Abstract
The human cytomegalovirus (HCMV) homolog of the Epstein-Barr virus (EBV) protein kinase (PK), UL97, is inhibited by maribavir (1263W94) and selected indolocarbazoles. Here we show that only one of these indolocarbazoles (K252a), but not maribavir, inhibits autophosphorylation of the EBV PK, BGLF4. However, maribavir and another indolocarbazole, NGIC-I, do inhibit EBV DNA synthesis, suggesting that although these last compounds inhibit both HCMV and EBV, they seem to operate through differ-ent pathways.
The family Herpesviridae contains approximately 130 herpesviruses from different animal species (28). Despite remarkable differences in biological properties among the members of the family, their genomes encode many conserved genes (28). This conservation may suggest important roles for the products of these genes in the viral life cycle. All known herpesvirus genomes contain genes that encode known or putative protein kinases (PKs) (4, 30), the functions of which are largely undetermined. The members of this family can be divided into two main groups; each group is exemplified by the most-studied members encoded by herpes simplex virus US3 (9, 19, 27) and UL13 (7, 23) genes. While alphaherpesviruses encode members of both PK groups, beta- and gammaherpesviruses encode only one PK, which is the homolog of herpes simplex virus UL13, a PK involved in modification of a number of viral (20, 21, 25, 26) and cellular (3, 14) proteins. Recent reports have linked the anti-human cytomegalovirus (HCMV) effects of the novel antiviral compound maribavir (6) to the inhibition of the virally encoded PK UL97 (2, 11, 33). The results suggest that both the inhibition of UL97 kinase activity and the deletion of the UL97 gene lead to a significant decrease in viral yield (16, 24), presumably due to inhibition of the nuclear egress of virions (16). The results also support previous data that suggest an important role for this kinase in the viral life cycle (32).
Epstein-Barr virus (EBV) PK encoded by the BGLF4 gene is a homolog of HCMV UL97. Previously, members of our group (34) and others (31) have reported that EBV is the only other human herpesvirus inhibited by maribavir; we have also shown that the hyperphosphorylation of EBV EA-D (encoded by the EBV BMRF1 gene), the viral DNA polymerase processivity factor, is correlated with PK expression, therefore implying a role for EBV PK in viral replication and a possible mechanism of action for the drug. However, although maribavir inhibited the level of hyperphosphorylated EA-D during viral reactivation in Akata cells, it did not directly inhibit the phosphorylation of EA-D by EBV PK in transient coexpression assays with these two viral genes (10).
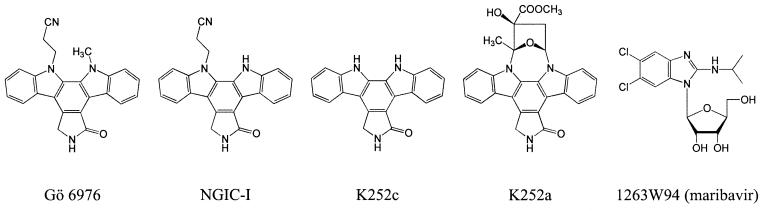
In the continuous search for more potent and selective antiviral compounds, unmodified indolocarbazole Arcyriaflavin A was proposed as a potent inhibitor of HCMV in vitro (29). Additional screening revealed a number of indolocarbazoles showing pronounced anti-HCMV effects (29, 35), presumably due to the specific targeting of HCMV UL97 (17, 18, 35). In the present study we tested the anti-EBV activity of maribavir and selected indolocarbazoles (K252a, K252c, Gö6976, and NGIC-I) (Fig. 1) in vivo as well as their effect on purified EBV PK in vitro.
FIG. 1.
Chemical structures of potential antiviral compounds and maribavir.
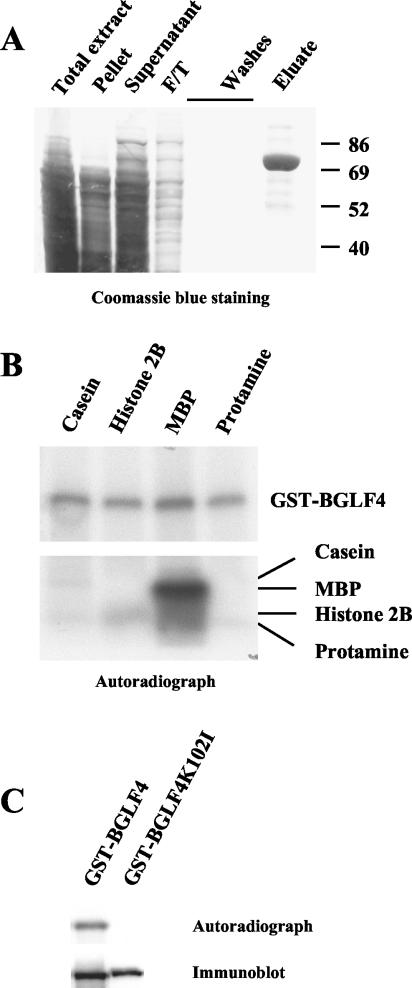
The EBV BGLF4 gene (genomic position, 110040 to 111404 [complement]; NCBI accession number AJ507799) or the gene encoding the K102I mutant of BGLF4, in which the invariant catalytic lysine was mutated, was cloned into a baculovirus genome as a fusion with sequence for glutathione S-transferase and His6 tags (12, 13) (the viruses were a gift from Y. Kawaguchi) and was expressed in insect cells by following standard protocols (22). The protein purification and in vitro kinase assay protocols were described previously (12). The purification efficiency in this study was routinely around 95% (by Coomassie staining) (Fig. 2A).
FIG. 2.
Purification and kinase activity of EBV PK. (A) EBV PK was expressed in insect cells infected with recombinant baculovirus and purified following a published protocol (12). The purified protein appears as a single band at ∼78 kDa detected by Coomassie staining. Numbers on the right side of the gel are standard molecular weights in thousands. (B) Kinase activity of the purified EBV PK was examined in in vitro kinase assays with casein, MBP, histone 2B, and protamine as substrates (lower panel) or in autophosphorylation assays (upper panel). The whole reaction mixtures were separated by SDS-PAGE (4 to 20% gradient gel), and dried gels were exposed to X-ray film. No labeled bands were detected in mock reactions (without kinase) (data not shown). (C) Specificity of the autophosphorylation was confirmed by use of the kinase-null K102I mutant of EBV BGLF4. The kinase assays were performed as described before, and the reaction mixtures were extensively washed, subjected to SDS-PAGE, and exposed to X-ray film (upper panel), followed by immunoblotting with anti-GST antibody (bottom panel).
First, we tested the kinase activity of the purified kinase or its catalytic knockout on nonspecific exogenous substrates and in autophosphorylation assays as well (Fig. 2B and C). Equal amounts (2 μg) of casein, histone 2B, myelin basic protein (MBP), and protamine were incubated in the kinase assays in the presence or absence of the kinase for 30 min at 37°C. The whole reaction mixtures were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels (4 to 20% gels), transferred onto a polyvinylidene difluoride membrane, and after staining with Ponceau S (Sigma), exposed to a film (Kodak) for autoradiography. The results show that EBV PK was highly efficient in phosphorylation of MBP and in autophosphorylation, which supports previous observations of basic substrate preference (histone versus casein) (5). Histone 2B was phosphorylated to a lesser extent (Fig. 2B). No phosphorylation was detected in the presence of the BGLF4 K102I mutant (Fig. 2C) and in the absence of the kinase (data not shown). Autophosphorylation was selected as a measure of the kinase activity.
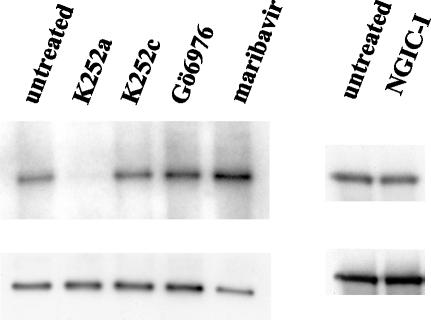
Second, we examined the effect of anti-HCMV UL97 compounds on EBV PK autophosphorylation. The compounds from an indolocarbazole group (K252a, K252c, Gö6976, and NGIC-I) were selected based on their effectiveness against HCMV UL97 (18, 35) and purchased from Calbiochem; maribavir was provided by GlaxoSmithKline (RTP). Concentrations ranged between 0.0001 and 1 μM, and the results presented are from the highest concentration. As shown in Fig. 3, only one of four compounds could effectively inhibit autophosphorylation of EBV PK. This compound, K252a, has a much broader range of targets (such as PKA, PKC, PKG, calmodulin kinase, myosin light-chain kinase, and tyrosine kinase) than the other compounds tested (which are mainly PKC inhibitors). These results support recent data, which showed that NGIC-I did not inhibit the phosphorylation of ganciclovir by EBV PK while it did inhibit phosphorylation of ganciclovir by HCMV UL97 (17, 18). Finally, these results are congruent with the failure of maribavir to inhibit phosphorylation of EA-D by EBV PK in transfected cells (10)
FIG. 3.
Inhibition of EBV PK kinase activity by selected compounds. A 1 μM concentration of each compound was added to the kinase assay samples. The control sample was treated with an equal amount of dimethyl sulfoxide. The reaction mixtures were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and exposed to X-ray film (upper panel) followed by probing with anti-glutathione S-transferase antibody (bottom panel).
Last, we examined the cytotoxicities of these compounds on latently infected Akata cells by a modified MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Promega) and their effects on EBV lytic replication by measuring EBV DNA levels. For the cytotoxicity assay, the cells were seeded at low density in 96-well plates (in triplicate) and treated with different concentrations (0.001 to 100 μM) of the selected compounds. Assay buffer was added 5 days after treatment, and measurements were performed according to the manufacturer's protocol. The results (Table 1) show that all tested indolocarbazoles are toxic to the cells at concentrations of ∼3 μM or less, with K252c being the least toxic (concentration resulting in 50% inhibition of cellular activity [CC50], ∼3.14 μM) and K252a being the most toxic (CC50, ∼0.18 μM). On the other hand, maribavir's toxicity was insignificant (less than 20% at 10 μM) at the concentration tested (CC50, ∼93 μM).
TABLE 1.
Antiviral activities of maribavir and selected indolocarbazoles against EBV and cytotoxicity in Akata cellsa
| Compound | CC50 (μM) | IC50 (μM) | Therapeutic index |
|---|---|---|---|
| Maribavir | 93 ± 1 | 0.63 ± 0.02 | 148 |
| K252a | 0.18 ± 0.03 | 0.02 ± 0.001 | 9 |
| K252c | 3.14 ± 0.07 | 1.11 ± 0.03 | 3 |
| Gö6976 | 0.41 ± 0.021 | 0.11 ± 0.02 | 4 |
| NGIC-I | 0.61 ± 0.02 | <0.004 | >153 |
Values are expressed as means ± standard deviations of three replicate assays. The assay value of <0.004 μM is assigned a value of 0.004 μM for the purpose of the calculation, and the mean value is designated by the symbol “<.” CC50, concentration of the compound that resulted in 50% inhibition of cellular activity in MTT assays; IC50, concentration of the compound that resulted in 50% reduction of viral DNA level; therapeutic index, CC50/IC50.
For the antiviral activity tests, cytolytic EBV replication was induced in latently infected Akata cells as described previously (10); simultaneously, the cells were treated with the compounds as described above, and total DNA was isolated 48 h after viral reactivation. Equal amounts of the total lysates were dot blotted onto nitrocellulose membranes and hybridized with 32P-labeled BGLF4 or BaRF1 genes used as probes. The hybridization efficiency was quantitated on a phosphorimager. Detected viral DNA represents largely newly synthesized DNA, since basal EBV DNA in the latently infected Akata cells is about 20 viral genomes (episomes) per cell (8). The results showed the following: (i) K252a, K252c, and Gö6976 were effective only at cytotoxic concentrations (therapeutic indices are 9, 3, and 4, respectively); (ii) maribavir and NGIC-I, on the other hand, effectively inhibited EBV DNA synthesis at levels significantly below cytotoxic concentrations (therapeutic indices are 148 and >153, respectively) (Table 1).
Several recent studies propose HCMV UL97 as an attractive target for antiviral therapy based on its importance in the viral life cycle (32) and the specific inhibition of UL97 kinase activity by several compounds with potential antiviral effects (1, 17, 18, 35). In the present study we examined the ability of selected compounds to inhibit the kinase activity of the UL97 homolog, EBV PK, encoded by the BGLF4 gene, in vitro and the ability of these compounds to inhibit EBV DNA synthesis in vivo. This study demonstrated that three of four indolocarbazoles that we tested (K252a, K252c, and Gö6976) were toxic for the Akata cells, and their “antiviral” effect in our system is merely the result of cytotoxicity (Table 1). However, the therapeutic index for NGIC-I, which also showed relatively high cytotoxicity, is >153, which makes this compound at least as efficient as maribavir in EBV inhibition (Table 1), and thus, it may warrant additional studies for its potential usefulness. In addition, the results show that the inhibitor of HCMV and EBV replication, maribavir, with a known inhibitory effect on HCMV UL97 kinase activity (1), has virtually no such effect on the activity of EBV PK (10) (Fig. 3); on the other hand, K252a, which inhibited both kinases (35) (Fig. 3) and HCMV replication (35), has an insignificant effect on EBV replication at noncytotoxic levels (Table 1). Therefore, our data imply that inhibition by maribavir of HCMV and EBV replication operates through different pathways. The recent discovery of maribavir-resistant HCMV bearing a mutation in the UL27 gene, which has no homolog in EBV, and no mutations in the UL97 gene (15) supports this statement. Thus, this and our previous studies (10) as well as reports from other groups clearly show that despite the homology between HCMV UL97 and EBV PK, these proteins might play distinct roles in the viral lytic cycle, and these roles are yet to be identified.
Acknowledgments
We thank Y. Kawaguchi (Tokyo Medical and Dental University) for providing EBV BGLF4 and the BGLF4 K102I mutant expressing recombinant baculoviruses.
This work was supported in part by grants AI042371 and HL64851 from the National Institutes of Health.
REFERENCES
- 1.Baek, M. C., P. M. Krosky, Z. He, and D. M. Coen. 2002. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase. importance of the P+5 position. J. Biol. Chem. 277:29593-29599. [DOI] [PubMed] [Google Scholar]
- 2.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, I. A. Smith, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruni, R., B. Fineschi, W. O. Ogle, and B. Roizman. 1999. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J. Virol. 73:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chee, M. S., G. L. Lawrence, and B. G. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. [DOI] [PubMed] [Google Scholar]
- 5.Chen, M. R., S. J. Chang, H. Huang, and J. Y. Chen. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 74:3093-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chulay, J., K. Biron, L. Wang, M. Underwood, S. Chamberlain, L. Frick, S. Good, M. Davis, R. Harvey, L. Townsend, J. Drach, and G. Koszalka. 1999. Development of novel benzimidazole riboside compounds for treatment of cytomegalovirus disease. Adv. Exp. Med. Biol. 458:129-134. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham, C., A. J. Davison, A. Dolan, M. C. Frame, D. J. McGeoch, D. M. Meredith, H. W. Moss, and A. C. Orr. 1992. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J. Gen. Virol. 73:303-311. [DOI] [PubMed] [Google Scholar]
- 8.Daibata, M., and T. Sairenji. 1993. Epstein-Barr virus (EBV) replication and expressions of EA-D (BMRF1 gene product), virus-specific deoxyribonuclease, and DNA polymerase in EBV-activated Akata cells. Virology 196:900-904. [DOI] [PubMed] [Google Scholar]
- 9.Frame, M. C., F. C. Purves, D. J. McGeoch, H. S. Marsden, and D. P. Leader. 1987. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J. Gen. Virol. 68:2699-2704. [DOI] [PubMed] [Google Scholar]
- 10.Gershburg, E., and J. S. Pagano. 2002. Phosphorylation of the Epstein-Barr virus (EBV) DNA polymerase processivity factor EA-D by the EBV-encoded protein kinase and effects of the l-riboside benzimidazole 1263W94. J. Virol. 76:998-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He, Z., Y. S. He, Y. Kim, L. Chu, C. Ohmstede, K. K. Biron, and D. M. Coen. 1997. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 71:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato, K., Y. Kawaguchi, M. Tanaka, M. Igarashi, A. Yokoyama, G. Matsuda, M. Kanamori, K. Nakajima, Y. Nishimura, M. Shimojima, H. T. Phung, E. Takahashi, and K. Hirai. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1δ (EF-1δ): EF-1δ is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457-1463. [DOI] [PubMed] [Google Scholar]
- 13.Kato, K., A. Yokoyama, Y. Tohya, H. Akashi, Y. Nishiyama, and Y. Kawaguchi. 2003. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J. Gen. Virol. 84:3381-3392. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1998. Eukaryotic elongation factor 1δ is hyperphosphorylated by the protein kinase encoded by the UL13 gene of herpes simplex virus 1. J. Virol. 72:1731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komazin, G., R. G. Ptak, B. T. Emmer, L. B. Townsend, and J. C. Drach. 2003. Resistance of human cytomegalovirus to the benzimidazole l-ribonucleoside maribavir maps to UL27. J. Virol. 77:11499-11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van den Bogaard, and T. Stamminger. 2002. Direct targeting of human cytomegalovirus protein kinase pUL97 by kinase inhibitors is a novel principle for antiviral therapy. J. Gen. Virol. 83:1013-1023. [DOI] [PubMed] [Google Scholar]
- 18.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van Den Bogaard, and T. Stamminger. 2001. Inhibitors of human cytomegalovirus replication drastically reduce the activity of the viral protein kinase pUL97. J. Gen. Virol. 82:1439-1450. [DOI] [PubMed] [Google Scholar]
- 19.McGeoch, D. J., and A. J. Davison. 1986. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 14:1765-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng, T. I., W. O. Ogle, and B. Roizman. 1998. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology 241:37-48. [DOI] [PubMed] [Google Scholar]
- 21.Ogle, W. O., T. I. Ng, K. L. Carter, and B. Roizman. 1997. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406-413. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly, D. R., L. K. Miller, and V. A. Luckow. 1992. Baculovirus expression vectors: a laboratory manual. W. H. Freeman and Company, New York, N.Y.
- 23.Overton, H. A., D. J. McMillan, L. S. Klavinskis, L. Hope, A. J. Ritchie, and P. Wong-kai-in. 1992. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology 190:184-192. [DOI] [PubMed] [Google Scholar]
- 24.Prichard, M. N., N. Gao, S. Jairath, G. Mulamba, P. Krosky, D. M. Coen, B. O. Parker, and G. S. Pari. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purves, F. C., and B. Roizman. 1992. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc. Natl. Acad. Sci. USA 89:7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purves, F. C., D. Spector, and B. Roizman. 1991. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 65:5757-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roizman, B. A. P. E. P. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Williams & Wilkins, Philadelphia, Pa.
- 29.Slater, M. J., S. Cockerill, R. Baxter, R. W. Bonser, K. Gohil, C. Gowrie, J. E. Robinson, E. Littler, N. Parry, R. Randall, and W. Snowden. 1999. Indolocarbazoles: potent, selective inhibitors of human cytomegalovirus replication. Bioorg. Med. Chem. 7:1067-1074. [DOI] [PubMed] [Google Scholar]
- 30.Smith, R. F., and T. F. Smith. 1989. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J. Virol. 63:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams, S. L., C. B. Hartline, N. L. Kushner, E. A. Harden, D. J. Bidanset, J. C. Drach, L. B. Townsend, M. R. Underwood, K. K. Biron, and E. R. Kern. 2003. In vitro activities of benzimidazole d- and l-ribonucleosides against herpesviruses. Antimicrob. Agents Chemother. 47:2186-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. USA 98:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf, D. G., A. Honigman, J. Lazarovits, E. Tavor, and A. Panet. 1998. Characterization of the human cytomegalovirus UL97 gene product as a virion-associated protein kinase. Arch. Virol. 143:1223-1232. [DOI] [PubMed] [Google Scholar]
- 34.Zacny, V. L., E. Gershburg, M. G. Davis, K. K. Biron, and J. S. Pagano. 1999. Inhibition of Epstein-Barr virus replication by a benzimidazole l-riboside: novel antiviral mechanism of 5,6-dichloro-2-(isopropylamino)-1-β-l-ribofuranosyl-1H-benzimidazole. J. Virol. 73:7271-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann, A., H. Wilts, M. Lenhardt, M. Hahn, and T. Mertens. 2000. Indolocarbazoles exhibit strong antiviral activity against human cytomegalovirus and are potent inhibitors of the pUL97 protein kinase. Antivir. Res. 48:49-60. [DOI] [PubMed] [Google Scholar]