Abstract
The airways of the mammalian lung are lined with highly specialized epithelial cell types that are the target of airborne toxicants and injury. Notch signaling plays an important role in the ontogeny of airway epithelial cells, but its contributions to recruitment, expansion or differentiation of resident progenitor/stem cells and repair and reestablishment of the normal composition of airway epithelium following injury have not been addressed. In this study, the role of a specific Notch receptor, Notch1, was investigated by targeted inactivation in the embryonic lung epithelium using the epithelial-specific Gata5-Cre driver line. Notch1-deficient mice are viable without discernible defects in pulmonary epithelial cell fate determination and differentiation. However, in an experimental model of airway injury, activity of Notch1 is found to be required for normal repair of the airway epithelium. Absence of Notch1 reduced the ability of a population of cells distinguished by expression of PGP9.5, otherwise a marker of pulmonary neuroendocrine cells, which appears to serve as a reservoir for regeneration of Clara cells. Hairy/Enhancer of Split-5 (Hes5) and a paired-box-containing gene 6 (Pax6) were found to be downstream targets of Notch1. Both Hes5 and Pax6 expressions were significantly increased in association with Clara cell regeneration in wild type lungs. Ablation of Notch1 reduced Hes5 and Pax6 and inhibited airway epithelial repair. Thus, although dispensable in developmental ontogeny of airway epithelial cells, normal activity of Notch1 is required for repair of the airway epithelium. The signaling pathway by which Notch1 regulates the repair process includes stimulation of Hes5 and Pax6 gene expression.
Keywords: Notch1, PGP9.5, UCHL1, Hes5, Lung morphogenesis, Epithelial repair, Naphthalene, Injury, Clara cells, Pulmonary Neuroendocrine, Stem cells, Progenitor cells, Differentiation, Cell fate
Introduction
The distal conducting airways of the mammalian lung, the bronchioles, are composed of three major epithelial cell types namely ciliated cells, non-ciliated Clara cells and the neuroendocrine cells. Precise information regarding their emergence and diversification during embryonic development is scant. Also, whether during embryonic development Clara, ciliated and neuroendocrine cells originate from the same progenitor remains largely unknown. The interrelationship amongst the three cell types and their interchangeability have been addressed by injury models that examine the dynamics of cell populations when one or another cell type is abolished in the airways.
Experimentally-induced airway injury by the toxicant naphthalene (NAPH), a commonly used injury model, abolishes Clara cells (1). Subsequently the surviving cells are thought to serve as progenitor/stem cells to restore the bronchiolar epithelium. There is evidence that at least two cell types serve in this capacity. First, the pulmonary neuroendocrine cells or PNE cells are distinguished mainly by expression of two markers, PGP9.5 and CGRP (2). The relationship and the timing of onset of the two markers on PNE cell ontogeny remain unknown. However, once they have emerged, PNE cells reside within a unique microenvironment known as neuroepithelial bodies and undergo expansion subsequent to NAPH injury (3). Second, a subset of NAPH-resistant Clara cells has been identified that seems to have the ability to self renew as well as generate other cell types (4). In transgenic mice expressing Thymidine Kinase from a CC10 promoter, Clara cell regeneration was blocked following treatment with ganciclovir (5). As ongoing maintenance of the airways and repair after injury are key to normal respiratory function, precise knowledge of which cell type(s) are recruited to reestablish airway homeostasis and the precise mechanics of how repair is controlled is of significant interest.
Notch, a receptor with key functions in mammalian cell-fate determination was initially discovered as a complex pleiotropic locus in Drosophila. Four mammalian Notch receptors Notch-1 thru Notch-4 have been identified. In the mouse lung, Notch1 expression is seen in cells of endodermal origin whereas Notch2 and 3 are expressed throughout the mesenchyme. Notch4 expression is confined to the pulmonary endothelium (6). There are five ligands (Jagged-1/-2, Delta-like 1 thru Delta-like 4). The expression patterns of Jagged 1 and Jagged 2 as well as Delta-like 1 have been elucidated in the embryonic mouse lung (7,8). Both receptors and ligands are heterodimeric type I membrane proteins that require cell–cell contact for activation. Notch signaling is a context-dependent regulator of cell proliferation, differentiation, and apoptosis. Ligand-receptor interactions trigger events the end product of which is the cleavage of the Notch intracellular domain (NICD) by Υ-secretase (9,10). Released from the cell membrane, the 97kDa NICD peptide translocates to the nucleus and binds to the transcriptional regulator Rbpjk to activate downstream target genes. These include the Hes and Hey families, homologues of Drosophila ‘Enhancer of Split’ (11).
Recent work on the role of Notch in the lung has revealed the importance of this signaling pathway in the ontogeny of Clara and PNE cells, the two dynamic cell populations in the NAPH-induced airway injury model (12,13). Loss of function mutations for Hes1, a downstream target of Notch signaling, increased PNE cell numbers and decreased Clara cells. Thus PNE and Clara cells may derive from a common progenitor, and commitment to PNE cell, or Clara cell differentiation may be dependent on Notch signaling (13,14). However, the disruption of NICD by γ-secretase inhibitors had little impact on Hes1 gene expression (7). In the Notch signal transduction pathway, deletion of Pofut1, which encodes an enzyme required for Notch-ligand binding & Notch-mediated signaling, blocked Clara cell differentiation (8). Finally, epithelial cells experiencing Notch signaling during lung development contribute to pools of Clara cells, but not PNE cells (12).
Notch signaling is also critical in progenitor/stem cells, contributing to tissue repair after injury by controlling the behavior of adult resident progenitor/stem cells in non-pulmonary tissues (15). Notch-activated transcription depends greatly upon specific cell and tissue types (9). However, precisely which specific target gene programs are activated in progenitor/stem cell differentiation remains unknown. Also, whether Notch signaling is involved in adult lung progenitor/stem cell maintenance or recruitment, and whether injury repair requires Notch activity are issues that as yet remain to be adequately addressed.
As Notch signaling is mediated via four heterodimeric type I membrane receptors, and as the signaling appears to be critical for Clara and PNE cell ontogeny during development, we examined the potential role of a specific Notch receptor gene, Notch1, by targeted gene inactivation in lung epithelial cells. Our objective was to address the following two queries: what is the role of Notch1 in the ontogeny of epithelial cell fate determination and differentiation; and what role, if any, does Notch1 play in the regeneration of airway epithelium in a Naphthalene injury model? The results show that Notch1 deficiency does not impact cell fate determination and differentiation in the bronchioles of mutant mice. However, signaling through Notch1 appears to be critical for effective recruitment of a population of cells, distinguished by the expression of PGP9.5 which during the repair phase of NAPH-induced airway injury act as putative progenitor/stem cells for Clara cell regeneration.
Materials & Methods
Mouse lines
Notch1flox/flox mice were generated as described (16) and purchased from Jackson Laboratories (Bar Harhor, ME). Generation of Gata5-Cre mice was previously reported (17). Notch1Δ/Δ mice were generated by crossing Notch1flox/flox mice with Gata5-Cre mice.
Histology and Immunohistochemistry (IHC)
Lungs were fixed in 4% paraformaldehyde in phosphate buffed saline (PBS; pH 7.0) and processed into serial paraffin sections using standard procedures. Immunostaining was performed as described (18). Antibodies against the following proteins were used: T1α (Developmental Studies Hybridoma Bank, Iowa City, Iowa), SP-B, SP-C, TTF-1 and CCSP (Seven Hill Bioreagents, Cincinnati, OH), NOTCH1 (Santa Cruz Biotechnology, INC. Santa Cruz, CA), β-tubulin (BioGenex, San Ramon, CA), PGP9.5 (AnaSpec, San Jose, CA), Ki67 (Thermo Scientific, Fremont, CA), HES5, CGRP and PAX6 (Millipore, Temecula, CA), activated NOTCH1 (Abcam, Cambridge, MA).
RNA extraction and Real-Time PCR
Total RNA was isolated from lungs using Trizol (GIBCO, Carlsbad, CA). cDNA was synthesized following the protocol by SuperScript™ (Invitrogen, Carlsbad, CA). Quantification of selected genes by real-time PCR was performed using LightCycler (Roche Diagnostics, Mannheim, Germany) as described (18). Sequences of the primers were as follows: Hey1: 5’-CATGAAGAGAGCTCACCCAGA-3’ and 5’-CGCCGAACTCAAGTTTCC-3’; Hay2: 5’-GTGGGGAGCGAGAACAATTA-3’ and 5’-GTTGTCGGTGAATTGGACCT -3’; Hes1: 5’-CAGCCAGTGTCAACACGA-3’ and 5’-TCGTTCATGCACTCGCTGA-3’; Hes5: 5’-GATGCTCAGTCCCAAGGAGA-3’ and 5’-AGCTTCAGCTGCTCTATGCTG-3’; Pax6: 5’-TGTCCTGTGGACTC-3’ and 5’-ACCGCCCTTGGTTAAAGTCT-3’. Primer sets for the following genes were used for semi-quantitative RT-PCR: Notch1: 5’-CGCACAAGGTGTCTTCCAG-3’ and 5’-AGGATCAGTGGCGTCGTG-3’; β-actin: 5’-GTCGTACCACAGGCATTGTGATGG-3’ and 5’-GCAATGCCTGGGTACATGGTGG-3’.
Western blot analysis
Cells or lung tissues were harvested and frozen in liquid nitrogen. Protein extracts were prepared in RIPA buffer (Sigma, St. Louis, MO) by homogenization, and equal amounts of protein were separated on 4–12% NuPAGE gels (Invitrogen, Carlsbad, CA). Proteins were transferred onto Immobilon-P transfer membranes (Millipore Corp. Billerica, MA) and analyzed by western blotting using antibodies to NOTCH1 and PGP9.5.
Naphthalene exposure
Adult female Notch1flox/flox and Notch1Δ/Δ mice were injected with 300mg/kg Naphthalene (Sigma) dissolved in Mazola corn oil. Animals were sacrificed on 2, 3, 5 and 7 days following Naphthalene exposure.
RESULTS
Epithelial specific inactivation of Notch1 in the murine lung
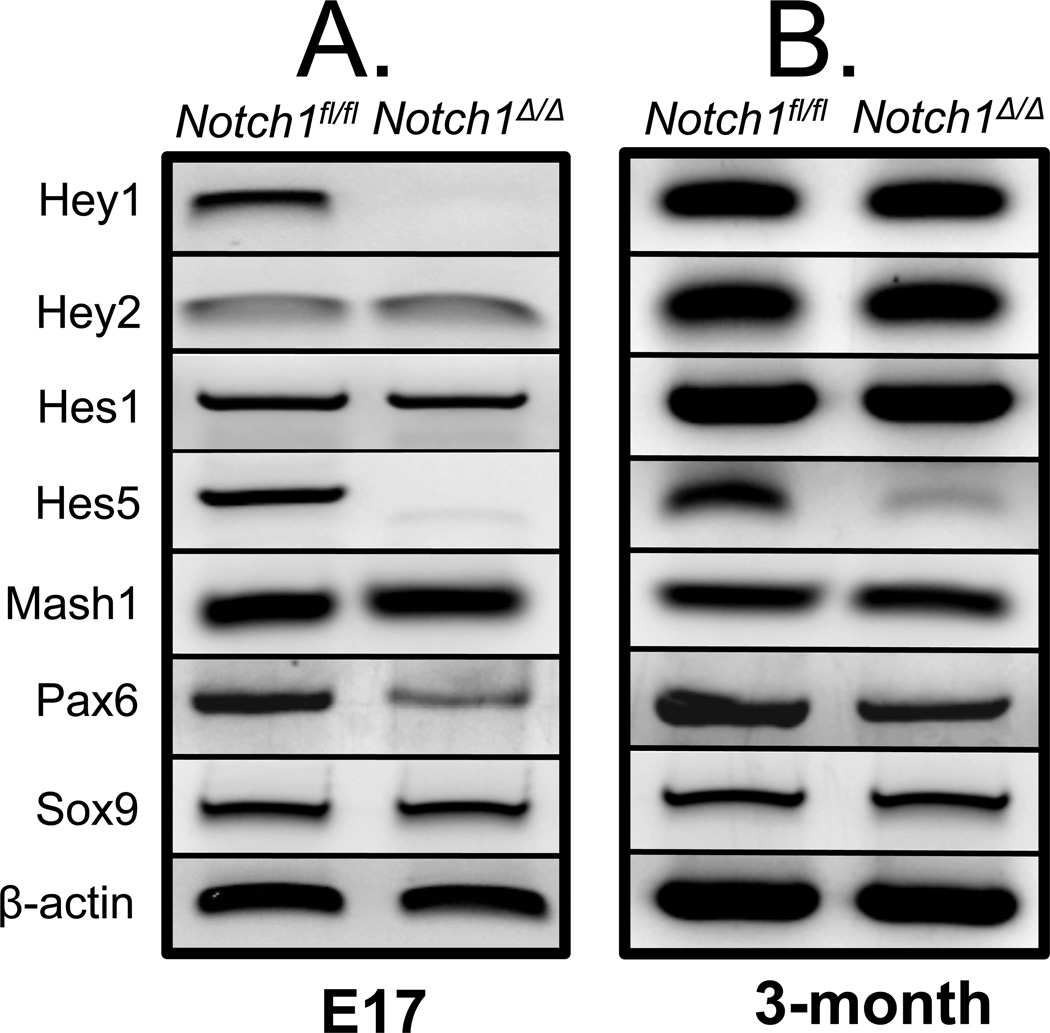
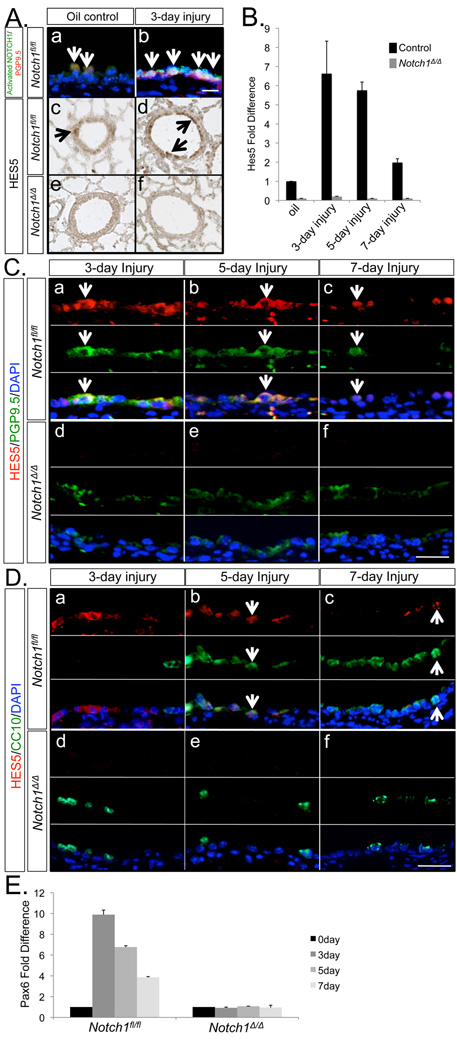
Analysis of RNA by RT-PCR showed Notch1 expression in the lung as early as E12.5 which continued into adulthood. Notch1 expression was documented in epithelial derived human lung carcinoma H441 and A549 cells, as well as in the SV40 immortalized murine alveolar type II, MLE15 cells. Notch1 mRNA was also detectable in MRC5 embryonic mesenchymal cells (Figure 1, Panel A). To investigate the potential role of epithelial Notch1 signaling in lung development and injury repair, we generated Notch1-deficient mice, referred to simply as Notch1Δ/Δ by crossing Notch1flox/flox mice with Gata5-Cre driver mice. As described previously the expression of GATA5 in the lung is uniform and limited to the endodermally derived epithelium (18). Notch1Δ/Δ mice which carry a homozygous deletion of exon 1 are born alive and are postnatally viable (Figure 1, Panel B). PCR, western blot analysis and immunohistochemistry showed high efficiency of Gata5-Cre-mediated recombination of the LoxP sites as NOTCH1 was not detected in the airway epithelium of Notch1Δ/Δ lungs (Figure 1, Panel C, D & E). We analyzed the expression of Notch signaling pathway components by real-time PCR in embryonic day 17 and 3-month old adults. In embryonic mutant lungs, the mRNA for three genes, Hey1, Hes5 and Pax6 appeared markedly reduced. The pattern in the adult lungs was different. Marked changes were only observed for Hes5 and Pax6 (Figure 2, Panel A & B).
Figure 1. Notch1 expression and generation of Notch1Δ/Δlungs.
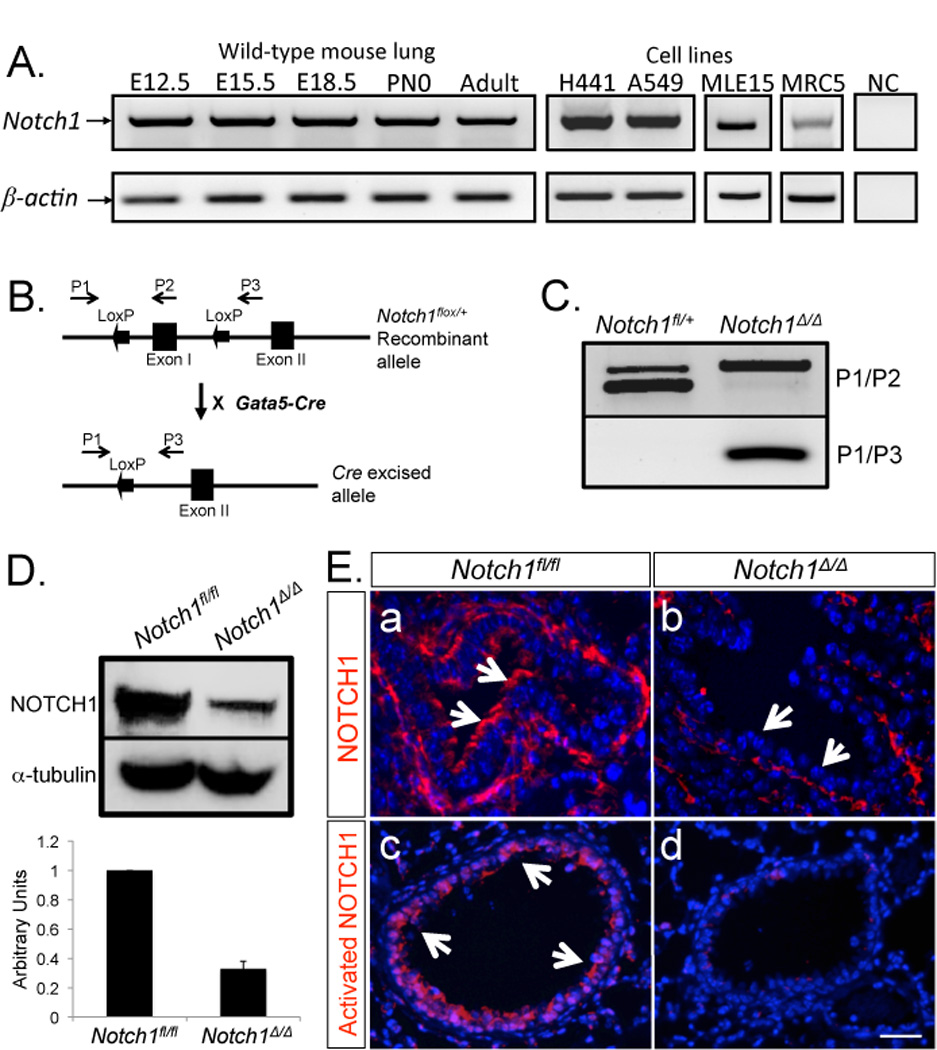
A: Notch1 expression during lung development was analyzed by RT-PCR. β-actin was used as control. B. Map of Notch1flox/+ and Notch1Δ/+ alleles. Primers for detection of recombination were designed as described by Yang et al., 2004. C. Tissue-specific inactivation of the Notch1Δ/Δ conditional allele by Gata5-Cre transgene was detected by PCR analysis. D. Western blot analysis & quantification of NOTCH1 in Notch1Δ/Δ & control lungs. α-tubulin was used as control. E. Immunolocalization of NOTCH1 and activated NOTCH1 (NICD) in E18.5 lungs. Arrows show spatial distribution of NOTCH1 & NICD in the airways. .
Figure 2. Analysis of gene expression in Notch1Δ/Δ lungs.
RNA isolated from E17 (A) and adult (B) Notch1fl/fl and Notch1Δ/Δ lungs was analyzed by real-time PCR. mRNA for Hes5 and Pax6 were reduced in both stages, whereas Hes1 remained unchanged.
Epithelial inactivation of Notch1 does not affect embryonic lung development
Based on a number of criteria, including branching, lobation and gross morphology, Notch1Δ/Δ lungs isolated from E15.5 and E18.5 embryos were nearly identical to the Notch1flox/flox controls, but consistently smaller in overall size (Supplemental Figure 1, Panel A, a & b; e & f). Histologically, the hematoxylin-eosin (H&E) stained sections of lungs from E15.5 and E18.5 Notch1Δ/Δ embryos appeared to be less mature than Notch1flox/flox controls, being more cellular and containing immature alveoli and reduced airspace in distal airways (Supplemental Figure 1, Panel A, c & d, g & h). These differences were not observed in the adult mice between mutant and control lungs and therefore not analyzed further (Supplemental Figure 2).
The major differentiated cellular constituents of the bronchial epithelium include Clara cells, ciliated cells and PNE cells. The distal respiratory epithelium of the adult lung consists of type I and type II cells. To determine whether epithelial deletion of Notch1 caused abnormalities in the composition of the pulmonary epithelium, the expression of cell-specific markers for Clara, ciliated, PNE, type I and type II cells was examined by immuno-histochemistry. Antibodies to CC10, β-tubulin, PGP9.5, T1α, Sp-C and Sp-B were used respectively. The expression levels and the pattern of the above markers showed no significant differences between Notch1Δ/Δ and the control lungs in E18.5 embryos (Supplemental Figure 1. Panel B & C). NKX2.1, a homeodomain transcription factor, is widely acknowledged as an early marker of lung endodermal cell specification. Distribution of NKX2.1 was also examined by immunohistochemistry which showed NKX2.1 to be expressed in both airway and alveolar epithelial cells in Notch1Δ/Δ and control lungs (Supplemental Figure 1. Panel C). Also, analysis of steady state levels of mRNA for CC10, β-tubulin, PGP9.5, Sp-B, Sp-C and Nkx2.1 by real-time PCR revealed no significant differences between Notch1Δ/Δand control lungs (Supplemental Figure 3). Therefore, epithelial inactivation of Notch1 does not interfere with normal epithelial cell differentiation during embryonic lung development.
Epithelial inactivation of Notch1 affects Clara cell regeneration in Naphthalene-induced airway injury
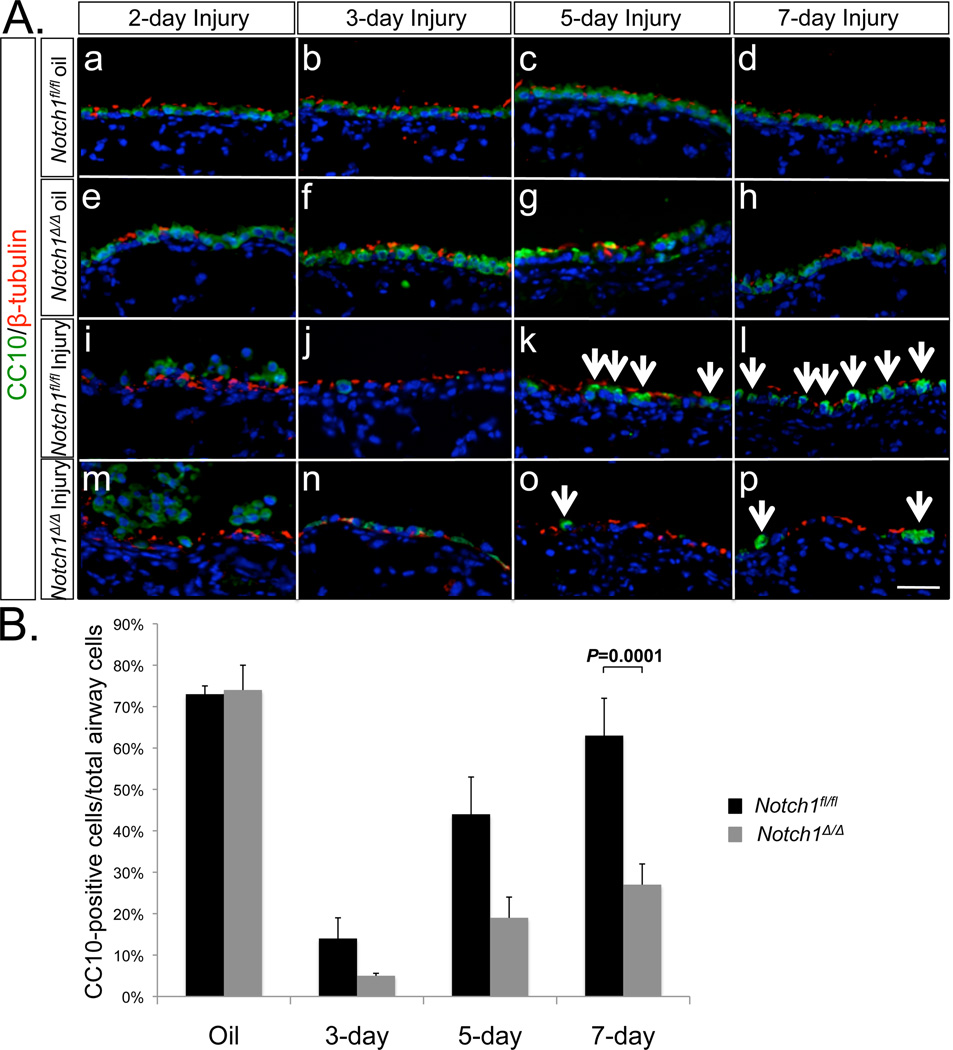
Intraperitoneal administration of Naphthalene to adult mice causes acute airway injury. Shortly after NAPH treatment, Clara cells in the airways die. Overtime however, Clara cells and the normal epithelial landscape of the airways are reconstituted. Thus, NAPH injury represents a simple and effective model to study the dynamics of Clara cell death and regeneration in the mouse airways. To determine whether epithelial deletion of Notch1 causes abnormalities in Clara cell dynamics, we exposed 3-month old Notch1Δ/Δand Notch1flox/flox control BL6 mice to Naphthalene (300mg/kg body weight). Mazola corn oil was used as negative control. Lung tissues from Notch1Δ/Δand control mice were analyzed on post-NAPH days 2, 3, 5 and 7. Antibodies to CC10 and β-tubulin were used to label Clara and ciliated cells respectively (Figure 3, Panel A). The ratio of CC10positive cells over the total number of airway cells was analyzed by direct cell counting. In mice treated with Mazola oil, the percentages of CC10positive cells were 73% in Notch1folx/flox (control) and 74% in Notch1Δ/Δlungs. As expected, by day 3 post-NAPH treatment, the majority of CC10positive cells were killed and released from the airway basement membrane in both Notch1Δ/Δand Notch1flox/flox lungs. On day 5, newly regenerated CC10positive cells comprised 44% of the total airway epithelium in Notch1flox/flox lungs. In contrast, fewer than 19% of the total airway epithelium of the Notch1Δ/Δlungs was CC10positive. Subsequently on day 7, CC10positive cells in the control lungs reached 63% of total airway cells, which compared to the Mazola oil control (no injury) demonstrated nearly complete Clara cell regeneration. In comparison, only 27% of the total airway cells in Notch1Δ/Δlungs were CC10positive (Figure 3, Panel B). In multiple experiments, we found no Notch1Δ/Δmice surviving after 14 days post-NAPH treatment, while mortality was nearly zero in the Notch1flox/flox mice.
Figure 3. Clara cell regeneration after Naphthalene injury.
A. Immunolocalization of CC10 and β-tubulin in Naphthalene injured lungs. CC10 expression on days 2, 3, 5 and 7 post-injury. Oil was used as injury control. B. Quantification of CC10positive cells. Percentages of CC10positive cells in total airway cells were counted on multiple random fields from 8 animals. P=0.0001.
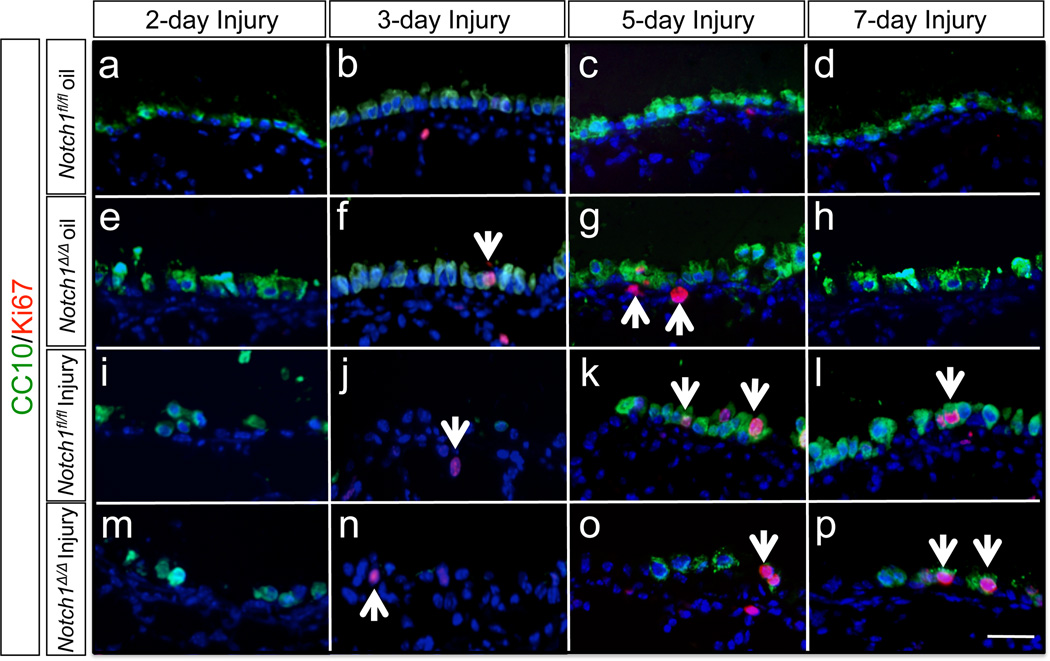
A simple plausible cause of failure in Clara cell regeneration in Notch1 mutants may be defects in cell proliferation. To examine this possibility we used an antibody to Ki67, a marker of cell proliferation, in lungs exposed to NAPH. We used female mouse lung tissues from day 2, 3, 5 and 7 post-NAPH exposure (Figure 4). Slightly increased Ki67 expression was found throughout the airways on day 5 and 7. However, there were no significant differences detected between Notch1Δ/Δ and control lungs (Supplemental Figure 6).
Figure 4. Cell proliferation after Naphthalene injury.
On days 2, 3, 5 and 7 post-injury proliferating cells were labeled with an anti-Ki67 antibody. CC10positive cells showed the timing pattern of Clara cell regeneration. Arrows indicate Ki67 positive cells.
Absence of NOTCH1 affects the ability of PNE cells to serve as reservoir for Clara cell regeneration
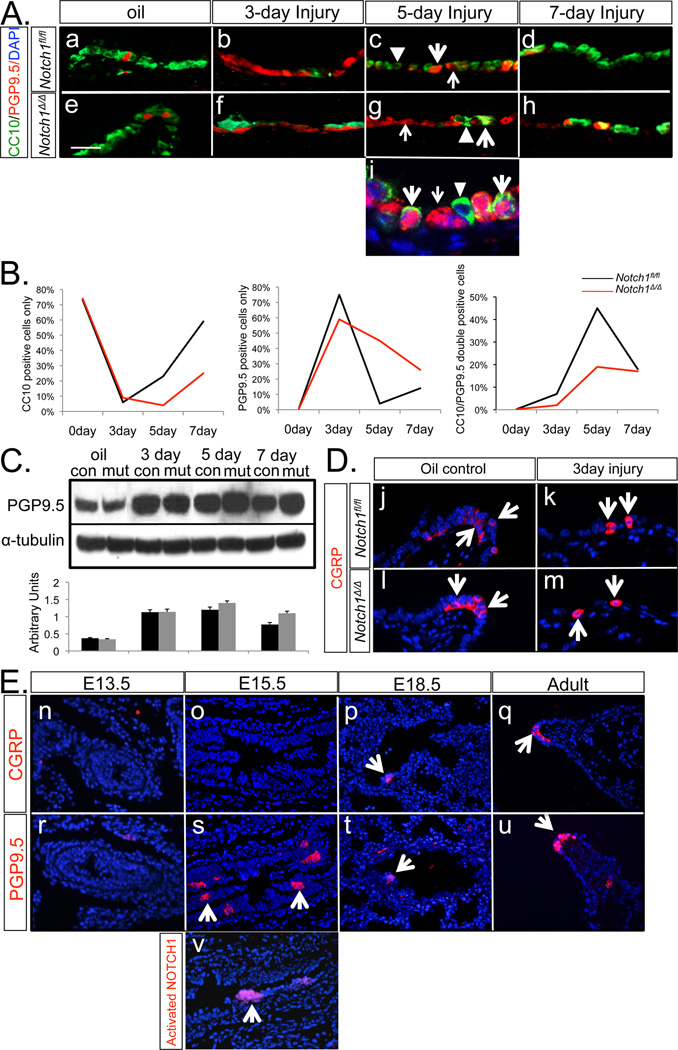
There is a significant body of data suggesting that Notch signaling regulates neural stem cells and neuroepithelial cell differentiation (19,20,21). PNE cells also play key roles in airway epithelial cell renewal and repair of injury (3). To determine whether PNE cells’ ability to serve as progenitor/stem cells is altered in the absence of Notch1 activity, we analyzed the behavior of PNE cells in response to Naphthalene injury in Notch1Δ/Δ and control lungs. Antibodies to PGP9.5 and CC10 were used to identify PNE and Clara cells respectively. Their numbers were determined by manual counting of positive cells on multiple samples (Figure 5, Panel A). On day 3, the airway surface area was covered by PGP9.5positive cells. Manual counting revealed 75% PGP9.5positive cells in control lungs compared to 59% in Notch1Δ/Δ lungs (Figure 5, Panel A, b & f; Panel B). On day 5 after NAPH exposure, two types of cells, PGP9.5positive cells (4%) and a large population of double positive, PGP9.5positive/CC10positive transitional cell intermediates (45%) were distinguishable in the control lungs. In contrast, examination of Notch1Δ/Δ lungs on day 5 revealed a markedly larger population of PGP9.5positive cells (45%) and a correspondingly lower number, only 19% of PGP9.5positive/CC10positive double positive cells (Figure 5, Panel A, c & g; Panel B). As airway recovery from NAPH injury progressed, the control lungs on day 7 contained a significantly reduced number of PGP9.5positive/CC10positive double positive cells (18%) and a concomitantly, increasing (59%) population of CC10positive, differentiated Clara cells. Significantly, in mutant lungs the number of PGP9.5positive cells on day 7 remained relatively high at 26%, while only 25% of the total airway cells were CC10positive (Figure 5, Panel A, d & h; Panel B). Analysis of the histological preparations by confocal laser scanning microscopy confirmed the presence of three distinct groups of cells on day 5 (Figure 5, Panel A, i). Consistent with the IHC results, western blot data showed that in the mutant lungs PGP9.5 levels were dramatically increased on day 3 and stayed high on day 5 and 7 (Figure 5, Panel C). Thus, our analysis of dynamic changes in cell populations subsequent to NAPH injury indicates a time-dependent progression from PNE cells to PNE/Clara cell transitional cell intermediates and eventually newly regenerated Clara cells. This highly orchestrated progression of PNE cells through the intermediate state eventually reestablished the normal airway epithelium. Genetic inactivation of Notch1 disrupted the transition of PNE cells to PNE/Clara intermediates and consequently blocked effective Clara cell regeneration.
Figure 5. PGP9.5positive & Clara cell dynamics after Naphthalene injury.
A. On days 3, 5 and 7 post-injury, PGP9.5positive & CC10positive cells were identified by immunostaining. Oil was used as injury control (Panels “a” through “h”). Panel “i” is confocal laser scanning microscopy of the airway cells on day 5. PGP9.5positive (red, thin arrow), PGP9.5positive/CC10positive (green/red, fat arrow) and CC10positive (green, triangle) cells. B. Quantification of PGP9.5positive, PGP9.5positive/CC10positive and CC10positive cells showing dynamics of cell changes in time. Percentages of three cell populations were determined by manual counting on multiple random fields (n= 8) on days 3, 5 and 7 post-injury. C. Western blot analysis of PGP9.5 in oil control and post-injury day 3, 5 and 7 lungs, α-tubulin was used as control. D. CGRP immunolocalization on day 3 post-injury. Oil was used as injury control. E. Temporal pattern of CGRP and PGP9.5 during wildtype lung development. Activated NOTCH1 (NICD) was also labeled in E15.5 wild-type lung (Panel “v”).
Calcitonin gene-related peptide (CGRP), a known pulmonary vasodilator (22) and bronchoconstrictor (23), is one of several neuropeptides secreted by PNE cells. In our study, antibody to CGRP detected PNE cells and neuroendocrine bodies in adult lung tissue. In contrast with PGP9.5, there were no increased CGRPpositive cells after NAPH exposure (Figure 5, Panel D). By IHC, we found the expression patterns of CGRP and PGP9.5 were very similar during lung development. However, PGP9.5 expression preceded that of CGRP, suggesting that PGP9.5 may serve as a marker for PNE cell progenitors (Figure 5, Panel E, o & s). A similar pattern of expression as PGP9.5 was found with the antibody to NICD, which detects activated Notch1 signaling (Figure 5, Panel E, s & v).
Hes5 expression is dependent on Notch1
Mammalian homologs of Drosophila ‘Enhancer of Split’, including the Hes and Hey families, are known downstream targets of Notch signaling. Real-time PCR analysis of total lung RNA showed that Hes5 expression was significantly reduced in Notch1Δ/Δlungs (Figure 2). We first examined whether Notch1 signaling was activated in the NAPH model of airway injury. Activated NOTCH1 antibody was used to identify the cleaved NOTCH1 intracellular domain (NICD). Based on our earlier data, we found both NOTCH1 and NICD proteins were strongly expressed in epithelial cells along the airways (Figure 1, Panel E). By IHC, we found co-localization of PGP9.5 and activated NOTCH1 in a select group of cells, suggesting that PNE cells are the target of Notch1 signaling. These PNECs which received Notch1 signaling were found in non-injury and post-injury day 3 wild type lungs (Figure 6, Panel A, a & b). As expected, activated NOTCH1 was scarcely detectable in Notch1Δ/Δ injured lungs (data not shown), suggesting a critical role specifically for Notch1 in repair of airway injury.
Figure 6. Hes5 and Pax6 expressions in injured and non-injured lungs.
A. Co-localization of activated NOTCH1 and PGP9.5 on control and injured lungs on day 3 (Panels “a” and “b”). Immunolocalization of HES5 in uninjured lungs and lungs after 3 days of naphthalene injury (Panels “c” to “f”). Arrows indicate double labeled cells. B. Real-time PCR of Hes5 mRNA. C. Co-localization of HES5 and PGP9.5 on days 3, 5 and 7 post-injury (arrows point at double labeled cells). D. Co-localization of HES5 and CC10 on days 3, 5 and 7 post-injury (arrows). E. Pax6 expression in the naphthalene model of airway injury. Real-time PCR analysis of Pax6 mRNA in Notch1fl/fl (control) and Notch1Δ/Δ lungs.
Furthermore, we asked whether Hes5 expression was associated with Notch1-regulated, PNE-to-Clara cell differentiation in NAPH injury repair. For this purpose, we examined the expression level and localization of Hes5/HES5 in NAPH-injured Notch1Δ/Δ and control lungs. Anti-HES5 was used to label HES5 positive cells in lung tissue. By IHC, low level of HES5 protein was found in airway epithelial and alveolar type II cells in control lungs. In contrast, and consistent with the real-time PCR data, little, if any HES5 immunoreactivity was detectable in Notch1Δ/Δ lungs (Figure 6, Panel A, e & f). Analysis of RNA from lungs exposed to NAPH by real-time PCR showed Hes5 mRNA increased more than six fold on day 3 after NAPH injury (Figure 6, Panel B). The observed increase was transient and by day 7 Hes5 levels had returned to normal in injured control lungs. Hes5 mRNA was markedly lower in uninjured Notch1Δ/Δ lungs (Figure 6, Panel B). Immunohistochemical analysis showed localization of HES5 protein to PGP9.5positive cells in injured control lungs. However, there was a small subpopulation of HES5positive cells that were PGP9.5negative. In these lungs, HES5positive/PGP9.5positive cell population increased in number by day 3 after NAPH injury, but gradually decreased on days 5 and 7. This pattern was the converse of that observed for the reappearance of CC10positive cells, which increased as a function of time (Figure 6, Panel C, a–c). Compared with control lungs, HES5positive cells were rarely found in Notch1Δ/Δ injured lungs (Figure 6, Panel C, d–f). Furthermore, in the control lungs on day 5 after NAPH injury, HES5 protein was localized to CC10positive cells, which as shown above were mostly transitional cell intermediates (Figure 6, Panel D, b & c). The population of HES5positive/CC10positive cells was largely decreased on day 7, consistent with the patterns of decreased intermediate, and increased new terminally differentiated Clara cells (Figure 6, Panel D). Based on the observed phenotype in Notch1Δ/Δ injured lungs, the above data suggested that Hes5 was required for PNE-to-Clara cell differentiation.
Another transcription factor whose expression was significantly reduced in Notch1Δ/Δ lungs is Pax6 (Figure 2). Pax6 is a paired- and homeodomain-containing transcription factor necessary for many different levels of specification and differentiation (24, 25). Pax6 is known to control the neuronal differentiation and multipotent status of progenitor cells in the central nervous system (26) and retina (27). Importantly, in wild type mice, Pax6 mRNA, similar to that of Hes5 increased on post-injury day 3 and gradually decreased on day 5 and 7. In contrast, Pax6 expression was not changed after injury in Notch1Δ/Δ lungs (Figure 6, Panel E). Thus, the accumulated data suggest a role also for Pax6 in the repair of the airway epithelium and the PNE-to-Clara cell differentiation process in the NAPH-induced airway injury model. This role is currently under detailed investigation.
Discussion
The purpose of the current study was to determine the precise role of endodermal-specific Notch1, one of four Notch receptors in cell fate determination and differentiation in lung development, injury and repair. The choice of Notch1 was based on its specific expression in the pulmonary epithelium (6). The other three mouse Notch receptors are expressed in the cells of mesodermal origin (6). Endodermal deletion of Notch1 did not interfere with normal lung morphogenesis or epithelial cell differentiation. In wild type mice, examination of airway epithelial regeneration in the Naphthalene-induced injury/repair model revealed a sequential process of cell population dynamics characterized by single and double expression of PGP9.5 and Clara cell markers that lead to regeneration of Clara cells. This well orchestrated process was interrupted in Notch1Δ/Δ mice and resulted in inhibition of Clara cell regeneration. Loss of Notch1 function reduced Hes5 gene expression, which in wild type lungs was increased significantly during the repair phase of airway injury. These studies provide novel evidence that signaling through Notch1, potentially mediated via Hes5 is required for re-establishment of a functional airway epithelium by regulating the step-wise transition of putative progenitor/stem cells to transitional cell intermediates and finally, to newly differentiated Clara cells.
The Notch signaling pathway is complex, consisting of multiple receptors and ligands (28). Activation of this pathway controls mammalian cell-fate determination through heterodimeric type I membrane proteins via cell-cell contact. Activation leads to γ-secretase-mediated cleavage of Notch receptor and generation of a 97kDa peptide known as the Notch intracellular domain (NICD) (9). Thus, intracellular detection of NICD by epitope-specific antibodies is evidence for activation of Notch pathway.
In the present study our first aim was to determine whether Notch1 loss of function impacts epithelial cell differentiation during lung development. There are convincing data that ontogeny of Clara cells is mediated through Notch signaling. First, cell lineage analysis shows that cells receiving Notch signaling, (NICDpositive) contribute to pools of Clara cells (12). Second, abrogation of Notch-ligand binding and signaling via inactivation of Pofut1 and RBPjk blocks Clara cell differentiation (8). Further evidence is derived from studies on Hes1, a transcription factor that in non-pulmonary tissues is downstream of Notch signaling. That Hes1 is required, in a binary fashion for ontogeny of Clara verses PNE cells is supported by dynamic changes in these cell populations in the airways of Hes1 loss-of-function mutants (13). In contrast, our current study showed that epithelial-specific inactivation of Notch1 does not impact Hes1 nor Clara cell ontogeny (Figure 2 & Supplemental Figure 3). The possibility that one or a combination of the other three Notch receptors may participate in Hes1 regulation or emergence of Clara cells was not addressed. However, this possibility is unlikely as disruption of NICD by γ-secretase inhibitors also had little impact on Hes1 gene expression (7). It appears that unlike other tissues, there may be other upstream regulators of Hes1 in the pulmonary epithelium. Thus Notch signaling and Hes1 regulate Clara cell ontogeny in an independent manner.
Our second aim was to determine whether Notch1 was necessary for regeneration of airway epithelial cells after injury. Notch signaling is critical in stem and progenitor cells, contributing to tissue repair after injury by controlling the behavior of adult resident progenitor/stem cells in non-pulmonary tissues (for review, 38). In the lung, basal cells residing within the tracheal epithelium are dependent on Notch signaling for their differentiation, but not for self-renewal (29). Whether Notch signaling is involved in other highly specialized epithelial cells types in the bronchiolar or alveolar compartments of the lung are queries that had not been hitherto addressed.
To address the potential role of Notch1 in the repair of the bronchiolar epithelium, we employed a well-characterized model of NAPH-induced airway injury. In this model, systemic (IntraPeritoneal or IP) administration of Naphthalene to wild type mice ablated nearly all Clara cells within a few hours. On day 3, examination of survived cells in the airway revealed a predominant population of PGP9.5positive cells, which comprised approximately 75% of the total airway epithelial cell population. Pgp9.5 encodes “Protein Gene Product 9.5” and is a member of a gene family whose products hydrolyze small C-terminal adducts of ubiquitin to generate the ubiquitin monomer. Expression of Pgp9.5 is highly specific to neurons and to cells of the diffuse neuroendocrine system and their tumors (30). Antibodies to PGP9.5 have been used to identify cells of neuroendocrine lineages in the pulmonary epithelium (31). However, antibodies to CGRP, another marker of PNE cells revealed no expansion of cells positive for this marker on day 3. PGP9.5 was not expressed by ciliated cells (Supplemental Figure 5), which undergo squamous change to protect the denuded airway within the first 72 hours after NAPH treatment (Figure 5, Panel A). To explore the identity of the PGP9.5positive cells we compared the pattern of PGP9.5 expression to that of CGRP during lung development. We found similar spatial pattern of expression (Figure 5, Panel E) as both markers identified cells with PNE cell phenotype, both as isolated cells as well as localized to neuroepithelial bodies. The only significant difference was in the temporal onset of PGP9.5, which preceded the emergence of CGRPpositive cells in the developing lung. These data support the likelihood that expression of PGP9.5 may represent an early stage of neuroendocrine differentiation. Further analysis in the NAPH injury model showed that cells expressing PGP9.5 were recipients of Notch signaling as PGP9.5 and the 97 kDa activated Notch peptide (NICD) were co-localized in the injured airway epithelium.
The analyses in the present study are consistent with the interpretation that PGP9.5positive cells may serve as a cellular reservoir for regenerating Clara cells and reestablishing a functional airway epithelium subsequent to injury. However, this interpretation must be viewed in the light of a significant caveat stemming from the unavailability of a genetic approach (e.g. inducible Pgp9.5-cre) that would enable a more definitive cell lineage analysis. In the absence of that, careful and quantitative analysis of cellular dynamics over time revealed a precise step-by-step progression of PGP9.5positive cells to transitional cell intermediates and finally to regenerated Clara cells (Figure 5). Targeted inactivation of Notch1 profoundly affected the transitional cell intermediate step without affecting the expansion of the PGP9.5positive population. In the wild type mice, the transitional cell intermediate population identified as PGP9.5positive/CC10positive comprised 45% of the total cell population on day 5 when the population was progressing to differentiated Clara cells.
In the Notch1 mutant airways, nearly 45% of the total cell population was comprised of PGP9.5positive cells. This difference clearly explains the significant inhibition in Clara cell regeneration and failure in re-establishment of a functional airway epithelium in the mutant mice. It is noteworthy that whereas all wild type mice survived the NAPH treatment, all mutant mice had died within 14 days after injury. The reason for this lethality remains unknown. Whether the demise of mutant mice can be explained by inhibition of airway epithelial repair remains to be investigated. A role in the inflammatory response for the major secretory product of Clara cells, CC10 has been noted. It is possible that reduced Clara cell numbers may expose the animals to life-threatening pathogens that overwhelm the immune system in the airways of the mutant mice.
Despite the recognized role of Notch pathway in progenitor/stem cell differentiation, the precise identity of the target genes that mediate this function remains unknown. Notch-activated transcription depends greatly upon specific cell and tissue types (9). It is generally thought that the Notch pathway regulates bHLH transcription factors during development (32). In mammals, one group of bHLH factors encoded by proneural genes such as Mash1 activates, whereas another group encoded by such genes as Hes1 represses differentiation towards a neuronal phenotype. Thus, the balance between Hes1 and Mash1 is deemed an important determinant of neuroendocrine versus non-neuroendocrine cell differentiation (13). In this study, although the loss of Notch1 function had no impact on Hes1 or Mash1 mRNAs, expression of Hes5, another Hes family member was significantly reduced (Figure 2). Analysis of Hes5 expression during the course of NAPH-induced airway injury revealed a number of novel findings. In wild type lungs, Hes5 mRNA increased robustly within the first 3 days of NAPH exposure and the protein was immunolocalized to PGP9.5positive cells (Figure 6). This increase was transient and by day 7 Hes5 levels had returned to normal. Hes5 mRNA decreased subsequent to its surge on day 3, coincident with emergence of PGP9.5positive/CC10postitive cell “intermediates” on day 5 after NAPH exposure (Figure 6, Panel C). These data are consistent with a model whereby early increase in Hes5 triggers the onset of transition from PGP9.5positive cells to PGP9.5positive/CC10positive cell intermediates on day 5. This model predicts that inactivation of lung epithelial Hes5 gene would interfere with the process of airway repair after NAPH injury. Our observation that in the Notch1Δ/Δ lungs Hes5 mRNA did not increase during the early phase of NAPH injury and that there was a correspondingly decreased number of PGP9.5positive/CC10positive intermediate cells provides de facto support for this model, although more convincing results await analysis of airway repair in Hes5 loss of function mutants. Previous studies have described a role for Hes5 in the choroid plexus by lineage tracing analysis in which Hes5 regulated non-neuronal versus neuronal cell fates (33).
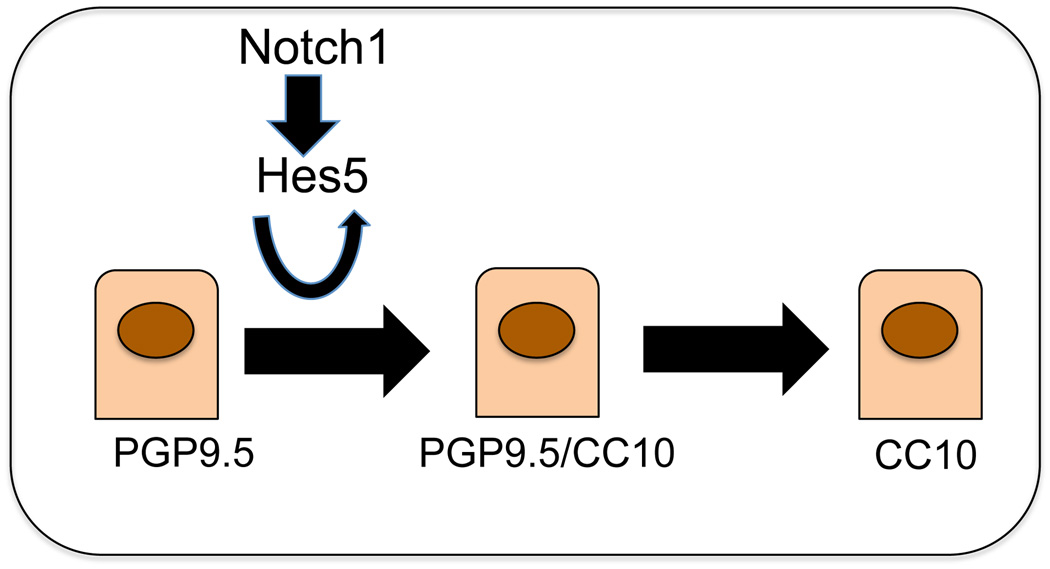
The present understanding of the cellular interrelationships and dynamics in airway epithelial repair after NAPH injury has emerged from robust cell lineage analyses (34). The results have revealed a number of important facts. First, different populations of epithelial cells serve as progenitor/stem cells in different compartments of the lung. In the distal or alveolar compartment alveolar type 2 cells appear to serve as a predominant progenitor/stem cell reservoir (35). In the trachea, there is strong evidence for the role of basal cells as stem cells (36). Of interest, these cells are enriched for transcripts of the Notch pathway including Notch1 (29). In the intermediate lung compartment, the bronchioles, NAPH-resistant cells have been found to contribute to regeneration of Clara cells using Scgb1a1-creER mice (37). In the latter study however, newly regenerated lineage-labeled Clara cells were examined at 3 weeks post NAPH-treatment, long after the repair of the airway had taken place, and the percentage of the regenerated lineage-labeled cells was not described. Thus, the contribution of other potential progenitors, including PGP9.5positive cells could have been missed. Similarly, in the studies by Reynolds et al (5) in which airway epithelial repair was blocked after ablation of Clara cells via treatment with ganciclovir in CCtk transgenic mice, the duration of treatment was continuous over a 12-day period. During this period, any cell, including PGP9.5positive/CC10postitive intermediates, in which CC10 expression was activated, would have been ablated, thus blocking the repair of the airway epithelium. Nevertheless, one collective conclusion from the above studies is that the range of epithelial cells that function as stem or long-term self-renewing progenitors is greater than previously thought. This maybe the reason that the Notch1Δ/Δ airways were not entirely devoid of Clara cells as Notch1 ablation did not affect the ability of other cell types, perhaps even including resistant Clara cells to serve as progenitor/stem cells. But the repair of the injured airway epithelium was clearly impaired in the absence of Notch1 activity. In sum, the results of this study suggest that, during mouse lung Naphthalene injury repair, Notch1 potentially via Hes5 is required for a population of cells expressing PGP9.5 to serve as a reservoir for Clara cell regeneration and re-establishment of the normal airway epithelium (Figure 7).
Figure 7. A simplified model illustrating the role of Notch1 in Clara cell regeneration.
PGP9.5positive cells are proposed to undergo a highly ordered progression to intermediate and new Clara cells. Notch1 signaling via Hes5 and potentially Pax6 are thought to be involved in this progression.
Supplementary Material
Acknowledgments
We thank Dr. Pilar Ruiz-Lozano for the Gata5-Cre mice; Dr. Raphael Kopan for the Notch1fl/fl mice. This work was supported by NIH/NHLBI and generous funds from the Hastings Foundation.
Footnotes
Authors’ contribution:
Yiming Xing: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing.
Aimin Li: Collection and/or assembly of data.
Zea Borok: Data analysis and interpretation, manuscript writing.
Changgong Li: Data analysis and interpretation.
Parviz Minoo: Conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Note added in proof: The recent findings by Tsao et al. (Development 138, 3533–3543, 2011) concerning the role of Notch signaling in the lung are consistent with the data presented in the present report.
Contributor Information
Yiming Xing, Department of Pediatrics, Division of Neonatology, University of Southern California, Keck School of Medicine, Los Angeles, CA.
Aimin Li, Department of Pediatrics, Division of Neonatology, University of Southern California, Keck School of Medicine, Los Angeles, CA.
Zea Borok, Will Rogers Institute Pulmonary Research Center, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Southern California, Keck School of Medicine, Los Angeles, CA.
Changgong Li, Department of Pediatrics, Division of Neonatology, University of Southern California, Keck School of Medicine, Los Angeles, CA.
Parviz Minoo, Department of Pediatrics, Division of Neonatology, University of Southern California, Keck School of Medicine, Los Angeles, CA.
REFERENCES
- 1.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000 Jan;156(1):269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wuenschell CW, Sunday ME, Singh G, Minoo P, Slavkin HC, Warburton D. Embryonic mouse lung epithelial progenitor cells co-express immunohistochemical markers of diverse mature cell lineages. J Histochem Cytochem. 1996;44(2):113–123. doi: 10.1177/44.2.8609367. [DOI] [PubMed] [Google Scholar]
- 3.Peake JL, Reynolds SD, Stripp BR, Stephens KE, Pinkerton KE. Alteration of pulmonary neuroendocrine cells during epithelial repair of naphthalene-induced airway injury. Am J Pathol. 2000;156(1):279–286. doi: 10.1016/S0002-9440(10)64728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001 Jun;24(6):671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional Clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1256–L1263. doi: 10.1152/ajplung.2000.278.6.L1256. [DOI] [PubMed] [Google Scholar]
- 6.Post LC, Ternet M, Hogan BL. Notch/Delta expression in the developing mouse lung. Mech Dev. 2000;98(1–2):95–98. doi: 10.1016/s0925-4773(00)00432-9. [DOI] [PubMed] [Google Scholar]
- 7.Tsao PN, Chen F, Izvolsky KI, Walker J, Kukuruzinska MA, Lu J, Cardoso WV. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J Biol Chem. 24. 2008;283(43):29532–29544. doi: 10.1074/jbc.M801565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136(13):2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33(3):416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 10.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011 Sep;138(17):3593–3612. doi: 10.1242/dev.063610. Review. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci. 2010;123(Pt 2):213–224. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127(18):3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- 14.Shan L, Aster JC, Sklar J, Sunday ME. Notch-1 regulates pulmonary neuroendocrine cell differentiation in cell lines and in transgenic mice. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L500–L509. doi: 10.1152/ajplung.00052.2006. [DOI] [PubMed] [Google Scholar]
- 15.Carlson ME, Conboy IM. Regulating the Notch pathway in embryonic, adult and old stem cells. Curr Opin Pharmacol. 2007;7(3):303–9. doi: 10.1016/j.coph.2007.02.004. Review. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269(1):81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Belmonte JC, Chien KR, Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A. 2005;102(51):18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xing Y, Li C, Li A, Sridurongrit S, Tiozzo C, Bellusci S, Borok Z, Kaartinen V, Minoo P. Signaling via Alk5 controls the ontogeny of lung Clara cells. Development. 2010;137(5):825–833. doi: 10.1242/dev.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson AP, Foraker J, Ylostalo J, Prockop D. Human Stem/Progenitor Cells from Bone Marrow Enhance Glial Differentiation of Rat Neural Stem Cells: a Role for TGFbeta and Notch Signaling. Stem Cells Dev. 2010 Jun;2010:24. doi: 10.1089/scd.2009.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapouton P, Skupien P, Hesl B, Coolen M, Moore JC, Madelaine R, Kremmer E, Faus-Kessler T, Blader P, Lawson ND, Bally-Cuif L. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J Neurosci. 2010;30(23):7961–7974. doi: 10.1523/JNEUROSCI.6170-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy BV, Rauskolb C, Irvine KD. Influence of fat-hippo and notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development. 2010;137(14):2397–2408. doi: 10.1242/dev.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormack DG, Mak JC, Coupe MO, Barnes PJ. Calcitonin gene-related peptide vasodilation of human pulmonary vessels. J Appl Physiol. 1989;67(3):1265–1270. doi: 10.1152/jappl.1989.67.3.1265. [DOI] [PubMed] [Google Scholar]
- 23.Gatto C, Lussky RC, Erickson LW, Berg KJ, Wobken JD, Johnson DE. Calcitonin and CGRP block bombesin- and substance P-induced increases in airway tone. J Appl Physiol. 1989;66(2):573–577. doi: 10.1152/jappl.1989.66.2.573. [DOI] [PubMed] [Google Scholar]
- 24.Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113(4):1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 25.St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387(6631):406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- 26.Bel-Vialar S, Medevielle F, Pituello F. The on/off of Pax6 controls the tempo of neuronal differentiation in the developing spinal cord. Dev Biol. 2007;305(2):659–673. doi: 10.1016/j.ydbio.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105(1):43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 28.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi: 10.1038/nrm2009. Review. [DOI] [PubMed] [Google Scholar]
- 29.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway Basal stem cells. Cell Stem Cell. 2011 Jun 3;8(6):639–648. doi: 10.1016/j.stem.2011.04.003. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theodorou E, Dalembert G, Heffelfinger C, White E, Weissman S, Corcoran L, Snyder M. A high throughput embryonic stem cell screen identifies Oct-2 as a bifunctional regulator of neuronal differentiation. Genes Dev. 2009;23(5):575–588. doi: 10.1101/gad.1772509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulsen TT, Naizhen X, Poulsen HS, Linnoila RI. Acute damage by naphthalene triggers expression of the neuroendocrine marker PGP9.5 in airway epithelial cells. Toxicol Lett. 2008;181(2):67–74. doi: 10.1016/j.toxlet.2008.06.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134(7):1243–1251. doi: 10.1242/dev.000786. Epub 2007 Feb 28. Review. [DOI] [PubMed] [Google Scholar]
- 33.Imayoshi I, Shimogori T, Ohtsuka T, Kageyama R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008;135(15):2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- 34.Rawlins EL, Okubo T, Que J, Xue Y, Clark C, Luo X, Hogan BL. Epithelial stem/progenitor cells in lung postnatal growth, maintenance, and repair. Cold Spring Harb Symp Quant Biol. 2008;73:291–295. doi: 10.1101/sqb.2008.73.037. 2008, Epub 2008 Nov 21. Review. [DOI] [PubMed] [Google Scholar]
- 35.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975 Feb;22(1):142–150. doi: 10.1016/0014-4800(75)90059-3. 1975. [DOI] [PubMed] [Google Scholar]
- 36.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 37.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4(6):525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson ME, Conboy IM. Regulating the Notch pathway in embryonic, adult and old stem cells. Curr Opin Pharmacol. 2007;7(3):303–309. doi: 10.1016/j.coph.2007.02.004. Review. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.