| Maxalt or Placebo (neutral information) | Correct | Correct |
| Maxalt (positive information) | Incorrect | Correct |
Table 2.
Mean pain scores in the 7 study attacks
| Number of attacks (n) and mean (SD) pain scores |
|||||||
|---|---|---|---|---|---|---|---|
| Available values |
Available values |
Available and imputed values |
|||||
| Pill | Label | n | 0.5 h | n | 2.5 h | n | 2.5 h |
| None | No treatment | 66 | 4.6 (1.8) | 63 | 5.3 (2.4) | 66 | 5.4 (2.4) |
| Placebo | Placebo | 65 | 5.9 (2.1) | 57 | 4.8 (3.0) | 63 | 5.0 (3.0) |
| Maxalt or Placebo | 66 | 5.7 (2.0) | 63 | 4.2 (2.6) | 66 | 4.3 (2.7) | |
| Maxalt | 66 | 5.7 (2.0) | 62 | 4.2 (2.9) | 65 | 4.2 (2.9) | |
| Maxalt | Placebo | 65 | 5.6 (2.1) | 63 | 3.3 (2.9) | 65 | 3.4 (3.0) |
| Maxalt or Placebo | 65 | 5.5 (2.0) | 64 | 2.4 (2.8) | 65 | 2.4 (2.8) | |
| Maxalt | 66 | 5.6 (1.9) | 63 | 2.5 (3.0) | 63 | 2.5 (3.0) | |
| Total number of attacks | 459 | 435 | 453 | ||||
Primary Endpoint
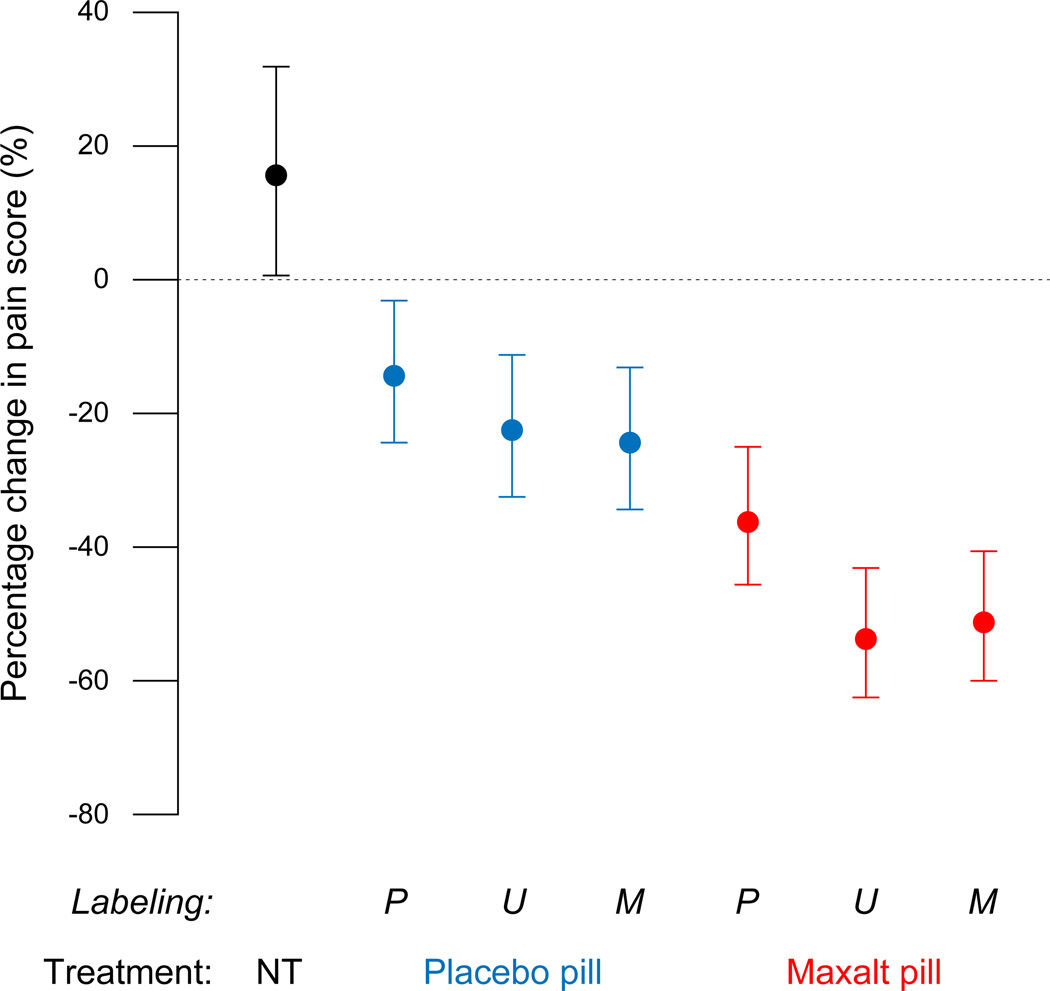
The primary outcome measure was the change in headache between the baseline pain score recorded 30 min after the onset of headache and the pain score recorded 2 h later. Baseline pain scores at time of treatment did not vary significantly (P = 0.75) among the 6 treated migraine attacks (Fig. 3). The typical 2.5-h pain score was significantly lower than the baseline values in the treated attacks (P ≤ 0.015) and significantly higher than baseline values in the untreated attack (P = 0.037),
Figure 3. Changes in headache intensity as a percentage of the 30-min pain score (estimate with 95% confidence intervals.
The within-subjects design of this study allowed each subject to serve as his or her own control, which substantially increased statistical power. Consequently, 95% confidence intervals could not be interpreted in the same manner as in a typical between-subjects study. Thus, two groups can differ significantly even when the mean for one group falls within the 95% confidence interval for the other group. NT = no treatment, P = ‘placebo’ labeling, U = unspecified (‘Maxalt or placebo’) labeling, M = ‘Maxalt’ labeling.
The difference between baseline pain score and the pain score recorded 2 h later was influenced significantly by the treatment pill (P < 0.001) and the labeling of the pill (P = 0.010). The interaction between the two factors was not significant (P = 0.37), which allowed the labeling effect to be summarized across treatments, and the treatment effect to be summarized across labels. Thus, the typical decrease in pain score as a function of labeling was 26.1% [confidence interval CI, 18.2 to 33.2%] with ‘Placebo’ label, 40.1% [CI, 32.1 to 47.0%] with ‘Maxalt or Placebo’ label, and 39.5% [CI, 31.7 to 46.5%] with ‘Maxalt’ label. The typical decrease in pain score as a function of treatment was 47.6% [CI, 41.5 to 53.0%] for Maxalt pill vs. 20.7% [CI, 14.3 to 26.7%] for placebo pill.
Exploratory analyses showed that placebo treatment that was labeled accurately was still associated with a typical 14.5% [CI, 2.9 to 24.6%] decrease in pain scores, which contrasted significantly (P = 0.001) with the typical increase of 15.4% [CI, 0.9 to 31.9%] in the untreated attack. The efficacies of Maxalt mislabeled as placebo (36.1%) and the efficacy of placebo mislabeled as Maxalt (24.6%) were not significantly different from each other (P = 0.127).
Relative to the untreated attack (15.4% average increase in pain) the placebo effect was quite robust under each labeling condition. Under ‘placebo’ label, the average effect of placebo was 30.9% (15.5% + 15.4%) compared to 51.6% (36.1% + 15.4%) in the corresponding maxalt treatment, that is, 60.0% (30.9/51.6) as large as the average effect of Maxalt treatment under the same label. Similarly, the placebo effect was 59.8% as large as the Maxalt effect under ‘Maxalt’ label and 55.3% as large under ‘Maxalt or placebo’ label.
Secondary Endpoint
A secondary measure of attack outcome was based on categorical classification of the pain freedom (pain score = 0) 2.5 h after onset of headache. The proportion of subjects who were pain-free at 2.5 h varied significantly (P < 0.001) among the 6 treated attacks (Fig. 4). The rate of pain freedom was influenced significantly by treatment (P < 0.001), but the effect of labeling did not reach the level of significance (P = 0.053), and there was no significant interaction between the two factors (P = 0.48). As a function of treatment, the typical percentage of participants rendered free of pain was 25.5% [CI, 17.2 to 36.0%] for all Maxalt-treated attacks vs. 6.6% [CI, 3.4 to 12.2%] for all placebo-treated attacks. As a function of treatment labeling, The typical percentage of subjects rendered pain-free was 16.6% [CI, 9.6 to 27.3%] for ‘Maxalt’ label vs. 9.2% [CI, 4.7 to 17.2%] for ‘placebo’ label (P = 0.082) and 15.5% [CI, 8.8 to 25.9%] for ‘Maxalt or placebo’ label.
Figure 4. Percentage of subjects who reported being pain free 2.5 h after onset of headache (estimate with 95% confidence intervals.
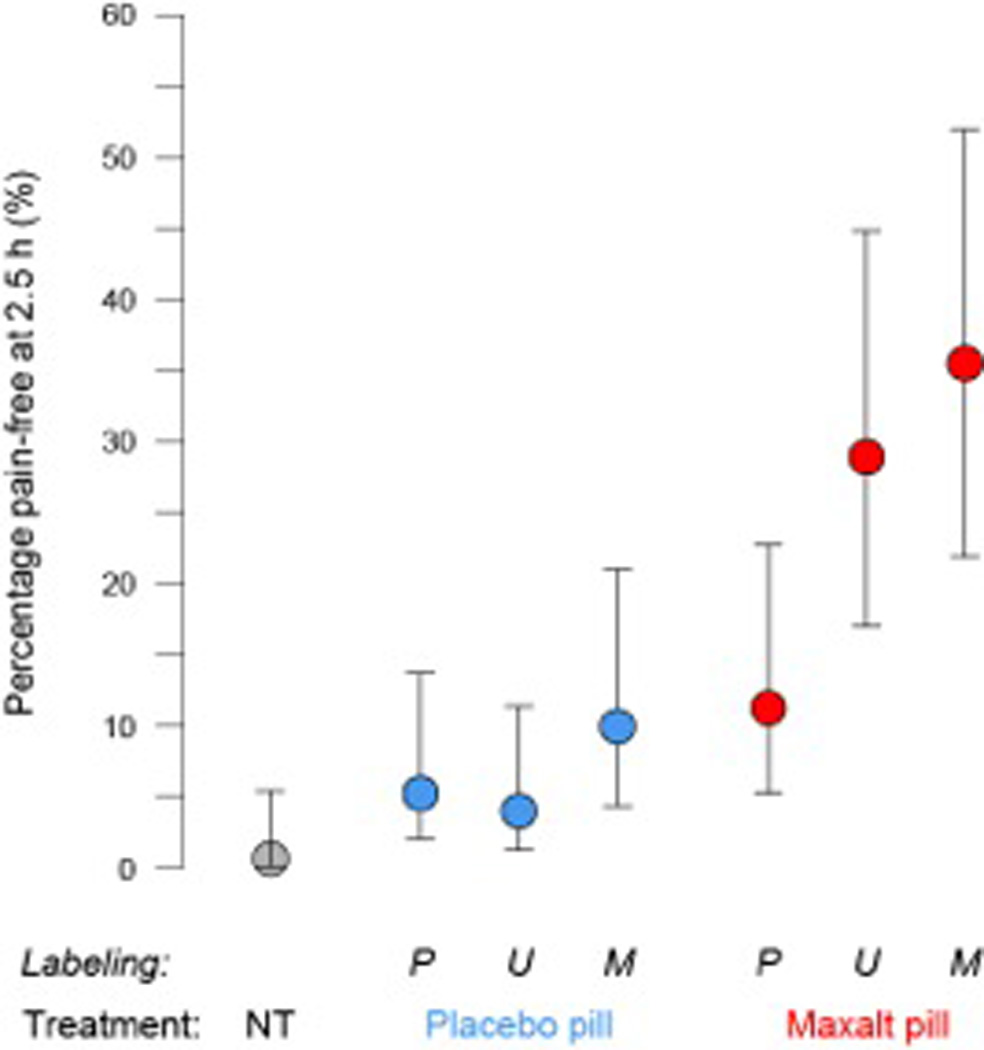
NT = no treatment, P = ‘placebo’ labeling, U = unspecified (‘Maxalt or placebo’) labeling, M = ‘Maxalt’ labeling.
Unlike the primary endpoint, the proportion of participants who were pain-free during the no-treatment condition (0.7%) was not statistically different from when participants took open-label placebo (5.7%). As with the primary endpoint, the proportion of subjects pain-free after treatment was not statistically different between Maxalt treatment mislabeled as placebo (14.6%) and placebo treatment mislabeled as Maxalt (7.7%). The therapeutic gain of maxalt treatment over placebo treatment (maxalt-associated rate of pain freedom minus placebo-associated rate of pain freedom) was 8.8 percentage points under ‘placebo’ label [Odds Ratio (OR), 2.80], 26.6 percentage points under ‘Maxalt or placebo’ labeling (OR, 7.19) and 24.6 percentage points under ‘Maxalt’ labeling (OR, 5.70).
DISCUSSION
By manipulating the information provided to subjects, our primary analysis showed that the magnitude of headache relief induced by Maxalt (10 mg rizatriptan), as well as placebo, was lowest when pills were labeled as placebo, and higher when pills had uncertain labeling or were labeled as active medication. The effect was monotonic for placebo and nearly monotonic for Maxalt. Two other findings were that (a) placebo treatment mislabeled as 10mg Maxalt reduced headache severity as effectively as Maxalt mislabeled as placebo, and (b) open-label placebo treatment was superior to no treatment. We conclude that raising the likelihood of receiving active treatment for pain relief significantly contributed to increased success rate of triptan therapy for migraine, that open-label placebo treatment may have an important therapeutic benefit, and that placebo and medication effects can be modulated by expectancies.
Although Maxalt was superior to placebo under each type of information, we were surprised that the efficacy of Maxalt mislabeled as placebo was not significantly better than the efficacy of placebo mislabeled as Maxalt. We were also surprised to find that open-label placebo treatment induced pain relief as compared to the worsening of pain during the untreated attack. A therapeutic benefit of open-label placebo vs. no treatment was also recently reported for subjects with irritable bowel syndrome in a randomized controlled study (8) and in a pilot study in depression (9).
One of our exploratory goals was to assess whether information provided to subjects can influence the net therapeutic gain of drug treatment (drug efficacy minus placebo efficacy). We found that the difference in pain-free outcome between Maxalt and placebo was reduced by negative information (9 percentage points) compared with neutral (27 percentage points) or positive information (25 percentage points). The results for the primary endpoint were in the same direction, but less dramatic (21.6, 30.8 and 26.9 percentage points for negative, neutral and positive information, respectively). The reduced therapeutic gain when negative information was provided to subjects appeared to reflect a decrease in the efficacy of the drug rather than an increase in the efficacy of the placebo treatment, a conclusion supported by a recent imaging study (10). In the context of triptan therapy for migraine, our data suggest that the therapeutic gains cannot be improved by decreasing positive information; in fact, lower expectations may reduce drug-placebo differences.
In the placebo literature, expectancy is usually defined as the subjective probability of the occurrence of a certain clinical outcome (11). Many placebo researchers would have considered our provision of positive, neutral and negative information as a method for manipulating expectancy (12, 13). However, we did not assess expectancies in our within-subjects experiment because we thought such queries might cause subjects to question the accuracy of the information we provided. Therefore, we cannot assert with certainty that our manipulation worked through changes in conscious or non-conscious expectations due to the information provided (14). We also did not assess blinding to avoid suspicions in a within-subjects design. Because our study used deception, its applicability to routine clinical care is limited, and the present findings are essentially a proof-of-concept. It would be important to expand our findings with experimental manipulations of expectancy considered ethical in clinical practice.
To our knowledge, only one between-subjects study previously examined clinical pain responses of placebo under three different information sets (13). Thoracotomized subjects (n=38), all of whom were treated post-surgically with a fixed dose of buprenorphine, also received a basal intravenous infusion of saline solution for the first 3 days post-surgery. The subjects received three kinds of information concerning the saline: (1) a rehydrating solution (no additional treatment control), (2) either a powerful medication or placebo (uncertain information) or (3) a powerful painkiller (positive information). Request for oral pain medication, the primary outcome measure, was lowest with positive information, highest in the no additional treatment condition, and intermediate in the uncertain condition. Our study improved on this prior study by using a within-subject design, including an uncompromised no-treatment control, and examining both placebo and active treatment responses.
We were surprised that when Maxalt was labeled "Maxalt or Placebo," it had a numerically greater effect on pain (though statistically not significant, P=0.385) than when it was labeled "Maxalt. " We had expected that greater certainty of receiving active medication would result in greater efficacy (as occurred for the placebo experiment described above and the placebo treatment in our experiment). Very few experiments have compared medication under different degrees of certainty. Some have found that certainty increases medication efficacy in pain and anxiety (15, 16), while other studies, for example, in Parkinson’s disease (17) and cancer pain (18) have shown that uncertainty enhances medication effects. A related experiment with 601 asthmatic subjects showed that positive expectations selectively affected placebo but not drug responses (19). Furthermore, anthropological investigations have noted that subjects assessing whether treatment is helpful report that the increased vigilance that comes with uncertainty may increase therapeutic efficacy (20). This conflicting evidence suggests that much research is needed in this domain.
In conclusion, positive information about active medication contributes to successful treatment of episodic migraine. Medication and information (which presumably influences expectancies) may be equally critical for pain relief. The benefits of placebo persist even if placebo treatment is honestly described. Further research is warranted to investigate the application of our findings to clinical practice and research design.
MATERIALS AND METHODS
Participants
Subjects were recruited from Beth Israel Deaconess Medical Center (BIDMC) outpatient clinics, signed an informed consent in a prescreening visit, and met with investigators (SKH and RB) to determine eligibility. Subjects were eligible if they met the criteria of the International Headache Classification Committee for Migraine (7), had suffered from episodic migraine attacks (with and without aura) in the past 3 years, and were at least 18 years old. Excluded were non-migraine headache, peripheral nervous system injuries, chronic pain conditions, opioid use, cardiovascular or cerebrovascular disorders, cardiac risk factors, or uncontrolled hypertension. Eligible subjects were informed that the purpose of the study was to understand the effects of 10 mg rizatriptan (orally disintegrating melting tablet, brand name Maxalt-MLT, Merck and Co., Inc., Whitehouse Station, NJ) and placebo treatment (microcrystalline cellulose, Merck) on acute migraine. The study was carried out according to the ethical standards of the BIDMC IRB in compliance with Helsinki Declaration, Federal Regulation 46.116c.
Study Design
The study followed migraine headache subjects over 7 migraine attacks. Baseline pain intensity in the absence of treatment was measured in an untreated first attack. Then subjects self-reported response to treatment over the next 6 attacks, for which they were given 1 of 2 treatments (Maxalt or placebo) labeled in 1 of 3 ways (Maxalt, placebo, Maxalt or placebo) with labels that were true (for 4 attacks) or false (for 2 attacks) (Fig. 2, Table 1).
Medications
At the end of the prescreening visit, each subject received 7 sealed white envelopes, one for each successive migraine attack. The envelope for the first attack (No-Treatment) contained a 5-page diary concerning details of symptomatology and a small brown envelope containing rescue medications (one 10 mg Maxalt and two 220 mg naproxen for relief 2.5 hours after attack onset) (Fig. 2). Envelopes for the subsequent 6 attacks contained a 4-page diary and two small brown envelopes, one labeled ‘Study Drug’ (to be taken . hour after headache onset) and the other ‘Rescue Medication’ (containing one 10 mg Maxalt and two 220 mg naproxen, to be taken 2.5 hours after headache onset if the study drug was ineffective). Subjects were instructed to open only one white envelope at the beginning of each attack, to do so in the exact order indicated on the envelopes (Treatment 1 through 6 in sequence), and to call the responsible physician anytime (SKH) to report any adverse event or if they had questions. Subjects were also told that, if needed, they could use the rescue medications 2.5 hours after the onset of the attack.
Intervention
The study intervention was manipulation of information about study pill identity to assess the effects of that information on treatment efficacy. At the time of study enrollment subjects were informed that the Study Drug envelope contained 10 mg Maxalt or placebo, that Maxalt is a medication for aborting migraine headache, that it works best when taken 30 minutes after onset of headache, and that the placebo pill looks and tastes the same as the Maxalt pill but contains no active medication. They were also informed that for the 6 study migraine attacks following the baseline attack, the brown Study Drug envelope would be labeled ‘Maxalt’, ‘placebo’, or ‘Maxalt or placebo’ for 3 randomly ordered pairs of treatments. It was not disclosed to them that 2 of the 6 study drug envelopes were labeled incorrectly: one envelope containing a placebo tablet was mislabeled as Maxalt (to maximize placebo effect), and one envelope containing a tablet of Maxalt was mislabeled as placebo (to minimize drug effect). Exemption from full transparency for the two attacks that involved deception was granted by the institutional review board in compliance with the 4 criteria specified by Federal Regulation 46.116c (http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html#46.116) for Protection of Human Subjects (accessed 5.31.12) because the deception involved minimal additional risk, did not adversely affect the rights and welfare of the subjects, was necessary to practically answer an important question, and was followed with a full debriefing at the end of the study.
Randomization
Subjects were randomly assigned in blocks of 8 to 8 sequence patterns that balanced the order of the 2 treatments and 3 types of information (Supplementary Material and Methods: table S5, table S6 and the section Randomization of Treatment and Treatment Labeling). A spreadsheet summarizing the randomization scheme for each subject was placed in a sealed envelope and given to the treating physician (SKH) to be opened in case of unexpected reaction or medical emergency. All study personnel were blind to treatment allocation.
Outcomes
The primary study outcome was the percentage change from baseline in headache intensity 2 h after treatment using self-reported pain assessments obtained from diary entries that subjects completed during each attack at home. The secondary study outcome was the proportion of subjects pain-free 2 h after treatment. Pain level was assessed on a discrete numerical scale ranging from 0 (no pain) to 10 (maximal pain). Subjects returned completed diaries after each attack.
Statistical Analysis
Our statistical analysis is detailed in Supplementary Material and Methods. Briefly, our main objective was to assess whether the effect on the primary and secondary endpoints differed among the three types of information and whether the effect differed between the two treatments. We also made exploratory comparisons. For the primary endpoint, change in headache intensity from baseline to 2 h after treatment, we used generalized linear mixed models with a normal random component and a logarithmic link function to analyze the pain scores. Because the systematic component of the model is on the logarithmic scale, estimates of differences in its parameters translate into estimates of ratios on the pain-score scale, which one can interpret as percentage increase or decrease. These approximate ratios of averages are not literally ratios of means (or means of ratios). Phrases such as “typical value” reflect this feature of the analysis; one can interpret “typical values” as similar to means. For the secondary endpoint, the proportion of subjects who were free from pain 2 h after treatment, we used a mixed-effects logistic regression model to analyze the individual dichotomous outcomes. Its systematic component on the log-odds scale yields estimates that translate into odds, probabilities, and odds ratios. Preliminary analysis of each endpoint verified that the 8 sequence patterns had no statistically significant effects on treatment outcomes and no interactions with labeling or with treatment. Seven participant baseline characteristics (age, sex, family history, years of migraine, attacks per month, migraine days per month, triptan history) were potential covariates, but, when added jointly to the primary and secondary models, none were statistically significant. In an exploratory manner, we examined several pre-specified pairwise comparisons (‘Maxalt labeled placebo’ versus ‘placebo labeled Maxalt’, ‘placebo labeled placebo’ versus ‘no treatment’) and whether the therapeutic gain (the difference between Maxalt and placebo) differed among the information conditions.
To deal with missing data, we compared background characteristics of participants who provided no data on any treated attack (dropouts) with those of participants who provided some or all data, using t-tests for continuous characteristics and Fisher’s exact test for dichotomous characteristics; continuous background variables were expressed as mean (SD) or as median and quartiles (when the data were clearly skewed), and missing data from subjects who did not contribute any data to the study was considered uninformative. When a pain score 2 h after treatment was missing because the subject resorted prematurely to rescue medications, the missing value was imputed using either the pain score at 30 min or a subsequent higher pain score. Missing pain scores not associated with premature rescue treatment (e.g., the subject fell asleep) were not imputed.
Supplementary Material
Acknowledgments
We thank Norma Nighelli for administrative, technical and logistic support.
Funding: Primary funding source: Merck and Co., Inc. Dr. Burstein received grant support from Merck and Co., Inc., and from the National Institutes of Health (R01 NS069847, R37 NS079678). Dr. Kaptchuk and Dr. Kelley were partially supported by NIH-NCAAM (K24 AT004095). Dr. Kaptchuk was partially funded by the Blue Guitar Foundation. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript both on the submission form and in the text of the manuscript.
Footnotes
Author contributions: Conception and design: T.J. Kaptchuk, R. Burstein, I. Kirsch, J.M. Kelley, M. Jakubowski.
Analysis and interpretation of the data: D.C. Hoaglin, M. Jakubowski, J.M. Kelley, I. Kirsch, T.J. Kaptchuk, R. Burstein.
Drafting of the article: R. Burstein, M. Jakubowski, T.J. Kaptchuk, D.C. Hoaglin, J.M. Kelley.
Critical revision of the article for important intellectual content: M. Jakubowski, T.J. Kaptchuk, R. Burstein, D.C. Hoaglin, J.M. Kelley, I. Kirsch.
Final approval of the article: R. Burstein, T.J. Kaptchuk, M. Jakubowski, J.M. Kelley, D.C. Hoaglin.
Provision of study materials or participants: S. Kam-Hansen, R. Burstein.
Statistical expertise: D.C. Hoaglin, J.M. Kelley, M. Jakubowski.
Obtaining of funding: R. Burstein, T.J. Kaptchuk.
Collection and assembly of data: J.M. Kelley, M. Jakubowski, S. Kam-Hansen, R. Burstein.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). RB received research support (grant) for this study from Merck and Co., Inc. He was a member of Merck’s speaker bureau and received honoraria for lectures on topics unrelated to the current study. RB also consults for Allergan. The other authors declare no competing interests.
REFERENCES
- 1.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010 Feb 20;375:686. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metrik J, et al. Balanced placebo design with marijuana: pharmacological and expectancy effects on impulsivity and risk taking. Psychopharmacology (Berl) 2012 Oct;223:489. doi: 10.1007/s00213-012-2740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002 Oct;164:61. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, et al. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neurolmage. 2006 Oct 1;32:1782. doi: 10.1016/j.neuroimage.2006.04.192. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell SH, Laurent CL, de Wit H. Interaction of expectancy and the pharmacological effects of d-amphetamine: subjective effects and self-administration. Psychopharmacology (Berl) 1996 Jun;125:371. doi: 10.1007/BF02246020. [DOI] [PubMed] [Google Scholar]
- 6.Hull JG, Bond CF., Jr Social and behavioral consequences of alcohol consumption and expectancy: a meta-analysis. Psychol Bull. 1986 May;99:347. [PubMed] [Google Scholar]
- 7.The International Classification of Headache Disorders. Cephalalgia. (2nd edition) 2004;24(Suppl 1):9. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 8.Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom. 2012;81:312. doi: 10.1159/000337053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaptchuk TJ, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. Plos One. 2010;5:el5591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenk LA, Sprenger C, Geuter S, Buchel C. Expectation requires treatment to boost pain relief: An fMRI study. Pain. 2013 Sep 26; doi: 10.1016/j.pain.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch I. Response expectancy as a determinate of experience and behavior. American Psychologist. 1985;40:1189. [Google Scholar]
- 12.Benedetti F, et al. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. Journal of Neuroscience. 2003;23:4315. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polio A, et al. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 14.Jensen KB, et al. Nonconscious activation of placebo and nocebo pain responses. Proc NatlAcadSci USA. 2012 Sep 25;109:15959. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amanzio M, Polio A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. Pain. 2001;90:205. doi: 10.1016/S0304-3959(00)00486-3. [DOI] [PubMed] [Google Scholar]
- 16.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. The Lancent Neurology. 2004;3:679. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- 17.Lidstone SC, et al. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Archives of general psychiatry. 2010 Aug;67:857. doi: 10.1001/archgenpsychiatry.2010.88. [DOI] [PubMed] [Google Scholar]
- 18.Bergmann JF, et al. A randomised clinical trial of the effect of informed consent on the analgesic activity of placebo and naproxen in cancer pain. Clinical trials and meta-analysis. 1994 Apr;29:41. [PubMed] [Google Scholar]
- 19.Wise RA, et al. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. The Journal of allergy and clinical immunology. 2009 Sep;124:436. doi: 10.1016/j.jaci.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaptchuk TJ, et al. "Maybe I made up the whole thing": placebos and patients' experiences in a randomized controlled trial. Culture, medicine and psychiatry. 2009 Sep;33:382. doi: 10.1007/s11013-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.