Abstract
Secreted phospholipase B is a proven virulence factor for the pathogenic fungus Cryptococcus neoformans and exhibits three phospholipase activities in the one protein. These are phospholipase B (PLB), lysophospholipase (LPL), and lysophospholipase transacylase (LPTA). Our aim was to investigate the feasibility of using this enzyme as a target for antifungal therapy. We determined in C. neoformans var. grubii strain H99 that 82% of PLB activity was secreted but that 64% of LPL activity and 70% of LPTA activity were cell associated. Cell-associated activities (cytosolic and membrane) were further characterized, since it is likely that any fungicidal effect would depend on inhibition of these enzymes. Four commercially available compounds with structural similarities to phospholipid substrates were tested as inhibitors. These were alexidine dihydrochloride (compound A), dioctadecyldimethylammonium bromide (compound O), 1,12 bis-(tributylphosphonium)dodecane dibromide (compound P), and decamethonium dibromide (compound D). The best phospholipase inhibitors (compounds A and P) were also the most potent antifungal agents by the standard broth microdilution test. Compound A was highly selective for secreted and cell-associated PLB activities and showed no inhibition of mammalian phospholipase A2 at 0.25 μM. Compound O, which was specific for secretory and cytosolic LPL and LPTA and membrane-associated PLB, was not antifungal. We conclude that inhibitors of cryptococcal phospholipases can be selective for fungal enzymes and intrinsically antifungal. They also provide tools for assessing the relative importance of the various enzyme activities in virulence. Our results enable further rational structure-function studies to validate the use of phospholipases as antifungal targets.
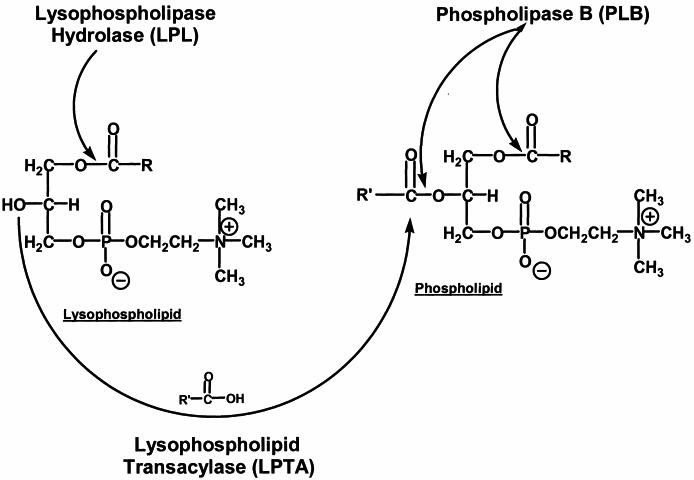
Cryptococcus neoformans is the most common cause of fungal meningitis, which is fatal if it is left untreated (8, 24). Pathogenic strains of cryptococci produce a number of so-called virulence factors, one of which is a secreted phospholipase termed phospholipase B (EC 3.1.1.5) (6, 11). This phospholipase has been purified and characterized as a single protein containing three separate activities (5, 7). These include phospholipase B (PLB), which removes both acyl chains simultaneously from phospholipids; lysophospholipase (LPL), which removes the single acyl chain from lysophospholipids; and lysophospholipase transacylase (LPTA), which adds an acyl chain to lysophospholipids to form phospholipids (Fig. 1). A second secreted phospholipase containing only LPL and LPTA activities has also been identified (L. C. Wright, unpublished data). This may be the product of a newly discovered gene, CnLYSO1 (10).
FIG. 1.
Sites of action of the three activities of cryptococcal phospholipase B: PLB, LPL, and LPTA.
The structure and mechanism of action of phospholipase B are not understood, and which of the secreted phospholipase activities is important in virulence is unknown. However, secreted phospholipase B is involved in the survival of cryptococci in macrophages (11) and in the destruction of lung tissue and the production of eicosanoids, which modulate phagocytic activity (29). This and the observation that phospholipase B is also related to virulence in other medically important fungi, such as Candida albicans and Aspergillus fumigatus (1, 27), make the secreted enzyme a target for antifungal therapy. Hanel et al. (20) tested the hypothesis that fungal phospholipases might be drug targets in a mouse model of C. albicans infection. Mice were treated with beta-blocker drugs and related compounds which inhibited secretory phospholipase activity measured by egg yolk plate assays. Some of the compounds were active per se, and others showed a synergistic effect with fluconazole. To the best of our knowledge, that is the only publication that has related inhibition of phospholipase to antifungal activity.
It was previously reported (33) that approximately 85% of the phospholipase B activity in C. neoformans is cell associated. Deletion of the PLB1 gene, which is responsible for production of secreted cryptococcal phospholipase B (11), did not significantly affect fungal growth in vitro. Thus, inhibition of the secreted enzyme, while reducing the levels of tissue invasion (32) and dissemination of infection from the lung (29, 32), would not kill the fungus. Antifungal therapy should therefore be directed at the cell-associated phospholipases, which may have housekeeping functions necessary for maintenance of cell membrane integrity and, hence, viability, as well as at the secreted enzyme.
In this study, we characterized the cell-associated (membrane and cytosolic) phospholipase B activities in C. neoformans to establish the optimal conditions for the testing of inhibitors and to establish whether the secreted and cell-associated enzymes can be targeted by the same compounds or if different ones are required. We also sought evidence for the feasibility of selective inhibition of the fungal phospholipase(s) relative to that of mammalian phospholipases A (PLAs) and selective inhibition of the three fungal enzyme activities, since if one of the activities was more critical for virulence, it would be the preferred target for inhibition. A number of compounds which differentially inhibit the various phospholipase activities were identified. These compounds constitute novel structural types for phospholipase inhibition. The results support the conclusions that (i) either the LPL and LPTA activities or the PLB activity can be selectively inhibited, (ii) some compounds can inhibit both secreted and cytosolic enzymes, and (iii) selective inhibition of the fungal enzyme compared with that of mammalian PLA2 is feasible. Since the most potent inhibitors were also strongly antifungal, we provide the first evidence of a causal link between specific inhibition of the fungal phospholipase(s) and antifungal activity.
MATERIALS AND METHODS
Fungal isolates and media.
A virulent clinical isolate of C. neoformans var. grubii (serotype A), isolate H99, which produces high levels of secreted phospholipase B activity was used for cell-associated phospholipase characterization and inhibition of phospholipase activities. Isolate H99 was kindly supplied by Gary Cox (Duke University Medical Center, Durham, N.C.) and was subcultured onto Sabouraud dextrose agar at 30°C.
Preparation of supernatants containing secreted phospholipase activities.
Isolate H99 was grown to confluence on Sabouraud dextrose agar in petri dishes (diameter, 16 cm) for 72 h at 30°C in air. Cells scraped from 10 to 20 dishes were washed sequentially with isotonic saline and imidazole buffer (10 mM imidazole, 2 mM CaCl2, 2 mM MgCl2, 56 mM d-glucose made up in isotonic saline [pH 5.5]), resuspended in a volume of this buffer that was about 10% of the cell volume, and incubated for 24 h at 37°C. The cell-free supernatant was separated by centrifugation as described previously (5) and stored at −70°C.
Cellular disruption to prepare membrane and cytosolic fractions.
The cell pellet obtained from the preparation of the supernatant as described above was also frozen at −70°C. After the cell pellet was washed twice with imidazole buffer, it was disrupted in the presence of a protease inhibitor cocktail [P 8215 for fungal and yeast cells, which consists of 100 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 500 mM 1,10-phenanthroline, 2.2 mM pepstatin A, and 1.4 mM E-64; Sigma, St. Louis Mo.] in a MiniBeadbeater-8 Cell Disrupter (MBB-8; Daintree Scientific, Tasmania, Australia) for three cycles of 1 min, alternating with a 1-min cooling period on ice. The homogenate was centrifuged at 14,000 × g for 15 min to obtain the membrane (pellet) and the cytosolic (supernatant) fractions. The cytosolic enzyme activities were stable during storage at −70°C for up to 3 months, whereas the membrane-associated activities were less stable (maximum, 5 weeks).
Radiometric assay method for phospholipases.
Enzyme activities were measured as described previously (5, 6, 7) in a final volume of 125 μl at 37°C. For the determination of secreted PLB activity, carrier dipalmitoyl phosphatidylcholine (DPPC; final concentration, 800 μM) and 1,2-di[1-14C]palmitoyl phosphatidylcholine (20,000 dpm) were dried under nitrogen and suspended in 125 mM imidazole acetate buffer (assay buffer [pH 4.0]) by sonication with a Branson 450 sonifier. The reaction time with 1 μg of total protein was 22 min, and PLB activity was determined by the rate of decrease of the radiolabeled phosphatidylcholine (PC) substrate with the appearance of the label in free fatty acids. Variations to these conditions for the cytosolic and membrane fraction assays are indicated throughout the text. This assay also simultaneously allows the determination of PLA, phospholipase C (PLC), and phospholipase D (PLD) activities. These activities were measured by the appearance of radiolabel from PC in lyso-phosphatidylcholine (lyso-PC), diacylglycerol, and phosphatidic acid, respectively.
Secreted LPL and LPTA activities were measured simultaneously in a reaction mixture containing 1-[14C]palmitoyl lyso-PC (25,000 dpm) and carrier lyso-PC (final concentrations, 200 μM) in assay buffer. The reaction time was 15 s with 1 μg of total protein, and LPL activity was measured by determination of the rate of loss of 1-[14C]palmitoyl lyso-PC with release of radiolabeled fatty acids. LPTA activity was estimated from the rate of formation of radiolabeled PC. Variations to these conditions for the membrane and cytosolic fractions are indicated throughout the text.
All reactions were terminated by adding 0.5 ml of chloroform-methanol (2:1; vol/vol). The reaction products were extracted by the method of Bligh and Dyer (2), separated by thin-layer chromatography, and quantified as described previously (5). In the case of PLC activity, the thin-layer chromatography plates were developed in petroleum ether (boiling point, 60 to 80°C)-diethyl ether-acetic acid (90:15:1; vol/vol/vol) instead of chloroform-methanol-water (65:25:4; vol/vol/vol).
Characterization of enzyme activities.
All experiments were carried out in duplicate or triplicate. The effects of pH on the various phospholipase activities were measured by using 50 mM final concentrations of imidazole-acetate buffer (pH range 3 to 5), morpholineethanesulfonic acid buffer (pH range 6 to 8), and glycine buffer (pH 9 to 10). Controls for nonenzymatic breakdown of substrates were included at all pHs. Cations, Triton X-100, and metal chelators were made up as stock solutions in water and diluted to the final concentration in the appropriate assay buffer.
Protein assays.
Total protein estimations were obtained by a Coomassie blue binding assay (for supernatants containing secreted enzymes) or the bicinchoninic acid assay (bicinchoninic acid assay kit; Pierce Chemical Co., Rockford, Ill.) for cell-associated fractions, with bovine serum albumin used as a standard.
Selection of potential phospholipase inhibitors.
Since no three-dimensional structural information on fungal phospholipases is available, selection of potential inhibitors was based on the traditional approach of testing compounds that are structurally related to the substrate, i.e., phospholipids. We sought commercially available compounds containing the two dominant features in phospholipids (one or two hydrophobic alkyl chains and a tetra-alkylated strongly positively charged nitrogen atom) that would be metabolically stable and sufficiently water soluble to avoid the use of solvents in the assays. Screening for suitable compounds was performed with the search tool SciFinder Scholar (Chemical Abstract Services-based database [3, 31]).
Preparation of inhibitors and use in assays.
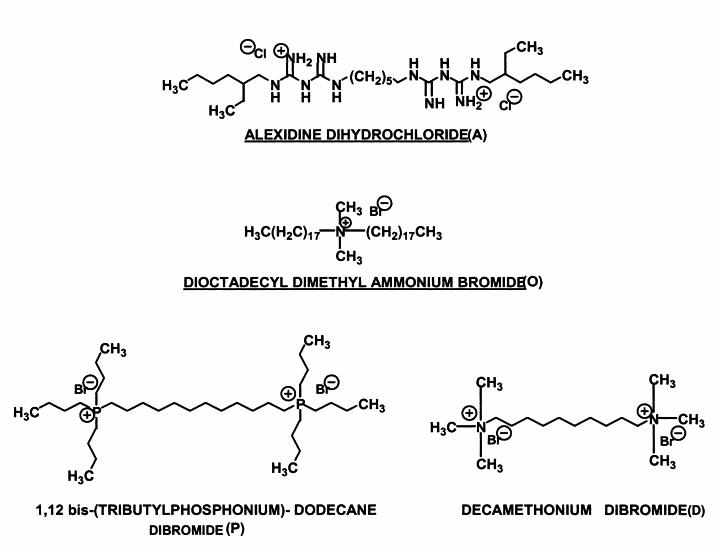
The following compounds were tested (for structures, see Fig. 2): compound O, dioctadecyldimethylammonium bromide (Fluka AG, Buchs, Switzerland); compound P, 1,12-bis(tributylphosphonium)dodecane dibromide (Fluka AG); compound A, alexidine dihydrochloride (Sigma); and compound D, decamethonium bromide (Sigma). All compounds were prepared as stock solutions of 700 μM in assay buffer containing 5 mM EDTA; the stock solutions were then serially diluted with buffer to obtain solutions of 70, 7, 0.7, and 0.07 μM. Each of these solutions was used at 45 μl in each assay; and the final volume of 125 μl was made up of substrate, enzyme, and buffer. The radiometric assay was carried out as described above. Inhibition was calculated as the percentage of substrates (DPPC or lyso-PC) remaining in the case of PLB and LPL activities or the amount of DPPC produced in the case of LPTA activity. The amounts converted or produced in the inhibitor-free control were normalized to 100%, and the level of inhibition was calculated against that amount. All assays were done in triplicate.
FIG. 2.
Structures of the four compounds tested as inhibitors.
Pancreatic phospholipase assay.
A porcine pancreatic PLA2 suspension in 3.2 M ammonium sulfate (2.9 mg of protein/ml; Sigma) was used for the pancreatic phospholipase assay. One part of well-mixed enzyme suspension was added to 4 parts of buffer (10 mM Tris-HCl [pH 8.2], 10 mM CaCl2) (13). The levels of activity and inhibition by the test compounds were then measured by the radiometric method described above for determination of fungal phospholipase B activity. However, 25 μl of enzyme solution was used and the reaction time was 1 h. These conditions resulted in ∼60% substrate conversion in the inhibitor-free control.
Hemolytic activities of compounds.
Human blood was collected and placed into 10-ml Vacutainer tubes containing potassium-EDTA as the anticoagulant. The blood from each Vacutainer tube was transferred to a 50-ml centrifuge tube, and the cells were washed three times with calcium- and magnesium-free phosphate-buffered saline (PBS; Gibco) by centrifugation at 2,000 × g for 15 min. The supernatant obtained after the third centrifugation was clear and colorless. Cells were stored in PBS for up to 2 weeks. A total of 0.5 ml of the cell suspension in PBS was mixed with 0.5 ml of the test substance stock solutions (final erythrocyte concentration, about 0.5 × 109 ml−1). The mixtures were incubated at 37°C for 1 h with gentle shaking and then centrifuged at 2,000 × g for 10 min, the supernatant was diluted 10-fold with PBS, and the optical density was measured at 540 nm. The values for 0 and 100% lysis were determined by incubating cells with PBS and 0.1% (wt/vol) Triton X-100 (in water), respectively. Assays were carried out in triplicate, and the reproducibility was >95%. The concentrations of the test compounds (made from stock solutions in PBS) in the assays were 350, 35, 3.5, and 0.35 μM.
Antifungal susceptibility testing.
The antifungal activities of the compounds were measured by a standard microdilution method (16, 28). The MIC of each compound was defined as the concentration which produced no visible growth after 48 h (Candida) and 72 h (Cryptococcus) of culture at 35°C. The fungal strains tested included C. neoformans H99, C. neoformans ATCC 90112, and C. albicans ATCC 10231. All tests were performed in duplicate.
RESULTS
Identification of PLB, LPL, and LPTA in cell-associated fractions.
The conditions chosen for cellular disruption (see Materials and Methods) were optimized such that the highest levels of phospholipase activity were measured in both membrane and cytosolic fractions. Assays of cell-associated phospholipase activities were performed at pH 4.0 with the substrates lyso-PC and DPPC, since these compounds were preferred substrates of the secreted enzyme in both its natural and purified states and enzyme activity was maximal at pH 4.0 (7, 33). Hydrolysis of the substrate lyso-PC by the cryptococcal membrane and cytosolic fractions resulted in the formation of free fatty acids and PC only, indicating the presence of both LPL and LPTA, as was found for the secreted enzyme. Similarly, DPPC, both acyl chains of which were radiolabeled, was degraded to produce free fatty acids only, indicating that the activity was due to PLB at pH 4.0.
Effects of protein concentration and time on phospholipase activity.
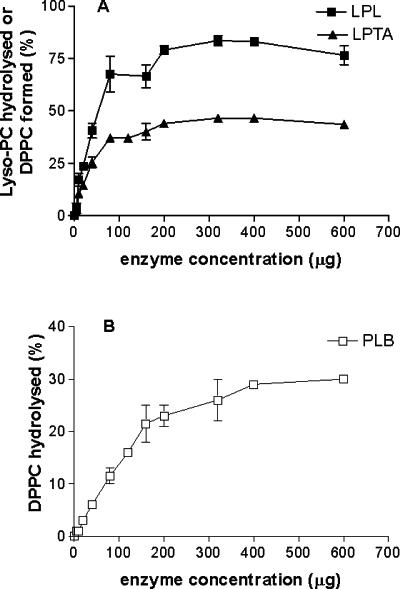
The activity of the cytosolic fraction with increasing protein concentrations was linear only to 1 μg for LPL and LPTA and 4 μg for PLB, similar to the values for the secreted enzyme (data not shown). In contrast, membrane-associated LPL and LPTA activities were linear with increasing protein concentrations to 80 μg, after which no further increase occurred (Fig. 3A). The PLB activity was linear with increasing protein concentrations to 160 μg (Fig. 3B). The optimal protein concentrations chosen for the assays were 1 and 4 μg for cytosolic LPL and LPTA activities and PLB activity, respectively, and 80 and 120 μg for membrane-associated LPL and LPTA activities and PLB activity, respectively.
FIG. 3.
Effects of protein concentration on membrane-associated phospholipase activities. Points are the means ± standard errors of the means of three assays.
The time course of both the cytosolic and the membrane activities resembled that of the secreted enzyme, with a linearity of LPL and LPTA activities only to 20 to 30 s, beyond which no further increase occurred. Membrane-associated PLB activity was linear to 30 min, whereas cytosolic activity was linear to 22 min (data not shown). The optimal incubation times chosen for the assays were 20 s and 18 min for cytosolic LPL and LPTA activities and PLB activity, respectively, and 30 s and 18 min for membrane-bound LPL and LPTA activities and PLB activity, respectively.
Effect of substrate concentration on enzyme activity.
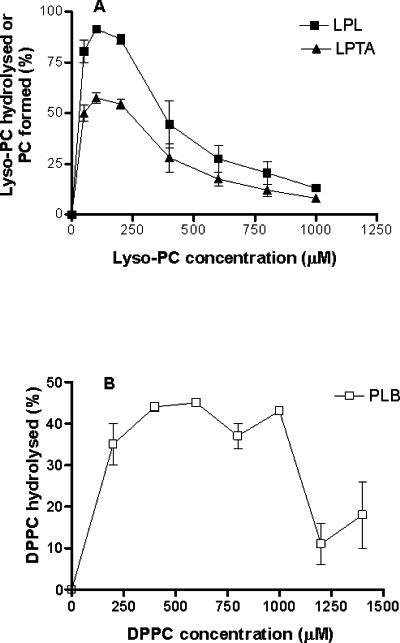
Cytosolic LPL and LPTA activities reached a maximum with between 50 and 200 μM lyso-PC, after which there was a rapid decline in activity with increasing substrate concentration (Fig. 4A). Cytosolic PLB activity reached saturation with 400 μM DPPC and declined at concentrations above 1,000 μM (Fig. 4B). Membrane-associated LPL and LPTA activities reached a maximum with about 50 μM lyso-PC but maintained the same level of activity up to 600 μM, after which it decreased (data not shown). The membrane-associated PLB activity reached a maximum with 200 μM DPPC and declined at concentrations above 800 μM (data not shown). The optimal substrate concentrations used for the assay were 200 and 1000 μM for cytosolic LPL-LPTA and PLB activities, respectively, and 600 and 800 μM for membrane-bound LPL-LPTA and PLB activities, respectively.
FIG. 4.
Effects of substrate concentration on cytosolic phospholipase activities. Points are means ± standard errors of the means of three assays.
Effects of pH on enzyme activity.
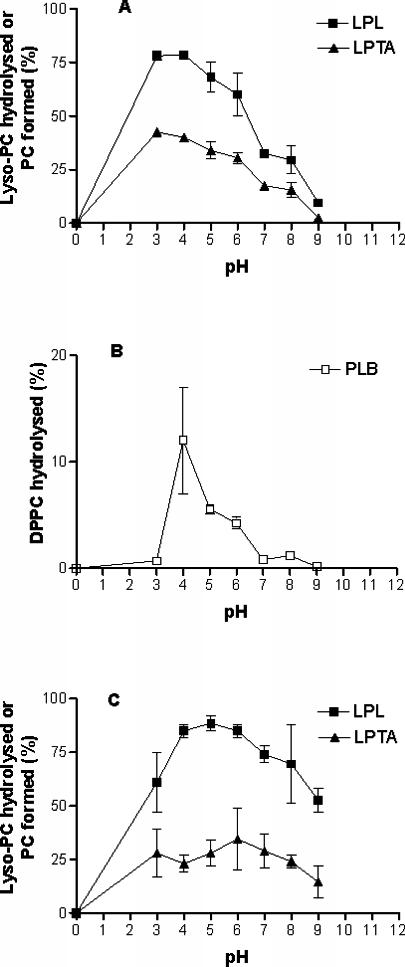
Variations in cell-associated phospholipase activity were observed over a pH range of 3 to 10 by use of the optimal assay conditions established for cytosolic and membrane-associated activities. Cytosolic LPL and LPTA activities exhibited two pH optima, at pH 4.0 and 6.0, with the activities decreasing to zero at pH 9.0 (data not shown). The cytosolic PLB pH profile (data not shown) was similar to that of membrane-associated PLB pH profile (Fig. 5B), although it was less sharp. The pH optimum was 3 to 4 for membrane-associated LPL and LPTA activities, and the activities gradually decreased to zero at pH 9.0 (Fig. 5A). The optimum pH for membrane PLB (pH 4.0) was very narrow (Fig. 5B), with virtually no activity at pH 7.0. For the inhibitor studies, all assays were performed at pH 4.0.
FIG. 5.
Effects of pH on membrane-associated phospholipase activities. LPL and LPTA activities were measured by using 30 s (A) and 10 min (C) of incubation. Points are means ± standard errors of the means of three assays.
The most obvious difference between the cell-associated (cytosolic and membrane-associated) activities and those of both the crude and purified secreted enzymes was the greater activity of the cell-associated enzymes at pH 6.0 (PLB) and 7 to 8 (LPL and LPTA) (5, 7). Interestingly, when the incubation time of the assay with LPL and LPTA was extended from 20 to 30 s to 10 min, the activity was increased over a pH range of 7 to 9, but not at lower pH values, in both membrane and cytosolic enzyme preparations (see the results in Fig. 5C for the membrane-associated enzyme). This phenomenon has also been noted for the secreted LPL and LPTA activities (Wright, unpublished).
Cellular distributions of phospholipase activities.
By taking 4.0 as the optimal pH for all three activities, it is clear that the distribution of PLB differs from those of LPL and LPTA, in that the greatest percentage of the total activity is secreted (Table 1). For LPL and LPTA most of the activity is cytosolic. The specific activities and percentages of all three activities were lowest in the membrane fraction (Table 1).
TABLE 1.
Cellular distribution of phospholipase activities measured at pH 4.0a
| Fraction | Sp act
|
Total activity
|
% Distribution
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| LPL | LPTA | PLB | LPL | LPTA | PLB | LPL | LPTA | PLB | |
| Secreted | 86.6 (7.2) | 53.9 (3.5) | 2.1 (0.29) | 650 (54) | 405 (26) | 16.2b,c (2.1) | 36.1 (1.4) | 29.9 (1.9) | 82.7b,c (10.7) |
| Cytosolic | 78.4 (12.6) | 68.9 (20.6) | 0.2 (0.15) | 955b (154) | 839b (251) | 2.5 (1.8) | 53.1b (8.6) | 62.0b (18.6) | 12.8 (9.2) |
| Membrane | 0.54 (0.28) | 0.31 (0.15) | 0.003 (0.001) | 195 (99) | 109 (53) | 0.9 (0.4) | 10.8 (5.5) | 8.1 (3.9) | 4.6 (2.0) |
Data are expressed as the means (standard errors of the means) of at least three assays, with activities calculated as micromoles of substrate degraded or product formed (LPTA) per minute per milligram of protein (specific activity) or per total amount of protein (total activity) in the cellular fraction.
P < 0.01 compared with membrane activity (analysis of variance).
P < 0.01 compared with cytosolic activity (analysis of variance).
PLA, PLC, and PLD.
The secreted forms of PLA, PLC, and PLD have not been identified in cryptococcal supernatants (5, 33). High levels of PLA were detected in the membrane preparations by the formation of radiolabeled lyso-PC from DPPC at pH 7 to 8 (Fig. 6A), but only trace amounts of PLA were found in the cytosolic fraction (data not shown). Small amounts of PLD activity were detected by the formation of radiolabeled phosphatidic acid from both the cytosolic and the membrane fractions at pH 7 to 8 (see Fig. 6B for membrane-associated PLD). No evidence of PLC was found at any pH by use of DPPC as the substrate.
FIG. 6.
Membrane-associated PLA and PLD. Assays were performed in the absence of added cations.
Modifying agents and cell-associated activities.
Calcium, magnesium, or FeCl3, each at a concentration of 10 mM and pH 4.0, had no significant effect on any of the cytosolic or membrane-associated activities. Triton X-100 (0.1%; wt/vol) produced some inhibition at pH 4.0, with cytosolic and membrane-associated LPTA (61%) and PLB (81%) activities being the most affected. When the activities were assayed at pH 7.0, all three of the cytosolic and membrane-associated activities were stimulated by calcium (1.7- to 5.9-fold). Cytosolic PLB and all three of the membrane-associated activities were also stimulated by magnesium (1.5- to 4.8-fold). At pH 7.0, FeCl3 and Triton X-100 were inhibitors of all three activities from both the cytosolic and the membrane-bound fractions (with 98% inhibition of membrane-associated PLB by FeCl3). Triton X-100 was an especially effective inhibitor of the LPL and LPTA activities (96 to 100%).
Selection and testing of potential phospholipase inhibitors.
Monomeric and dimeric ammonium and phosphonium compounds, cationic heterocyclic compounds, phosphocholines, guanidines, and biguanidines were screened. Compounds belonging to classes known to inhibit mammalian PLAs, for example, all the so-called stable amide (14) and ether-bonded (17, 23) phospholipids, were excluded, as their micellar structures can cause false-positive assay results (19). Other compounds that form micelles were also excluded, as they tend to cause lysis of mammalian cells and are therefore cytotoxic. The structures and common names of the four compounds selected are shown in Fig. 2. These compounds retained the key features of a strong positive charge and fatty acid-like hydrophobicity.
Assays were performed at pH 4 in the absence of added cations and under the optimized conditions established as described above. Under these conditions only PLB, LPL, and LPTA activities (both secreted and cell-associated activities) were measured. Initially, compounds deemed to be potential inhibitors were assayed at 25 and 250 μM. Those showing some inhibitory activity were then also assayed at 2.5 and 0.25 μM.
Compound A.
The most potent compound was the dicationic symmetrical bis-alkylbiguanido alkane alexidine dihydrochloride (compound A), which showed a strong preference for PLB, especially the secreted and cytosolic enzymes. The LPL and LPTA activities were inhibited to a lesser extent in all three fractions, especially the secretory and cytosolic enzymes (Table 2). At 2.5 μM, compound A had selectivity for secreted PLB and cytosolic LPL and LPTA. The higher concentrations of compound A also inhibited porcine pancreatic PLA2 (Table 3). The toxicity of compound A for mammalian cells, as determined by measurement of the percent hemolysis, was zero at concentrations up to 3.5 μM and 20 and 100% at concentrations of 35 and 350 μM, respectively.
TABLE 2.
Inhibition of the activities of C. neoformans strain H99 phospholipases by three test compounds
| Fraction and compounda | % Inhibition by the following compound at the indicated concn (μM)b
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPL
|
LPTA
|
PLB
|
||||||||||
| 0.25 | 2.5 | 25 | 250 | 0.25 | 2.5 | 25 | 250 | 0.25 | 2.5 | 25 | 250 | |
| Secretory enzyme | ||||||||||||
| A | 0 | 18.0c (0.6) | 52.0c (2.0) | 61.0c (4.0) | 0 | 16.0c (2.1) | 50.7c (3.5) | 60.3c (2.4) | 59.7c (3.8) | 83.0c (0.6) | 88.0c (0.6) | 94.7c (1.5) |
| P | 0 | 0 | 0 | 6.3c (0.8) | 0 | 0 | 0 | 3.7c (0.6) | 0 | 0 | 35.2c (1.5) | 55.0c (1.5) |
| O | ND | 0 | 39.0c (2.1) | 91.7c (2.0) | ND | 0 | 41.0c (2.1) | 94.7c (1.5) | ND | 0 | 0 | 0 |
| Cytosolic enzyme | ||||||||||||
| A | 0 | 15.8c (2.0) | 16.6c (1.6) | 56.4c (1.3) | 0 | 20.8c (1.7) | 27.1c (2.0) | 71.0c (1.7) | 0 | 0 | 21.0c (1.9) | 74.6c (0.9) |
| P | ND | 0 | 0 | 12.7c (0.9) | ND | 0 | 0 | 0 | ND | 0 | 0 | 41.3c (2.3) |
| O | ND | 0 | 8.0c (0.6) | 39.7c (3.4) | ND | 0 | 9.0c (0.6) | 44.3c (3.7) | ND | 0 | 0 | 13.7c (0.9) |
| Membrane-bound enzyme | ||||||||||||
| A | 0 | 0 | 0 | 4.7c (1.4) | 0 | 0 | 0 | 24.3c (1.4) | 0 | 0 | 0 | 63.2c (2.3) |
| P | ND | 0 | 0 | 0 | ND | 0 | 0 | 0 | ND | 0 | 0 | 0 |
| O | ND | 0 | 0 | 12.0c (3.1) | ND | 0 | 0 | 18.0c (3.5) | ND | 0 | 0 | 42.3c (2.6) |
The structures and abbreviations for these compounds are shown in Fig. 2. Compound D did not inhibit any of the activities of any enzyme at these concentrations.
Data are expressed as the means (standard errors of the means) of at least three assays. ND, not determined.
Significantly different (P < 0.01) from the results for the inhibitor-free controls by the Dunnett multiple-comparison test.
TABLE 3.
Effects of three compounds tested against the cryptococcal phospholipases on porcine pancreatic PLA2
| Concn (μM) | % Inhibition of activity by test compounda:
|
|||||
|---|---|---|---|---|---|---|
| A
|
P
|
O
|
||||
| PLA2 | PLB | PLA2 | PLB | PLA2 | LPLb | |
| 250 | 94.0c (1.0) | 94.7c (1.4) | 96.5c (0.3) | 55.0c (1.5) | 0 | 91.7c (2.0) |
| 25 | 88.6c (2.0) | 88.0c (0.6) | 62.2c (1.7) | 35.2c (1.5) | 0 | 39.0c (2.1) |
| 2.5 | 54.3c (2.3) | 83.0c (0.6) | 4.9c (1.3) | 0 | 0 | 0 |
| 0.25 | 0 | 59.7c (3.8) | 0 | 0 | ND | 0 |
| 0.025 | ND | 30.0c (1.7) | ND | ND | ND | ND |
Data are expressed as the means (standard errors of the means) of at least three assays. The structures and abbreviations of the compounds are given in Fig. 2. ND, not determined.
This compound does not inhibit PLB activity but inhibits LPL and LPTA activities equally strongly (Table 2).
Significantly different (P < 0.01) from the results for the inhibitor-free controls by the Dunnett multiple-comparison test.
Compound P.
Another dicationic compound, namely, 1,12-bis-(tributylphosphonium)dodecane (compound P) inhibited secreted and cytosolic PLB but not membrane-associated PLB, albeit to a lesser extent than the biguanide. Furthermore, this compound did not inhibit LPL or LPTA (Table 2) and was a more effective inhibitor of pancreatic PLA2 than of PLB (Table 3). Compound P showed remarkably low levels of toxicity for mammalian cells, with a concentration of 350 μM required to produce 15% hemolysis.
Compound D.
A third dicationic compound, 1,10-bis(trimethylammonium)decane, commonly known as decamethonium (compound D), was also tested but was not inhibitory (data not shown).
Compound O.
In contrast to the bicationic compounds, the monocationic compound dioctadecyldimethylammonium bromide (compound O) inhibited the secreted and cytosolic LPL and LPTA activities almost exclusively but affected only the membrane PLB activity (Table 2). Even at high concentrations, this monocationic compound did not inhibit mammalian PLA2 (Table 3).
Antifungal activities of inhibitors.
All compounds mentioned above were assayed by a standardized serial dilution sensitivity test for their antifungal activities against two strains of C. neoformans and one strain of C. albicans (Table 4). The two stronger phospholipase inhibitors (compounds A and P) were quite potent, with MICs in the range of 0.5 to 10 μM, whereas the noninhibitory decamethonium compound (compound D) had a much higher MIC (88 to 350 μM). Notably, the monocationic compound (compound O) that inhibited secretory and cytosolic LPL and LPTA activities but not PLB activity had an MIC of >350 μM for all three strains (Table 4).
TABLE 4.
MICs of the four compounds tested as antifungal agents for C. neoformans strain H99, C. neoformans reference strain ATCC 90112, and C. albicans reference strain ATCC 10231a
| Compound | FWb |
C. neoformans H99
|
C. neoformans ATCC 90112
|
C. albicans ATCC 10231
|
|||
|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | MIC (μM) | MIC (μg/ml) | MIC (μM) | MIC (μg/ml) | MIC (μM) | ||
| A | 581.0 | 0.58 | 1.0 | 0.29 | 0.50 | 0.58 | 1.0 |
| P | 732.8 | 0.80 | 1.1 | 1.83 | 2.5 | 6.45 | 8.8 |
| O | 631.0 | 110.4 | 175 | 220.8 | 350 | 220.8 | 350 |
| D | 418.3 | 146.4 | 350 | 36.8 | 88 | 146.4 | 350 |
The MIC of the benchmark positive control, amphotericin B (formula weight, 924.0), for these fungi is 0.25 to 0.5 μg/ml (0.27 to 0.54 μM).
FW, formula weight.
DISCUSSION
Characterization of the cell-associated phospholipases of C. neoformans.
Under the assay conditions tested, phospholipase B was the major phospholipase present in the cytosol and secreted from C. neoformans. In contrast, PLA and PLD were membrane associated, with some PLD activity in the cytosol. It is of interest that the pH optimum for PLB activity, whether secreted, membrane bound, or cytosolic, was always acidic (pH 4.0), whereas the cytosolic LPL and LPTA activities were bimodal (pH 4.0 and 6.0). PLA and PLD activities were detected only at pH 7 to 8. These observations are consistent with the roles of secreted PLB activities in cryptococcal virulence, since the putative sites of action of PLB are the acidic vacuoles of macrophage-like cell lines (11, 29) and mouse macrophages in vivo (32). We reported previously that cryptococci create their own extracellular acidic microenvironment both in vitro and in vivo by secreting large amounts of acetic acid (21, 35). This enables the secreted PLB to have optimal activity at the Cryptococcus-host tissue interface and may aid invasion by the fungus.
Since the phospholipase B protein, encoded by the CnPLB1 gene, contains PLB, LPL, and LPTA activities, the observation that LPL and LPTA activities are mostly cytosolic yet PLB activity is mostly secreted suggests that there could be a second LPL-LPTA enzyme. Recently, we discovered a novel gene, CnLYSO1, in C. neoformans (10) and have purified a secreted protein with LPL and LPTA activities but no PLB activity which is stimulated by calcium and which is active at pH 7.0 (Wright, unpublished). It now appears that a similar enzyme is present in both the cytosolic and the membrane-associated fractions. The membrane-associated LPL and LPTA activities were stimulated by both calcium and magnesium. The unexpected stimulation of cell-associated PLB activity by calcium and magnesium at pH 7.0 was probably due in part to PLA activity, although this has not been proven.
In addition to the possibility of a second enzyme, the predominant activity (PLB or LPL and LPTA) of the phospholipase B protein in a particular cellular compartment may be dependent on the physiological conditions existing in that compartment. The protein may contain several active sites which could be differentially exposed as a result of either environmental conditions or posttranslational events. Previously we showed that modifiers affected secreted PLB and LPL-LPTA differentially at pH 4.0. The detergent Triton X-100 inhibited both crude and purified secreted LPL and LPTA activities but not PLB activity (7, 33), whereas FeCl3 strongly inhibited all three activities. In contrast, Triton X-100 inhibited all three cell-associated activities to some extent, whether cytosolic or membrane associated, especially at pH 7.0, which is the pH of the cytosol. FeCl3 showed little or no inhibition of cytosolic or membrane-associated activities at pH 4.0 but showed significant inhibition at pH 7.0.
Inhibition studies.
The characterization studies demonstrated that assay at pH 4.0 in the absence of added cations measures both secreted and cell-associated phospholipase B activities rather than PLA, PLD, or PLC activity. Three of the four compounds tested (compounds A, P, and O) selectively inhibited PLB and LPL-LPTA activities at concentrations in the low-micromolar range. These results indicate that the binding profile for PLB differs from that of LPL-LPTA and that the enzyme has two different active sites or two different binding features in a single active site. This conclusion is supported by the characterization of cell-associated phospholipase B activities and agrees with previous findings for the secreted enzymes (5, 7, 33). It will therefore be possible to establish which activity is more relevant for cell viability and virulence and will provide direction for the future design of inhibitors and further target validation.
Both cytosolic and membrane-associated enzymes were more resistant to inhibition by FeCl3 and Triton X-100 than the secreted enzyme when they were tested at pH 4.0 (5, 7, 33). However, the compounds analogous to phospholipid substrates (compounds A, O, and P) that inhibited either the PLB activity or the LPL and LPTA activities, or both, in the secreted enzyme showed the same inhibition pattern with the cytosolic enzyme, although the inhibition was about five times less potent. This indicates that the binding properties of the two enzymes are remarkably similar and that a single compound can be used to target both enzymes. In contrast, although the membrane-bound enzyme had low overall activity, it was relatively resistant to phospholipase inhibition, and its PLB activity was most strongly inhibited by the compound that was selective for the LPL and LPTA activities in the cytosolic and secreted enzymes. These data indicate that the membrane-bound enzyme has a different binding site.
A novel finding was that the most potent inhibitors of PLB or both PLB and LPL-LPTA also demonstrated antifungal activity in a standard microdilution assay. The most active compound (compound A), a bis-biguanidinium compound, was as potent as the marketed antifungal drug amphotericin B. The weaker PLB inhibitors exerted a weaker antifungal effect. Compound O, which selectively inhibited LPL and LPTA activities, had an MIC >350 μM.
Our data indicate that antifungal activity is correlated with inhibition of PLB but does not prove a causal relationship. Three of the compounds tested are nontoxic to humans and have already been approved for use in other situations: compound O as a component in liposomes for drug delivery (12), compound D as an anticholinergic (26), and compound A as a bactericidal agent for the treatment of gingivitis (9). Compound P is known as an ion-pairing reagent (30). We found compound P to have surprisingly low hemolytic activity (only 15% at a concentration 100-fold higher than the MIC). This is the first report that a bis-(trialkylphosphonium)alkan exhibits antifungal activity, and its low level of toxicity suggests that this class of compounds, including the corresponding bis-ammonium analogues, could be attractive molecules for further development as antifungals. Since the bis-biguanido structure offers the most promise for selectivity and also shows antifungal activity, it is suitable as a platform for a directed synthesis program aimed at understanding the structural basis for selectivity between fungal and mammalian phospholipases and for the correlation of enzyme inhibition and antifungal activity.
It is noteworthy that a viral phospholipase has been reported to be critical for infectivity (18). Moreover, compounds closely related structurally to our inhibitors are bactericidal (25) and have demonstrated antimalarial properties (4). Bacterial phospholipases are well characterized (22, 34), and the consensus sequence for phospholipases is present in the genome of Plasmodium falciparum (15). Phospholipase activity has also been detected (36). Microbial phospholipases may therefore be suitable targets for anti-infectives in general.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (grant 211040).
REFERENCES
- 1.Birch, M., G. Robson, D. Law, and D. W. Denning. 1996. Evidence of multiple extracellular phospholipase activities of Aspergillus fumigatus. Infect. Immun. 64:751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bligh, E. C., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 3.Bremner, J. B., K. Castle, R. Griffith, P. A. Keller, and D. D. Ridley. 2002. Mining the chemical abstracts database with pharmacophore-based queries. J. Mol. Graphics Model 21:185-194. [DOI] [PubMed] [Google Scholar]
- 4.Calas, M., M. L. Ancelin, G. Cordina, P. Portefaix, G. Piquet, V. Vidal-Sailhan, and H. Vial. 2000. Antimalarial activity of compounds interfering with Plasmodium falciparum phospholipid metabolism: comparison between mono- and bisquaternary ammonium salts. J. Med. Chem. 43:505-516. [DOI] [PubMed] [Google Scholar]
- 5.Chen, S. C., L. C. Wright, R. T. Santangelo, M. Muller, V. R. Moran, P. W. Kuchel, and T. C. Sorrell. 1997. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect. Immun. 65:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, S. C., M. Muller, J. Z. Zhou, L. C. Wright, and T. C. Sorrell. 1997. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J. Infect. Dis. 175:414-420. [DOI] [PubMed] [Google Scholar]
- 7.Chen, S. C., L. C. Wright, J. C. Golding, and T. C. Sorrell. 2000. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem. J. 347:431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuck, S. L., and M. A. Sande. 1989. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N. Engl. J. Med. 321:794-799. [DOI] [PubMed] [Google Scholar]
- 9.Coburn, R. A., P. J. Baker, R. T. Evans, R. J. Genco, and S. L. Fischman. 1978. In vitro antiplaque properties of a series of alkyl bis(biguanides). J. Med. Chem. 21:828-829. [DOI] [PubMed] [Google Scholar]
- 10.Coe, J. G. S., C. F. Wilson, T. C. Sorrell, N. G. Latouche, and L. C. Wright. 2003. Cloning of CnLYSO1, a novel extracellular lysophospholipase of the pathogenic fungus Cryptococcus neoformans. Gene 316:67-68. [DOI] [PubMed] [Google Scholar]
- 11.Cox, G. M., H. C. McDade, S. C. Chen, S. C. Tucker, M. Gottfredsson, L. C. Wright, T. C. Sorrell, S. D. Leidich, A. Cassadevall, M. A. Ghannoum, and J. R. Perfect. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39:166-175. [DOI] [PubMed] [Google Scholar]
- 12.Dass, C. R., T. L. Walker, and M. A. Burton. 2002. Liposomes containing cationic dimethyl dioctadecyl ammonium bromide: formulation, quality control, and lipofection efficacy. Drug Deliv. 9:11-18. [DOI] [PubMed] [Google Scholar]
- 13.De Haas, G. H., M. G. Van Oort, R. Dijkman, and R. Verger. 1989. Phospholipase A2 inhibitors: monoacyl, monoacylamino-glycero-phosphocholines. Biochem. Soc. Trans. 17:274-276. [DOI] [PubMed] [Google Scholar]
- 14.Dijkman, R., N. Dekker, and G. H. de Haas. 1990. Competitive inhibition of lipolytic enzymes. II. Preparation of “monoacylamino” phospholipids. Biochim. Biophys. Acta 1043:67-74. [DOI] [PubMed] [Google Scholar]
- 15.Gardner, M. J., H. Tettelin, D. J. Carucci, L. M. Cummings, L. Aravind, E. V. Koonin, S. Shallom, T. Mason, K. Yu, C. Fujii, J. Pederson, K. Shen, J. Jing, C. Aston, Z. Lai, D. C. Schwartz, M. Pertea, S. Salzberg, L. Zhou, G. G. Sutton, R. Clayton, O. White, H. O. Smith, C. M. Fraser, M. D. Adams, J. C. Venter, and S. L. Hoffman. 1998. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science 282:1126-1132. [DOI] [PubMed] [Google Scholar]
- 16.Ghannoum, M. A., A. S. Ibrahim, Y. Fu, M. C. Shafiq, J. E Edwards, Jr., and R. S. Criddle. 1992. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J. Clin. Microbiol. 30:2881-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert, A., and R. Bittman. 1998. The inhibition of cell signalling pathways by antitumor ether lipids. Biochim. Biophys. Acta 1390:85-102. [DOI] [PubMed] [Google Scholar]
- 18.Girod, A., C. E. Wobus, Z. Zadori, M. Ried, K. Leike, P. Tijssen, J. A. Kleinschmidt, and M. Hallek. 2002. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 83:973-978. [DOI] [PubMed] [Google Scholar]
- 19.Glaser, K. B. 1995. Regulation of phospholipase A2 enzymes: selective inhibitors and their pharmacological potential. Adv. Pharmacol. 32:31-66. [DOI] [PubMed] [Google Scholar]
- 20.Hanel, H., R. Kirsch, H.-L. Schmidts, and H. Kottmann. 1995. New systematically active antimycotics from the beta-blocker category. Mycosis 38:251-264. [DOI] [PubMed] [Google Scholar]
- 21.Himmelreich, U., C. Allen, S. Dowd, R. Malik, B. P. Shehan, C. Mountford, and T. C. Sorrell. 2002. Identification of metabolites of importance in pathogenesis of pulmonary cryptococcoma using nuclear magnetic resonance spectroscopy. Microbes Infect. 5:285-290. [DOI] [PubMed] [Google Scholar]
- 22.Kang, J.-H., J.-H. Lee, J.-H. Park, S.-H. Huh, and I.-S. Kong. 1998. Cloning and identification of a phospholipase gene from Vibrio mimicus. Biochim. Biophys. Acta 1394:85-89. [DOI] [PubMed] [Google Scholar]
- 23.Kazi, A. B., S. Shidmand, and J. Hajdu. 1999. Stereospecific synthesis of functionalized ether phospholipids. J. Org. Chem. 64:9337-9347. [Google Scholar]
- 24.Kwon-Chung K. J., and J. E. Bennett. 1992. Cryptococcosis, p. 397-446. In Medical mycology, 16th ed. Lea & Febiger, Philadelphia, Pa.
- 25.Massi, L., F. Guittard, S. Geribaldi, R. Levy, and Y. Duccini. 2003. Antimicrobial properties of highly fluorinated bis-ammonium salts. Int. J. Antimicrob. Agents 21:20-26. [DOI] [PubMed] [Google Scholar]
- 26.Michelson, M., and E. Zaimal. 1973. Acetylcholine, an approach to the molecular mechanism of action. Pergamon Press, Oxford, United Kingdom.
- 27.Mirbod, F., Y. Banno, M. A. Ghannoum, A. S. Ibrahim, S. Nagashima, Y. Kitajhima, G. T. Gole, and Y. Nozava. 1995. Purification and characterization of lysophospholipase-transacylase (h-LPTA) from a highly virulent strain of Candida albicans. Biochim. Biophys. Acta 1257:181-188. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 1992. Reference method for broth dilution susceptibility testing of yeast: proposed standard. NCCLS document M27-P. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 29.Noverr, M. C., G. M. Cox, J. R. Perfect, and G. B. Huffnagle. 2003. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 71:1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohki, A., J. Okamoto, K. Naka, and S. Maeda. 1991. Ion-pair chromatography of a dianionic species dionium reagent. Chromatographia 32:73-78. [Google Scholar]
- 31.Ridley, D. D. 2002. Information retrieval: SciFinder and SciFinder Scholar. John Wiley & Sons, Inc., New York, N.Y.
- 32.Santangelo, R., H. Zoellner, T. Sorrell, C. Wilson, C. Donald, J. Djordjevic, Y. Shounan, and L. Wright. 2004. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 72:2229-2239. [DOI] [PMC free article] [PubMed]
- 33.Santangelo, R. T., M. H. Nouri-Sorkhabi, T. C. Sorrell, M. Cagney, S. C. Chen, P. W. Kuchel, and L. C. Wright. 1999. Biochemical and functional characterisation of secreted phospholipase activities from Cryptococcus neoformans in their naturally occurring state. J. Med. Microbiol. 48:731-740. [DOI] [PubMed] [Google Scholar]
- 34.Snijder, H. J., and B. W. Dijkstra. 2000. Bacterial phospholipase A: structure and function of an integral membrane phospholipase. Biochim. Biophys. Acta 1488:91-101. [DOI] [PubMed] [Google Scholar]
- 35.Wright, L., W. Bubb, J. Davidson, R. Santangelo, M. Krockenberger, U. Himmelreich, and T. Sorrell. 2002. Metabolites released by Cryptococcus neoformans var. neoformans and var. gattii differentially affect human neutrophil function. Microbes Infect. 4:1427-1438. [DOI] [PubMed] [Google Scholar]
- 36.Zidovetzki, R., I. W. Sherman, and L. O'Brian. 1993. Inhibition of Plasmodium falciparum phospholipase A2 by chloroquine, quinine and arteether. J. Parasitol. 79:565-570. [PubMed] [Google Scholar]