Abstract
Candida glabrata can become resistant to fluconazole, causing persistent colonization and invasive infection during prolonged exposure to the drug. To determine the mechanism of resistance in this setting, weekly oropharyngeal cultures for C. glabrata were obtained over a 2-year period from hematopoietic stem cell transplant recipients who were receiving fluconazole prophylaxis. In 20 patients from whom at least two isolates of the same karyotype were obtained more than two weeks apart, fluconazole MICs doubled every 31 days on average. The mechanism of fluconazole resistance in isolates from the 14 of the 20 patients studied in whom MICs changed at least fourfold was studied. Cellular resistance was accompanied by increased drug efflux as measured by decreased accumulation of fluconazole and rhodamine 6G and increased abundance of transcripts from two drug transporters, CgCDR1 and PDH1. The rapidity and regularity of the rising resistance indicated that C. glabrata is able to upregulate drug efflux without losing the ability to maintain colonization.
Fluconazole prophylaxis of hematopoietic stem cell transplant recipients decreased the incidence of candidemia at the Fred Hutchinson Cancer Research Center from 11% in 1980 to 1986 to 3% in 1994 to 1997 (13). The Candida species most commonly invading the bloodstream also changed. The prevalence of Candida albicans in blood cultures decreased from 64 to 3%, whereas that of Candida glabrata increased from 4 to 47%. Colonization with C. glabrata occurred in 88 of 585 (15%) patients receiving fluconazole prophylaxis. Colonization was present on admission in 43% of the patients and appeared after admission in 57% of the patients, with the median time of onset being day 36. C. glabrata sepsis occurred by posttransplant day 120 in 9.8% of colonized patients.
The fluconazole MIC for C. glabrata is approximately 16 times higher than that for C. albicans (18), accounting for the selection of this species during fluconazole prophylaxis. We addressed the question whether resistance increased during fluconazole prophylaxis for stem cell recipients and, if so, whether this change was due to the acquisition of a new isolate or a change in an existing isolate. We also asked whether increased fluconazole resistance in an existing isolate might be due to increased drug efflux and increased activity of the ABC transporters CgCDR1 and PDH1, which have been associated with fluconazole resistance in prior reports (15, 20).
Both genes were noted to have increased transcription in isolates which were more fluconazole resistant than a patient's prior isolate. In both reports, increased resistance occurred in the same strain in the patient rather than by acquisition of a new strain. To provide an estimate of drug efflux, we measured fluconazole and rhodamine 6G accumulation in pairs of isolates that had been obtained from the same patient and had the same karyotype. Both compounds have been reported to have less accumulation in fluconazole-resistant C. glabrata due to increased drug efflux (5, 17).
MATERIALS AND METHODS
Isolates.
Weekly oropharyngeal cultures for fungus were obtained from all patients undergoing hematopoietic stem cell transplantation at the Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Wash. All Candida species were identified, and single colonies from the initial culture plates were stored at −70°C in 10% glycerol. All cultures from patients for whom more than one oropharyngeal isolate was identified as C. glabrata between 13 July 1995 and 18 July 1997, comprising 104 isolates from 29 patients, were included in this study. Information on antifungal drug usage during and prior to isolate collection was obtained by examination of computerized records. All patients received prophylactic fluconazole at 400 mg daily during the first 90 days posttransplantation except during periods when they were receiving intravenous amphotericin B for suspected or proven invasive mycosis. This protocol was approved by the FHCRC Institutional Review Board. Multiple other C. glabrata isolates were examined for validation of the molecular typing technique.
Frozen stocks were also prepared from 38 additional isolates, all from primary cultures of different patients, from three sources; 18 isolates were obtained from the National Institutes of Health Clinical Center, 10 blood culture isolates were obtained from the Johns Hopkins Hospital (courtesy of William Merz), and 10 blood culture isolates were obtained from an international surveillance study. The last group of cultures were selected from Sentry program specimens obtained in geographically separated sites (courtesy of Michael Pfaller) in order to increase heterogeneity (18).
All procedures to be described in this study were done with isolates recovered directly from frozen stocks to decrease the effects of serial passage in culture. The identification of C. glabrata was confirmed by API 20C Aux strips (BioMerieux Vitek Inc., Marcy l'Etoile, France). Fluconazole susceptibility was measured by the NCCLS M27A microdilution method with an end point at 48 h (16).
Molecular typing.
Contour-clamped homogenous electric field (CHEF) gel electrophoresis was used to obtain ethidium bromide-stained chromosomal bands as described previously (3). Agarose gels (1%) were run for 24 h at 13°C with a CHEF Dr II power supply (Bio-Rad, Richmond, Calif.) at 200 V with a 60- to 120-s ramp. Digitized images of the ethidium bromide-stained gels were analyzed with a Molecular Analyst (Bio-Rad) by using the Dice coefficient and UPGMA clustering to construct a dendrogram and calculate percentages of identity. A single isolate with a clear CHEF pattern (isolate 19456) was used in each run to normalize patterns between runs. The ability of the CHEF pattern to discriminate between isolates was determined by the discrimination index (DI) as described by Hunter and Gaston (7). Isolates were given a Roman numeral type.
Northern analysis.
C. glabrata cultures grown overnight in yeast extract-peptone (YEPD) broth (Bacto Peptone 1% [Difco, Detroit, Mich.], yeast extract 1% [Difco], 2% dextrose) were centrifuged, resuspended in fresh YEPD broth at 106 cells/ml as determined by hemocytometer count, and shaken at 30°C for 1 h. RNA was extracted by the FastRNA Red kit (Qbiogene, Vista, Calif.). Northern analysis was conducted by using standard methods (2). Hybond nylon membranes (Amersham Biosciences, Piscataway, N.J.) were used for blotting. For the PDH1 probe, a 1.1-kb PCR product was obtained by using the plasmid pCglib18 (15) as a template, Taq polymerase (Stratagene, La Jolla, Calif.), and the oligonucleotides 5′-TTTGGCTCTGTGGGAAGAAGGG-3′ and 5′-ACATGGTTGGCGAAGTCCTCTC-3′ as primers. For the CgCDR1 probe, a 1.1-kb PCR product was obtained by using genomic DNA from C. glabrata strain NCCLS 84 as a template, Taq polymerase, and the oligonucleotides 5′-GGCACACACACAAACAAACAATGC-3′ and 5′-GACTTCAGCAATGGAGACACGC-3′ as primers. Both amplifications used 25 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 70 s followed by an extension period of 10 min at 72°C. The PCR product was cloned into pPCR-Script Amp SK(+) (Stratagene) and excised from the vector with SacI and EcoRI endonucleases, and the fragment was extracted from the agarose gel with GENECLEAN (Bio101, Vista, Calif.). The ACT1 probe was prepared as previously described (15). Probes were randomly labeled with [32P]dCTP by using the Prime It II kit (Stratagene). Unincorporated 32P was removed with NucTrap (Stratagene). Filters which had been hybridized with labeled probes were exposed to Storage Phosphor screens (Molecular Dynamics, Sunnyvale, Calif.). Images were scanned on a PhosphorImager 445SI with STORM 860 (Molecular Dynamics) and analyzed with Imagequant software (Molecular Dynamics). Relative transcription was calculated as the ratio of PDH1 or CgCDR1 message to ACT1 message on the same membrane.
Rhodamine 6G accumulation.
Rhodamine 6G (Sigma, St. Louis, Mo.) accumulation was measured by flow cytometry with a FACS Calibur fluorescence-activated cell cytometer (Becton Dickinson, San Jose, Calif.). Cells were shaken overnight at 30°C in YEPD broth, transferred to fresh yeast nitrogen base broth, adjusted to 106 cells/ml with a hemocytometer, and shaken for an additional 2 h. Rhodamine 6G was added at a final concentration of 100 μg/ml and incubated with shaking for an additional 30 min. After incubation, 1 ml of culture solution was transferred to an equal volume of ice-cold phosphate-buffered saline (PBS; pH 7.0) and immediately tested. A total of 20,000 cells were scanned with the 488-nm laser and FL-2 filter. Data were analyzed with CellQuest (Becton Dickinson). Geometric mean fluorescence was used for calculation.
[3H]fluconazole accumulation.
C. glabrata cultures were grown at 30°C overnight in yeast nitrogen base (Difco) with 2% dextrose on a rotary shaker at 225 rpm. Cells were diluted with fresh culture medium to 108/ml, as determined by optical density at 600 nm. Fluconazole accumulation was measured by using drug that had been tritiated by gas exchange to a specific activity of 629 GBq/mM (Amersham Biosciences). [3H]fluconazole was added to 8-ml cell suspensions at a final concentration of 7.4 kBq/ml (0.2 μCi/ml, 3.6 ng/ml). After 60 min of incubation with rotation at 225 rpm (30°C), triplicate 2.5-ml samples were filtered on a vacuum manifold (Millipore, Bedford, Mass.) with 24-mm-diameter GF/C glass fiber filters (Whatman, Maidstone, Kent, United Kingdom) which had been presoaked in 100 μM unlabeled fluconazole in PBS (pH 7.0). Zero time samples were chilled in ice for 30 min before the addition of tritiated fluconazole and kept on ice until they were filtered. Cells were washed on the filters twice with 8 ml of PBS containing 100 μM unlabeled fluconazole. The filters were dried at 37°C for 1 h, placed in Hydrofluor scintillation fluid (National Diagnostics, Atlanta, Ga.), and allowed to stand overnight, and the cells were counted with a 2200CA Tricarb liquid scintillation counter (Packard, Downer's Grove, Ill.). Results were expressed as differences in counts per minute per cell between 0 and 60 min.
RESULTS
Molecular typing of C. glabrata isolates.
Analysis of CHEF gel patterns distinguished 16 types among the 142 C. glabrata isolates on the basis of 90% identity, and 30 types were distinguished at 95% identity. Discrimination between isolates at 90% identity was clear and reproducible (Fig. 1), but at 95% identity, reproducibility was impaired by two factors. Nonlinearity of band positions between runs introduced errors in Molecular Analyst's algorithm for normalizing patterns. The program also had difficulty discriminating between single and closely approximated double bands. Band patterns designated types III and VI predominated and accounted for initial isolates from 23 of the 29 FHCRC patients (79%) and 25 of the 38 other patients (66%). The DIs were 0.74 at 90% identity and 0.91 at 95% identity when all 67 first isolates from different patients were considered. The 29 first isolates from the FHCRC patients were somewhat less diverse (DI = 0.70) than the 38 isolates from other sources (DI = 0.77). At 90% identity, only one of 29 FHCRC patients had an isolate of more than one type. This patient had type X C. glabrata with an MIC of 32 μg/ml on posttransplant days 15, 49, and 77 but type XVI with an MIC of 4 μg/ml on posttransplant day 93, 21 days after his last dose of fluconazole. With the exception of this single patient, the patients remained colonized with the same C. glabrata types over several months' time.
FIG. 1.
CHEF gel showing representatives of 16 karyotypes (I to XVI), distinguished at 90% identity.
Fluconazole resistance.
Fluconazole MICs were analyzed for 20 patients who had more than one isolate of the same genotype recovered at least 14 days apart while receiving fluconazole. Isolates from the other nine patients did not meet this criterion, usually because fluconazole prophylaxis had been interrupted prior to the culture, and isolates from these patients were included only for analysis of karyotypic change over time. The interval from the first to the last culture in the 20 patients ranged from 15 to 70 days. Resistance tended to increase over time, with the MIC doubling every 31 days on average (Fig. 2). Correlation between log MIC and days of fluconazole administration was highly significant (Pearson correlation, P < 0.001).
FIG. 2.
Fluconazole MICs during fluconazole prophylaxis of 20 patients. Each line represents a single patient. The dashed line is the best-fit line. mcg, micrograms
Rhodamine 6G and fluconazole accumulation.
Accumulation of Rhodamine 6G was measured in the most fluconazole susceptible and the most fluconazole resistant isolates from 14 patients whose isolate pairs differed at least fourfold and were of the same karyotype (Fig. 3). Geometric mean fluorescence was highly correlated with log fluconazole MIC (Spearman rank correlation, P = 0.0001). Fluconazole accumulation was inversely correlated with fluconazole MIC in the 14 pairs studied (Spearman rank correlation, P < 0.002) (Fig. 4). Only 2 of 14 pairs of isolates showed greater fluconazole accumulation in the more resistant pair. One of these two isolates also showed a negligible fall in rhodamine accumulation as resistance rose, as shown by the dashed lines in Fig. 3 and 4. Not surprisingly, fluconazole accumulation also correlated with rhodamine 6G accumulation (Spearman rank correlation, P = 0.04) (data not shown). Decreased accumulation, implying increased drug efflux, occurred consistently as strains became more fluconazole resistant.
FIG. 3.
Rhodamine 6G accumulation in 14 pairs of isolates as a function of fluconazole MIC. Two lines overlap and appear as one line. The dashed line represents the same patient as the dashed line in Fig. 4. mcg, micrograms.
FIG. 4.
Fluconazole accumulation in 14 pairs of isolates as a function of fluconazole MIC. The dashed line represents the same patient as the dashed line in Fig. 3.
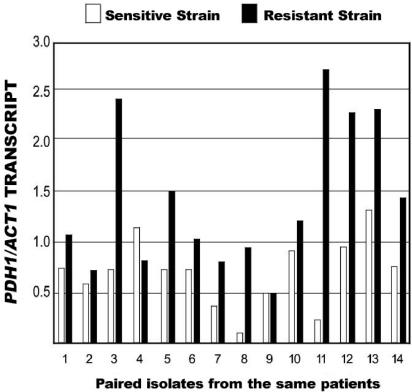
Transcription of the multidrug resistance transporters CgCDR1 and PDH1.
The abundance of CgCDR1 and PDH1 transcripts was compared to the amount of ACT1 transcript in 14 pairs of isolates from the same patients. The radioactivity levels of the ACT1 probe differed between the two experiments shown in Fig. 5 and 6, so the amounts of PDH1 and CgDR1 transcript cannot be compared. In 12 of 14 pairs, the more fluconazole resistant isolate had a greater transcript abundance than the more susceptible isolate, though in several of the pairs the differences were not large. The exceptions were patient 4 for both transcripts, patient 9 for PDH1, and patient 10 for CgDR1.
FIG. 5.
Abundance of PDH1 transcript divided by that of ACT1 transcript in paired susceptible and resistant isolates from 14 patients.
FIG. 6.
Abundance of CgCDR1 transcript divided by that of ACT1 transcript in paired susceptible and resistant isolates from 14 patients.
DISCUSSION
Increased fluconazole resistance has been noted in oral C. albicans isolates from patients with very advanced AIDS who have been receiving fluconazole over long periods. Both the acquisition of new strains and increased resistance in prior strains have been noted (4, 6, 14). Multiple mechanisms of resistance have been reported, including mutations in the gene coding for the azole target enzyme, C14 sterol demethylase, and increased transcription of multidrug efflux transporters (19, 21). As in the case for AIDS, one of us has previously identified two transplant recipients whose C. albicans isolates became resistant to fluconazole and who developed deep infections (11, 12). The isolates for these patients exhibited increased expression of efflux pumps (12). Isolates from one of these patients developed drug resistance in a little over 2 weeks, similar to the findings reported here for C. glabrata, for which the MIC doubled an average of every 31 days. Although we did not attempt to assess the contribution of mutations in C14 sterol demethylase to fluconazole resistance, for all but one or two of the pairs reported here, the more resistant isolate had less rhodamine 6G accumulation, less fluconazole accumulation, and increased transcript abundance of the ABC transporters CgCDR1 and PDH1. This result is consistent with adaptive upregulation of drug transporters in C. glabrata.
Electrophoretic karyotyping of C. glabrata has been found to provide sufficient discrimination for epidemiologic purposes (9, 10). The DI for Barchiesi's karyotyped isolates from 29 patients (3) can be calculated as 0.75, similar to the DI we found for 38 isolates from outside the FHCRC. Up to 13 to 14 chromosomal bands on CHEF gels of C. glabrata have been reported, although we usually resolved about 11 bands. The Génolevure Consortium found 13 chromosomes in their sequencing of C. glabrata isolate GBS13 (http://cbi.labri.fr/Genolevures/C_glabrata.php), consistent with karyotyping results. One or two bands larger than 1.4 Mb have been reported to be particularly variable, perhaps due to a variable number of ribosomal DNA repeats (1). Sequential isolates from the same patient have usually been reported to yield the same karyotype (3, 9). Consistent with these reports, all of the FHCRC patients whose isolates became more resistant kept the same C. glabrata genotype. One patient acquired a new, more susceptible strain after fluconazole was discontinued.
Congruence between the changes in CgCDR1 and PDH1 transcript abundances as isolates became more fluconazole resistant was expected. The genes have overlapping functions, including fluconazole and rhodamine 6G efflux, and share transcriptional regulators (8). One pair of isolates, shown by the dashed lines in Fig. 3 and 4, showed little or no decrease in either rhodamine 6G accumulation or fluconazole uptake between the paired isolates. It is more likely that the increase in fluconazole resistance seen in our 20 patients resulted from several mechanisms, only one of which was the upregulation of ABC transporters.
Acknowledgments
This study was supported in part by NIAID award no. AI01571 to K.A.M.
REFERENCES
- 1.Asakura, K., S. I. Iwaguchi, M. Homma, T. Suae, K. Higashide, and K. Tanaka. 1991. Electrophoretic karyotypes of clinically isolated yeasts of Candida albicans and C. glabrata. J. Gen. Microbiol. 137:2531-2538. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1998. Current protocols in molecular biology. John Wiley & Sons, Somerset, N.J.
- 3.Barchiesi, F., L. Falconi Di Francesco, D. Arzeni, F. Caselli, D. Gallo, and G. Scalise. 1999. Electrophoretic karyotyping and triazole susceptibility of Candida glabrata clinical isolates. Eur. J. Clin. Microbiol. Infect. Dis. 18:184-187. [DOI] [PubMed] [Google Scholar]
- 4.Barchiesi, F., D. Arzeni, M. S. Del Prete, A. Sinicco, L. F. Di Fancesco, M. B. Pasticci, L. Lamura, M. M. Nuzzo, F. Burzacchini, S. Coppola, F. Chiodo, and F. Scalise. 1998. Fluconazole susceptibility and strain variation of Candida albicans isolates from HIV-infected patients with oropharyngeal candidosis. J. Antimicrob. Chemother. 41:541-548. [DOI] [PubMed] [Google Scholar]
- 5.Clark, F. S., T. Parkinson, C. A. Hitchcock, and N. A. R. Gow. 1996. Correlation between rhodamine 123 accumulation and azole sensitivity in Candida species: possible role for drug efflux in resistance. Antimicrob. Agents Chemother. 40:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guennec, R. L., J. Reynes, M. Mallié, C. Pujol, F. Janbon, and J.-M. Bastide. 1995. Fluconazole- and itraconazole-resistant Candida albicans strains from AIDS patients: multilocus enzyme electrophoresis analysis and antifungal susceptibilities. J. Clin. Microbiol. 33:2732-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 226:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izumikawa, K., H. Kakeya, H.-F. Tsai, B. Grimberg, and J. E. Bennett. 2003. Function of Candida glabrata ABC transporter, PDH1. Yeast 20:249-261. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann, C. S., and W. G. Merz. 1989. Electrophoretic karyotypes of Torulopsis glabrata. J. Clin. Microbiol. 27:2165-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khattak, M. N., J. P. Burnie, R. C. Matthews, and B. A. Oppenheim. 1992. Clamped homogeneous elective field typing of Torulopsis glabrata isolates causing nosocomial infections. J. Clin. Microbiol. 30:2211-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marr, K. A., T. White, J.-A. H. van Burik, and R. A. Bowden. 1997. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing bone marrow transplantation. Clin. Infect. Dis. 25:908-910. [DOI] [PubMed] [Google Scholar]
- 12.Marr, K. A., C. N. Lyons, K. Ha, T. R. Rustad, and T. C. White. 2001. Inducible azole resistance associated with a heterogenous phenotype in Candida albicans. Antimicrob. Agents Chemother. 45:52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marr, K. A., K. Seidel, T. C. White, and R. A. Bowden. 2000. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. Clin. Infect. Dis. 181:309-316. [DOI] [PubMed] [Google Scholar]
- 14.Millon, L., A. Manteaux, G. Reboux, C. Drobacheff, M. Monod, T. Barale, and R. Michel-Briand. 1994. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: persistence of Candida albicans strains with the same genotype. J. Clin. Microbiol. 32:1115-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazaki, H., Y. Miyazaki, A. Geber,T. Parkinson, C. Hitchcock, D. Falcone, K. Marsden, and J. E. Bennett. 1998. Fluconazole resistance associated with drug efflux and increased transcription of a drug transporter gene, PDH1, in Candida glabrata. Antimicrob. Agents Chemother. 42:1695-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 1995. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Parkinson, T., D. F. Falconer, and C. A. Hitchcock. 1995. Fluconazole resistance due to energy-dependent drug efflux in Candida glabrata. Antimicrob. Agents Chemother. 39:1696-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and J. D. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 20.Sanglard, D., F. Ischer, D. Calabrese, P. A. Majcherczyk, and J. Bille. 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 43:2753-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanglard, D., K. Kuchler, F. Ischer, J.-L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific drug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]