Abstract
Objective
To investigate the effectiveness of a home-based multiple-speed treadmill training program to improve gait performance in persons with a transfemoral amputation (TFA).
Design
Repeated measures.
Setting
Research laboratory.
Participants
Individuals with a TFA (N=8) who had undergone a unilateral amputation at least 3 years prior as a result of limb trauma or cancer.
Intervention
Home-based treadmill walking for a total of 30 minutes a day, 3 days per week for 8 weeks. Each 30-minute training session involved 5 cycles of walking for 2 minutes at 3 speeds.
Main Outcome Measures
Participants were tested pretraining and after 4 and 8 weeks of training. The primary measures were temporal-spatial gait performance (symmetry ratios for stance phase duration and step length), physiological gait performance (energy expenditure and energy cost), and functional gait performance (self-selected walking speed [SSWS], maximum walking speed [MWS], and 2-minute walk test [2MWT]).
Results
Eight weeks of home-based training improved temporal-spatial gait symmetry at SSWS but not at MWS. A relative interlimb increase in stance duration for the prosthetic limb and proportionally greater increases in step length for the limb taking shorter steps produced the improved symmetry. The training effect was significant for the step length symmetry ratio within the first 4 weeks of the program. Energy expenditure decreased progressively during the training with nearly 10% improvement observed across the range of walking speeds. SSWS, MWS, and 2MWT all increased by 16% to 20%.
Conclusions
Home-based treadmill walking is an effective method to improve gait performance in persons with TFA. The results support the application of training interventions beyond the initial rehabilitation phase, even in individuals considered highly functional.
Keywords: Amputees, Energy metabolism, Exercise, Locomotion, Rehabilitation
Restoring a person's ability to walk is a primary goal and a measure of success for physical rehabilitation in persons with a lower extremity amputation (LEA). Evidence suggests that rudimentary walking ability can be achieved in most individuals with an LEA by the completion of physical rehabilitation.1 However, fewer than half of all persons with a transfemoral amputation (TFA) meet or exceed their preamputation level of mobility within 1 year.2,3 Moreover, the number of individuals dependent on a wheelchair for mobility increases from 13% to 39% within 5 years postamputation.4 In this context, additional physical rehabilitation interventions are needed to maximize and maintain gait performance.
Home-based exercise training offers a potentially feasible strategy to continue the rehabilitation process over the longer term. Previous training interventions in persons with LEA, none of which were home-based, relied primarily on bicycle ergometers for training.5-9 Bicycle ergometry was likely selected because it minimizes weight-bearing stresses on the residual limb and reduces the potential for falls. The studies all demonstrated improvements in cardiorespiratory fitness.5-9 Several of the studies also examined the carryover effect of bicycle ergometry training on gait performance. James,9 who included unstructured walking on days alternate to the bicycle training, found nonsignificant increases in the self-selected walking speed (SSWS) and maximum walking speed (MWS), and a trend toward a more symmetrical gait after 12 weeks. Pitetti et al5 reported that 15 weeks of training improved walking economy. Chin et al6 suggested, based on unpublished data, that the SSWS for persons with TFA could be increased to speeds similar to nonamputees within 6 weeks of bicycle training.
Although evidence demonstrates bicycle ergometry training can be beneficial, it is not clear if bicycle training would be preferable if the primary goal is to improve the walking of persons with LEA. Task specificity in training suggests optimal performance will be achieved by using training conditions that most closely reflect the desired task performance.10 As such, a walking exercise program would be a more appropriate training method to improve gait performance than a program using a bicycle ergometer. Moreover, treadmills offer a convenient task-specific method to control walking conditions and may inherently provide additional benefits, including enhanced temporal-spatial gait symmetry.11
A number of studies have investigated the use of treadmill-based interventions for persons with impaired gait performance associated with neurologic disorders.12-14 However, research investigating the effectiveness of treadmill-based training is lacking in persons with LEA. Only 2 case reports describe the exclusive use of a walking program.15,16 One reported improved aerobic exercise tolerance for an individual with bilateral LEA and significant cardiopulmonary disease.16 The other described a 3-week training intervention using clinician-guided real-time visual feedback on gait kinematics in a person with a TFA.15 The training produced improvements in frontal plane kinematic motion and perhaps most significantly, decreases of up to 23% in oxygen consumption for a given walking speed. As promising as the results were, it is currently unlikely that the gait training program described could be made widely available in a home environment. To our knowledge, no study has investigated a home-based treadmill training intervention in persons with LEA.
Therefore, the purpose of this study was to investigate the effectiveness of a task-specific home-based treadmill exercise program focused on improving gait performance in persons with TFA. A secondary objective was to evaluate the rate of improvement over the course of the training program. We hypothesized that the training program would improve functional gait performance by facilitating a more symmetrical gait and reducing the metabolic energy expenditure (EE).
Methods
Participants
Volunteers with a longstanding (>1y) unilateral TFA, a microprocessor knee unit, and an SSWS ranging from approximately .67 to 1.12m/s were recruited for the study. The inclusion of an SSWS range served to identify individuals who could physically tolerate the training and testing protocols but exclude highly functioning individuals who might have less potential for improvement. Participants provided medical clearance, and the prosthetic limb was verified to fit and function properly. The study was approved by the local institutional review board at the University of Iowa. Written informed consent was obtained prior to admittance to the study.
Training protocol
The training protocol involved treadmill walking 3 times a week for 8 weeks. Each training session included multiple cycles of incrementally increased walking speeds. Cycles consisted of 2-minute stages at .89, 1.12, and 1.34m/s. The top speed could be adjusted higher based on the individual's capabilities but did not exceed 1.56m/s. In this manner, walking speeds above the SSWS were achieved, and acclimation to a single speed was avoided. The training cycle was repeated 5 times to produce a total length of approximately 30 minutes for each training session. An analogous slower speed protocol (speeds of .67, .89, and 1.12m/s) was used for 1 participant who was unable to complete the protocol using the .89m/s starting speed. The participants were permitted to divide the 30 minutes of training for a given day into multiple shorter bouts, if necessary.
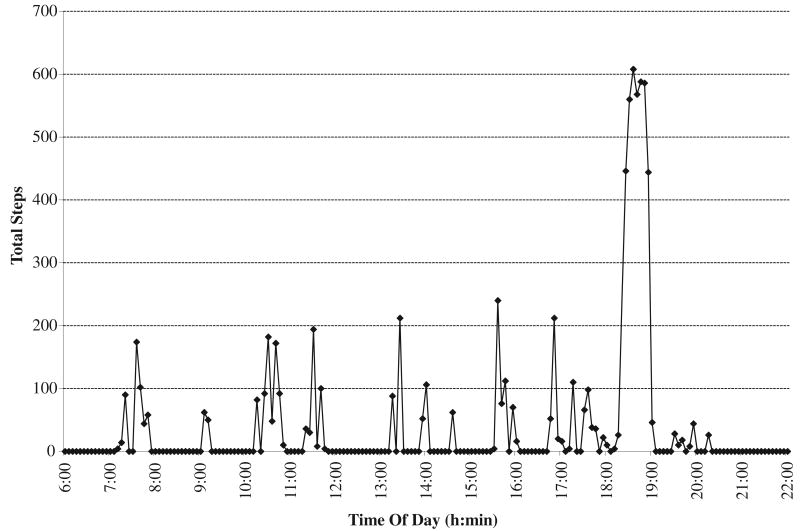
A daily physical activity checklist and a triaxial accelerometrybased physical activity monitora were used to assess compliance with the training. The activity monitor was secured to the shank of the prosthetic limb for the duration of an individual's participation in the study to record the average number of steps per day in 5-minute epochs. Figure 1 illustrates the activity profile generated by completing a training session.
Fig 1.
Activity monitor data for a participant from a single period. Treadmill training occurred between 18:00 and 19:00. Abbreviations: h, hour; min, minute.
An orientation to the treadmill training protocol and a chance to practice the prescribed training were provided at the conclusion of the pretraining test session in order to facilitate the participant's ability to carry out the program. In addition, a handout summarizing the training protocol was provided as a reference. Weekly contact was made with each subject to assess any difficulties with completing the program and to encourage compliance.
Outcome measures
Measures of temporal-spatial, physiological, and functional gait performance were used to evaluate the effectiveness of the intervention. Testing was completed at pretraining, at an intermediate time (after 4wk of training), and at posttraining (8wk).
Temporal-spatial gait performance
Primary temporal-spatial measures of interest were stance phase duration symmetry ratio (stance phase duration symmetry ratio = prosthetic limb/intact limb) and step length symmetry ratio (step length symmetry ratio = longer step length/shorter step length). The ratios were calculated from data collected as participants walked over a 5.18-m-long GAITRite instrumented walkwayb placed at the midpoint of an 18.3-m overground segmental walking course. The participants were instructed to walk at a comfortable pace that could be sustained for a prolonged period in order to assess the parameters at the SSWS. Three minutes of continuous walking were completed to allow the participant to acclimate and adopt a steady speed. After this, 5 passes over the walkway were recorded as trials. The middle 3 trials were averaged and analyzed. For the trials at the MWS, participants were instructed to safely walk the overground course as fast as possible. Two practice trials were completed, followed by 5 recorded trials. The average of the 3 fastest trials was used in the statistical analyses. No feedback on speed or actual performance was provided during testing.
Physiological gait performance
A Medgraphics Cardio2 metabolic cartc with an interfaced electrocardiograph radiotelemetry system measured oxygen consumption and heart rate during a submaximal multispeed treadmill walking test. Prior to testing, the system was allowed to warm-up for at least 1 hour. The pneumotachometer and gas analyzer were then calibrated according to the manufacturer guidelines. The starting speed for the treadmill test was .45m/s and increased in increments of .22m/s to a maximum of 1.56m/s. Each stage lasted 4 minutes to ensure adequate time to reach a physiological steady state. Averaged breath-by-breath data from the final minute of each stage were used to calculate the primary measures of interest, EE (W/kg) and the energy cost for walking (EC) (J/kg·m). The EC represents the walking economy and is calculated as a ratio of the EE to the walking speed.
Functional gait performance
SSWS and MWS were primary measures of functional gait performance and were obtained using the procedures previously described for collecting temporal-spatial measures. A 2-minute walk test (2MWT) was also completed on a separate 137-m hallway course. Participants were provided instructions based on recommendations by the American Thoracic Society,17 and a measuring wheeld was used to determine the distance walked. The total distance covered during the timed walk test provided an indicator of overall walking capacity.17
Statistical analysis
Descriptive statistics (mean ± SD) were calculated for all variables. Shapiro-Wilk tests were used to confirm that data were normally distributed. One-factor (time) repeated-measures analysis of variance models were used to test temporal-spatial and functional gait performance data. Two-factor (time and speed) repeated-measures analysis of variance models were used for physiological gait performance data because of the incorporation of multiple speeds in the treadmill test. A priori Tukey adjusted t tests were used to analyze intervals between the pretraining, intermediate, and posttraining tests for all outcome measures. Statistical testing was performed using the SASe mixed model procedure. The alpha level was set at P<.05 for determining statistical significance.
Results
Table 1 provides group demographics for the 8 individuals (5 men and 3 women; mean age ± SD, 41.4±12.1y) enrolled in the study. Each participant wore a suction socket with an Ottobock C-legf and an energy storing dynamic response prosthetic foot. All participants completed the 3 testing sessions and 8 weeks of home-based treadmill training with a 90% compliance rate based on physical activity monitor data and physical activity checklists.
Table 1. Participant demographics.
| Subject | Sex | Age (y) | Height (cm) | BMI (kg/m2) | Time Since Amputation (y) | Residual Limb Length (cm) |
|---|---|---|---|---|---|---|
| 1 | M | 51 | 176 | 25.52 | 5 | 37 |
| 2 | F | 45 | 166 | 34.77 | 5 | 38 |
| 3 | M | 50 | 178 | 33.72 | 31 | 45 |
| 4 | M | 26 | 173 | 30.65 | 3 | 43 |
| 5 | M | 28 | 184 | 21.67 | 23 | 30 |
| 6 | F | 53 | 176 | 24.11 | 24 | 42 |
| 7 | M | 27 | 198 | 24.07 | 23 | 26 |
| 8 | F | 51 | 163 | 26.88 | 8 | 43 |
| Mean ± SD | 41.4±12.1 | 176.7±10.7 | 27.64±4.80 | 15.3±11.1 | 37.9±6.8 |
Abbreviations: BMI, body mass index; F, female; M, male.
Temporal-spatial gait performance
Table 2 lists the results for temporal-spatial characteristics and symmetry at SSWS and MWS. Participants displayed shorter stance phase durations with the prosthetic limb at their SSWS throughout the study. The pretraining group means for intact and prosthetic limbs were .844 and .723 seconds, respectively. At the intermediate test, the stance phase durations shortened for the intact limb by .057 seconds (P<.05) and the prosthetic limb by .047 seconds (P<.05). Because the shortening of the stance phase durations was similar between limbs, the stance phase duration symmetry ratio did not change between the pretraining test and the intermediate test. For the intermediate to posttraining interval, a decrease in stance phase duration of .028 seconds in the intact limb exceeded the .004-second decrease in the prosthetic limb. Similar to the pretraining to intermediate test comparison, the stance phase duration symmetry ratio for the intermediate test was not statistically different than the posttraining test ratio (P=.069). However, a significant improvement in the stance phase duration symmetry ratio (closer to 1 or perfect symmetry) was found for the pretraining to posttraining comparison (P<.05). This interval reflected an overall net effect resulting from the full training program.
Table 2. Mean and SE values for measures of temporal-spatial gait performance at pretraining, intermediate, and posttraining tests.
| Parameters | Pretraining | Intermediate | Posttraining |
|---|---|---|---|
| SSWS* | |||
| Stance phase duration (s) | |||
| Prosthetic limb | 0.723±0.011 | 0.677±0.014‡ | 0.672±0.015§ |
| Intact limb | 0.844±0.034 | 0.787±0.033‡ | 0.759±0.029§ |
| Stance phase duration symmetry ratio | 0.860±0.030 | 0.870±0.030 | 0.890±0.020§ |
| Step length duration (m) | |||
| Shorter step length | 0.550±0.030 | 0.610±0.030‡ | 0.610±0.030§ |
| Longer step length | 0.680±0.030 | 0.710±0.030 | 0.710±0.020 |
| Step length symmetry ratio | 1.250±0.030 | 1.170±0.040‡ | 1.170±0.040§ |
| Cadence | 97.900±2.600 | 102.900±2.600‡ | 105.200±2.600§ |
| MWS† | |||
| Stance phase duration (s) | |||
| Prosthetic limb | 0.562±0.024 | 0.534±0.023 | 0.494±0.032§ |
| Intact limb | 0.636±0.040 | 0.603±0.039‡ | 0.567±0.045§‖ |
| Stance phase duration symmetry ratio | 0.890±0.030 | 0.890±0.030 | 0.880±0.030 |
| Step length duration (m) | |||
| Shorter step length | 0.700±0.050 | 0.750±0.050‡ | 0.770±0.050§ |
| Longer step length | 0.800±0.040 | 0.850±0.040‡ | 0.860±0.040§ |
| Step length symmetry ratio | 1.140±0.030 | 1.130±0.040 | 1.120±0.030 |
| Cadence | 123.500±5.700 | 129.200±5.600‡ | 136.500±7.100§ |
Note. Values are mean ± SE.
SSWS: pretraining .96m/s; intermediate 1.09m/s; posttraining 1.13m/s.
MWS: pretraining 1.53m/s; intermediate 1.7m/s; posttraining 1.79m/s.
Pretraining to intermediate a priori comparison is significant.
Pre- to posttraining a priori comparison is significant.
Intermediate to posttraining a priori comparison is significant.
Step length data were assessed based on the longer step lengths and shorter step lengths because improved symmetry was a goal of the training (5 of the 8 participants exhibited longer step lengths with the intact limb). Pretraining test of the limb exhibiting a shorter step length averaged a step length of .55m. The limb with a longer step length averaged a step length of .68m. During the first 4 weeks of training, the shorter step length increased .06m at the SSWS (P=.001); however, step length did not increase significantly over the next 4 weeks. For the longer step length, neither a .02-m increase at the intermediate test nor an additional .01-m increase at the posttraining test was found to be statistically significant. Accordingly, the pretraining step length symmetry ratio of 1.25 improved to 1.17 at the intermediate test (P<.01); however, no further improvement was exhibited at the posttraining test. The overall pretraining to posttraining test interval was significantly improved (P<.025).
Temporal-spatial parameters at the MWS demonstrated a shortening of the stance phase duration and a lengthening of the step lengths when the participants selected faster walking speeds for each test. Unlike the SSWS, the altered gait characteristics did not change the symmetry ratios for any of the time intervals.
Physiological gait performance
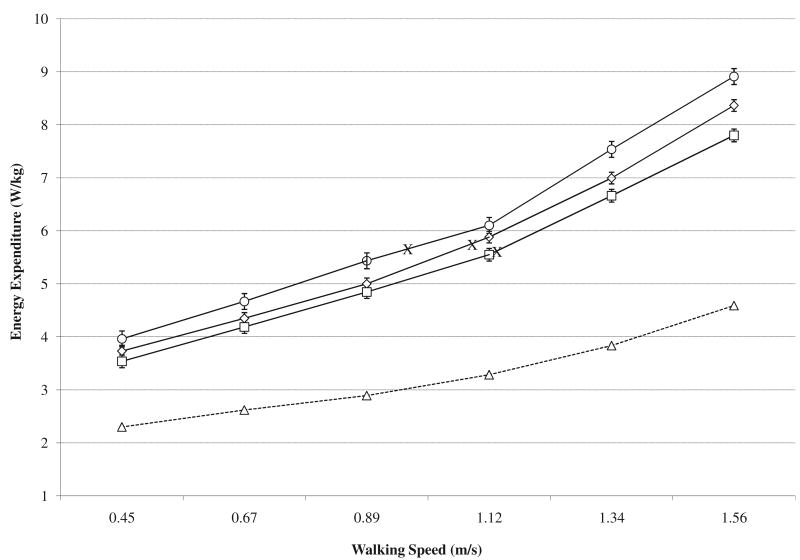
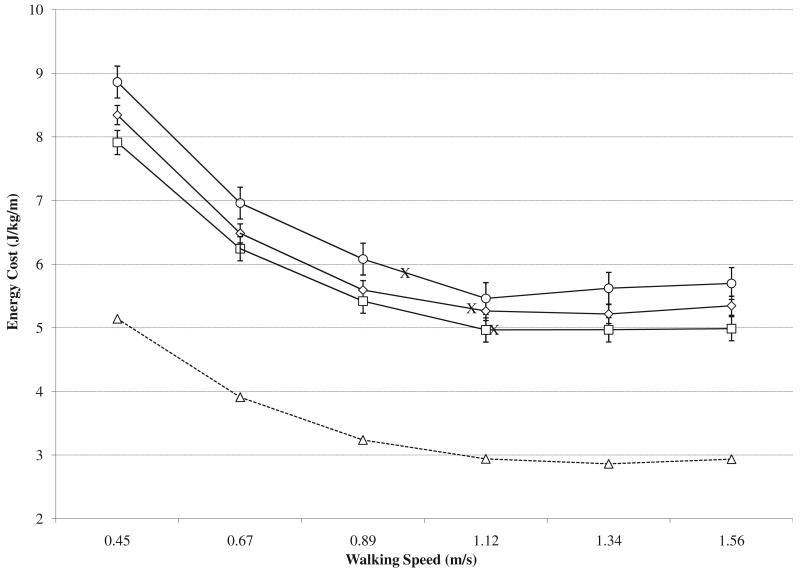
A systematic downward displacement in the EE (fig 2) and EC (fig 3) curves was observed over the 8-week training intervention for walking speeds between .45 and 1.56m/s. The mean values for the EE and EC curves are listed in table 3 and indicate that the improvement in EE averaged .31W/kg at the intermediate test and an additional .26W/kg at the posttraining test. For the EC, the decrease (improved economy) averaged .40J/(kg·m)m at the intermediate test and a further .20J/(kg·m) at the posttraining test. Statistically, no evidence for a time × speed interaction effect was found. Pairwise comparisons for the pretraining to intermediate test interval did not reach statistical significance for EE (P=.054) but were statistically significant for the EC (P<.01). The intermediate to posttraining test interval (EE: P<.05; EC: P<.025) and the overall pre- to posttraining interval (EE: P<.01; EC: P<.001) were statistically significant.
Fig 2.
Mean and SE values for EE across walking speeds at pretraining, intermediate, and posttraining tests. Circle data markers represent results from the pretraining test, diamond data markers represent results from the intermediate test, square data markers represent results from the posttraining test, triangle data markers represent results from a nonpathologic group of individuals previously assessed in the laboratory,26 and X data markers represent an estimate of the EE for the mean SSWS of the group at each test.
Fig 3.
Mean and SE values for energy cost across walking speeds at pretraining, intermediate, and posttraining tests. Circle data markers represent results from the pretraining test, diamond data markers represent results from the intermediate test, square data markers represent results from the posttraining test, triangle data markers represent results from a nonpathologic group of individuals previously assessed in the laboratory,26 and X data markers represent an estimate of the EC for the mean SSWS of the group at each test.
Table 3. Mean and SE values for measures of physiological gait performance at pretraining, intermediate, and posttraining tests.
| Parameters | Pretraining | Intermediate | Posttraining |
|---|---|---|---|
| EE (W/kg) | 6.07±0.15 | 5.76±0.11 | 5.50±0.12†‡ |
| EC J/(kg·m) | 6.43±0.22 | 6.03±0.22* | 5.83±0.22†‡ |
Note. Values are means for time main effect ± SE.
Pretraining to intermediate a priori comparison is significant.
Pre- to posttraining a priori comparison is significant.
Intermediate to posttraining a priori comparison is significant.
Functional gait performance
All functional gait performance measures increased over the course of the 8 weeks of treadmill training (table 4). At the intermediate test, the SSWS and MWS increased .13 and .17m/s, respectively. After the full 8 weeks, the increase was .17m/s in the SSWS and .26m/s in the MWS. Statistical comparisons for the pretraining test with the intermediate and posttraining tests were significant for the SSWS (P<.001 for each interval) and the MWS (P<.01 for each interval). The intermediate to posttraining comparison was not significant for the SSWS (P=.064) or the MWV (P=.114).
Table 4. Mean and SE values for measures of functional gait performance at pretraining, intermediate, and posttraining tests.
| Parameters | Pretraining | Intermediate | Posttraining |
|---|---|---|---|
| SSWS (m/s) | 0.96±0.09 | 1.09±0.10* | 1.13±0.11† |
| MWS (m/s) | 1.53±0.19 | 1.70±0.19* | 1.79±0.19† |
| 2MWT (m) | 151.53±17.01 | 168.48±18.78 | 180.71±19.72†‡ |
Note. Values are mean ± SE.
Pretraining to intermediate a priori comparison is significant.
Pre- to posttraining a priori comparison is significant.
Intermediate to posttraining a priori comparison is significant.
The pretraining 2MWT distance of 151.53m increased 16.95m at the intermediate test and an additional 12.23m at the post-training test (29.18m overall). The increases during each time interval were statistically significant (P<.05).
Discussion
All participants experienced an amputation several years prior to this study. In the time since the amputation, each participant achieved an ability to walk unassisted in the community at a level exceeding basic walking skills. Nevertheless, pretraining test data suggest that all participants displayed impaired temporal-spatial, physiological, and functional gait performance. The results indicate that the training intervention was effective in facilitating a more symmetrical overground walking pattern at the SSWS. Moreover, a concomitant lowering of the metabolic requirements for walking may have contributed to an improved functional ability. Overall, these results support the use of a structured home-based treadmill walking program to improve gait performance.
In the current study, 8 weeks of home-based treadmill training resulted in a faster SSWS, which was achieved through a higher cadence coupled with shorter stance phase durations and longer step lengths with each limb. Related to the temporal-spatial goals of the training, participants displayed a more symmetrical overground gait pattern at the SSWS. The improved SRstance appeared to result from a relative interlimb increase in stance time on the prosthetic limb. The step length symmetry ratio appeared to improve as a result of proportionally longer increases in step length, with the limb taking shorter steps. Relative to prior research, the improvements in gait symmetry observed in the current study were comparable with the gains from a specialized 10-month physical rehabilitation program focusing on gait training.18 Furthermore, a statistically significant improvement in the step length symmetry ratio at the intermediate test suggests the training effect can occur with relatively small amounts of training. It is unclear whether extending the training would have produced further improvement in temporal-spatial symmetry.
The average decrease in EE approached 10% over the 8 weeks. Improvements of ≥10% have been suggested as representing a clinically meaningful change.19 Furthermore, a plateau in improvement was not observed at the posttraining test. A greater cumulative training effect may have been achieved if the training program had extended beyond 8 weeks. Nevertheless, the improvements equaled or exceeded the training effect reported for longer training programs of 12 to 15 weeks using lower extremity bicycle ergometers alone5 or coupled with an unstructured walking program.9 This further substantiates the importance of training specificity (walking vs bicycling) as an important consideration in designing a training program. Only the case study evaluating clinician-directed realtime kinematic feedback during gait training produced larger improvements in EE. One may speculate, based on a comparison with the case report,15 that adding real-time feedback during treadmill walking could provide greater benefit. Further research is needed because, to our knowledge, no published study has directly compared treadmill training with and without feedback. Regardless, a home-based program may be more practical because providing feedback, as described in the case study,15 requires far greater resources in equipment, personnel, and clinical expertise than the home-based program described in the current study.
The training intervention resulted in clear improvements in functional gait performance. Increases of 16% to 18% in SSWS and MWS almost doubled the reported minimal clinically significant difference for walking speed.20,21 The increases in walking speeds were larger than the 2.5% (SSWS) and 3.7% (MWS) increases reported for the training intervention by James,9 but were less than the 50% (SSWS) and 31% (MWS) increases reported with 10 months of rehabilitation focused on gait training by Sjodahl et al.22 However, the larger gains may have resulted in part from the extended length of the training intervention and the inclusion of direct supervision with feedback on performance.
The EE and EC results suggest improved physiological gait performance potentially contributed to the improved SSWS and overall walking capacity (2MWT). As figure 2 illustrates, a relatively consistent EE value was noted for the mean SSWS of each test, even though the SSWS increased. This finding agrees with previous research suggesting that faster walking speeds will be self-selected when the EE is lower.23 The contribution to improved functional performance may also be viewed in terms of the EC. The SSWS normally matches the most economical walking speed in nonamputees (walking speed coinciding with the low point of the EC curve) but is slower than the most economical speed in persons with a TFA.24,25 Likewise, results from the pretraining test suggest that the participants chose an SSWS slower than the most economical walking speed (see fig 3). As such, it is clinically meaningful that the training program resulted not only in walking that was more economical for a given speed (downward displacement of EC curve) but also an SSWS approximating the most economical walking speed (shift right along the EC curve).
Study limitations
Several issues should be considered when interpreting the results of the current study. The small study sample consisted only of individuals with an LEA as a result of trauma or tumors. Caution should be taken in applying the results to individuals with greater or less walking ability, or those persons with an LEA because of dysvascular reasons. A second potential limitation is the lack of a control group. However, we believe the participants would not exhibit any significant spontaneous changes because the amputation had occurred >1 year prior. Finally, the physical activity monitor data were stored in 5-minute epochs. A 5-minute epoch provided a clear demonstration of when the training occurred and the total number of steps taken (see fig 1), but it did not provide the resolution to assess if the training program was performed exactly as designed.
Conclusions
The home-based training program produced clinically relevant benefits in temporal-spatial, physiological, and functional gait performance in persons with a TFA. Moreover, the results support that improved temporal-spatial and physiological gait performance likely contributed to improved functional performance. In light of the need to promote a healthy, active lifestyle in all persons, developing and evaluating new potentially advantageous physical rehabilitation techniques remains more relevant than ever. The results provide valuable information that may be useful toward optimizing long-term functionality in persons with an LEA.
Acknowledgments
We thank Dale Berry, CP of Hanger Clinics and Don Shurr, CPO, PT of American Prosthetics who assisted in recruiting participants for the study.
Supported by a clinical and translational science award from the National Center for Advancing Translational Sciences (award no. KL2TR000057).
List of abbreviations
- EC
energy cost
- EE
energy expenditure
- LEA
lower extremity amputation
- MWS
maximum walking speed
- SSWS
self-selected walking speed
- TFA
transfemoral amputation
- 2MWT
2-minute walk test
Footnotes
Presented to the American Physical Therapy Association, February 6-9, 2008, Nashville, TN.
No commercial party having a direct financial interest in the results of the research supporting this article has conferred or will confer a benefit on the authors or on any organization with which the authors are associated.
Suppliers
- AMP 331; Dynastream Innovations, 100 Grande Blvd, Ste 201, Cochrane, AB T4C 0S4, Canada.
- CIR Systems Inc, 376 Lafayette Ave, Ste 202, Sparta, NJ 07871.
- Medical Graphics Corp, 350 Oak Grove Pkwy, St Paul, MN 55127.
- Model 6012; Meter-Man By Komelon, 301 Commerce Place, Waukesha, WI 53186.
- Version 9.1; SAS Institute, 100 SAS Campus Dr, Cary, NC 27513.
- Otto Bock, 2 Carlson Pkwy N, Ste 100, Minneapolis, MN 55447.
References
- 1.Munin MC, Espejo-De Guzman MC, Boninger ML, Fitzgerald SG, Penrod LE, Singh J. Predictive factors for successful early prosthetic ambulation among lower-limb amputees. J Rehabil Res Dev. 2001;38:379–84. [PubMed] [Google Scholar]
- 2.Taylor SM, Kalbaugh CA, Blackhurst DW, et al. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg. 2005;42:227–35. doi: 10.1016/j.jvs.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Czerniecki JM, Turner AP, Williams RM, Hakimi KN, Norvell DC. The effect of rehabilitation in a comprehensive inpatient rehabilitation unit on mobility outcome after dysvascular lower extremity amputation. Arch Phys Med Rehabil. 2012;93:1384–91. doi: 10.1016/j.apmr.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McWhinnie DL, Gordon AC, Collin J, Gray WR, Morrison JD. Rehabilitation outcome 5 years after 100 lower-limb amputations. Br J Surg. 1994;81:1596–9. doi: 10.1002/bjs.1800811110. [DOI] [PubMed] [Google Scholar]
- 5.Pitetti KH, Snell PG, Stray-Gunderson J, Gottschalk FA. Aerobic training for individuals who had amputations of the lower limb. J Bone Joint Surg Am. 1987;69:914–21. [PubMed] [Google Scholar]
- 6.Chin T, Sawamura S, Fujita H, et al. Effect of endurance training program based on anaerobic threshold (AT) for lower limb amputees. J Rehabil Res Dev. 2001;38:7–11. [PubMed] [Google Scholar]
- 7.Chin T, Sawamura S, Fujita H, et al. Physical fitness of lower limb amputees. Am J Phys Med Rehabil. 2002;81:321–5. doi: 10.1097/00002060-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Bostom B, Mazzarella B, Adler J. Ergometer modification for combined arm-leg use by lower extremity amputees in cardiovascular testing training. Arch Phys Med Rehabil. 1987;68:244–7. [PubMed] [Google Scholar]
- 9.James U. Effect of physical training in healthy male unilateral above-knee amputees. Scand J Rehabil Med. 1973;5:88–101. [PubMed] [Google Scholar]
- 10.Van Peppen RP, Kwakkel G, Wood-Dauphinee S, Hendriks HJ, Van der Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: what's the evidence? Clin Rehabil. 2004;18:833–62. doi: 10.1191/0269215504cr843oa. [DOI] [PubMed] [Google Scholar]
- 11.Harris-Love ML, Forrester LW, Macko RF, Silver KH, Smith GV. Hemiparetic gait parameters in overground versus treadmill walking. Neurorehabil Neural Repair. 2001;15:105–12. doi: 10.1177/154596830101500204. [DOI] [PubMed] [Google Scholar]
- 12.Mehrholz J, Friis R, Kugler J, Twork S, Storch A, Pohl M. Treadmill training for patients with Parkinson's disease. Cochrane Database Syst Rev. 2010;(1):CD007830. doi: 10.1002/14651858.CD007830.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2012;(11):CD006676. doi: 10.1002/14651858.CD006676.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2005;(4):CD002840. doi: 10.1002/14651858.CD002840.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Darter BJ, Wilken JM. Gait training with virtual reality-based realtime feedback: improving gait performance following transfemoral amputation. Phys Ther. 2011;91:1385–94. doi: 10.2522/ptj.20100360. [DOI] [PubMed] [Google Scholar]
- 16.Adler JC, Mazzarella N, Puzsier L, Alba A. Treadmill training program for a bilateral below-knee amputee patient with cardiopulmonary disease. Arch Phys Med Rehabil. 1987;68:858–61. [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 18.Sjodahl C, Jarnlo GB, Soderberg B, Persson BM. Kinematic and kinetic gait analysis in the sagittal plane of trans-femoral amputees before and after special gait re-education. Prosthet Orthot Int. 2002;26:101–12. doi: 10.1080/03093640208726632. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin JE, King GA, Howley ET, Bassett DR, Jr, Ainsworth BE. Validation of the COSMED K4 b2 portable metabolic system. Int J Sports Med. 2001;22:280–4. doi: 10.1055/s-2001-13816. [DOI] [PubMed] [Google Scholar]
- 20.Palombaro KM, Craik RL, Mangione KK, Tomlinson JD. Determining meaningful changes in gait speed after hip fracture. Phys Ther. 2006;86:809–16. [PubMed] [Google Scholar]
- 21.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful changes and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 22.Sjodahl C, Jarnlo GB, Persson BM. Gait improvement in unilateral transfemoral amputees by a combined psychological and physiotherapeutic treatment. J Rehabil Med. 2001;33:114–8. doi: 10.1080/165019701750165934. [DOI] [PubMed] [Google Scholar]
- 23.Waters RL, Perry J, Antonelli D, Hislop H. Energy cost of walking of amputees: the influence of level of amputation. J Bone Joint Surg Am. 1976;58:42–6. [PubMed] [Google Scholar]
- 24.Jaegers SM, Vos LD, Rispens P, Hof AL. The relationship between comfortable and most metabolically efficient walking speed in persons with unilateral above-knee amputation. Arch Phys Med Rehabil. 1993;74:521–5. doi: 10.1016/0003-9993(93)90117-s. [DOI] [PubMed] [Google Scholar]
- 25.Wezenberg D, van der Woude LH, Faber WX, de Haan A, Houdijk H. Relation between aerobic capacity and walking ability in older adults with a lower-limb amputation. Arch Phys Med Rehabil. 2013;94:1714–20. doi: 10.1016/j.apmr.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Rohrig WL. Submaximal exercise testing treadmill and floor walking. Iowa City: Univ of Iowa; 1978. [Google Scholar]