Abstract
A colorimetric assay based on the reduction of a tetrazolium salt {2,3-bis[2-methyloxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide (XTT)} for rapidly determining the susceptibility of Pseudomonas aeruginosa isolates to bactericidal antibiotics is described. There was excellent agreement between the tobramycin and ofloxacin MICs determined after 5 h using the XTT assay and after 18 h using conventional methods. The data suggests that an XTT-based assay could provide a useful method for rapidly determining the susceptibility of P. aeruginosa to bactericidal antibiotics.
The significant increase in the number of antibiotic-resistant microorganisms causing clinical infection highlights the need for rapid and accurate antimicrobial susceptibility tests. Current standard methods, such as the broth microdilution methods approved by the National Committee for Clinical Laboratory Science (12) and the British Society for Antimicrobial Chemotherapy (BSAC) (1), require at least 18 h before the final antimicrobial susceptibility results are known. When a patient presents with a severe life-threatening infection such as Pseudomonas aeruginosa bacteremia in clinical practice, antibiotic treatment is usually commenced prior to obtaining these results to give the patient the best chance of recovery. Such treatment usually involves the administration of broad-spectrum antibiotics which may be inappropriate (7). The development of rapid susceptibility tests would allow specific and cheaper narrow-spectrum antibiotics to be used, resulting in improved patient care and cost effectiveness and a decrease in the development of antibiotic resistance.
Promising alternative colorimetric methods have been proposed as rapid methods for antifungal susceptibility testing of yeasts (8, 13-15) and Aspergillus species (9-11) and for detection of drug resistance in Mycobacterium tuberculosis (4-6). These methods utilize the reduction of a tetrazolium salt {2,3-bis[2-methyloxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-car-boxanilide (XTT)} by metabolically active cells to a colored-water-soluble formazan derivative that can be easily quantified colorimetrically. In the study described here, we developed an XTT-based assay for antimicrobial susceptibility testing which provided results within 5 h. The assay was used to determine the susceptibility of clinical P. aeruginosa isolates to a range of antibiotics, and the drug MICs obtained after 5 h using this method were compared with the MICs obtained after 18 h using the conventional NCCLS and BSAC broth microdilution methods.
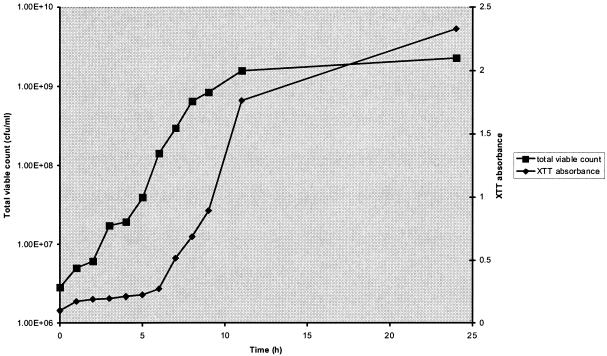
An initial set of experiments were performed with 4A, a clinical P. aeruginosa isolate, to optimize the quantities of XTT (Sigma Chemical Co, Dorset, United Kingdom) and menadione (Aldrich, Dorset, United Kingdom) required to quantify bacterial metabolic activity. XTT was prepared as saturated solutions at 0.5 and 1 mg/ml in phosphate-buffered saline, filter sterilized, and stored at −70°C. Menadione was prepared as a 10 mM stock solution in acetone and stored at −70°C. Prior to each assay, aliquots of XTT were thawed and menadione was added to give the desired final concentration. The planktonic growth of strain 4A over 24 h in Isosensitest broth was measured with the XTT assay. In brief, at each time point six 1-ml samples were removed and centrifuged and 1-ml aliquots of XTT with menadione were added to the resultant pellet to obtain final concentrations of 0.5 and 1.0 mg of XTT/ml and 50, 100, and 200 μM menadione. The samples were incubated in the dark for 1 h at 37°C, after which a colorimetric change in the XTT reduction assay was measured using a microtiter plate reader (Tecan Sunrise absorbance reader; Tecan UK, Reading, United Kingdom) at 492 nm. To determine the relationship between XTT reduction and viable counts, total viable counts were also performed at each time point. We demonstrated that XTT at a concentration of 0.5 mg/ml and menadione at a concentration of 50 μM gave the optimal change in absorbance after 1 h of incubation over the 24-h period of the planktonic growth curve. There was a direct correlation between the viable count results (ranging from 1 × 106 to 2 × 109 CFU/ml) determined by plating and XTT colorimetric readings (Fig. 1), with a correlation coefficient (R2) of 0.9943. The excellent correlation between XTT readings and total viable counts was also demonstrated for a number of additional clinical strains (R2 > 0.98 for all strains tested; results not shown).
FIG. 1.
Relationship between total viable counts determined by plating and XTT-colorimetric readings at an XTT concentration of 0.5 mg/ml and a menadione concentration of 50 μM. Results were analyzed statistically to determine the correlation coefficient (R2 = 0.9943).
The MICs of ofloxacin (Sigma) (MIC range, 0.0625 to 16 μg/ml), tobramycin (Sigma) (0.5 to 128 μg/ml), piperacillin (as Pipril; Cyanamid Ltd., Gosport, United Kingdom) (2 to 512 μg/ml), meropenem (as Meronem; AstraZeneca UK Ltd., Luton, United Kingdom) (0.0625 to 16 μg/ml), and ceftazidime (as Fortum; GSK, Uxbridge, United Kingdom) (0.5 to 128 μg/ml) for 15 P. aeruginosa isolates were determined using the NCCLS broth microdilution method (12) and for a further 15 isolates were determined using the BSAC broth microdilution method (1). The P. aeruginosa isolates used in the study were all clinical strains isolated from the sputum of cystic fibrosis patients attending cystic fibrosis clinics at either the University of Calgary Medical Clinic (NCCLS method) or the Belfast City Hospital (BSAC method). Quality assurance testing for the NCCLS and BSAC methods was performed using reference strains ATCC 27853 and NCTC 12934, respectively.
After incubation, the MIC was read as the lowest concentration of each antimicrobial agent that inhibited the visible growth of the isolate. Determinations of the MIC of each antibiotic for each isolate were carried out in triplicate, and the results were taken in cases in which there was agreement between the results obtained with at least two out of three wells. The microtiter plates were also scanned in the microtiter plate reader at 540 nm; for all isolates tested, a 90% reduction in optical density (compared with control growth results) led to the determination of the same drug MIC. Therefore, a 90% reduction in optical density compared with control growth results was used to determine the drug MIC in the XTT assay for a number of isolates with which visual determination of the MIC by the unaided eye was difficult.
The MICs were also determined colorimetrically using XTT in a modified broth microdilution method. The susceptibility trays were prepared as for the NCCLS and BSAC methods, with 50 μl of serially diluted antibiotic added to each well of the tray. Initial experiments were then performed using strain 4A and tobramycin to determine the inoculum density to be used and the earliest time at which an MIC reading could be determined. Inoculum densities of 105 (standard BSAC inoculum) and 108 CFU/ml were prepared in Isosensitest broth, and 50 μl was added to the wells of the microtiter trays. After 2, 4, and 6 h of incubation at 37°C, the trays were removed and 100 μl of fresh XTT was added. After incubation for 1 h at 37°C, the MIC was read as the lowest concentration of antimicrobial agent at which no color change occurred.
For isolates with which visual determination of the drug MIC by the unaided eye was difficult, the plates were scanned in the microtiter plate reader at 492 nm and the MIC was determined as the drug concentration that gave a 90% reduction in optical density compared with control growth results. The MIC obtained was then compared with the MIC obtained using the BSAC broth microdilution method following 18 h of incubation. Results from these experiments demonstrated that no drug MIC readings could be determined (using a standard inoculum of 105 CFU/ml) after 2, 4, and 6 h. When the higher inoculum density of 108 CFU/ml was used, however, drug MICs determined at 4 and 6 h corresponded to the MIC obtained using the BSAC broth microdilution method at 18 h. Therefore, subsequent MIC determinations for all antibiotics and isolates were performed with an inoculum density of 108 CFU/ml, and the results were read after addition of XTT to trays which had been incubated for 4 h.
The MICs of piperacillin, meropenem, and ceftazidime could not be determined using the XTT assay at 4 h, as there was no difference in growth results between test and control wells (results not shown). No comparison could therefore be made with the MICs determined by the broth microdilution method at 18 h. The MICs of tobramycin and ofloxacin were determined using the XTT assay at 4 h, however, and there was good correlation between these MICs and the MICs determined using the NCCLS and BSAC broth microdilution methods after 18 h (Table 1). The inability of the XTT assay to differentiate between growth levels in control and test wells for piperacillin, meropenem, and ceftazidime at 4 h is probably related to the mode of action of these β-lactam antibiotics. β-Lactams exhibit time-dependent killing of pathogens. For such antibiotics, killing is concentration independent and proceeds relatively slowly with time; the length of time that the concentration of the antibiotic at the site of infection is maintained above the antibiotic MIC for the pathogen is critical to bacterial eradication.
TABLE 1.
Comparison of drug MICs determined for P. aeruginosa strains by the XTT assay and conventional NCCLS and BSAC broth microdilution methods
| Method and strain | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Ofloxacin
|
Tobramycin
|
|||
| XTT (5 h) | NCCLS (18 h) | XTT (5 h) | NCCLS (18 h) | |
| NCCLS | ||||
| ATCC 27853 | 2 | 2 | 1 | 2 |
| 1 | 1 | 4 | 0.5 | 4 |
| 2 | >16 | >16 | 8 | 2 |
| 3 | >16 | >16 | >128 | 32 |
| 4 | 2 | 2 | 0.5 | 1 |
| 5 | 1 | 2 | 0.5-1 | 1 |
| 6 | 8 | 8 | 8 | 8 |
| 7 | 1 | 2 | 1 | 1 |
| 8 | 2 | 2 | 0.5 | 0.5 |
| 9 | >16 | 16 | 0.5 | 0.5 |
| 10 | 16 | 16 | 4-8 | 4 |
| 11 | 16 | 8 | 8-16 | 4 |
| 12 | 8 | 8 | >128 | >128 |
| 13 | >16 | >16 | 2 | 2 |
| 14 | 0.5 | 1 | 2-4 | 2 |
| BSAC | ||||
| NCTC 12934 | 1 | 2 | <0.5 | <0.5 |
| 1A | 8 | 8 | <0.5 | <0.5 |
| 2A | >16 | 16 | 32 | 2 |
| 3A | 1 | 0.5 | <0.5 | <0.5 |
| 4A | 1 | 1 | <0.5 | 1 |
| 5A | >16 | 16 | 64 | 64 |
| 6A | 1 | 1 | 1 | 0.5 |
| 7A | >16 | 16 | 128 | 64 |
| 8A | 16 | 8 | 2 | <0.5 |
| 9A | 16 | 4 | 4 | 2 |
| 10A | 2 | 2 | <0.5 | <0.5 |
| 11A | 2 | 1 | 1 | 1 |
| 12A | >16 | 16 | 64 | 64 |
| 13A | >16 | 16 | 128 | 32 |
| 14A | 8 | 8 | <0.5 | <0.5 |
In contrast, tobramycin (an aminoglycoside) and ofloxacin (a quinolone) both exhibit concentration-dependent killing of pathogens; for such antibiotics, the peak concentration achieved at the site of infection is critical to bacterial eradication (3). To more closely analyze the agreement between the XTT assay and the conventional broth microdilution methods, the definitions of the NCCLS (12) and the BSAC (1) were used to convert the MICs obtained by each method to interpretive categories of susceptible, intermediate, and resistant. Interpretive category results were then compared by calculating the number of minor, major, and very major errors (2) and the number of strains for which there was absolute and essential interpretive agreement. There was excellent agreement between the NCCLS method and the XTT assay, with 93 and 100% absolute and essential agreement, respectively, for ofloxacin and 80 and 93% absolute and essential agreement, respectively, for tobramycin. There was similarly excellent agreement between the BSAC method and the XTT assay, with 87 and 100% absolute and essential agreement, respectively, for both ofloxacin and tobramycin. There were three minor errors between the NCCLS method and the XTT assay and four minor errors between the BSAC method and the XTT assay. Only one major error (when isolate 11 was categorized as resistant by the XTT assay but was categorized as sensitive by the NCCLS method) was noted.
In conclusion, we have developed a simple and inexpensive assay that allows more rapid determination of the susceptibility of P. aeruginosa isolates to bactericidal antibiotics. Although the XTT assay developed employs a higher inoculum density than the conventional NCCLS and BSAC broth microdilution methods, results obtained are comparable. The XTT assay could therefore be used to rapidly determine the susceptibility of P. aeruginosa to bactericidal antibiotics, thereby ensuring the earlier commencement of appropriate antibiotic therapy. However, further studies with a number of different antibiotics and with additional P. aeruginosa isolates and isolates from other bacterial species would be required to confirm the applicability of the XTT assay for rapidly determining antimicrobial susceptibility in routine clinical practice.
REFERENCES
- 1.Andrews, J. M. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48(Suppl. 1):5-16. [DOI] [PubMed] [Google Scholar]
- 2.Baker, C. N., and F. C. Tenover. 1996. Evaluation of Alamar colorimetric broth microdilution susceptibility testing method for staphylococci and enterococci. J. Clin. Microbiol. 34:2654-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess, D. S. 1999. Pharmacodynamic principles of antimicrobial therapy in the prevention of resistance. Chest 115:19S-23S. [DOI] [PubMed] [Google Scholar]
- 4.De Logu, A., P. Uda, M. L. Pellerano, M. C. Pusceddu, B. Saddi, and M. L. Schivo. 2001. Comparison of two rapid colorimetric methods for determining resistance of Mycobacterium tuberculosis to rifampin, isoniazid, and streptomycin in liquid medium. Eur. J. Clin. Microbiol. Infect. Dis. 20:33-39. [DOI] [PubMed] [Google Scholar]
- 5.De Logu, A., R. Borgna, P. Uda, A. Sanna, M. L. Pellerano, and B. Saddi. 2003. The 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay as rapid colorimetric method for determination of antibiotic susceptibility of clinical Mycobacterium tuberculosis isolates in liquid medium. Clin. Lab. 49:357-365. [PubMed] [Google Scholar]
- 6.De Logu, A., M. L. Pellerano, A. Sanna, M. C. Pusceddu, P. Uda, and B. Saddi. 2003. Comparison of the susceptibility testing of clinical isolates of Mycobacterium tuberculosis by the XTT colorimetric method and the NCCLS standards method. Int. J. Antimicrob. Agents 21:244-250. [DOI] [PubMed] [Google Scholar]
- 7.Granato, P. A. 1993. The impact of same-day tests versus traditional overnight testing. Diagn. Microbiol. Infect. Dis. 16:237-243. [DOI] [PubMed] [Google Scholar]
- 8.Hawser, S. P., H. Norris, C. J. Jessup, and M. A. Ghannoum. 1998. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J. Clin. Microbiol. 36:1450-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawser, S. P., C. Jessup, J. Vitullo, and M. A. Ghannoum. 2001. Utility of2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenyl-amino)carbonyl]-2H-tetrazolium hydroxide (XTT) and minimum effective concentration assays inthe determination of antifungal susceptibility of Aspergillus fumigatus to the lipopeptide class compounds. J. Clin. Microbiol. 39:2738-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, B. A. Bouman, P. J. Donnelly, P. E. Verweij, and EUROFUNG Network. 2001. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, {2,3-bis[2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide}, for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, B. A. Bouman, J. P. Donnelly, P. E. Verweij, and EUROFUNG Network. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—fifth edition. Approved standard. NCCLS document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Ramage, G., W. K. VandeWalle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramage, G., K. VandeWalle, S. P. Bachmann, B. L. Wickes, and J. L. Lopez-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tellier, R., M. Krajden, G. A. Grigoriew, and I. Campbell. 1992. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob. Agents Chemother. 36:1619-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]