Abstract
Purpose.
We describe a particular form of autosomal recessive generalized choriocapillaris dystrophy phenotype associated with ABCA4 mutations.
Methods.
A cohort of 30 patients with identified ABCA4 mutations and a distinct phenotype was studied. A retrospective review of history, fundus photographs, electroretinography, visual field testing, dark adaptometry, and optical coherence tomography was performed. Genetic analyses were performed by ABCA4 microarray analysis, high resolution melting, and/or next generation sequencing of all protein-coding sequences of the ABCA4 gene.
Results.
The earliest recorded manifestation of ABCA4-associated disease was a central bull's eye type of macular dystrophy that progressed to chorioretinal atrophy of the macula with coarse rounded hyperpigmentations and expanding involvement of the periphery. The mean age at first presentation was 10.3 years, the longest follow-up was 61 years. All patients had two ABCA4 mutations identified, confirming the molecular genetic diagnosis of an ABCA4-associated disease. Most patients harbored at least one mutation classified as “severe,” the most common of which was the p.N965S variant that had been found previously at a high frequency among patients with ABCA4-associated retinal dystrophies in Denmark.
Conclusions.
Generalized choriocapillaris dystrophy is a progressive ABCA4-associated phenotype characterized by early-onset macular dystrophy that disperses and expands to widespread end-stage chorioretinal atrophy with profound visual loss. All cases in this study were confirmed as harboring two ABCA4 mutations. Most of the ABCA4 mutations were classified as “severe” explaining the early onset, panretinal degeneration, and fast progression of the disease.
Keywords: chorioretinal dystrophy, ABCA4, phenotype–genotype
This study delineates a particular type of generalized choriocapillaris dystrophy caused by mostly deleterious mutations in the ABCA4 gene. The disease presents with early-onset macular dystrophy and progresses into an end-stage of widespread choriocapillaris atrophy.
Introduction
The retina-specific adenosine triphosphate (ATP)-binding cassette transporter (ABCA4) protein is a transporter of vitamin A derivatives in the visual cycle, a process that is vital for the maintenance of healthy photoreceptors. Mutations in the ABCA4 gene are responsible for a wide range of retinal degeneration phenotypes, including, Stargardt disease,1 cone-rod dystrophy (CRD), and retinitis pigmentosa (RP).2,3
A substantial fraction of patients in clinical practice present with a phenotype that differs from Stargardt disease and fundus flavimaculatus4 by the lack of flecks in the early stages, and by a characteristic end-stage with generalized choriocapillaris dystrophy. The end-stage is characterized by severe retinal dystrophy in addition to the choriocapillaris dystrophy, but differs from the primary photoreceptor dystrophies, RP, and CRD by the significant involvement of the choriocapillaris layer. Other studies of the phenotypes associated with mutations in the ABCA4 gene have reported similar cases with phenotypes resembling end-stage generalized choriocapillaris dystrophy, but these were classified as CRD,5–8 RP-like,9–11 and retinal dystrophy,12 and did not describe the early stages. A study of several families of Arabic descent from a single village included a few cases resembling the early stages described in this study.13
Based on a follow-up study over a long time span of a relatively large selected group of patients with the distinct phenotype of generalized choriocapillaris dystrophy, we outlined the natural history of this subset within the spectrum of ABCA4-related retinopathies.
Methods
Patients
Patients were enrolled from the Danish Retinitis Pigmentosa Registry (DRPR) and the ABCA4 mutation database, which are located at the National Eye Clinic for the Visually Impaired at the Kennedy Center, Denmark. The mutation database holds information on all of the pathogenic mutations found in previous genetic studies of the ABCA4 gene in Danish patients with ABCA4-related retinopathies, including Stargardt disease, CRD, and RP.14,15 The DRPR comprises all patients in Denmark with generalized retinal and chorioretinal dystrophies.16 The charts of patients registered with generalized choriocapillaris dystrophy and/or atypical RP were reviewed in the DRPR, and cases with two ABCA4 mutations were included from the mutation database. The study adhered to the tenets of the Declaration of Helsinki II. According to Danish law, no ethical approval was required for this retrospective clinical study that used anonymized data.
Clinical Evaluation
All patients had undergone a complete ophthalmic examination by a medical retina specialist, including best-corrected visual acuity (BCVA) testing, slit-lamp examination, and direct and indirect ophthalmoscopy. The available records generally included color fundus photographs (from 1970, most photographs presented here were made using the TRC 50 DX camera [Topcon, Tokyo, Japan]), Goldmann perimetry plots, Goldmann-Weekers dark adaptometry curves, and electroretinograms (ERG). The Ganzfeld full field ERGs were performed according to the ISCEV standard guidelines with a Burian-Allen electrode. The recording equipment varied throughout the period. Patients seen more recently often also had undergone fundus autofluorescence photography and optical coherence tomography (OCT; Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany) and wide-field scanning laser ophthalmoscopy (Optos 200Tx; Optos PLC, Dunfermline, UK). The initial review identified 42 patients with diagnoses related to chorioretinal dystrophy. A second review of the clinical records classified 12 patients as presenting with CRD, central areolar atrophy, pattern dystrophy, or serpiginous choroiditis, which left 30 patients with a mutual retinal appearance and course of disease who were strictly diagnosed with generalized choriocapillaris dystrophy. In a few patients with end-stages resembling generalized choriocapillaris dystrophy, no possibly disease-associated mutations were identified in the ABCA4 gene. These patients were not included in the present study. The examinations were performed between 1952 and May 2013.
The mutations found in 10 of the 30 patients included in the present study (patients D137, D109, D147, D022, D112, D108, D135, D117, D186, and D173) were included in past reports on ABCA4 disease14,15 that described the cross-sectional genetic and clinical spectrum of ABCA4 disease in Denmark, with a more detailed description of the clinical history and end-stage imaging of one patient (D137).15 To our knowledge, this is the first large study to define the longitudinal characteristics of this particular form of generalized choriocapillaris dystrophy.
Genetic Analysis
DNA was isolated from EDTA-treated blood samples and the ABCA4 gene was examined using ABCA4 microarray analyses, high-resolution melting (HRM) following direct sequencing of heteroduplex positive bands or next-generation sequencing (NGS) methods. DNA samples from 23 patients were analyzed in 2002 to 2004 for known ABCA4 mutations using a commercial APEX microarray (Asper Biotech AS, Tartu, Estonia),17 which then included 386 pathogenic ABCA4 variants.15 DNA samples from 8 patients were analyzed using ABCA4 microarray and HRM methods.14 Finally, DNA samples from 6 patients were analyzed using NGS sequencing of the ABCA4 gene, and 3 of these samples had already been analyzed with the ABCA4 microarray analysis.18
All missense ABCA4 variants, except for the known frequent polymorphisms, were analyzed with algorithms, such as Align GVGD,19 Sorting Intolerant from Tolerant (SIFT; available in the public domain at http://sift.jcvi.org/), Polymorphism Phenotyping v2 (PolyPhen2; available in the public domain at http://genetics.bwh.harvard.edu/pph2/), and MutationTaster,20 to predict the impact of variants on the ABCA4 function and, consequently, on disease susceptibility. Variants detected in the vicinity to exons/intron boundaries were analyzed with splice site prediction programs MaxEntScan,21 GeneSplicer (available in the public domain at http://www.cbcb.umd.edu/software/GeneSplicer), NNSPLICE (available in the public domain at http://www.fruitfly.org/seq_tools/splice.html), and Human Splicing Finder.22 All the prediction programs were accessed via Alamut 2.2 software (available in the public domain at http://www.interactive-biosoftware.com).
Results
The mean duration of follow-up in the 30 patients (14 males, 16 females) was 27.9 years (range, 9.4–60.7 years; Table 1). The mean age of clinical presentation was 10.3 years (range 4–18 years), and the year of presentation ranged from 1952 to 1996. Six patients were from the same family, GCCD0801, and the remaining patients were unrelated. All families were Caucasian, with four families of mixed descent (Danish and Swedish/Greek/Polish), while one family was from Portugal, and the rest of the families were from Denmark. None of the patients had high myopia.
Table 1.
Demographic Characteristics of Patients With Generalized Choriocapillaris Dystrophy
|
Patient |
Pedigree |
Sex |
Refraction |
Age at Presentation |
Date of Presentation |
Most Recent Date of Examination |
Period of Observation, y |
| D513 | RP02270 | M | Plano | 10 | January 19, 1952 | February 25, 1983 | 31.1 |
| D514 | STG04071 | M | Plano | 8 | March 16, 1984 | April 27, 2011 | 27.1 |
| D516 | STG1 | M | Plano | 6 | May 27, 1992 | October 9, 2010 | 18.4 |
| D517 | STG2 | M | −3.5/−4.7 | 12 | October 6, 1970 | December 8, 2010 | 40.2 |
| D137 | STG04078 | M | Plano | 18 | January 16, 1968 | September 3, 2002 | 34.7 |
| D801 | GCCD0801 | M | Plano | 7 | April 29, 1987 | September 21, 2001 | 14.4 |
| D802 | GCCD0801 | M | Plano | 7 | May 30, 1990 | February 23, 2000 | 9.8 |
| D109 | GCCD0801 | M | Plano | 18 | April 14, 1969 | September 16, 1997 | 28.5 |
| D803 | GCCD0801 | F | −1.0/−1.0 | 6 | May 9, 1969 | April 20, 1994 | 24.9 |
| D804 | GCCD0801 | M | Plano | 14 | February 7, 1969 | October 26, 2012 | 43.7 |
| D805 | GCCD0801 | F | −0.5/−0.5 | 15 | February 1, 1971 | January 14, 2005 | 33.9 |
| D040 | GCCD0804 | F | −1.5/−2.0 | 4 | August 23, 1985 | March 7, 2013 | 27.6 |
| D159 | STG00215 | M | N/A | 7 | May 25, 1984 | March 22, 2004 | 19.8 |
| D129 | STG04042 | F | −4.0/−4.0 | 13 | March 12, 1964 | August 31, 2000 | 36.4 |
| D115 | STG04073 | M | Plano | 10 | April 16, 1953 | November 19, 1996 | 43.6 |
| D033 | STG04012 | F | −1.0/−1.0 | 11 | September 11, 1975 | May 16, 2012 | 36.7 |
| D023 | STG00205 | F | Plano | 9 | January 21, 1985 | May 5, 2013 | 28.3 |
| D001 | STG04003 | F | Plano | 8 | November 19, 1996 | August 28, 2012 | 15.7 |
| D147 | STG04069 | F | −3.0/−3.0 | 11 | August 14, 1981 | January 8, 1991 | 9.4 |
| D162 | STG00216 | F | −1.0/−1.5 | 18 | September 4, 1992 | November 11, 2003 | 11.0 |
| D022 | STG04092 | M | Plano | 9 | September 15, 1983 | June 6, 2003 | 19.7 |
| D112 | STG04077 | F | −2.5/−1.2 | 9 | November 27, 1984 | February 28, 2011 | 26.2 |
| D108 | STG04088 | F | Plano | 11 | August 15, 1977 | February 5, 2008 | 30.5 |
| D107 | STG04087 | F | −0.7/−0.5 | 13 | September 7, 1990 | December 3, 2003 | 13.1 |
| D070 | STG04023 | F | Plano | 16 | March 10, 1975 | August 17, 2011 | 36.4 |
| D116 | STG04081 | M | Plano | 10 | February 11, 1977 | April 29, 2013 | 36.2 |
| D135 | STG04074 | M | +1.2/+3 | 7 | September 27, 1950 | March 16, 1995 | 44.5 |
| D117 | STG04082 | F | Plano | 7 | August 22, 1984 | January 22, 2003 | 18.4 |
| D186 | GCCD0810 | F | Plano | 12 | June 2, 1975 | April 4, 1991 | 15.8 |
| D173 | GCCD4067 | F | +1.5/+1 | 13 | May 29, 1952 | January 28, 2013 | 60.7 |
Clinical Characteristics of Stage 1
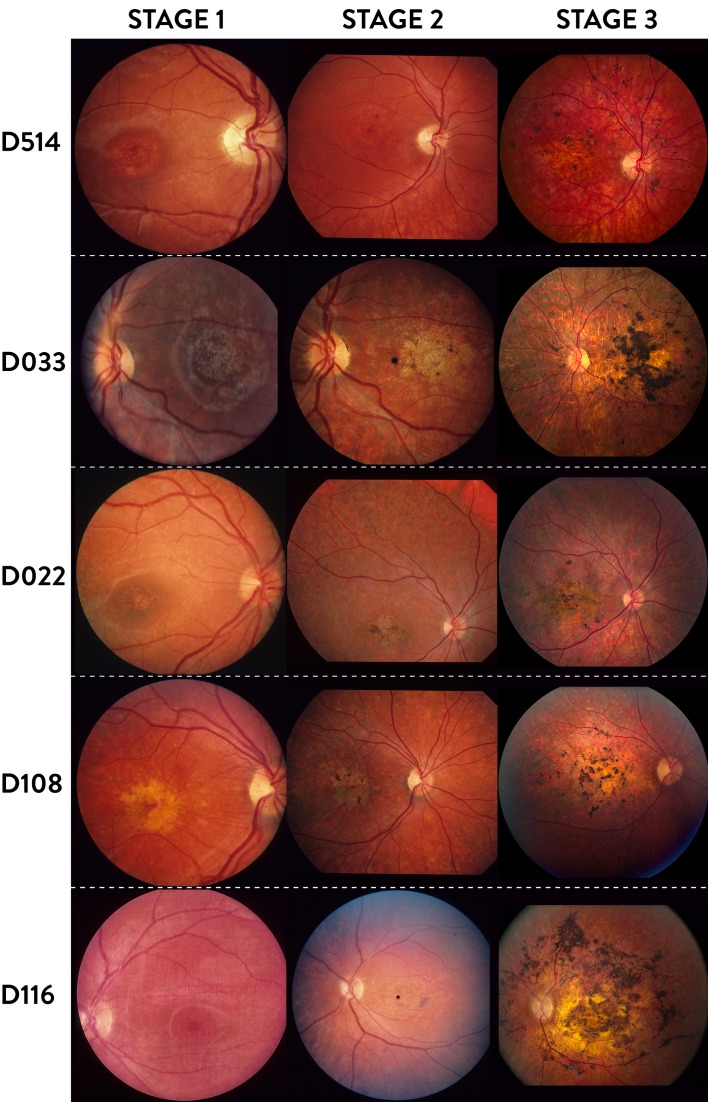
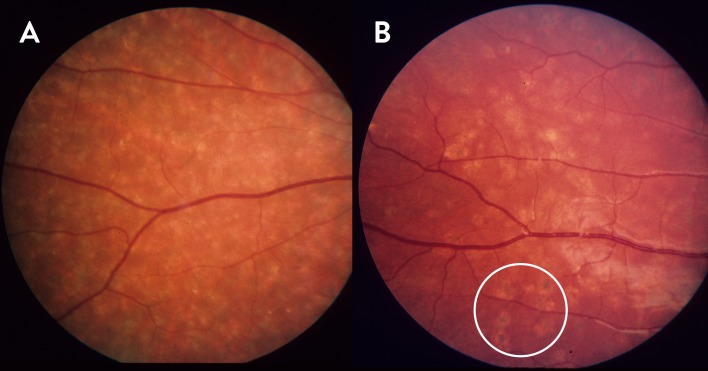
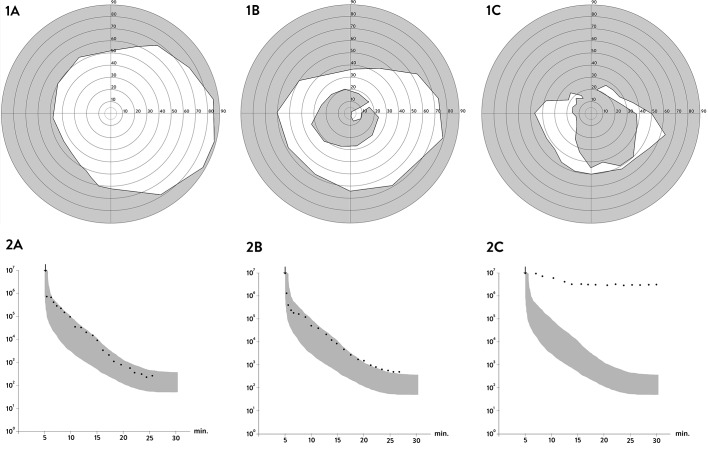
The clinical course of generalized choriocapillaris dystrophy found in all patients presented in this study falls into three stages (Fig. 1). The initial stage (stage 1) is characterized by the development of bull's eye macular dystrophy without flecks characteristic for Stargardt disease or fundus flavimaculatus (Fig. 1, Stage 1), and in some cases by an additional speckled mid peripheral pattern with small, coalescent, round, or oblong atrophies resembling faded flavimaculatus flecks (Fig. 2A), and/or small, round atrophies with a punctate central pigmentation (Fig. 2B, white circle). At this stage the full-field dark-adapted ERG had rod responses within normal limits, while the 30 Hertz flicker cone responses had normal to subnormal amplitudes and moderately prolonged implicit times (0%–20%) depending on the duration of the disease. Half of the patients, however, did not have any available early ERG recordings. The symptoms in this stage were dominated by central visual loss, and in some cases dyschromatopsia. The mean BCVA in this stage was 0.31 (SD = 0.20), with early measurements available for 16 patients. Some patients also had difficulties adapting from light to dark surroundings. Most patients had normal dark adaptations and visual fields at stage 1 (Figs. 3-1A, 3-2A).
Figure 1.
Three stages of generalized choriocapillaris dystrophy in 5 patients. Stage 1: Bull's-eye maculopathy. Stage 2: Expanding maculopathy with indistinct margins, central pigmentations, and early peripheral atrophy. Stage 3: End-stage disease with central atrophy and coarse round pigmentations plus widespread peripheral chorioretinal atrophy. The dark spot in the maculae of patients D514 and D116 at Stage 2 is a camera artifact.
Figure 2.
Peripheral/mid peripheral atrophy in the initial/middle stages of generalized choriocapillaris dystrophy. (A) Patient D117 at the age of 18, with small, partially confluent, round and oblong patches of unpigmented atrophy, and (B) patient D112 at the age of 14, with less confluent peripheral round patches of atrophy, some of which have a small, round central pigmentation (white circle).
Figure 3.
Goldman visual fields for object IV of patient D112 (10 years old) in stage 1 (1A), and patient D514 in stage 2 ([1B], 20 years old) and stage 3 ([1C], 36 years old). Dark adaptations for patient D107 in stage 1 ([2A], 15 years old), patient D514 in stage 2 ([2B], 20 years of age), and patient D804 in stage 3 ([2C], 58 years of age).
Clinical Characteristics of Stage 2
In the intermediary stage, the central atrophy started to disperse and spread centrifugally (Fig. 1, Stage 2). The scattered isles of chorioretinal atrophy increased in size and fused with time. Pigmentation clumps began to form in the center. The symptoms at this stage were more severe and were characterized by worsening of the central vision. In some cases, the peripheral vision and night vision began to deteriorate, with objective progression of the visual field scotomas and in some cases a subnormal dark adaptation (Figs. 3-1B, 3-2B). The mean BCVA for available 27 patients at this stage was 0.1 (SD = 0.05). The ERG recordings typically showed reduced cone and rod responses by approximately 50%. The patients mostly reached this stage in their 20s.
Clinical Characteristics of Stage 3
The final, end-stage (stage 3) was reached at the age of 30 to 50 years. This stage was the most characteristic for this phenotype with widespread diffuse chorioretinal atrophy. The choroidal space appeared clearly in the fundus with a gray-pink color. Distinct, coarse, rounded hyperpigmentations were present in the center (Fig. 1; D022 and D108, Stage 3), or in the entire retina (Fig. 1; D514, D033, and D116, Stage 3). The pigmentations were located superficially in the retina, in some cases enveloping the retinal vessels. The optic nerve was relatively well vascularized and the vessels were less attenuated compared to what is observed in the classical form of RP. In this stage, the symptoms were characterized by further worsening of the central vision, with BCVA of ≤2/60 in most cases. The mean BCVA was 0.05 (SD = 0.01), with measurements available for 29 patients. Dark adaptation was delayed and highly prolonged with a typically approximately linear middle phase. The visual fields showed further progression with visual field constriction and increasing central scotomas (Figs. 3-1C, 3-2C). The ERG recordings at this stage were either extinct or demonstrated severely reduced cone and rod responses.
In patients with advanced disease, the fundus was characterized by very severe or total areolar outer retinal atrophy with ophthalmoscopically visible sclera and confluent hyperpigmentation in the macula, whereas isolated or clustered lesions were seen in the periphery (Fig. 4). The 200° color images (Fig. 4A) show the coarse pigmentations in the macula and the presence of pigmented plaques in the outer periphery. Spectral domain OCT (Fig. 4D) revealed abnormal structural changes with widespread photoreceptor loss and complete absence of the photoreceptor layer in the center. Intraretinal hyperreflective deposits corresponding to larger pigmentation clumps observed in the fundus also were evident (Fig. 4D2, white arrow).
Figure 4.
Fundus photographs in color (A), autofluorescence (B, C), and infrared ([D], left), and OCTs ([D], right) from two patients (upper block No. 1, D116 at the age of 47; lower block No. 2, D023 at the age of 39) with end-stage generalized choriocapillaris dystrophy characterized by semiconfluent multifocal areolar outer retinal atrophy, the severity of which decreases with increasing eccentricity. Central patches of visible sclera are surrounded by coarse confluent hyperpigmentation. The periphery shows isolated or clustered hyperpigmentation. In and around the fovea there is pronounced atrophy of the retinal pigment epithelium, the photoreceptor layer, and the outer nuclear layer and intraretinal hyperreflective material corresponding to the hyperpigmentation ([D2], white arrow).
Mutations
Among the 52 mutations found in the 26 patients with generalized choriocapillaris dystrophy and unique genotypes (only including one affected family member from each of the two generations in family GCCD0801), 34 mutations (65%) were missense (Tables 2, 3), and 11 (42%) of the 26 subjects with unique genotypes patients had two missense mutations. The remaining mutations were 4 nonsense mutations (found in 15% of the patients), 6 deletions (found in 23% of the patients), 6 splice-site mutations (found in 23% of the patients), and 2 other intron mutations. Most of the variants were already known pathogenic mutations; however, the c.5169C>G; p.Y1723* variant found by NGS is a novel and likely deleterious mutation, since it generates a stop codon that terminates translation at codon 1723 of ABCA4. The most frequent mutations in the cohort were the p.N965S Danish founder mutation (21%), p.G863A, c.2408delG, and p.L541P/A1038V. It should be noted that the segregation and phase of the mutations were evaluated in only a few cases that had relatives available to analyze. Predicted severity and consequences of each mutation are provided in Table 3.
Table 2.
Summary of Detected Potential Pathogenic Variants (Known and Novel [in Bold Face]) Found in the ABCA4 Gene of Patients With Generalized Choriocapillaris Dystrophy
|
Patient |
Method |
Mutation 1 |
Mutation 2 |
||
|
Nucleotide |
Protein |
Nucleotide |
Protein |
||
| D513 | NGS | c.203C>T | p.P68L | c.2894A>G | p.N965S |
| D514 | Microarray, NGS | c.2894A>G | p.N965S | c.5461-10T>C | - |
| D516 | NGS | c.4926C>G | p.S1642R | c.5041_5055del | p.V1681_C1685del |
| D517 | NGS | c.5169C>G | p.Y1723* | c.6079C>T | p.L2027F |
| D137 | Microarray, NGS | c.2894A>G | p.N965S | c.2894A>G | p.N965S |
| D801 | Microarray, NGS | c.6386+1G>A | Aberrant splicing | c.4234C>T | p.Q1412* |
| D109 | Microarray | c.2894A>G | p.N965S | c.4234C>T | p.Q1412* |
| D040 | Microarray | c.6229C>T | p.R2077W | c.6229C>T | p.R2077W |
| D159 | Microarray | c.3113C>T | p.L541P/A1038V | c.3113C>T | p.L541P/A1038V |
| D129 | Microarray | c.2894A>G | p.N965S | c.3322C>T | p.R1108C |
| D115 | Microarray | c.2894A>G | p.N965S | c.3113C>T | p.L541P/A1038V |
| D033 | Microarray | c.2894A>G | p.N965S | c.2041C>T | p.R681* |
| D023 | Microarray | c.203C>T | p.P68L | c.3329-2A>G | Aberrant splicing |
| D001 | Microarray | c.666_678del | p.K223_R226delfs | c.4667+2T>C | Aberrant splicing |
| D147 | Microarray, HRM | c.2894A>G | p.N965S | c.2408delG | p.G803fs |
| D162 | Microarray | c.3329-2A>G | Aberrant splicing | c.6089G>A | p.R2030Q |
| D022 | Microarray, HRM | c.4462T>C | p.C1488R | c.4102C>T | p.R1368C |
| D112 | Microarray, HRM | c.2894A>G | p.N965S | c.1529T>G | p.L510R |
| D108 | Microarray, HRM | c.1648G>A | p.G550R | c.4102C>T | p.R1368C |
| D107 | Microarray | c.666_678del | p.K223_R226delfs | c.2588G>C | p.G863A |
| D070 | Microarray | c.2588G>C | p.G863A | c.2588G>C | p.G863A |
| D116 | Microarray | c.2300T>A | p.V767D | c.5461-10T>C | - |
| D135 | Microarray, HRM | c.2894A>G | p.N965S | c.2408delG | p.G803fs |
| D117 | Microarray, HRM | c.3191-2A>G | Aberrant splicing | c.2408delG | p.G803fs |
| D186 | Microarray, HRM | c.3322C>T | p.R1108C | c.6386+1G>A | Aberrant splicing |
| D173 | Microarray, HRM | c.4469G>A | p.C1490Y | c.2915C>A | p.T972N |
Table 3.
In Silico Analysis of ABCA4 Variants Detected in This Study Using Alamut 2.2 Software
|
cDNA Variant |
Protein Variant |
Effect on Protein Function |
AGVGD Class |
SIFT Prediction |
Effect on Protein PPH2 Prediction |
Effect on Protein TASTER Prediction |
Effect on Splicing |
| Missense variants | |||||||
| c.203C>T | p.P68L | C65 | Deleterious | Probably damaging | Disease causing | ||
| c.1529T>G | p.L510R | C65 | Deleterious | Benign | Polymorphism | ||
| c.1622T>C | p.L541P | Reduced ATP binding mislocali- zation26,27 | C65 | Deleterious | Probably damaging | Disease causing | |
| c.1648G>A | p.G550R | C65 | Deleterious | Possibly damaging | Disease causing | New acceptor site | |
| c.2300T>A | p.V767D | Reduced protein28 | C65 | Deleterious | Benign | Disease causing | |
| c.2588G>C | p.G863A | Reduced protein level, reduced ATP binding, reduced ATPase activity26 | C55 | Deleterious | Possibly damaging | Disease causing | Predicted change at acceptor site 1 bp upstream: −11.1%, creating a new stronger acceptor 3 bp downstream |
| c.2894A>G | p.N965S | Reduced ATP binding26 | C45 | Deleterious | Probably damaging | Disease causing | New acceptor site |
| c.2915C>A | p.T972N | C55 | Deleterious | Probably damaging | Disease causing | ||
| c.3113C>T | p.A1038V | Reduced ATP binding, reduced ATP hydrolysis26 | C65 | Deleterious | Benign | Disease causing | |
| c.3322C>T | p.R1108C | Reduced ATP binding26 | C65 | Deleterious | Probably damaging | Disease causing | |
| c.4102C>T | p.R1368C | C65 | Deleterious | Probably damaging | Disease causing | ||
| c.4462T>C | p.C1488R | C65 | Deleterious | Possibly damaging | Disease causing | ||
| c.4469G>A | p.C1490Y | Misfolding, mislocali- zation27 | C65 | Deleterious | Probably damaging | Disease causing | Cryptic donor strongly activated |
| c.4926C>G | p.S1642R | C25 | Deleterious | Benign | Disease causing | ||
| c.6079C>T | p.L2027F | Reduced ATP binding26,29 | C15 | Deleterious | Probably damaging | Disease causing | |
| c.6089G>A | p.R2030Q | C35 | Deleterious | Probably damaging | Disease causing | ||
| c.6229C>T | p.R2077W | Reduced ATP binding26 | C65 | Deleterious | Probably damaging | Disease causing | |
| Deletion/frame-shift/stop variants | |||||||
| c.666_678del | p.K223_ R226delfs |
||||||
| c.2041C>T | p.R681* | ||||||
| c.2408delG | p.G803fs | ||||||
| c.4234C>T | p.Q1412* | ||||||
| c.5041_5055del | p.V1681_ C1685del |
||||||
| c.5169C>G | p.Y1723* | ||||||
| Splicing affecting variants | |||||||
| c.3191-2A>G | Predicted change at acceptor site 2 bps downstream: −100% | ||||||
| c.3329-2A>G | Predicted change at acceptor site 2 bps downstream: −100% | ||||||
| c.4667+2T>C | Predicted change at donor site 2 bps upstream: −100% | ||||||
| c.4773+3A>G | Predicted change at donor site 3 bps upstream: −46.5% | ||||||
| c.6386+1G>A | Predicted change at donor site 1 bp upstream: −100% | ||||||
| Variant of unknown effect | |||||||
| c.5461-10T>C | Predicted change at acceptor site 10 bps downstream: −4.3% | ||||||
Nucleotide positions and protein translation correspond to CCDS747.1 and NP_000341.2, respectively. References to the effect on ABCA4 protein function are given in parentheses. bp, base pair; cDNA, complementary DNA; AGVGD, Align Grantham Variation Grantham Deviation; PPH2, polymorphism phenotyping 2.
Family GCCD0801
A single large family with generalized choriocapillaris dystrophy contributed 6 cases to this study (Fig. 5). The affected family members in generation III reached the end-stage of the disease between 40 and 50 years of age with visual acuities reduced to 1/60, reduced cone and rod responses on ERG recordings, prolonged dark adaptation times, large central scotomas, and fundi with widespread chorioretinal atrophy and coarse hyperpigmentations. Both of the affected brothers in generation IV had an early onset at 7 years of age, with visual acuity loss as the initial symptom and with fundi showing bull's eye maculopathy. The oldest brother (Fig. 5, 4:8) was last seen in his early 20s with a visual acuity reduced to 1/60, prolonged dark adaptation and ERG recordings showing severely reduced cone and rod responses. His fundus at that time was characterized by widespread chorioretinal atrophy and coarse, round hyperpigmentations in the center and periphery. The younger brother suffered from congenital brain damage in addition to generalized choriocapillaris dystrophy, and advanced examinations, such as ERG, had not been possible. He was last seen at the age of 25, and his visual acuity was reduced to finger counting. His fundus examination revealed widespread chorioretinal atrophy with hyperpigmentations in the entire retina.
Figure 5.
Family GCCD0801 with ABCA4 mutations and fundus photographs of affected family members from two generations.
Mutational screening was performed with ABCR600 microarray and NGS sequencing of the ABCA4 gene. Two ABCA4 mutations were detected in patient D109 from generation III; a common Danish missense mutation p.N965S and a nonsense mutation p.Q1412*. In the fourth generation, the array screening identified only the p.Q1412* mutation, which was presumably inherited from the unaffected father. We found the second, at that time novel, splice mutation c.6386+1G>A with NGS, suggesting that the mother of the two affected brothers must have been a carrier of this third mutation, explaining the occurrence of this recessive disorder in two successive generations and a pseudo-dominant inheritance pattern.
Discussion
This retrospective review outlines a particular, hitherto poorly described disease phenotype within the group of generalized choriocapillaris dystrophies.23 The clinical picture develops from an early bull's eye type of maculopathy into a severe central and peripheral chorioretinal dystrophy with a profound visual loss. The characteristic end-stage was described before the era of molecular genetics within the mixed group of “diffuse choroidal atrophies,”23 “primary choroidal sclerosis,”24 and “diffuse choroidal sclerosis.”25
Given the wide variation of phenotypes associated with ABCA4 and the length of time that is required to gain an overview of the stages covered during the development of the disease, it is not surprising that the longitudinal characteristics of this particular form of generalized choriocapillaris dystrophy have not been described in detail before. The end-stage is likely to have been reported in single cases under the clinical definitions of CRD,5–8 RP-like,9–11 and retinal dystrophy,12 and a family-based study has shown fundus images that resemble the earlier stages.13 The phenotype, however, differs from the photoreceptor dystrophies RP, cone-dystrophy, and CRD, by the primary and significant involvement of the choriocapillaris and retinal pigment epithelium layers, emphasized by a normal or slightly subnormal ERG at the initial stage, and the severe fundus appearance at later stages. While generalized choriocapillaris dystrophy in its advanced stage may resemble fundus flavimaculatus stage IV,4 none of our patients ever demonstrated the characteristics of early Stargardt disease or fundus flavimaculatus flecks. The small, rounded, peripheral atrophies that can be present in the initial stages were mistaken in some of our patients, though, for flavimaculatus flecks. Because most of our cases presented several decades ago, we do not have modern image studies that can tell whether patients with generalized choriocapillaris dystrophy have the occasional hallmarks of early Stargardt disease, such as vermillion fundus, fundus hyperautofluorescence, and a dark choroid on fluorescein angiograms. The differential diagnoses include CRD, RP, central areolar atrophy, pattern dystrophy, and serpiginous choroiditis.
In all but two patients with the typical phenotype of generalized choriocapillaris dystrophy, we found at least one, and in most patients two, severe mutations in the ABCA4 gene. The Danish p.N965S founder mutation was among the most frequent mutations found in our patients, and this mutation is believed to lead to moderate-to-severe retinal phenotypes, because ABCA4 protein dysfunction results in impaired ATP hydrolysis. The variant also is deemed pathogenic by all predictive programs and, in addition to a protein defect, it also may affect splicing (Table 3).15,26 This cohort harbored a higher than usual fraction (10/60, ∼17%) of deleterious variants (deletions and stop codons), which are postulated to result in a completely dysfunctional protein. In addition, most other, missense, mutations are deemed severe either by in silico or indirect functional analyses (Table 3), or from data presented in previous studies even if the exact effect of the variant on the protein is unknown (e.g., the c.5461-10T>C variant).26–29 The p.G863A mutation found in some of our patients is a common mutation in Stargardt disease patients of mainly Dutch descent,30 and it also has been observed in patients with CRD.31 This mutation is considered to be relatively mild and claimed not to be disease-causing in a homozygous state32; however, the patient D070 (Table 2) who is homozygous for this mutation, is one of a few examples of two mild mutations leading to generalized choriocapillaris dystrophy. Since the entire ABCA4 gene was not sequenced in this patient, we cannot exclude the possibility that allelic severe ABCA4 mutation(s), including a large deletion, remained undetected. It also may be possible that these patients have additional modifier alleles, or epigenetic or environmental factors that have a particularly prominent effect. Whole exome or genome sequencing, or genome-wide association studies (GWAS) would be needed to determine if the patients in this cohort share other common or rare variants in other genes that may influence the phenotype.
In summary, we delineated a relatively large cohort of patients (26 of a mixed group of 108 patients (24%) with both pathogenic ABCA4 alleles identified)14,15 presenting a severe ABCA4-associated phenotype characterized by three stages with early-onset central retinopathy that disperses and expands to widespread end-stage generalized choriocapillaris dystrophy. This phenotype, which apparently is not rare among the ABCA4-associated retinopathies in Denmark, is caused by mostly deleterious mutations in the ABCA4 gene, including the high prevalence of the p.N965S mutation (Table 3), which likely explains the early onset, panretinal degeneration, and fast progression of the disease. A combination of early onset of symptoms, a characteristic fundus appearance, and specific severe mutations can be used to improve the prediction of the development of generalized choriocapillaris dystrophy in a large number of cases.
Acknowledgments
The authors thank the patients who participated in this study, as well as the ophthalmologists and optometrists who assisted with completing the study materials, notably Hajer Ahmad Al-Abaiji, BSc Optometry, Hanne Jensen, MD, Niels Beck, MD, and Flemming Klie, MD.
Supported by The Danish Eye Research Foundation, Fight for sight and, in part, by Grants EY021163, EY019861, and EY019007 from the National Eye Institute/National Institutes of Health (NIH, Bethesda, MD, USA; Core Support for Vision Research), Foundation Fighting Blindness (Owings Mills, MD, USA), and unrestricted funds from Research to Prevent Blindness (New York, NY, USA) to the Department of Ophthalmology, Columbia University. The authors alone are responsible for the content and writing of the paper.
Disclosure: M. Bertelsen, None; J. Zernant, None; M. Larsen, None; M. Duno, None; R. Allikmets, None; T. Rosenberg, None
References
- 1. Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997; 15: 236–246 [DOI] [PubMed] [Google Scholar]
- 2. Cremers FP, van de Pol DJ, van DM, et al. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet. 1998; 7: 355–362 [DOI] [PubMed] [Google Scholar]
- 3. Martinez-Mir A, Paloma E, Allikmets R, et al. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998; 18: 11–12 [DOI] [PubMed] [Google Scholar]
- 4. Fishman GA. Fundus flavimaculatus. A clinical classification. Arch Ophthalmol. 1976; 94: 2061–2067 [DOI] [PubMed] [Google Scholar]
- 5. Xi Q, Li L, Traboulsi EI, Wang QK. Novel ABCA4 compound heterozygous mutations cause severe progressive autosomal recessive cone-rod dystrophy presenting as Stargardt disease. Mol Vis. 2009; 15: 638–645 [PMC free article] [PubMed] [Google Scholar]
- 6. Klevering BJ, Deutman AF, Maugeri A, Cremers FP, Hoyng CB. The spectrum of retinal phenotypes caused by mutations in the ABCA4 gene. Graefes Arch Clin Exp Ophthalmol. 2005; 243: 90–100 [DOI] [PubMed] [Google Scholar]
- 7. Yzer S, van den Born LI, Zonneveld MN, et al. Molecular and phenotypic analysis of a family with autosomal recessive cone-rod dystrophy and Stargardt disease. Mol Vis. 2007; 13: 1568–1572 [PubMed] [Google Scholar]
- 8. Valverde D, Riveiro-Alvarez R, Aguirre-Lamban J, et al. Spectrum of the ABCA4 gene mutations implicated in severe retinopathies in Spanish patients. Invest Ophthalmol Vis Sci. 2007; 48: 985–990 [DOI] [PubMed] [Google Scholar]
- 9. Klevering BJ, Maugeri A, Wagner A, et al. Three families displaying the combination of Stargardt's disease with cone-rod dystrophy or retinitis pigmentosa. Ophthalmology. 2004; 111: 546–553 [DOI] [PubMed] [Google Scholar]
- 10. Klevering BJ, van DM, van de Pol DJ, Pinckers AJ, Cremers FP, Hoyng CB. Phenotypic variations in a family with retinal dystrophy as result of different mutations in the ABCR gene. Br J Ophthalmol. 1999; 83: 914–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukui T, Yamamoto S, Nakano K, et al. ABCA4 gene mutations in Japanese patients with Stargardt disease and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002; 43: 2819–2824 [PubMed] [Google Scholar]
- 12. Singh HP, Jalali S, Hejtmancik JF, Kannabiran C. Homozygous null mutations in the ABCA4 gene in two families with autosomal recessive retinal dystrophy. Am J Ophthalmol. 2006; 141: 906–913 [DOI] [PubMed] [Google Scholar]
- 13. Beit-Ya'acov A, Mizrahi-Meissonnier L, Obolensky A, et al. Homozygosity for a novel ABCA4 founder splicing mutation is associated with progressive and severe Stargardt-like disease. Invest Ophthalmol Vis Sci. 2007; 48: 4308–4314 [DOI] [PubMed] [Google Scholar]
- 14. Duno M, Schwartz M, Larsen PL, Rosenberg T. Phenotypic and genetic spectrum of Danish patients with ABCA4-related retinopathy. Ophthalmic Genet. 2012; 33: 225–231 [DOI] [PubMed] [Google Scholar]
- 15. Rosenberg T, Klie F, Garred P, Schwartz M. N965S is a common ABCA4 variant in Stargardt-related retinopathies in the Danish population. Mol Vis. 2007; 13: 1962–1969 [PubMed] [Google Scholar]
- 16. Haim M, Holm NV, Rosenberg T. A population survey of retinitis pigmentosa and allied disorders in Denmark. Completeness of registration and quality of data. Acta Ophthalmol (Copenh). 1992; 70: 165–177 [DOI] [PubMed] [Google Scholar]
- 17. Jaakson K, Zernant J, Kulm M, et al. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum Mutat. 2003; 22: 395–403 [DOI] [PubMed] [Google Scholar]
- 18. Zernant J, Schubert C, Im KM, et al. Analysis of the ABCA4 gene by next-generation sequencing. Invest Ophthalmol Vis Sci. 2011; 52: 8479–8487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tavtigian SV, Deffenbaugh AM, Yin L, et al. Comprehensive statistical study of 452 BRCA1 missense substitutions with classification of eight recurrent substitutions as neutral. J Med Genet. 2006; 43: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010; 7: 575–576 [DOI] [PubMed] [Google Scholar]
- 21. Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol. 2004; 11: 377–394 [DOI] [PubMed] [Google Scholar]
- 22. Desmet FO, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009; 37: e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krill AE, Archer D. Classification of the choroidal atrophies. Am J Ophthalmol. 1971; 72: 562–585 [DOI] [PubMed] [Google Scholar]
- 24. Duke-Elder S, Perkins ES. System of Ophthalmology. London, UK: Henry Kimpton; 1966: 702–706 [Google Scholar]
- 25. Franceschetti A, Francois J, Babel J. Les Heredo-degenerescences Chorio-retiniennes. Paris, France: Masson et Cie.; 1963: 635–639 [Google Scholar]
- 26. Sun H, Smallwood PM, Nathans J. Biochemical defects in ABCR protein variants associated with human retinopathies. Nat Genet. 2000; 26: 242–246 [DOI] [PubMed] [Google Scholar]
- 27. Wiszniewski W, Zaremba CM, Yatsenko AN, et al. ABCA4 mutations causing mislocalization are found frequently in patients with severe retinal dystrophies. Hum Mol Genet. 2005; 14: 2769–2778 [DOI] [PubMed] [Google Scholar]
- 28. Shroyer NF, Lewis RA, Yatsenko AN, Wensel TG, Lupski JR. Cosegregation and functional analysis of mutant ABCR (ABCA4) alleles in families that manifest both Stargardt disease and age-related macular degeneration. Hum Mol Genet. 2001; 10: 2671–2678 [DOI] [PubMed] [Google Scholar]
- 29. Biswas EE, Biswas SB. The C-terminal nucleotide binding domain of the human retinal ABCR protein is an adenosine triphosphatase. Biochemistry. 2000; 39: 15879–15886 [DOI] [PubMed] [Google Scholar]
- 30. Maugeri A, Flothmann K, Hemmrich N, et al. The ABCA4 2588G>C Stargardt mutation: single origin and increasing frequency from South-West to North-East Europe. Eur J Hum Genet. 2002; 10: 197–203 [DOI] [PubMed] [Google Scholar]
- 31. Klevering BJ, Blankenagel A, Maugeri A, Cremers FP, Hoyng CB, Rohrschneider K. Phenotypic spectrum of autosomal recessive cone-rod dystrophies caused by mutations in the ABCA4 (ABCR) gene. Invest Ophthalmol Vis Sci. 2002; 43: 1980–1985 [PubMed] [Google Scholar]
- 32. Maugeri A, van Driel MA, van de Pol DJ, et al. The 2588G-->C mutation in the ABCR gene is a mild frequent founder mutation in the Western European population and allows the classification of ABCR mutations in patients with Stargardt disease. Am J Hum Genet. 1999; 64: 1024–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]