Abstract
Resistance to diminazene aceturate (Berenil) is a severe problem in the control of African trypanosomiasis in domestic animals. It has been speculated that resistance may be the result of reduced diminazene uptake by the parasite. We describe here the mechanisms by which [3H]diminazene is transported by Trypanosoma brucei brucei bloodstream forms. Diminazene was rapidly accumulated through a single transporter, with a Km of 0.45 ± 0.11 μM, which was dose dependently inhibited by pentamidine and adenosine. The Ki values for these inhibitors were consistent with this transporter being the P2/TbAT1 adenosine transporter. Yeast expressing TbAT1 acquired the ability to take up [3H]diminazene and [3H]pentamidine. TbAT1-null mutants had lost almost all capacity for [3H]diminazene transport. However, this cell line still displayed a small but detectable rate of [3H]diminazene accumulation, in a nonsaturable manner. We conclude that TbAT1 mediates [3H]diminazene transport almost exclusively and that this explains the observed diminazene resistance phenotypes of TbAT1-null mutants and field isolates.
African trypanosomiasis, caused by infection with any of several members of the genus Trypanosoma, both as sleeping sickness and as the livestock disease nagana, is currently resurgent across much of tropical Africa, reaching epidemic levels in many places (25, 27, 28). Although this is partly due to lack of surveillance and vector control in some countries, control of both the human and the veterinary condition is severely affected by resistance to many of the first-line drugs (17, 18). For human patients it is resistance to melarsoprol, for decades the drug of choice for late-stage sleeping sickness (20), which is particularly alarming. Treatment failure has exceeded 30% in some foci (6, 23). In contrast, treatment failure with the diamidine pentamidine, the first-line treatment for early-stage West-African sleeping sickness (20), does not appear to be a problem at present (5). Nagana is most commonly treated with Berenil, the active ingredient of which is the diamidine diminazene, marketed as the diaceturate salt. The only other drugs on the market are isometamidium and homidium, which also have prophylactic properties. Resistance to each of these drugs is a severe problem, particularly in eastern and southern Africa (1, 17).
Resistance to common drugs is a severe and increasing problem in the treatment of many infectious diseases. For African trypanosomiasis, resistance to particular drugs is often associated with reduced uptake of the drug (11, 15, 16). In particular, the Trypanosoma brucei P2 transporter, encoded by the TbAT1 gene (21), has been implicated in the transport of the melaminophenyl arsenical and diamidine classes of trypanocides (3, 4, 8, 9). Recent research has focused on linking changes in TbAT1 activity to resistance phenotypes. In laboratory-derived strains the evidence strongly supports a correlation between loss of P2 activity and drug resistance: point mutations have been described in TbAT1 alleles from an arsenical-resistant T. brucei brucei strain (21), P2 activity was lost from a diminazene-adapted T. equiperdum line (3). Moreover, P2 substrates such as adenosine and adenine protect trypanosomes against lysis by melaminophenyl arsenicals in vitro (9) and inhibit this transporter with high affinity (9, 12). Moreover, some drug-resistant laboratory strains have either lost the TbAT1 gene altogether or no longer express it (R. Burchmore and M. P. Barrett, unpublished data). However, no clear correlation between TbAT1 mutations and melarsoprol treatment failures could be established in one clinical study (22), and we have now established that, whereas P2 is involved in arsenical transport, deletion of TbAT1 causes only a minor loss of sensitivity to these drugs (24). It is now clear that an additional transport activity is involved in melarsoprol uptake and that the loss of both transporters is necessary for high levels of resistance (24). A similar situation exists with pentamidine, which is actually taken up by three distinct transporters in T. brucei brucei (10, 5).
It is therefore clear that a simple model of drug resistance arising from the loss of a single plasma membrane transporter protein is often too simplistic for trypanosomes, and so the situation for each individual drug must be assessed carefully. We describe here the first study assessing the transport of diminazene by trypanosomes. Whereas diminazene is capable of inhibiting the P2 transporter activity (3, 12) and this activity was lost in one diminazene-adapted trypanosome line (3), this does not formally prove that TbAT1 transports diminazene, nor does it establish whether transporter(s) in addition to P2 could be involved in diminazene uptake. Using a [3H]diminazene of high specific activity, we confirm here that diminazene is indeed a permeant for TbAT1 in T. brucei brucei and for TbAT1 expressed in Saccharomyces cerevisiae. No saturable uptake of [3H]diminazene was observed in Tbat1−/− trypanosomes.
MATERIALS AND METHODS
Trypanosomes.
Trypanosomes of the following strains were propagated in adult female Wistar rats by intraperitoneal injection: T. brucei brucei strain 427 and the Tbat1−/− line derived thereof (24). Blood from infected rats was collected at peak parasitemia by cardiac puncture under terminal anesthesia. The parasites were isolated by using a DE52 (Whatman, Maidstone, United Kingdom) anion-exchange column (19) and washed twice in assay buffer (33 mM HEPES, 98 mM NaCl, 4.6 mM KCl, 0.55 mM CaCl2, 0.07 mM MgSO4, 5.8 mM NaH2PO4, 0.3 mM MgCl2, 23 mM NaHCO3, 14 mM glucose [pH 7.3]) prior to use in transport experiments.
Diminazene transport in bloodstream trypanosomes.
[ring-3H]Diminazene (83 Ci/mmol) was synthesized by Amersham Pharmacia Biotech UK. Transport assays for [3H]diminazene were performed exactly as described for pentamidine (10, 24) by using a rapid oil-stop protocol. Briefly, cells at 108 cells/ml were incubated with the radioligand in the presence or absence of competitive inhibitor for a predetermined time as described in Results. Incubations were stopped by the addition of 1 ml of ice-cold 1 mM diminazene in assay buffer (stop solution) and spun through oil (30 s, 12,000 × g). The radioactivity in the cell pellet was determined, after solubilization in 2% sodium dodecyl sulfate, by liquid scintillation counting. For the determination of nonspecific association with the cell pellet, cells and stop solution were added simultaneously, and the cells were spun immediately through oil. The observed radioactivity was subtracted from all other samples.
Transport assays in S. cerevisiae.
The S. cerevisiae ade2 mutant strain RH2884 was transformed with the pRS416-Met25 vector containing the TbAT1 gene as described by Mäser et al. (21) and grown at 30°C in complete minimal medium lacking uracil and containing 20 g of glucose/liter to a density of 1 to 2 optical density units at 600 nm. Yeast transport of [3H]diminazene were performed as described for [3H]hypoxanthine (7) and essentially the same as for trypanosomes, with yeast cells resuspended in assay buffer at ∼3 × 108 cells/ml.
RESULTS
High-affinity transport of [3H]diminazene aceturate by bloodstream forms of T. brucei brucei.
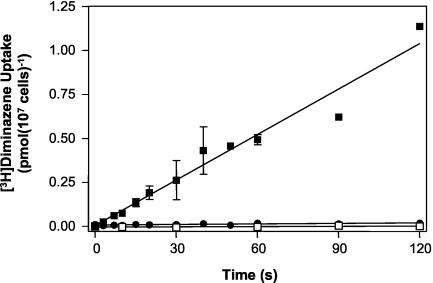
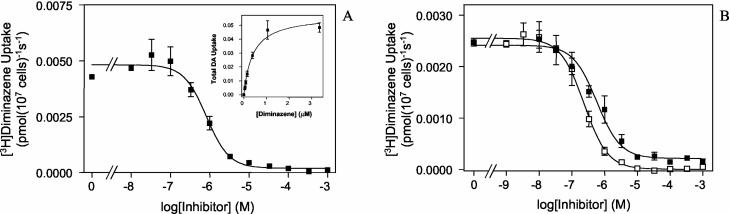
Bloodstream trypanosomes were isolated from infected rat blood and incubated with a final concentration of 50 nM [3H]diminazene. Uptake was rapid and linear over at least 120 s (Fig. 1). The accumulation of [3H]diminazene was clearly transporter mediated since it was completely inhibited by 1 mM unlabeled diminazene aceturate (Fig. 1). Incubation with various concentrations of unlabeled diminazene aceturate (10 nM to 1 mM) yielded a classic sigmoid inhibition plot with a Hill slope of approximately −1 and submicromolar 50% inhibitory concentration (IC50) values (Fig. 2A). Michaelis-Menten kinetics yielded a Km value of 0.45 ± 0.11 μM and a Vmax of 0.049 ± 0.010 pmol 107 cells−1 s−1 (n = 4) (Fig. 2A, inset). [3H]Diminazene transport was also potently inhibited by pentamidine and adenosine, with Ki values of 0.21 ± 0.02 (n = 3) and 0.25 ± 0.08 μM (n = 4), respectively (Fig. 2B). These observations are all consistent with the great majority of [3H]diminazene being taken up by the P2 adenosine/adenine transporter. Adenosine and pentamidine, at 1 mM, also inhibited [3H]diminazene transport to a very similar extent, with apparent residual accumulations over 30 s measured at (1.7 ± 0.4) × 10−4 and (1.2 ± 0.4) × 10−4 pmol 107 cells−1 s−1, respectively (n = 5; P > 0.05 [paired t test]), presumably via a nonsaturable uptake mechanism (see below).
FIG. 1.
[3H]diminazene aceturate uptake in bloodstream form T. brucei brucei. The uptake of 50 nM [3H]diminazene aceturate by T. brucei brucei 427 (boxes) or Tbat1−/− (circles) in the presence (solid symbols) or absence (open symbols) of 1 mM unlabeled diminazene aceturate was determined over various time intervals as indicated. Uptake by strain 427 in the absence of unlabeled excess permeant was 0.0086 ± 0.0005 pmol 107 cells−1 s−1 (linear regression, r2 = 0.96). In the presence of 1 mM diminazene aceturate, uptake was not significantly different from zero (P = 0.26 [F-test]). Although the slope for diminazene transport by Tbat1−/− was significantly different from zero (P < 0.01), the rate of transport over 120 s was 98.9% reduced in this line.
FIG. 2.
Inhibition plots of [3H]diminazene aceturate transport in T. brucei brucei 427. (A) Transport of 50 nM [3H]diminazene aceturate in the presence of increasing concentrations of unlabeled diminazene aceturate. (Inset) Conversion to Michaelis-Menten plot. The Km value for this experiment was 0.40 ± 0.07 μM; the Vmax 0.057 ± 0.003 pmol 107 cells−1 s−1. (B) Inhibition of 20 nM [3H]diminazene aceturate by up to 1 mM adenosine (▪) or pentamidine (□). The IC50 values were 0.58 ± 0.11 and 0.24 ± 0.02 μM, respectively.
The IC50 values reported here for the inhibition of [3H]diminazene uptake by adenosine and pentamidine are very similar to those reported previously for the inhibition of [3H]adenosine transport by P2 (8, 9, 12). However, the Km value for [3H]diminazene transport was lower than previously reported Ki values for diminazene inhibition of P2 (2.4 ± 0.5 μM in T. brucei brucei [12] or 3.9 μM in T. equiperdum [3]). The apparent Ki values, however, can be strongly influenced by different translocation rates for the substrate-transporter and substrate-inhibitor complex, in which case Ki values do not equal Km (14). Since diminazene is a di-cation and is adenosine neutral, different translocation rates across a lipid bilayer would not be unexpected.
In order to verify that [3H]diminazene transport in T. brucei brucei is indeed mediated by P2, we made use of a line in which the encoding gene, TbAT1, has been deleted (24). Uptake of 50 nM [3H]diminazene was barely detectable in TbAT1-null mutants (Fig. 1) with a rate of (9.7 ± 2.7) × 10−5 pmol 107 cells−1. This is only 1.1% of the rate in the wild-type control, but it was significantly nonzero (P < 0.01) and not significantly different in the presence of 1 mM unlabeled diminazene aceturate.
Uptake of diamidines by yeast expressing TbAT1.
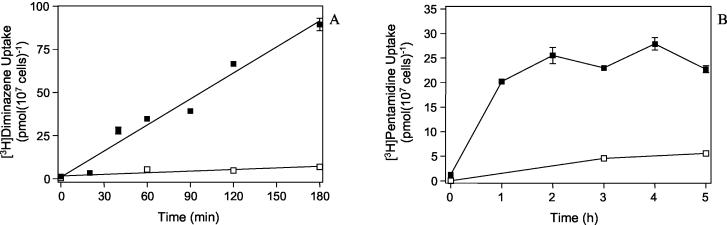
The TbAT1 gene encoding the P2 transporter was expressed in S. cerevisiae. An earlier study was unable to demonstrate the transport of the related diamidine trypanocide pentamidine in yeast expressing TbAT1 (21), even though P2 clearly mediates pentamidine transport in T. brucei brucei (8, 10). This led to speculation that TbAT1 recognition of diamidines requires a cofactor that is not present in yeast. We found that diamidines and, in particular, pentamidine appear to bind to the outside of the yeast cell, regardless whether it expresses TbAT1, and that this leads to a very high background, against which small increases are impossible to measure. This seems to amount to 1.5 to 2 pmol 107 cells−1 as determined from the radioactivity associated with the cell pellet when cells and stop solution were simultaneously added to the label (all ice-cold) and immediately spun through oil. We therefore measured the accumulation of radiolabeled diamidines over longer periods of time, which do not necessarily represent true initial rates of transport, and subtracted binding at 0°C. Nevertheless, 2.5 μM [3H]diminazene was clearly accumulated by yeast cells expressing TbAT1, with a rate of 0.0083 ± 0.0007 pmol 107 cells−1 s−1, and this process was completely inhibited by the presence of 1 mM unlabeled diminazene aceturate (Fig. 3A). In a parallel experiment with control cells transformed with the same vector but without the TbAT1 insert, no significant uptake of [3H]diminazene was observed over 3 h (P > 0.4, linear regression [data not shown]). [3H]pentamidine was similarly accumulated by yeast expressing TbAT1 (Fig. 3B) but not in the parallel experiment expressing empty vector (not shown). However, a slight accumulation was observed even in the presence of 1 mM unlabeled permeant (Fig. 3B), possibly due to diffusion or an endogenous yeast transporter.
FIG. 3.
Uptake of diamidines by S. cerevisiae expressing TbAT1. (A) Yeast cells of strain RH2884 transformed with pRS416-MET25 were incubated with 2.5 μM [3H]diminazene in the presence (□) or absence (▪) of 1 mM unlabeled diminazene. Uptake rates were calculated by linear regression and found to be not significantly different from zero (P = 0.12) or very significantly different from zero (P < 0.0001), respectively, in the presence or absence of excess permeant. (B) Accumulation of 2.5 μM [3H]pentamidine by RH2884/pRS416-MET25 in the presence (□) or absence (▪) of 1 mM unlabeled pentamidine.
Nonsaturable uptake of [3H]diminazene.
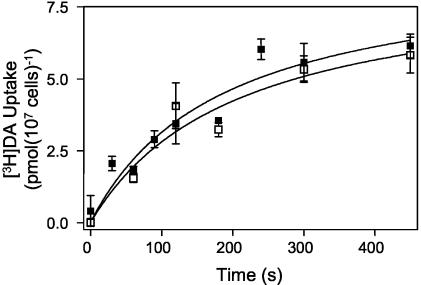
The experiments described above clearly establish that TbAT1 efficiently transports diminazene with high affinity and that low concentrations of this trypanocide are salvaged exclusively by this carrier. However, these studies used very low concentrations of radiolabeled permeant, and any low-affinity uptake mechanism might not be detectable under these conditions. We therefore conducted an experiment with 2 μM [3H]diminazene and increasing concentrations of unlabeled diminazene aceturate. TbAT1-mediated uptake has reached saturation under these conditions; thus, a lower-affinity transporter might be detectable under these conditions if its capacity were sufficient to distinguish it from background levels. No evidence for such a transport component was found (data not shown). However, it was still conceivable that a minor flux of [3H]diminazene was masked by TbAT1 under these conditions. In order to test uptake in an absolutely TbAT1-deficient background, further studies were performed with the T. brucei brucei Tbat1−/− line. At 20 μM [3H]diminazene, some diminazene uptake was evident, but this was not inhibited by 1 mM unlabeled diminazene aceturate (Fig. 4). It was concluded that any non-TbTA1-mediated uptake of diminazene either occurs by nonspecific processes such as diffusion or endocytosis or is mediated by a transporter with such low affinity for diminazene as to make it virtually irrelevant at therapeutic concentrations in plasma.
FIG. 4.
[3H]Diminazene transport in TbAT1-deficient trypanosomes. Transport of 20 μM [3H]diminazene by Tbat1−/− in the presence (□) or absence (▪) of 1 mM unlabeled diminazene. The two curves were not significantly different.
DISCUSSION
We describe here the mechanism by which trypanosomes may become resistant to diminazene. It has been speculated that resistance is the result of loss of a particular purine transporter, P2/TbAT1 (3, 11). The same transporter was implicated in the transport of the main sleeping sickness drugs, melarsoprol and pentamidine (8-10, 13, 26, 29). In the proposed model, the loss of TbAT1 would induce cross-resistance to all three drugs (4). Although such a phenotype is sometimes observed, it has become clear that cross-resistance does not always occur (2, 5, 18) and that pentamidine was transported by two additional T. brucei brucei transporters, HAPT1 and LAPT1 (10), making resistance much less likely to occur (5). Recently, we have also shown that deletion of TbAT1 caused only a two- to threefold resistance to melaminophenyl arsenicals and that an additional, as-yet-unidentified transporter is capable of accumulating melaminophenyl arsenicals, albeit less efficiently than P2 (24). Although P2/TbAT1 is clearly involved in the transport of melaminophenyl arsenicals and pentamidine, it is evidently not the only route of uptake for these compounds. Since the loss of TbAT1 did yield relatively high levels of diminished sensitivity to diminazene aceturate, it seemed likely that P2/TbAT1 is the principal route of entry of this compound.
We have now thoroughly investigated the transport of [3H]diminazene by T. brucei brucei and found that this is overwhelmingly mediated by TbAT1. TbAT1 clearly mediated the uptake of both diminazene and pentamidine when expressed in yeast, and [3H]diminazene transport was almost completely absent in Tbat1−/− trypanosomes. However, [3H]diminazene did enter the cells, at a very low rate, in a TbAT1-independent fashion. The mechanism for this is currently unclear, but it was not saturable by 1 mM unlabeled diminazene. We would speculate that, in the absence of TbAT1, diminazene enters the cell at a very slow rate through a transporter for which it is has very low affinity (>1 mM). This model explains why TbAT1-null mutants display a much higher resistance to diminazene than to pentamidine (19- versus 2.4-fold) (24) but do remain sensitive to high diminazene concentrations in vitro. However, we acknowledge that other instances of nonreciprocal cross-resistance between diminazene and pentamidine or melaminophenyl arsenicals could be the result of alterations in intracellular targets rather than (or in addition to) transport.
In summary, we demonstrated that diminazene is almost exclusively accumulated by the T. brucei brucei P2 transporter and that loss of this single transport activity is sufficient to explain high levels of resistance observed in laboratory strains and veterinary isolates.
Acknowledgments
This study was supported by the Wellcome Trust and the BBSRC (17/C13486).
We thank Tom Seebeck (University of Bern, Bern, Switzerland) for the T. brucei brucei Tbat1−/− strain. We thank Howard Riezman, Department of Biochemistry, University of Geneva, Geneva, Switzerland, for the yeast strain RH2884 and Elmar Schiebel, Paterson Institute for Cancer Research, Manchester, United Kingdom, for the yeast expression vector pRS416-MET25.
REFERENCES
- 1.Anene, B. M., D. N. Onah, and Y. Nawa. 2001. Drug resistance in pathogenic African trypanosomes: what hopes for the future? Vet. Parasitol. 96:83-100. [DOI] [PubMed] [Google Scholar]
- 2.Bacchi, C. J. 1993. Resistance to clinical drugs in African trypanosomes. Parasitol. Today 9:190-193. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, M. P., Z. Q. Zhang, H. Denise, C. Giroud, and T. Baltz. 1995. A diamidine-resistant Trypanosoma equiperdum clone contains a P2 purine transporter with reduced substrate affinity. Mol. Biochem. Parasitol. 73:223-229. [DOI] [PubMed] [Google Scholar]
- 4.Barrett, M. P., and A. H. Fairlamb. 1998. The biochemical basis of arsenical-diamidine cross-resistance in African trypanosomes. Parasitol. Today 15:136-140. [DOI] [PubMed] [Google Scholar]
- 5.Bray, P. G., M. P. Barrett, S. A. Ward, and H. P. de Koning. 2003. Pentamidine uptake and resistance in pathogenic protozoa: past, present and future. Trends Parasitol. 19:232-239. [DOI] [PubMed] [Google Scholar]
- 6.Brun, R., R. Schumacher, C. Schmid, C. Kunz, and C. Burri. 2001. The phenomenon of treatment failures in human African trypanosomiasis. Trop. Med. Int. Health 6:906-914. [DOI] [PubMed] [Google Scholar]
- 7.Burchmore, R., L. J. M. Wallace, D. Candlish, M. I. Al-Salabi, P. Beal, M. P. Barrett, S. A. Baldwin, and H. P. de Koning. 2003. Cloning, heterologous expression and in situ characterization of the first high-affinity nucleobase transporter from a protozoan. J. Biol. Chem. 278:23502-23507. [DOI] [PubMed] [Google Scholar]
- 8.Carter, N. S., B. J. Berger, and A. H. Fairlamb. 1995. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 270:28153-28157. [DOI] [PubMed] [Google Scholar]
- 9.Carter, N. S., and A. H. Fairlamb. 1993. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature 361:173-175. [DOI] [PubMed] [Google Scholar]
- 10.De Koning, H. P. 2001. Uptake of pentamidine in Trypanosoma brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol. Pharmacol. 59:586-592. [DOI] [PubMed] [Google Scholar]
- 11.De Koning, H. P. 2001. Transporters in African trypanosomes: role in drug action and resistance. Int. J. Parasitol. 31:512-522. [DOI] [PubMed] [Google Scholar]
- 12.De Koning, H. P., and S. M. Jarvis. 1999. Adenosine transporters in bloodstream forms of Trypanosoma brucei brucei: substrate recognition motifs and affinity for trypanocidal drugs. Mol. Pharmacol. 56:1162-1170. [DOI] [PubMed] [Google Scholar]
- 13.De Koning, H. P., A. MacLeod, M. P. Barrett, B. Cover, and S. M. Jarvis. 2000. Further evidence for a link between melarsoprol resistance and P2 transporter function in African trypanosomes. Mol. Biochem. Parasitol. 106:181-185. [DOI] [PubMed] [Google Scholar]
- 14.Deves, R. 1991. Kinetics of transport: characterising the interaction of substrates and inhibitors with carrier systems, p. 3-18. In D. L. Yudilivich (ed.), Cell membrane transport. Plenum Press, Inc., New York, N.Y.
- 15.Frommel, T. O., and A. E. Balber. 1987. Flow cytometric analysis of drug accumulation by multidrug resistant Trypanosoma brucei brucei and T. brucei rhodesiense. Mol. Biochem. Parasitol. 26:183-191. [DOI] [PubMed] [Google Scholar]
- 16.Fulton, J. D., and W. Yorke. 1942. Further observations on the stability of drug resistance in trypanosomes. Ann. Trop. Med. Parasitol. 35:221-227. [Google Scholar]
- 17.Geerts, S., P. H. Holmes, O. Diall, and M. C. Eisler. 2001. African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol. 17:25-28. [DOI] [PubMed] [Google Scholar]
- 18.Kaminsky, R., and P. Mäser. 2000. Drug resistance in African trypanosomes. Curr. Opin. Anti-infect. Investig. Drugs 2:76-82. [Google Scholar]
- 19.Lanham, S. M. 1968. Separation of trypanosomes from the blood of infected rats and mice by anion-exchangers. Nature 218:1273-1274. [DOI] [PubMed] [Google Scholar]
- 20.Legros, D., G. Ollivier, M. Gastellu-Etchegorry, C. Paquet, C. Burri, J. Jannin, and P. Büscher. 2002. Treatment of human African trypanosomiasis: present situation and needs for research and development. Lancet Infect. Dis. 2:437-440. [DOI] [PubMed] [Google Scholar]
- 21.Mäser, P., C. Sütterlin, A. Kralli, and R. Kaminsky. 1999. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 285:242-244. [DOI] [PubMed] [Google Scholar]
- 22.Matovu, E., F. Geiser, V. Schneider, P. Mäser, J. C. K. Enyaru, R. Kaminsky, S. Gallati, and T. Seebeck. 2001. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol. Biochem. Parasitol. 117:73-81. [DOI] [PubMed] [Google Scholar]
- 23.Matovu, E., T. Seebeck, J. C. K. Enyaru, and R. Kaminsky. 2001. Drug resistance in Trypanosoma brucei spp., the causative agents of sleeping sickness in man and nagana in cattle. Microbes Infect. 3:763-770. [DOI] [PubMed] [Google Scholar]
- 24.Matovu, E., M. Stewart, F. Geiser, R. Brun, P. Mäser, L. J. M. Wallace, R. J. Burchmore, J. C. K. Enyaru, M. P. Barrett, R. Kaminsky, T. Seebeck, and H. P. De Koning. 2003. The mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2:1003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepin, J., and H. A. Meda. 2001. The epidemiology and control of human African trypanosomiasis. Adv. Parasitol. 49:71-132. [DOI] [PubMed] [Google Scholar]
- 26.Scott, A. G., A. Tait, and C. M. R. Turner. 1997. Trypanosoma brucei: lack of cross-resistance to melarsoprol in vitro by cymelarsan-resistant parasites. Exp. Parasitol. 86:181-190. [DOI] [PubMed] [Google Scholar]
- 27.Stanghellini, A., and T. Josenando. 2001. The situation of sleeping sickness in Angola: a calamity. Trop. Med. Int. Health 6:330-334. [DOI] [PubMed] [Google Scholar]
- 28.Stich, A., Barrett, M. P., and Krishna, S. 2003. Waking up to sleeping sickness. Trends Parasitol. 19:195-197. [DOI] [PubMed] [Google Scholar]
- 29.Suswam, E. A., D. W. Taylor, C. A. Ross, and R. J. Martin. 2001. Changes in properties of adenosine transporters in Trypanosoma evansi and modes of selection of resistance to the melaminophenyl arsenical drug, MelCy. Vet. Parasitol. 102:193-208. [DOI] [PubMed] [Google Scholar]