Abstract
The ErbB tyrosine kinases (epidermal growth factor receptor (EGFR), ErbB2/HER2, ErbB3, and ErbB4) are cell surface growth factor receptors widely expressed in many developing mammalian tissues, including in the intestinal tract. Signaling elicited by these receptors promotes epithelial cell growth and survival, and ErbB ligands have been proposed as therapeutic agents for intestinal diseases of pediatric populations, including inflammatory bowel diseases (IBD), necrotizing enterocolitis (NEC), and inflammation associated with total parenteral nutrition (TPN). Furthermore, emerging evidence points to reduced ErbB ligand expression and thus reduced ErbB activity in IBD, NEC, and TPN models. This review will discuss the current understanding of the role of ErbB receptors in the pathogenesis and potential treatment of pediatric intestinal inflammation, with focus on the altered signaling in disease and the molecular mechanisms by which exogenous ligands are protective.
Inflammatory disorders of the intestine are a major source of morbidity and mortality in the pediatric population. A significant proportion of inflammatory bowel disease (IBD) diagnoses are in children and adolescents, with an apparent increase in incidence over the last two decades 1,2. Necrotizing enterocolitis (NEC) affects up to 10% of premature infants 3 and has up to 30% mortality 4. Sepsis and infection are major complications of parenteral nutrition 5, which in rodent models are associated with tumor necrosis factor (TNF)-driven intestinal inflammation 6. Together, these conditions represent a major burden on public health in the developed world.
The most widely-used therapeutic approaches for these disorders generally focus on anti-inflammatory agents (e.g., corticosteriods or anti-TNF biologicals for IBD) and supportive care. However, currently available agents are not effective in all patients, and relapses are common. Furthermore, significant long-term safety concerns with a number of drugs (possible growth retardation with corticosteriods 7, risk of lymphoma with biologicals 8, etc.) limit options with a significant number of patients. Thus, new approaches to treating pediatric intestinal inflammation are desperately needed.
The mixed effectiveness of exclusively anti-inflammatory agents in IBD and NEC may, in part, be because a major element of disease pathophysiology—damage to the intestinal epithelium—is not a direct target of these approaches. For example, mucosal healing is a desirable endpoint in IBD, and in some studies it predicts long-term remission 9,10, but it is generally expected to occur through endogenous mechanisms secondary to the inhibition of inflammation. Thus, strategies aimed specifically at protecting or repairing the epithelium are attractive options to augment anti-inflammatory treatment in these diseases. A major class of molecules which has been studied in this regard are the ErbB family of receptor tyrosine kinases (RTKs) and their cognate ligands, which promote intestinal epithelial cell growth 11,12, survival 13,14, and restitution/wound healing 15,16. They may therefore be good models for therapeutic agents for intestinal diseases of pediatric populations. Intriguingly, accumulating evidence makes it clear that defects in ErbB signaling occur, and may be causal, in multiple intestinal inflammatory conditions 17–20. Thus, replacement or reactivation of the ligands and receptors might be effective at directly promoting mucosal healing.
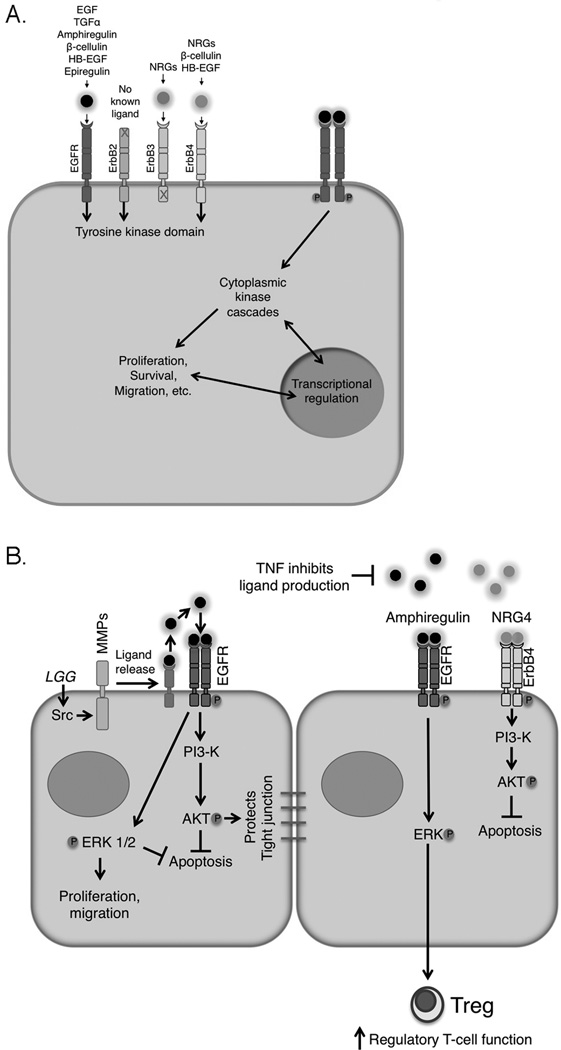
The ErbB family includes the prototypic member epidermal growth factor (EGF) receptor (R)/ErbB1 as well as ErbB2, ErbB3, and ErbB4 (Figure 1A). ErbBs transduce signals from outside the cell by recognizing, with varying affinities and specificities, growth factors from the EGF and heregulin/neuregulin (NRG) families. Most ErbB ligands are detectable in the intestine 6,17. After ligand binding, ErbBs signal as dimers (both homo- and hetero-dimers) through increased kinase activity and autophosphorylation on c-terminal cytoplasmic tyrosines; these phosphotyrosine residues then provide docking sites for downstream substrates and adapter proteins. The four ErbBs all share significant homology, but have distinct individual properties. EGFR and ErbB4 each have exclusive (EGF and NRG4 respectively, for example) as well as shared (betacellulin and HB-EGF, for example) ligands, while ErbB3 binds a subset of the NRG family. ErbB2 has no known ligand 21, and ErbB3 has greatly attenuated kinase activity relative to ErbB 1, 2, and 4 22, and thus these family members are thought to signal primarily in hetero-dimers. These distinct properties, as well as a variable set of downstream molecules that can attach to c-terminal phosphotyrosine docking sites 23 provide intricate networks of ligand/receptor/target combinations that are just beginning to be understood.
Figure 1.
ErbB receptors and their ligands play key roles in intestinal epithelial integrity. (A) The ErbB family consists of four receptors which bind a suite of ligands with varying specificity. Ligand binding promotes homo- and hetero-dimer formation, autophosphorylation, and downstream signaling involving both cytoplasmic kinases and transcriptional changes. (B) Multiple protective mechanisms are triggered by ErbB activation, including ERK MAPK and PI3K/Akt signaling. These cascades restrict apoptosis, promote proliferation and migration, maintain barrier integrity, and contribute to immune system regulation. Metalloproteinase-dependent ErbB ligand cleavage and release can be triggered by extracellular signals such as probiotic/commensal bacteria (Lactobacillus rhamnosus GG shown as example). However, inflammatory cytokines can inhibit expression of multiple ErbB ligands, potentially limiting responses in the absence of exogenous ligand.
ErbB activation in cultured intestinal cells promotes cellular outcomes that would be expected to be protective during inflammation (Figure 1B). For example, in colon epithelial cells EGFR stimulates proliferation 12,24, reduction in cytokine-induced apoptosis 13, and migration/wound healing 15,16, both in vitro and in vivo. Selective responses can be invoked by particular ligand/receptor combinations; NRG4, which exclusively activates ErbB4 25, signals for mouse colonocyte survival but not proliferation or migration 17. This suggests that, with better understanding of the relative effects of different ligands and receptors, a significant degree of selectivity in response can be obtained.
Inflammatory bowel disease
EGFR’s capacity to promote healing of gastrointestinal ulcers has been appreciated since the 1980s 26. Since then, a number of studies using rodent models have demonstrated a protective role in intestinal inflammation. Mice with defective EGFR activity 27,28 or lacking the EGFR ligand TGF-α 29 are more susceptible to experimental colitis, while mice over-expressing TGF-α 30 or rats treated with exogenous EGF 31 are resistant. EGFR is furthermore responsible for the effects of some other treatments; for example, proteins derived from the probiotic organism Lactobacillus rhamnosus GG ameliorate dextran sulfate sodium colitis in mice by an EGFR- and Akt-dependent mechanism 32. In that model, the mechanism involved stimulation of metalloproteinase-mediated ligand release 33, which is a common theme for EGFR transactivation by other pathways such as G protein coupled receptors 34 or toll-like receptor/MyD88 signaling 35.
Multiple cellular effects of EGFR signaling contribute to its protective effects in colitis. In addition to promoting epithelial cell survival, proliferation, and restitution, EGF rescues colitis-associated perturbations of transepithelial ion transport 36. Furthermore, ErbB signaling is not only a target of inflammation but also plays an immunomodulatory role; for example, amphiregulin signals through EGFR to support regulatory T cell function 37.
ErbB2-4 also play protective but distinct roles in murine colitis models. Epithelial-specific knockout of ErbB2 or ErbB3 does not show profound effect on the onset of injury in DSS colitis, but long-term recovery is dramatically impaired in these mice 38. Additionally, ErbB4 activation, by i.p administration of its selective NRG4 ligand, blocks cytokine-induced colonocyte apoptosis and reduces injury in acute DSS colitis 17. This response is dependent on PI3K/Akt signaling similar to results seen with EGF. However, unlike EGF, NRG4 has no effect on cell proliferation or migration, apparently acting strictly through an anti-apoptotic pathway. Together these results indicate that ErbB family members play distinct but complementary roles in protecting or repairing the intestinal mucosa.
Evidence from several studies shows that ErbB signaling is altered in IBD, though the results have not always been in agreement. Multiple studies of clinical specimens have reported reduced levels of EGFR or EGFR ligands in ulcerative colitis (UC) or Crohn’s disease (CD) patients 19,39. For example, a recent study of over fifty patients each in healthy control, CD, and UC groups showed reduced serum EGF in the IBD patients 20. We have also noted a reduction in the ErbB4-specific NRG4 ligand in active UC and CD compared to uninflamed controls 17. On the other hand, some groups have described increased expression of specific ligands such as amphigregulin in inflamed tissue 40 or increased EGFR 41 and EGFR ligand 42 in rodent colitis models. These apparently contradictory results may be in part a result of assaying expression at different phases of disease (e.g., acute vs. chronic vs. recovery), but may also reflect the natural complexity and interdependency of the different ErbBs and their ligands. For example, we find increased ErbB4 receptor expression in colitis 43 but downregulation of its ligand NRG4, which in IL-10−/− mice actually results in a relative decrease in ErbB4 activation 17. A thorough understanding of coordinate changes in multiple ligands and receptors, as has recently been attempted in the context of TPN 6, will likely be informative and necessary.
Importantly, EGF was effective in a 2003 double-blind clinical trial with ulcerative colitis 44. Twelve patients per group were given either EGF or vehicle daily (by enema) for two weeks in combination with oral mesalamine. By two weeks, 10/12 of the EGF patients compared to 1/12 controls were in remission. Remission was maintained for >3 months. As proof of concept, this trial demonstrates the potential effectiveness of ErbB ligands for treating IBD.
Nectotizing Enterocolitis
EGF is present in human milk 45, which is protective against NEC, and premature infants with NEC have reduced intestinal and serum EGF 46. Two EGFR ligands, EGF and HB-EGF, have been extensively tested in rodent models of NEC. Most studies have used the “formula feeding/hypoxia” (FFH) rat model, in which prematurely delivered rat pups are fed by formula and subjected to several rounds of hypoxia and cold stress, with or without variations including LPS exposure along with formula 47,48. This protocol induces a reproducible and highly penetrant NEC-like pathology.
Luminal EGF treatment is protective in FFH NEC 49, reducing disease onset and severity and blocking ileal epithelial apoptosis and histological damage 50. It also improves many of the model’s physiological symptoms and molecular signatures. NEC-associated increase in bile acids is ameliorated by EGF 51, as are the loss of barrier integrity and dissolution of tight junctions within the epithelium 52, and the overexpression of IL-18 53. Furthermore, a recent study showed that in both human and mouse NEC, EGFR levels are significantly reduced, but amniotic fluid is protective against mouse NEC by dampening TLR4 signaling in an EGFR-dependent manner 18. Thus there is ample evidence that loss of EGF-driven signaling is associated with NEC development, and replacing this signal may be therapeutic. In fact, a limited 2007 trial with recombinant EGF in severe NEC showed improvement in several clinical parameters 54.
Similar to EGF, HB-EGF administration reduces experimental NEC incidence, severity, and barrier defects 55 in rat pups. This is accompanied by reductions in epithelial apoptosis 56 and increased proliferation and migration 11. Microcirculation in the intestine, normally compromised in the FFH model, is preserved 57, as are repair-inducing signaling pathways such as MAPK and PI3K 58. Similar to the rat data, FFH NEC experiments with transgenic mice either over-expressing 59 or deficient 60 in HB-EGF confirmed a protective role for this growth factor.
There has been some disagreement in the literature over the relative effectiveness of EGF vs. HB-EGF 47,48, which may in part be due to differences in the experimental conditions used, for example the presence or absence of LPS added in the FFH model. This variability with apparently small changes suggests the need to expand the study of ErbB ligands in NEC to additional models such as the recently described dithizone/Klebsiella mouse protocol 61, in order to test which compounds work well over a range of experimental systems and thus maximize translatability.
Parenteral Nutrition
Intestinal atrophy and barrier dysfunction as a result of TPN appear particularly amenable to improvement with EGF. Treatment rescues the crypt proliferative index 62, reduces mucosal atrophy and bacterial translocation 63, and partly reverses loss of Gln uptake 64 in rats given TPN. The trophic effects of EGF can apparently be enhanced by combined administration of other growth factors such as GLP-2 65. As a leaky barrier and associated sepsis are major complications of TPN in pediatric patients 66, these results definitely suggest a role for ErbB signaling in improving outcomes of patients who cannot tolerate enteral nutrition. For example, TPN adds additional complexity to the problem of managing short bowel syndrome after massive resection, and EGF has been shown to be effective in supporting intestinal adaptation in rodent models of SBS with TPN 67,68.
A recent string of papers from the Teitelbaum lab using a mouse model of TPN have demonstrated not only a protective role for EGF in the model, but also a causative role for loss of EGFR activity in the onset of pathology. In this model, animals fed only parenteral nutrition develop intestinal inflammation characterized by decreased proliferation and increased apoptosis in the epithelium, and at the molecular level by reduced signaling through the PI3K/Akt cascade 69. Forced activation of Akt improved disease, as did supplementation with glutamine 70. Since both Akt phosphorylation 71 and glutamine transport 64 are targets of EGFR signaling, these results suggest a defect in that pathway, which is what was found. In TPN mice, there is a TNF-driven, TNFR1-dependent loss of EGFR signaling 6. This was accompanied by decreased expression of most ErbB ligands including EGF, TGF-α, HB-EGF, NRG1, and NRG4. Exogenous EGF improved pathology and survival, interestingly in a TNFR2-dependent manner.
The observation that ligands for ErbB3 and ErbB4 (e.g., NRG1, NRG4) are also altered in TPN suggests that signaling through these receptors is likely dysregulated in disease as well. Loss of NRG4 and an accompanying reduction in ErbB4 signaling would be consistent with our own observations in colitis 17 and NEC models (Castle, McElroy, and Frey, unpublished observations). However, the specific role that altered ErbB3 or ErbB4 signaling might play in TPN is still an open question.
Potential issues with targeting ErbBs in inflammation
Despite numerous in vitro and animal studies over the last two and a half decades and a few small but promising clinical trials, only limited progress has been made towards translating the mucosal protective effects of ErbB RTK signaling into therapeutic use. In large part this is due to reluctance to chronically promote the activity of receptors which, when mutated or over-expressed, may be oncogenic. This is certainly a valid concern, especially given the predisposition of patients with inflammation of the intestine to colorectal cancer development 72,73. However, several lines of evidence argue against CRC promotion being a major issue for GF therapy: the fundamental difference between mutation of a pathway and activation of the endogenous wild type signaling, possible immuno-modulatory effects of GF treatment, and the idea that the anti-tumorigenic effects of wiping out inflammation may be much greater than any tumor promoting effects.
EGFR, ErbB2, and ErbB3 are indeed over-expressed or expressed in the form of constitutively active mutants in many cases of intestinal neoplasia 74–76. The role of ErbB4 in CRC is as yet less clear, but its expression has been reported in tumors with high levels in a subset 77. Thus, the ErbB family members are at least candidate proto-oncogenes. However, there are likely differences between expression at supra-physiologic levels or expression of constitutively active mutants versus activation of wild-type receptor with ligand. Mutant or hyper-expressed receptors exhibit defects in location or duration of signaling 78, and ErbB mutations which inhibit ligand-stimulated down-regulation resulting in inappropriately sustained signaling have been described 79. In contrast, application of exogenous ligand to wild-type receptor triggers a more acute, physiological response, which in the case of wild type receptors is terminated over time 78. Furthermore, it was recently shown that loss of endogenous EGFR signaling can in fact promote colon carcinogenesis in mice. When crossed with the EGFR hypomorphic Waved-5 allele, IL-10−/− animals displayed, increased inflammation, colonic crypt hyperproliferation, increased DNA damage in enterocytes, and dramatically accelerated onset of colitis-associated tumors 28. Similar results were observed in the AOM-DSS model. Thus, while excessive, “always-on” EGFR activity may contribute to tumorigenesis, insufficient signaling through this pathway contributes to chronic inflammation and, in the end, is also tumorigenic. As ErbB signaling levels appear to be reduced in IBD patients 17,19,39, application of ErbB ligands may actually be anticarcinogenic in this setting. Further study into this complex issue is warranted.
In addition to concerns regarding unintended effects of growth factor signaling, protein growth factors may present difficulties with bioavailability, stability, and convenient administration. In the single clinical trial of EGF for ulcerative colitis published to date, it was given in an enema preparation daily for 2 weeks 44, which is not a convenient means for wide-scale treatment. Alternative approaches using coated beads designed to release growth factor only in the lower intestinal tract are possible alternatives. Additionally, promoting ErbB signaling through indirect mechanisms may be a useable approach. For example, EGFR transactivation by Lactobacillus rhamnosus GG-derived soluble proteins is effective in mouse models of colitis 32, as is Lactobacillus-fermented milk 80. Evidence suggests that the protective effects of these probiotic-derived proteins is through stimulating local release of EGFR ligand.
Conclusions
Since the observation, decades ago, that urogastrone/EGF is a trophic factor for the intestinal epithelium, growth factor treatment of intestinal inflammatory disorders has been a theoretical possibility. Recent advances defining how EGF-like growth factors and their ErbB receptors affect—and are affected by—inflammatory signaling suggest that this signaling axis still has great potential to treat the epithelial damage in settings such as IBD, NEC, or TPN, perhaps in combination with anti-inflammatory agents to form a two-pronged attack on disease. Ongoing work towards understanding signaling specificity and selective activation mechanisms will likely open the way towards clinical use of ErbB signaling.
ACKNOWLEDGMENT
We thank Shivesh Punit, who assisted with manuscript revisions and figure preparation.
STATEMENT OF FINANCIAL SUPPORT: This work was supported by National Institutes of Health grants R01DK095004 (MRF), R03DK090295 (MRF), and R01DK056008 (DBP), and by Senior Research Awards from the Crohn’s and Colitis Foundation of America (MRF and DBP).
Footnotes
DISCLOSURE: Mark Frey has a patent application pending on the possible therapeutic use of NRG4 in intestinal inflammation.
REFERENCES
- 1.Martin-de-Carpi J, Rodriguez A, Ramos E, Jimenez S, Martinez-Gomez MJ, Medina E. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996–2009): the SPIRIT registry. Inflamm Bowel Dis. 2013;19:73–80. doi: 10.1002/ibd.22980. [DOI] [PubMed] [Google Scholar]
- 2.Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519–525. doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC) Pediatr Res. 2008;63:117–123. doi: 10.1203/PDR.0b013e31815ed64c. [DOI] [PubMed] [Google Scholar]
- 4.Blakely ML, Lally KP, McDonald S, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg. 2005;241:984–989. doi: 10.1097/01.sla.0000164181.67862.7f. discussion 9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–511. doi: 10.1097/00000658-199205000-00013. discussion 11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Y, Teitelbaum DH. Epidermal growth factor/TNF-alpha transactivation modulates epithelial cell proliferation and apoptosis in a mouse model of parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2012;302:G236–G249. doi: 10.1152/ajpgi.00142.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezri J, Marques-Vidal P, Nydegger A. Impact of disease and treatments on growth and puberty of pediatric patients with inflammatory bowel disease. Digestion. 2012;85:308–319. doi: 10.1159/000336766. [DOI] [PubMed] [Google Scholar]
- 8.Cucchiara S, Escher JC, Hildebrand H, Amil-Dias J, Stronati L, Ruemmele FM. Pediatric inflammatory bowel diseases and the risk of lymphoma: should we revise our treatment strategies? J Pediatr Gastroenterol Nutr. 2009;48:257–267. doi: 10.1097/mpg.0b013e31818cf555. [DOI] [PubMed] [Google Scholar]
- 9.Af Bjorkesten CG, Nieminen U, Sipponen T, Turunen U, Arkkila P, Farkkila M. Mucosal healing at 3 months predicts long-term endoscopic remission in anti-TNF-treated luminal Crohn's disease. Scand J Gastroenterol. 2013;48:543–551. doi: 10.3109/00365521.2013.772230. [DOI] [PubMed] [Google Scholar]
- 10.Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010;138:463–468. doi: 10.1053/j.gastro.2009.09.056. quiz e10-1. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2007;42:214–220. doi: 10.1016/j.jpedsurg.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 12.Frey MR, Dise RS, Edelblum KL, Polk DB. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J. 2006;25:5683–5692. doi: 10.1038/sj.emboj.7601457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaoka T, Yan F, Cao H, et al. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc Natl Acad Sci USA. 2008;105:11772–11777. doi: 10.1073/pnas.0801463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frey MR, Hilliard VC, Mullane MT, Polk DB. ErbB4 promotes cyclooxygenase-2 expression and cell survival in colon epithelial cells. Lab Invest. 2010;90:1415–1424. doi: 10.1038/labinvest.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology. 1998;114:493–502. doi: 10.1016/s0016-5085(98)70532-3. [DOI] [PubMed] [Google Scholar]
- 16.Yamaoka T, Frey MR, Dise RS, Bernard JK, Polk DB. Specific epidermal growth factor receptor autophosphorylation sites promote mouse colon epithelial cell chemotaxis and restitution. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00327.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard JK, McCann SP, Bhardwaj V, Washington MK, Frey MR. Neuregulin-4 is a survival factor for colon epithelial cells both in culture and in vivo. J Biol Chem. 2012 doi: 10.1074/jbc.M112.400846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good M, Siggers RH, Sodhi CP, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci USA. 2012;109:11330–11335. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander RJ, Panja A, Kaplan-Liss E, Mayer L, Raicht RF. Expression of growth factor receptor-encoded mRNA by colonic epithelial cells is altered in inflammatory bowel disease. Dig Dis Sci. 1995;40:485–494. doi: 10.1007/BF02064355. [DOI] [PubMed] [Google Scholar]
- 20.Oikonomou KA, Kapsoritakis AN, Kapsoritaki AI, et al. Downregulation of serum epidermal growth factor in patients with inflammatory bowel disease. Is there a link with mucosal damage? Growth Factors. 2010;28:461–466. doi: 10.3109/08977194.2010.527967. [DOI] [PubMed] [Google Scholar]
- 21.Brennan PJ, Kumagai T, Berezov A, Murali R, Greene MI. HER2/Neu: mechanisms of dimerization/oligomerization. Oncogene. 2002;21:328. doi: 10.1038/sj.onc.1205119. [DOI] [PubMed] [Google Scholar]
- 22.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA. 2010;107:7692–7697. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaushansky A, Gordus A, Budnik BA, Lane WS, Rush J, MacBeath G. System-wide investigation of ErbB4 reveals 19 sites of Tyr phosphorylation that are unusually selective in their recruitment properties. Chem Biol. 2008;15:808–817. doi: 10.1016/j.chembiol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finney KJ, Ince P, Appleton DR, Sunter JP, Watson AJ. A trophic effect of epidermal growth factor (EGF) on rat colonic mucosa in organ culture. Cell Tissue Kinet. 1987;20:43–56. doi: 10.1111/j.1365-2184.1987.tb01081.x. [DOI] [PubMed] [Google Scholar]
- 25.Harari D, Tzahar E, Romano J, et al. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- 26.Skov Olsen P, Poulsen SS, Therkelsen K, Nexo E. Oral administration of synthetic human urogastrone promotes healing of chronic duodenal ulcers in rats. Gastroenterology. 1986;90:911–917. doi: 10.1016/0016-5085(86)90867-x. [DOI] [PubMed] [Google Scholar]
- 27.Egger B, Buchler MW, Lakshmanan J, Moore P, Eysselein VE. Mice harboring a defective epidermal growth factor receptor (waved-2) have an increased susceptibility to acute dextran sulfate-induced colitis. Scand J Gastroenterol. 2000;35:1181–1187. doi: 10.1080/003655200750056664. [DOI] [PubMed] [Google Scholar]
- 28.Dube PE, Yan F, Punit S, et al. Epidermal growth factor receptor inhibits colitis-associated cancer in mice. J Clin Invest. 2012;122:2780–2792. doi: 10.1172/JCI62888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger B, Procaccino F, Lakshmanan J, et al. Mice lacking transforming growth factor alpha have an increased susceptibility to dextran sulfate-induced colitis. Gastroenterology. 1997;113:825–832. doi: 10.1016/s0016-5085(97)70177-x. [DOI] [PubMed] [Google Scholar]
- 30.Egger B, Carey HV, Procaccino F, et al. Reduced susceptibility of mice overexpressing transforming growth factor alpha to dextran sodium sulphate induced colitis. Gut. 1998;43:64–70. doi: 10.1136/gut.43.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Procaccino F, Reinshagen M, Hoffmann P, et al. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994;107:12–17. doi: 10.1016/0016-5085(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 32.Yan F, Cao H, Cover TL, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan F, Polk DB. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes. 2012;3:25–28. doi: 10.4161/gmic.19245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCole DF, Truong A, Bunz M, Barrett KE. Consequences of direct versus indirect activation of epidermal growth factor receptor in intestinal epithelial cells are dictated by protein-tyrosine phosphatase 1B. J Biol Chem. 2007;282:13303–13315. doi: 10.1074/jbc.M700424200. [DOI] [PubMed] [Google Scholar]
- 35.Brandl K, Sun L, Neppl C, et al. MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc Natl Acad Sci USA. 2010;107:19967–19972. doi: 10.1073/pnas.1014669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCole DF, Rogler G, Varki N, Barrett KE. Epidermal growth factor partially restores colonic ion transport responses in mouse models of chronic colitis. Gastroenterology. 2005;129:591–608. doi: 10.1016/j.gastro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Zaiss DM, van Loosdregt J, Gorlani A, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Dube PE, Washington MK, Yan F, Polk DB. ErbB2 and ErbB3 regulate recovery from dextran sulfate sodium-induced colitis by promoting mouse colon epithelial cell survival. Lab Invest. 2012;92:437–450. doi: 10.1038/labinvest.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hormi K, Cadiot G, Kermorgant S, et al. Transforming growth factor-alpha and epidermal growth factor receptor in colonic mucosa in active and inactive inflammatory bowel disease. Growth Factors. 2000;18:79–91. doi: 10.3109/08977190009003235. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura T, Andoh A, Inatomi O, et al. Amphiregulin and epiregulin expression in neoplastic and inflammatory lesions in the colon. Oncol Rep. 2008;19:105–110. [PubMed] [Google Scholar]
- 41.Hoffmann P, Reinshagen M, Zeeh JM, et al. Increased expression of epidermal growth factor-receptor in an experimental model of colitis in rats. Scand J Gastroenterol. 2000;35:1174–1180. doi: 10.1080/003655200750056655. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann P, Zeeh JM, Lakshmanan J, et al. Increased expression of transforming growth factor alpha precursors in acute experimental colitis in rats. Gut. 1997;41:195–202. doi: 10.1136/gut.41.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frey MR, Edelblum KL, Mullane MT, Liang D, Polk DB. The ErbB4 growth factor receptor is required for colon epithelial cell survival in the presence of TNF. Gastroenterology. 2009;136:217–226. doi: 10.1053/j.gastro.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350–357. doi: 10.1056/NEJMoa013136. [DOI] [PubMed] [Google Scholar]
- 45.Warner BW, Warner BB. Role of epidermal growth factor in the pathogenesis of neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:175–180. doi: 10.1053/j.sempedsurg.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Shin CE, Falcone RA, Jr, Stuart L, Erwin CR, Warner BW. Diminished epidermal growth factor levels in infants with necrotizing enterocolitis. J Pediatr Surg. 2000;35:173–176. doi: 10.1016/s0022-3468(00)90005-8. discussion 7. [DOI] [PubMed] [Google Scholar]
- 47.Dvorak B, Khailova L, Clark JA, et al. Comparison of epidermal growth factor and heparin-binding epidermal growth factor-like growth factor for prevention of experimental necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 2008;47:11–18. doi: 10.1097/MPG.0b013e3181788618. [DOI] [PubMed] [Google Scholar]
- 48.Radulescu A, Zorko NA, Yu X, Besner GE. Preclinical neonatal rat studies of heparin-binding EGF-like growth factor in protection of the intestines from necrotizing enterocolitis. Pediatr Res. 2009;65:437–442. doi: 10.1203/PDR.0b013e3181994fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dvorak B, Halpern MD, Holubec H, et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. American journal of physiology Gastrointestinal and liver physiology. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 50.Clark JA, Lane RH, Maclennan NK, et al. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. American journal of physiology Gastrointestinal and liver physiology. 2005;288:G755–G762. doi: 10.1152/ajpgi.00172.2004. [DOI] [PubMed] [Google Scholar]
- 51.Halpern MD, Holubec H, Saunders TA, et al. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology. 2006;130:359–372. doi: 10.1053/j.gastro.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark JA, Doelle SM, Halpern MD, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 53.Halpern MD, Dominguez JA, Dvorakova K, et al. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr. 2003;36:126–133. doi: 10.1097/00005176-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan PB, Lewindon PJ, Cheng C, et al. Intestinal mucosa remodeling by recombinant human epidermal growth factoR1-48) in neonates with severe necrotizing enterocolitis. J Pediatr Surg. 2007;42:462–469. doi: 10.1016/j.jpedsurg.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 55.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41:144–149. doi: 10.1016/j.jpedsurg.2005.10.018. discussion -9. [DOI] [PubMed] [Google Scholar]
- 56.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2006;41:742–747. doi: 10.1016/j.jpedsurg.2005.12.020. discussion -7. [DOI] [PubMed] [Google Scholar]
- 57.Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137:221–230. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen CL, Yu X, James IO, et al. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest. 2012;92:331–344. doi: 10.1038/labinvest.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radulescu A, Zhang HY, Yu X, et al. Heparin-binding epidermal growth factor-like growth factor overexpression in transgenic mice increases resistance to necrotizing enterocolitis. J Pediatr Surg. 2010;45:1933–1939. doi: 10.1016/j.jpedsurg.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radulescu A, Yu X, Orvets ND, Chen Y, Zhang HY, Besner GE. Deletion of the heparin-binding epidermal growth factor-like growth factor gene increases susceptibility to necrotizing enterocolitis. J Pediatr Surg. 2010;45:729–734. doi: 10.1016/j.jpedsurg.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang C, Sherman MP, Prince LS, et al. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech. 2012;5:522–532. doi: 10.1242/dmm.009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodlad RA, Lee CY, Wright NA. Cell proliferation in the small intestine and colon of intravenously fed rats: effects of urogastrone-epidermal growth factor. Cell Prolif. 1992;25:393–404. doi: 10.1111/j.1365-2184.1992.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 63.McAndrew HF, Lloyd DA, Rintala R, van Saene HK. The effects of intravenous epidermal growth factor on bacterial translocation and central venous catheter infection in the rat total parenteral nutrition model. Pediatr Surg Int. 2000;16:169–173. doi: 10.1007/s003830050715. [DOI] [PubMed] [Google Scholar]
- 64.Wang JY, Zhang LH, Song WL. Epidermal growth factor regulates intestinal glutamine uptake during total parenteral nutrition. Clin Nutr. 1996;15:21–23. doi: 10.1016/s0261-5614(96)80256-1. [DOI] [PubMed] [Google Scholar]
- 65.Kitchen PA, Goodlad RA, FitzGerald AJ, et al. Intestinal growth in parenterally-fed rats induced by the combined effects of glucagon-like peptide 2 and epidermal growth factor. JPEN J Parenter Enteral Nutr. 2005;29:248–254. doi: 10.1177/0148607105029004248. [DOI] [PubMed] [Google Scholar]
- 66.Pierro A, van Saene HK, Donnell SC, et al. Microbial translocation in neonates and infants receiving long-term parenteral nutrition. Arch Surg. 1996;131:176–179. doi: 10.1001/archsurg.1996.01430140066018. [DOI] [PubMed] [Google Scholar]
- 67.Eizaguirre I, Aldazabal P, Barrena MJ, et al. Effect of growth hormone, epidermal growth factor, and insulin on bacterial translocation in experimental short bowel syndrome. J Pediatr Surg. 2000;35:692–695. doi: 10.1053/jpsu.2000.6008. [DOI] [PubMed] [Google Scholar]
- 68.Stern LE, Erwin CR, O'Brien DP, Huang F, Warner BW. Epidermal growth factor is critical for intestinal adaptation following small bowel resection. Microsc Res Tech. 2000;51:138–148. doi: 10.1002/1097-0029(20001015)51:2<138::AID-JEMT5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 69.Feng Y, McDunn JE, Teitelbaum DH. Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G833–G841. doi: 10.1152/ajpgi.00030.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nose K, Yang H, Sun X, et al. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interferon Cytokine Res. 2010;30:67–80. doi: 10.1089/jir.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiu T, Santiskulvong C, Rozengurt E. EGF receptor transactivation mediates ANG II-stimulated mitogenesis in intestinal epithelial cells through the PI3-kinase/Akt/mTOR/p70S6K1 signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2005;288:G182–G194. doi: 10.1152/ajpgi.00200.2004. [DOI] [PubMed] [Google Scholar]
- 72.Laukoetter MG, Mennigen R, Hannig CM, et al. Intestinal cancer risk in Crohn's disease: a meta-analysis. J Gastrointest Surg. 2011;15:576–583. doi: 10.1007/s11605-010-1402-9. [DOI] [PubMed] [Google Scholar]
- 73.Higashi D, Futami K, Ishibashi Y, et al. Clinical course of colorectal cancer in patients with ulcerative colitis. Anticancer Res. 2011;31:2499–2504. [PubMed] [Google Scholar]
- 74.Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554–568. doi: 10.1081/cnv-100103852. [DOI] [PubMed] [Google Scholar]
- 75.Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Exp Cell Res. 2010;316:1083–1100. doi: 10.1016/j.yexcr.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 76.Maurer CA, Friess H, Kretschmann B, et al. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol. 1998;29:771–777. doi: 10.1016/s0046-8177(98)90444-0. [DOI] [PubMed] [Google Scholar]
- 77.Leung SP, Griffith OL, Masoudi H, et al. Clinical utility of type 1 growth factor receptor expression in colon cancer. Am J Surg. 2008;195:604–610. doi: 10.1016/j.amjsurg.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 78.Sundvall M, Korhonen A, Paatero I, et al. Isoform-specific monoubiquitination, endocytosis, and degradation of alternatively spliced ErbB4 isoforms. Proc Natl Acad Sci USA. 2008;105:4162–4167. doi: 10.1073/pnas.0708333105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shtiegman K, Kochupurakkal BS, Zwang Y, et al. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene. 2007;26:6968–6978. doi: 10.1038/sj.onc.1210503. [DOI] [PubMed] [Google Scholar]
- 80.Yoda K, Miyazawa K, Hosoda M, Hiramatsu M, Yan F, He F. Lactobacillus GG-fermented milk prevents DSS-induced colitis and regulates intestinal epithelial homeostasis through activation of epidermal growth factor receptor. Eur J Nutr. 2013 Mar 7; doi: 10.1007/s00394-013-0506-x. Epub ahead of print on. [DOI] [PMC free article] [PubMed] [Google Scholar]