Abstract
A new series of ether lipid esters of cidofovir (CDV) were evaluated against vaccinia and cowpox viruses. Activity was dependent on number of atoms in the alkyl or alkoxyalkyl chain, the linker moiety, and the presence of a double bond in the alkoxyalkyl chains linked to the phosphonate moiety of CDV.
The threat of an intentional or unintentional spread of poxvirus infections to a vulnerable population has led to increased efforts to find safe, rapidly deployable treatments against such infections. Although vaccination is now being offered to some healthcare workers and other “first responders,” there are valid concerns about potential vaccine risks (3, 9). Vaccination is not recommended for those with eczema and other exfoliative skin disorders or those with immunodeficiencies or for pregnant women. Therefore, the use of antiviral therapy in the event of a poxvirus outbreak or in the treatment of vaccination complications against smallpox virus (4) points to the continued need to examine available antiviral therapies as well as to develop new and more efficient treatment.
Cidofovir (CDV) and cyclic CDV (cCDV) have been shown to be potent inhibitors of poxvirus replication in vitro (1, 7, 8, 13) and in animal model studies (5, 10, 12); however, these compounds are inactive when given orally.
Previous in vitro studies have shown that multiple-log increases in antiviral activity against orthopoxvirus replication (8), as well as enhanced inhibition of cytomegalovirus and herpesvirus replication by these esters (2), were observed with hexadecyloxypropyl (HDP) and octadecyloxyethyl (ODE) derivatives of CDV and cCDV (HDP-CDV, HDP-cCDV, ODE-CDV, and ODE-cCDV) compared to the results seen with the parent compounds. HDP-CDV, ODE-CDV, and oleyloxypropyl-CDV (OLP-CDV) have oral bioavailabilities of 88 to 93% in mice (6) and have oral activity against vaccinia virus (VV) and cowpox virus (CV) infections in mice (11).
In this study, the unmodified acyclic nucleoside phosphonates CDV and cCDV (along with a new series of analogs synthesized by esterification of these compounds with an alkyl chain with or without the propoxy- or ethoxy-linker moieties) were evaluated (using methodologies described previously) (7) for activity (plaque reduction assay) against VV and CV and for cytotoxicity (neutral red uptake assay) in human foreskin fibroblast (HFF) cells. To determine efficacy, briefly, HFF cells seeded in 6-well plates 2 days prior to use were infected with either VV or CV by the addition of 20 to 30 PFU per well. After a 1-h incubation period, various concentrations of drug were added to triplicate wells and plates were incubated at 37°C for 3 days. Toxicity was evaluated using HFF cells seeded in 96-well plates incubated with various concentrations of drug for 7 days at 37°C. After incubation, cell monolayers were stained with a 0.01% solution of neutral red. The compounds were synthesized as reported previously (8).
As presented in Table 1, the most active ether lipid esters of CDV were OLE-CDV, ODBG-CDV, TDP-CDV, OLP-CDV, and ODP-CDV, with 50% effective concentrations (EC50s) of 0.06 to 1.2 μM for VV and 0.07 to 1.9 μM for CV (a 20- to 600-fold increase compared to the results seen with the parent compound). Most of the cCDV analogs followed a comparable but lower pattern of activity compared to their CDV counterparts, with 3- to 150-fold increases over the parent compound cCDV. The selectivity index (SI) values, which measure a compound's activity by evaluating the 50% cytotoxic concentration (CC50) divided by efficacy (EC50), ranged from 85 to 933 for OLE-CDV, OLE-cCDV and OLP-CDV, and OLP-cCDV with both viruses. Comparatively, SI values for HDP-CDV, HDP-cCDV and ODE-CDV, and ODE-cCDV were in the 40 to 140 range.
TABLE 1.
Efficacy and cytotoxicity of ether lipid esters of CDV and cCDV
| Compound | Abbreviation | CC50 (μM)a | VV (Copenhagen strain)
|
CV (Brighton strain)
|
||
|---|---|---|---|---|---|---|
| EC50 (μM)a | SIb | EC50 (μM)a | SIb | |||
| Cidofovir series | ||||||
| Cidofovir | CDV | >317 ± 0 | 31 ± 5.4 | >10 | 42 ± 5.4 | >7.5 |
| Propanediol linkers | ||||||
| Octyloxypropyl (12)c | OP-CDV | 80 ± 32 | >20 ± 0 | >20 ± 0 | ||
| Dodecyloxypropyl (16) | DDP-CDV | >190 ± 0 | 6.6 ± 0.9 | >29 | 16 ± 1.6 | >12 |
| Tetradecyloxypropyl (18) | TDP-CDV | >100 ± 0 | 0.5 ± 0.2 | >200 | 0.8 ± 0.5 | >125 |
| Hexadecyloxypropyl (20) | HDP-CDV | 29 ± 2.3 | 0.6 ± 0.4 | 48 | 0.5 ± 0.3 | 58 |
| Octadecyloxypropyl (22) | ODP-CDV | 44 ± 14 | 1.2 ± 0.5 | 37 | 1.9 ± 0.6 | 23 |
| Oleyloxypropyl (22:1) | OLP-CDV | 87 ± 15 | 0.4 ± 0.2 | 218 | 0.6 ± 0.3 | 145 |
| Eicosyloxypropyl (24) | ECP-CDV | 92 ± 1.4 | 2.0 ± 0.9 | 46 | 2.4 ± 0.7 | 38 |
| Ethanediol linkers | ||||||
| Octadecyloxyethyl (21) | ODE-CDV | 21 ± 8.8 | 0.2 ± 0.1 | 105 | 0.2 ± 0.2 | 105 |
| Oleyloxyethyl (21:1) | OLE-CDV | 56 ± 29 | 0.06 ± 0.02 | 933 | 0.07 ± 0.02 | 800 |
| Glycerol linker | ||||||
| 1-O-Octadecyl-2-O-benzyl-glyceryl | ODBG-CDV | 47 ± 24 | 0.4 ± 0.1 | 118 | 0.3 ± 0.01 | 157 |
| No linker | ||||||
| Octyl (8) | O-CDV | >100 | >20 ± 0 | >20 ± 0 | ||
| Dodecyl (12) | DD-CDV | >100 | >20 ± 0 | >20 ± 0 | ||
| Hexadecyl (16) | HD-CDV | >157 ± 58 | 3.1 ± 0.1 | >51 | 6.0 ± 2.0 | >26 |
| Eicosyl (20) | EC-CDV | 45 ± 8.5 | 1.6 ± 1.3 | 28 | 1.5 ± 0.9 | 30 |
| Docosyl (22) | DC-CDV | 73 ± 8.8 | 10 ± 1.7 | 7.3 | 13 ± 0.1 | 5.6 |
| Tetracosyl (24) | TC-CDV | >100 | >17 ± 4.2 | >20 ± 0 | ||
| Cyclic cidofovir series | ||||||
| Cyclic cidofovir | cCDV | >331 ± 0 | 37 ± 10 | >9.0 | 45 ± 9.4 | >7.4 |
| Propanediol linkers | ||||||
| Octyloxypropyl (12) | OP-cCDV | >84 ± 16 | >20 ± 0 | >20 ± 0 | ||
| Dodecyloxypropyl (16) | DDP-cCDV | 63 ± 2.7 | 4.9 ± 2.7 | 13 | 6.1 ± 2.7 | 10 |
| Hexadecyloxypropyl (20) | HDP-cCDV | 17 ± 0.1 | 1.3 ± 1.0 | 13 | 1.1 ± 0.5 | 16 |
| Octadecyloxypropyl (22) | ODP-cCDV | 77 ± 3.7 | 12 ± 1.1 | 6.4 | 14 ± 4.2 | 5.5 |
| Oleyloxypropyl (22:1) | OLP-cCDV | 46 ± 0.6 | 0.5 ± 0.1 | 92 | 0.4 ± 0.1 | 115 |
| Ethanediol linkers | ||||||
| Octadecyloxyethyl (21) | ODE-cCDV | 44 ± 8.0 | 0.3 ± 0.1 | 147 | 0.4 ± 0.1 | 110 |
| Oleyloxyethyl (21:1) | OLE-cCDV | 34 ± 11 | 0.4 ± 0.02 | 85 | 0.3 ± 0.06 | 113 |
| Glycerol linker | ||||||
| 1-O-Octadecyl-2-O-benzyl-glyceryl | ODBG-cCDV | 77 ± 32 | 8.6 ± 1.5 | 9.0 | 5.8 ± 3.7 | 13 |
| No linker | ||||||
| Hexadecyl (16) | HD-cCDV | 83 ± 20 | >20 ± 0 | >20 ± 0 | ||
| Eicosyl (20) | EC-cCDV | >93 ± 9.8 | 27 ± 12 | >3.4 | 36 ± 29 | >2.6 |
| Docosyl (22) | DC-cCDV | >100 | >100 | >100 | ||
| Tetracosyl (24) | TC-cCDV | >100 | >62 ± 27 | >100 ± 0 | ||
Values are the means of two or more assays (± standard deviations).
SI = CC50/EC50.
Values in parentheses are the numbers of atoms beyond the phosphonate oxygen; the number after the colon is the number of double bonds in the alkyl chain.
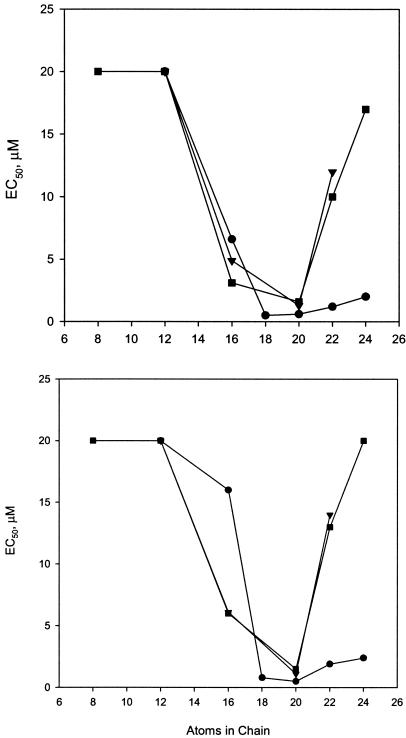
The majority of the new analogs tested were more active than the parent compounds, but four CDV and four cCDV analogs were inactive at concentrations of up to 20 μM or higher. Structure-activity analysis revealed that the less-active compounds were alkoxyalkyl or alkyl esters of CDV having chains shorter than 16 atoms beyond the phosphonate moiety of CDV. Of note was an interesting new analog, tetradecyloxypropyl-CDV (TDP-CDV), which showed activity levels equal to those of HDP-CDV but which had a much higher SI. Figure 1 shows the comparative activity levels of the CDV derivatives with no linker and with the oxypropyl linker. CDV esters of alkanols with no linker have optimal chain lengths of 20 atoms beyond the phosphonate, with activity declining sharply at 22 and 24 atoms. Cyclic CDV analogs show a sharply defined chain length optimum for antiviral activity at 20 atoms, with activity declining sharply as the chain is lengthened to 22 atoms. The presence of a 9,10 cis double bond in the eighteen-carbon alkyl chain (oleyl) increased the activity about 3-fold relative to that of the saturated alkyl chain (octadecyl) in the CDV series and 24-fold in the cCDV analogs. The oxyethyl analogs of CDV (ODE- and OLE-CDV) were generally more active than their oxypropyl counterparts (ODP- and OLP-CDV) even though they differ in the overall numbers of atoms by only one methylene. OLE-CDV was the most active and selective derivative of CDV in these studies, with an EC50 of 0.06 μM and a SI of 933 for VV and an EC50 of 0.07 μM and a SI of 800 for CV (Table 1). ODE and OLE esters were also the most active and selective compounds in the cCDV series, with EC50 values of 0.3 to 0.4 μM, respectively.
FIG. 1.
Effect of alkyl or alkoxyalkyl chain length on the antiviral activity of CDV and cCDV analogs against VV and CV in vitro. Top panel, VV; bottom panel, CV. Symbols: circles, CDV-oxypropyl-R; squares, CDV-no linker-R, where R is an alkyl chain; triangles, cCDV-oxypropyl-R.
Several of these new analogs have enhanced activity and selectivity against orthopoxvirus replication in vitro and warrant further investigation of their efficacy in animal models of orthopoxvirus disease. Quenelle et al. have reported that HDP-CDV and ODE-CDV were very effective in reducing mortality and viral replication in target organs of mice infected with CV and VV (11). These two compounds have been selected for preclinical pharmacokinetic, distribution, and toxicological studies. In addition, OLE-CDV (which was the most active and selective analog in vitro) is also being evaluated in animal studies.
Acknowledgments
These studies were supported by contract NO1-AI-85347 from the Antiviral Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, to the University of Alabama at Birmingham (E.R.K.) and by National Institutes of Health grants EY11832 and AI21694 to the San Diego Veterans Medical Research Foundation and Department of Defense grant DAMD17-01-2-0071 from the U.S. Army Medical Research and Material Command (K.Y.H.).
REFERENCES
- 1.Baker, R. O., Bray, M., and J. W. Huggins. 2003. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antivir. Res. 57:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booss, J., and L. E. Davis. 2003. Smallpox and smallpox vaccination. Neurological implications. Neurology 60:1241-1245. [DOI] [PubMed] [Google Scholar]
- 4.Bray, M. 2003. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antivir. Res. 58:101-114. [DOI] [PubMed] [Google Scholar]
- 5.Bray, M., M. Martinez, D. F. Smee, D. Kefauver, E. Thompson, and J. W. Huggins. 2000. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 181:10-19. [DOI] [PubMed] [Google Scholar]
- 6.Ciesla, S. L., J. Trahan, K. L. Winegarden, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 59:163-171. [DOI] [PubMed] [Google Scholar]
- 7.Keith, K. A., M. J. M. Hitchcock, W. A. Lee, A. Holý, and E. R. Kern. 2003. Evaluation of nucleoside phosphonates and their analogs and prodrugs for inhibition of orthopoxvirus replication. Antimicrob. Agents Chemother. 47:2193-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lane, J. M., and J. Goldstein. 2003. Evaluation of 21st-century risks of smallpox vaccination and policy options. Ann. Intern. Med. 138:488-493. [DOI] [PubMed] [Google Scholar]
- 10.Quenelle, D. C., D. J. Collins, and E. R. Kern. 2003. Efficacy of multiple- and single-dose cidofovir against vaccinia and cowpox virus infections of mice. Antimicrob. Agents Chemother. 47:3275-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quenelle, D. C., D. J. Collins, W. B. Wan, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2004. Oral treatment of cowpox or vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smee, D. F., and R. W. Sidwell. 2003. A review of compounds exhibiting anti-orthopoxvirus activity in animal models. Antivir. Res. 57:41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snoeck, R., A. Holý, C. Dewolf-Peeters, J. Van Den Oord, E. De Clercq, and G. Andrei. 2002. Antivaccinia activities of acyclic nucleoside phosphonate derivatives in epithelial cells and organotypic cultures. Antimicrob. Agents Chemother. 46:3356-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]