Abstract
The reverse transcriptase inhibitor 9-[2-(phosphonomethoxy)propyl]adenine (PMPA; tenofovir) was previously found to offer strong prophylactic and therapeutic benefits in an infant macaque model of pediatric human immunodeficiency virus (HIV) infection. We now summarize the toxicity and safety of PMPA in these studies. When a range of PMPA doses (4 to 30 mg/kg of body weight administered subcutaneously once daily) was administered to 39 infant macaques for a short period of time (range, 1 day to 12 weeks), no adverse effects on their health or growth were observed; this included a subset of 12 animals which were monitored for more than 2 years. In contrast, daily administration of a high dose of PMPA (30 mg/kg subcutaneously) for prolonged periods of time (>8 to 21 months) to 13 animals resulted in a Fanconi-like syndrome (proximal renal tubular disorder) with glucosuria, aminoaciduria, hypophosphatemia, growth restriction, bone pathology (osteomalacia), and reduced clearance of PMPA. The adverse effects were reversible or were alleviated following either complete withdrawal of PMPA treatment or reduction of the daily regimen from 30 mg/kg to 2.5 to 10 mg/kg subcutaneously. Finally, to evaluate the safety of a prolonged low-dose treatment regimen, two newborn macaques were started on a 10-mg/kg/day subcutaneous regimen; these animals are healthy and have normal bone density and growth after 5 years of daily treatment. In conclusion, our findings suggest that chronic daily administration of a high dose of PMPA results in adverse effects on kidney and bone, while short-term administration of relatively high doses and prolonged low-dose administration are safe.
In recent years, the discovery of novel potent antiretroviral drugs has led to significant advances in the clinical management of human immunodeficiency virus (HIV)-infected patients (9). Although combination therapy is able to suppress virus replication in many patients, a number of problems, including incomplete suppression of virus replication, the emergence of drug-resistant viral mutants, the difficulty of patients' adherence to complicated dosage regimens, and the occurrence of intolerable side effects, preclude prolonged therapy. Relative to adult HIV infection, these problems associated with drug therapy are even more accentuated in pediatric HIV infection (60). Accordingly, there is an urgent need for better and safer antiviral drugs with less complex dosage regimens for use by children and adults.
Simian immunodeficiency virus (SIV) infection of newborn and infant macaques is a practical animal model of pediatric HIV infection that allows researchers to test the efficacies of novel antiviral drugs. Studies with this animal model demonstrated that a once-daily dosage regimen of the acyclic nucleoside phosphonate 9-[2-(phosphonomethoxy)propyl]adenine (PMPA; tenofovir) was highly efficacious for prophylaxis and therapy, most remarkably, even in the presence of viral mutants with reduced susceptibility to PMPA in vitro (49-53, 55, 56).
The focus of the present review is to summarize and update the toxicity and safety data from these PMPA studies with infant and juvenile macaques. Our findings suggest that short-term administration of PMPA, even at relatively high doses (30 mg/kg of body weight per day administered subcutaneously), does not lead to any long-term adverse events. In contrast, prolonged daily treatment with a high dose of PMPA (30 mg/kg subcutaneously) resulted in a Fanconi-like syndrome (proximal renal tubular disorder), characterized by glucosuria, aminoaciduria, hypophosphatemia, and a bone mineralization defect; these effects were completely or largely reversible following drug withdrawal or dosage reduction, respectively. Finally, an important finding was that chronic administration of a low dose of PMPA starting at birth was not associated with any adverse health effects after 5 years of daily treatment.
MATERIALS AND METHODS
Animals, virus inoculation, and PMPA administration.
All animals were rhesus macaques (Macaca mulatta) from the type D retrovirus- and SIV-free colony at the California National Primate Research Center. Newborn macaques were hand reared in a primate nursery in accordance with the guidelines of the American Association for Accreditation of Laboratory Animal Care Standards. We strictly adhered to the Guide for the Care and Use of Laboratory Animals, prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (36). When necessary, animals were immobilized with ketamine HCl (Parke-Davis, Morris Plains, N.J.) at 10 mg/kg injected intramuscularly. Blood samples were regularly collected to monitor viral and immunological parameters, as described previously (54, 57). Complete blood counts and flow cytometry techniques (including analyses for CD3, CD4, CD8, and CD20) were performed with EDTA-anticoagulated blood samples from all animals. Samples were analyzed by using an automated electronic cell counter (Baker 9000; Serono Baker Diagnostics); differential cell counts were determined manually.
In this report animals described as being in the “short-term PMPA treatment” group included all animals that received PMPA treatment for ≤3 months in seven experiments performed from 1997 to 2000. The animals in the “chronic PMPA treatment” group included all animals which were given PMPA treatment for ≥4 months in five experiments started from 1995 to 1998. Most of these animals were inoculated with SIV at birth. More detailed descriptions of the virus stocks and the virological and immunological data for most of these studies have been published previously (49-53, 55, 56). The virus levels in these animals were determined by quantitative virus isolation and branched DNA assay, as described previously (55).
PMPA was supplied by Gilead Sciences, Inc., Foster City, Calif., and was typically of >97% purity. PMPA was suspended in distilled water, dissolved by addition of NaOH to a final pH of 7.0 at a final concentration of 40 or 60 mg/ml, and filter sterilized (pore size, 0.2 μm; Nalgene, Rochester, N.Y.). On the basis of the available data, the pH range for maximum stability is from approximately 6 to 10, in which the shelf life (t90, time to 10% degradation) at 30°C is at least 2 years. PMPA was administered subcutaneously into the back of the animal once per day at dosages ranging from 2.5 to 30 mg/kg of body weight. The PMPA dosages were adjusted weekly according to body weight. The untreated animals did not receive daily sham inoculations.
Some animals were administered a phosphate supplement twice daily; depending on the animal's weight, each dose consisted of one or two packages of Neutraphos (250 mg of phosphor, 164 mg of sodium, and 278 mg of potassium per package; Baker Norton Pharmaceuticals, Miami, Fla.) dissolved in 100 ml of a solution of Tang (Kraft Foods). Some animals were administered once daily a pediatric vitamin tablet with 200 mg of calcium (CentrumKids Extra Calcium; Lederle, Madison, N.J.) and/or a quarter of an Amino Fuel tablet (Twin Laboratories Inc., Ronkonkoma, N.Y.).
Serum chemistry panels.
Standard chemistry panels (including tests for sodium, potassium, chloride, calcium, total CO2, anion gap, calcium, phosphorus, creatinine, blood urea nitrogen [BUN], glucose, alanine aminotransferase, alkaline phosphatase (ALP), total protein, albumin, gamma-glutamyltransferase (GGT), creatine phosphokinase, aspartate aminotransferase, total bilirubin, lactate dehydrogenase, cholesterol, and triglycerides) were done with the Dacos system (Coulter Electronics, Hialeah, Fla.) or the Hitachi 717 system (Boehringer Mannheim, Indianapolis, Ind.). For the measurement of lactate and pyruvate in whole-blood supernatants, blood was collected in tubes with gray stoppers (sodium fluoride, potassium oxalate) and treated with perchloric acid, according to the instructions of the manufacturer (LabCorp, Research Triangle Park, N.C.). Alkaline phosphatase isoenzyme determination was performed by LabCorp by isoelectrofocusing techniques.
Urine analysis.
Urine was mostly collected by cystocentesis. Animals were placed in a metabolism chamber for 24-h urine collections. Urine was analyzed for specific gravity with a refractometer; the pH and the presence of protein, glucose, ketone, bilirubin, and occult blood were determined by using Multistix strips (Bayer Corporation, Elkhart, Ind.). For protein detection, these urine strips mainly detect albumin and have a sensitivity of 15 to 30 mg of albumin per dl. Urine sediments were examined microscopically. For some urine samples, creatinine and protein levels were determined with the Hitachi 717 system and the Microprotein-PR reagent (Sigma, St. Louis, Mo.), respectively; the Micro-Protein-PR assay has a sensitivity of ∼1 mg/dl. Fractional excretion values for nutrients relative to the fractional excretion of creatinine were calculated by the formula {([x]u/[x]s) × ([Cr]s/[Cr]u)} × 100, where x is the substance in question, Cr is creatinine, and s and u represent serum and urine, respectively.
For amino acid analysis, urine samples were diluted with an equal volume of 6% 5-sulfosalicylic acid (275 mmol/liter) to precipitate the proteins. After centrifugation the supernatant was diluted 1:1 or 1:10 or greater with lithium citrate buffer (pH 2.2) for the control and the experimental samples, respectively, and prepared with an internal standard. An equivalent of 40 μl of urine was injected onto the ion-exchange column. The quantity of each amino acid was determined colorimetrically by using ninhydrin for color development (model 6300 amino acid analyzer; Beckman Instruments, Palo Alto, Calif.).
Radiographs and DXA.
Radiographs were taken with a Thompson CGR X-ray unit (model SPG 515S) with the following settings: 100 mA, 0.10 s, and 46 to 54 kVp.
Dual-energy X-ray absorptiometry (DXA) scans were performed with a Norland Eclipse Compact DXA system. Animals were placed in standardized positions for scans. Bone mineral content (BMC), bone mineral area (BMA), and bone mineral density (BMD; where BMD is equal to BMC/BMA) were determined for each site. Measurement sites included lumbar vertebrae 2 to 4, distal radius and ulna, femoral neck, and global proximal femur.
In vivo fluorochrome labeling of bone and bone biopsy.
Calcein (Sigma) was slowly administered intravenously at 10 mg/kg of body weight at 3 weeks and 1 week prior to biopsy of the dorsocranial corner of the right iliac crest (20).
The biopsy specimens were embedded in methylmethacrylate, sectioned, and mounted unstained or stained with toluidine blue for histomorphometric analysis. Histomorphometric evaluations were performed in a blinded manner by using OsteoMeasure software (OsteoMetrics, Inc., Atlanta, Ga.) interfaced with a Nikon light and epifluorescent microscope and video subsystem.
Histomorphometric measurements consisted of (i) structural evaluation (including measurement of bone area, tissue area, and bone perimeters), (ii) label analysis (including measurement of interlabel width by a random sampling method and measurement of total label length), and (iii) surface histological analysis (including measurement of osteoid, eroded osteoclastic perimeters, osteoid width and area, and wall width). Histomorphometric methods, calculations, and nomenclature were consistent with the standardized system of the American Society for Bone Mineral Research (37).
PMPA pharmacokinetics.
Blood was collected by venipuncture at various time intervals after subcutaneous PMPA administration. The levels of PMPA in plasma or serum were determined either by a validated high-pressure liquid chromatography (HPLC) method with fluorescence derivatization with a limit of quantitation (LOQ) of 25 ng/ml (43) or by a validated HPLC method with mass spectrophotometric detection (liquid chromatography-mass spectrometry-mass spectrometry) with an LOQ of 1 ng/ml (unpublished data). The values of the pharmacokinetic parameters were derived by noncompartmental analysis with WinNonlin software (version 2.1 or 3.1; Pharsight Corporation, Mountain View, Calif.).
Necropsy collection and preparation of tissue samples.
A complete necropsy was performed for the animals that were euthanized. Tissues collected at necropsy were fixed in 10% buffered formalin, embedded in paraffin, sectioned at a thickness of 6 μm, stained with hematoxylin and eosin, and examined by light microscopy. Bone was first decalcified overnight in DECAL solution (DECAL Corp., Congers, N.Y.).
For the majority of animals, a special histologic analysis of the tibial diaphyses was performed to assess microdamage and bone remodeling. The specimens were bulk stained sequentially with 1% basic fuchsin in 70, 80, 90, and 100% ethyl alcohol under vacuum. Following staining, the specimens were embedded in methylmethacrylate and serially sectioned into 100-μm-thick sections transverse to the long axis at the middiaphysis. The sections were mounted on glass slides and viewed under a light microscope. Osteoid seam width, crack density, resorption cavity density, and porosity were calculated for each specimen. Standardized nomenclature for histomorphometric parameters was used (37).
RESULTS
Safety of short-term PMPA treatment regimens.
As summarized in Table 1, a total of 39 animals belonging to different research projects had been given various short-term PMPA treatment regimens starting either at birth or at a later age. None of these animals demonstrated clinical signs of toxicity; short-term PMPA treatment did not lead to growth restriction or bone abnormalities. The duration of the PMPA treatment regimens ranged from 1 day to 3 months, and the dose ranged from 4 to 30 mg of PMPA per kg of body weight, which was administered subcutaneously once daily (Table 1). Some of these animals were treated with a short-term course of PMPA more than once. Thirty-three of these animals were inoculated with SIV shortly after birth, but 15 animals which did not become infected were then reinoculated with SIV at a later age. The ages over which these 39 animals were monitored ranged from 7 weeks to 3 years.
TABLE 1.
Short-term PMPA treatments of newborn, infant, and juvenile macaques
| Monkey no. | Sexa | PMPA dose (mg/kg)b | Age at start of treatment with specified PMPA dose | Duration of PMPA treatment with specified regimen | Age at time of euthanasia or status | Glucose in urine |
|---|---|---|---|---|---|---|
| 30576 | M | 4 | 2 days | 2 days | Healthy at 2 yr | Negative at 15 mo |
| 30577 | F | 4 | 2 days | 2 days | Healthy at 2 yr | Negative at 15 mo |
| 30581 | M | 4 | 1 day | 2 days | Healthy at 2 yr | Negative at 15 mo |
| 30842 | M | 4 | 3 days | 2 days | Healthy at 2 yr | Negative at 14 mo |
| 30845 | M | 4 | 3 days | 2 days | Healthy at 2 yr | Negative at 14 mo |
| 30339 | M | 30 | 3 days | 2 days | Healthy at 3 yr | Negative at 24 mo |
| 30340 | M | 30 | 3 days | 2 days | Healthy at 3 yr | Negative at 18 mo and afterwards |
| 30338 | M | 30 | 3 days | 2 days | NDc | |
| 30338 | M | 10 | 16 mo | 14 days | ND | |
| 30338 | M | 30 | 18 mo | 21 days | Healthy at 3 yr | Negative at end of treatment and afterwards |
| 30343 | F | 30 | 2 days | 2 days | ND | |
| 30343 | F | 10 | 16 mo | 14 days | ND | |
| 30343 | F | 30 | 18 mo | 21 days | Healthy at 3 yr | Negative at 25 mo |
| 30478 | F | 30 | 1 day | 1 day | ND | |
| 30478 | F | 10 | 12 mo | 14 days | ND | |
| 30478 | F | 30 | 14 mo | 21 days | Healthy at 3 yr | Negative at end of treatment and afterwards |
| 29361 | F | 30 | 3 days | 14 days | ND | |
| 29361 | F | 30 | 8 mo | 14 days | 13 mo | ND |
| 29363 | F | 30 | 3 days | 14 days | ND | |
| 29363 | F | 8 mo | 14 days | 13 mo | ND | |
| 29364 | M | 30 | 3 days | 14 days | ND | |
| 29364 | M | 8 mo | 14 days | 13 mo | ND | |
| 29365 | M | 30 | 3 days | 14 days | 11 mo | ND |
| 29445 | M | 30 | 7 mo | 14 days | 12 mo | ND |
| 29596 | F | 30 | 7 mo | 14 days | 12 mo | ND |
| 29601 | F | 30 | 7 mo | 14 days | 12 mo | ND |
| 30020 | F | 30 | 8 days | 14 days | 6 mo | ND |
| 30022 | M | 30 | 8 days | 14 days | 6 mo | ND |
| 30023 | F | 30 | 8 days | 14 days | 6 mo | Negative at euthanasia |
| 30024 | F | 30 | 8 days | 14 days | 6 mo | Negative at euthanasia |
| 30007 | F | 10 | 18 mo | 14 days | ND | |
| 30007 | F | 30 | 20 mo | 21 days | Healthy at 3 yr | Negative at end of treatment and afterwards |
| 30155 | M | 30 | 1 day | 28 days | 15 mo | Negative at euthanasia |
| 30162 | M | 30 | 1 day | 28 days | ND | |
| 30162 | M | 10 | 18 mo | 14 days | ND | |
| 30162 | M | 30 | 20 mo | 21 days | Healthy at 3 yr | 1,000 mg/dl at end of treatment course, but repeatedly negative afterwards |
| 30300 | M | 30 | 1 day | 28 days | 14 mo | Negative at euthanasia |
| 30302 | F | 30 | 1 day | 28 days | 5 mo | Negative at euthanasia |
| 30306 | M | 30 | 0 day | 28 days | 14 mo | Negative at euthanasia |
| 30053 | M | 30 | 8 days | 60 days | 6 mo | Negative at euthanasia |
| 30054 | F | 30 | 8 days | 60 days | 6 mo | Negative at euthanasia |
| 30055 | M | 30 | 8 days | 60 days | 6 mo | Negative at euthanasia |
| 30061 | M | 30 | 6 days | 60 days | 6 mo | Negative at euthanasia |
| 30074 | M | 30 | 6 days | 60 days | 66 days | Negative at euthanasia |
| 30077 | F | 30 | 6 days | 60 days | 66 days | Negative at euthanasia |
| 31839 | M | 30/10d | 4 mo | 12 wk | 7 mo | Negative at end of treatment |
| 31840 | M | 30/10 | 4 mo | 12 wk | 7 mo | Negative during and at end of treatment |
| 31848 | F | 30/10 | 4 mo | 12 wk | 7 mo | Negative during and at end of treatment |
| 31852 | M | 30/10 | 4 mo | 12 wk | 7 mo | Negative during and at end of treatment |
| 31862 | M | 30/10 | 4 mo | 12 wk | 7 mo | Negative at end of treatment |
| 31863 | F | 30/10 | 4 mo | 12 wk | 7 mo | Negative during and at end of treatment |
M, male; F, female.
All PMPA doses were given subcutaneously once daily.
ND, not determined.
30/10, treatment for 4 weeks at 30 mg/kg, followed by treatment for 8 weeks at 10 mg/kg.
Of these 39 animals, 12 animals were healthy at more than 2 years of age, without PMPA-related adverse effects, as assessed by clinical examination, radiographic evaluation of the skeleton, and clinical pathology. The remaining 27 of these 39 animals have been euthanized. The indication for euthanasia was either the development of immunodeficiency (AIDS) due to SIV infection or a scheduled euthanasia as part of the experimental design to collect lymphoid tissues. Of these 27 euthanized animals, 8 animals were euthanized immediately at the end of their PMPA treatment courses. Of these eight animals, two infant macaques (animals 30074 and 30077) were started on PMPA (30 mg/kg subcutaneously) at 6 days of age and were euthanized after 60 days of daily PMPA treatment (52); there was no evidence of abnormalities in bone mineralization. Another six infant macaques, which were started on PMPA treatment at approximately 4 months of age, were euthanized at the end of a 12-week PMPA treatment course (4 weeks of treatment with 30 mg/kg and then 8 weeks of treatment with 10 mg/kg once daily subcutaneously). Necropsy of these six animals at the end of their PMPA treatment courses did not reveal any gross morphological or histopathological findings indicative of potential PMPA toxicity. Of the 27 euthanized animals, 19 animals were euthanized and necropsied between 3.5 and 14 months after PMPA treatment was stopped; there was no evidence of any late PMPA toxicity on the basis of clinical signs, gross morphology, or histopathology.
Serum chemistry analyses for these animals which received short-term PMPA treatment did not show any biologically important changes. In particular, the serum phosphorus, ALP, and BUN values remained within the age-matched reference intervals (data not shown).
For 10 animals, urinalysis data were available for urine samples collected immediately after the animals received PMPA subcutaneously at 30 mg/kg of body weight daily for 21 to 60 days: 9 of these 10 animals had normal urine parameters, including the absence of glucose. These nine animals without glucosuria while on short-term PMPA treatment included the two infant macaques (animals 30074 and 30077) at the end of a 60-day PMPA treatment course (30 mg/kg per day subcutaneously) (Table 1). The 10th animal (Table 1, animal 30162) had glucosuria (1,000 mg of glucose/dl) after 3 weeks of daily treatment with a subcutaneous 30-mg/kg dosage regimen at 20 months of age, but urine samples regularly collected from this animal after withdrawal of this high dosage regimen were always negative for glucose. No proteinuria was documented in any of these animals. Urine data were available for six infant macaques (which were approximately 4 months of age at the start of PMPA treatment) that received a 12-week PMPA treatment course (4 weeks of treatment with 30 mg/kg and then 8 weeks of treatment with 10 mg/kg once daily subcutaneously); none of these animals had glucosuria or proteinuria during or at the end of this treatment period (Table 1). For 22 animals, urine was available for time points ranging from 4 months to 3 years after their initial short-term PMPA treatment regimens had stopped; none of these 22 animals had glucosuria. Radiographs and DXA scans were collected for 14 and 12 animals, respectively, and there were no significant differences in the findings compared to those for untreated, uninfected age-matched animals.
Altogether, these data suggest that short-term subcutaneous PMPA treatment, even with the relatively high dosage regimen of 30 mg/kg per day for up to 60 days, did not lead to any detectable adverse effects on the kidney, bone, or other organ system during a drug-free period of up to 3 years.
Toxicity following prolonged high-dose (30-mg/kg) PMPA treatment. (i) Observation and characterization of chronic toxicity.
As summarized in Table 2, 15 animals received a daily subcutaneous injection of 30 mg of PMPA per kg for at least 4 months. Two of these animals (Table 2, animals 29360 and 29419, which were infected with viral mutants with reduced in vitro susceptibility to PMPA [51]) had been on PMPA treatment for only 4 months when they were euthanized due to the development of SIV-induced disease. Gross morphological and histopathological examinations of the organs of these two animals revealed lesions that are expected as a result of the SIV-induced immunosuppression and the occurrence of opportunistic infections (51). None of the lesions could be attributed to potential PMPA toxicity. Radiographs of these animals were not available for evaluation.
TABLE 2.
Regimens used for prolonged administration of a high dose (30 mg/kg) of PMPA
| Monkey no. | Sexa | SIV infection status | PMPA dose (mg/kg)b | Age at start of treatment with specified dose | Duration of treatment with specified dose | Age at time of euthanasia or status | Serum phosphorus level (mg/dl)c | Serum ALP level (U/liter)c | Urine glucose level (mg/dl)c | Bone lesions or other symptoms |
|---|---|---|---|---|---|---|---|---|---|---|
| 29360 | F | + | 30 | 3 wk | 4 mo | 5 mo | 5.2-5.4 | 1,245-1,279 | NDd | AIDS; no gross evidence of bone lesions |
| 29419 | F | + | 30 | 3 wk | 4 mo | 5 mo | 2.9-4.0 | 1,906-2,046 | ND | AIDS; no gross evidence of bone lesions |
| 29283 | F | + | 30 | 3 wk | 8 mo | 9 mo | 2.9-3.6 | 1,290-1,337 | 0 | AIDS; radiologic evidence of bone lesions |
| 27999 | M | + | 30 | 28 mo | 11 mo | 1.8 | 2,505 | 1,000-≥2,000 | Severe bone weakness | |
| 10 | 39 mo | 1 mo | 40 mo | 1.3 | 2,762 | ND | Euthanized because of severe bone lesions | |||
| 28214 | F | + | 30 | 18 mo | 11 mo | 30 mo | 1.8 | 3,939 | ND | Severe bone lesions |
| 29278 | M | + | 30 | 3 wk | 13 mo | 1.2-2.3 | 4,637-9,751 | ≥2,000 | Moderate to severe bone lesions | |
| 30 (2×/wk) | 13.5 mo | 8 mo | 21 mo | 2.1-4.0 | 2,684-4,176 | 100->2,000 | AIDS | |||
| 29279 | M | + | 30 | 3 wk | 15 mo | 1.6-3.3 | 1,200-1,358 | 250 | Moderate bone lesions | |
| 10 | 16 mo | 6 mo | 22 mo | 3.8-5.8 | 597-1,705 | 0 | Improvement of bone lesions; AIDS | |||
| 29276 | F | + | 30 | 3 wk | 15 mo | 0.9-1.3 | 1,790-3,080 | >2,000 | Moderate to severe bone lesions | |
| 10 | 16 mo | 7 mo | 1.8-4.0 | 1,392-4,884 | 500->2,000 | Improvement | ||||
| 5 | 23 mo | 42 mo | 1.7-4.8 | 575-2,388 | 250-1,000 | Improvement | ||||
| 2.5 | 65 mo | >25 mo | Alive at 7.5 yr | 2.3-5.7 | 194-469 | 0-2,000 | Stable condition | |||
| 29049 | M | − | 30 | 3 days | 16 mo | 16 mo | 1.8-7.2 | 815-3,181 | 2,000 | Severe bone lesions |
| 28006 | M | + | 30 | 22 mo | 17 mo | 39 mo | 0.5 | 2,150 | 1,000 | Moderate radiographic lesions; histological evidence of reduced bone mineralization; AIDS |
| 29055 | M | + | 30 | 3 wk | 19 mo | 20 mo | 1.1 | 815 | 500 | Terminal simian AIDS; radiographic evidence of bone lesions |
| 29003 | M | + | 30 | 3 wk | 21 mo | 1.4 | 5,343 | ≥2,000 | Severe bone lesions | |
| No treatment | 22 mo | 19 mo | 41 mo | 3.4-6.9 | 878-8,894 | Decreasing from ≥2,000 to 0 in 5 mo | Resolution of bone lesions; AIDS | |||
| 29008 | M | + | 30 | 3 wk | 25 mo | 1.1-2.0 | 802-1,831 | 1,000 | Moderate bone lesions | |
| 10 | 26 mo | 15 mo | 41 mo | 1.2-3.5 | 305-2,228 | 0-1,000 | Some improvement, but development of AIDS | |||
| 29045 | M | + | 30 | 3 wk | 24 mo | 1.5-2.0 | 1,844-2,611 | 250 | Moderate bone lesions | |
| 10 | 25 mo | 42 mo | 1.7-3.7 | 943-3,912 | 0-500 | Improvement | ||||
| 5 | 67 mo | 7 mo | 2.5-5.7 | 1,121-1,375 | 100-1,000 | Improvement | ||||
| 2.5 | 74 mo | 10 mo | Alive at 7 yr | 1.8-2.8 | 417-710 | 100-2,000 | Improvement | |||
| 29046 | M | − | 30 | 3 days | 25 mo | 1.7-6.7 | 827-1,945 | 250 | Moderate bone lesions | |
| 10 | 25 mo | 53 mo | 1.7-3.2 | 1,030-2,147 | 0-1,000 | Improvement | ||||
| 5 | 78 mo | >24 mo | Alive at >8 yr | 2.1-3 | 289-647 | 100-250 | Improvement |
F, female; M, male.
All PMPA doses were given subcutaneously once daily unless otherwise specified.
Values are single measurements or ranges.
ND, not done.
For the other 13 animals that were receiving daily subcutaneous PMPA treatment at 30 mg/kg for at least 8 months, this high dosage regimen led to adverse effects. When this toxicity was discovered in the first animals, these 13 animals, which were in different studies, had been receiving chronic PMPA treatment for various lengths of time (∼8 to 21 months), and accordingly, the degree of toxicity ranged from mild to severe. Thus, the initial investigation consisted mainly of a cross-sectional analysis of all animals at that time. Longitudinal data were subsequently collected for each animal.
The initial clinical finding for these 13 animals that received chronic high doses of PMPA was growth restriction. While infection with virulent SIV isolates is known to have a negative impact on growth, the finding of growth restriction was unexpected in the uninfected animals (animals 29046 and 29049) and the SIV-infected animals that had low virus levels due to PMPA treatment. Radiographs of all 13 animals revealed bone changes with various degrees of severity. The long bone metaphyses were incompletely ossified; there was a large lucent zone in the physis adjacent to the metaphysis. In addition, most animals had overall decreased bone opacity (Fig. 1). The physes and metaphyses of the vertebral bodies were similarly affected. In the most severely affected animals (such as animals 29049, 28214, 27999, and 29003) there were bone deformations (bending) and/or pathological fractures, mainly in the metaphyseal area of the long bones. The available data suggest that the extent of these symptoms, the time of onset, and the rate of progression were highly variable among these animals and that there were no apparent differences by sex. For some animals (e.g., animals 29276 and 29045), the bone deformations were mostly localized in the limbs, and as these lesions progressed the main clinical sign was reduced activity in their cages. For other animals (e.g., animals 27999 and 29003), the osteopenia defect became especially pronounced in the ribs, leading to increased rib flexibility and deformation; inward bending of the metaphyseal areas of the ribs, creating anterior protrusion of the sternum (“pigeon-breast” deformity); rib cage collapse; and respiratory distress. Some of the most severely affected animals had been receiving PMPA for only approximately 1 year, while other animals which had been receiving PMPA treatment for 2 years mainly showed radiological evidence of reduced bone ossification. Longitudinal analysis of the radiographs also suggested that while the bone mineralization defect usually progressed slowly, a rapid deterioration could occur within a few months' time, leading to clinical signs of bone weakness (e.g., animals 29003 and 27999).
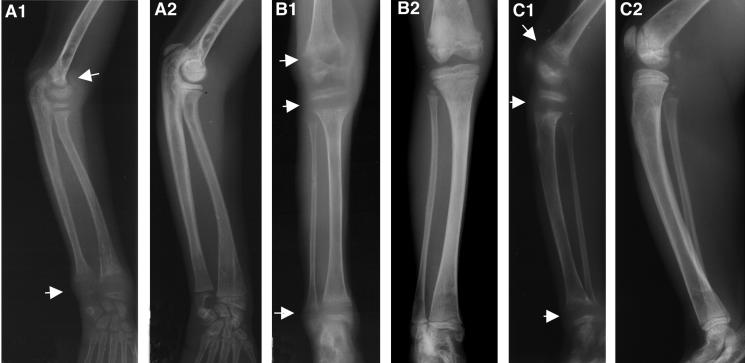
FIG. 1.
Induction and reversibility of bone changes following chronic high-dose PMPA treatment. Animal 29003 was started on chronic high-dose PMPA treatment (30 mg/kg per day subcutaneously) at 3 weeks of age for a duration of 21 months. (A) Lateral view of right arm; (B) anterior-posterior view of left leg; (C) lateral view of right leg. For each of these three sets (A to C), the number 1 radiographs were taken at the end of the 21 months of PMPA treatment. Notice the reduced bone opacity and the abnormally widened growth plates (arrows). The number 2 radiographs are from the same animal and were obtained 5 months after PMPA treatment was stopped. Notice the improved bone opacity and normalization of the growth plates.
A standard chemistry panel analysis was performed with serum samples. The only consistent abnormal finding for all 13 animals which had been on this high dose of PMPA (30 mg/kg per day subcutaneously) for at least 8 months was a decline in serum phosphorus levels to <3 mg/dl (animal 29283) or <2 mg/dl (all other 12 animals) (reference range for age-matched untreated animals, 4.3 to 7 mg/dl). Longitudinal data for the two uninfected animals (animals 29046 and 29049) demonstrated that a gradual decline in serum phosphorus levels had started after about 6 to 9 months of daily subcutaneous treatment with PMPA at 30 mg/kg. For 11 of the 12 animals, there was a rise in total ALP activity, with peak values of 1,358 to 9,751 U/liter (age-matched control values, <1,000 U/liter; Table 2). An exception was SIV-infected animal 29055, which maintained normal ALP levels. The raised serum ALP activity in association with normal GGT activity in these animals indicates that the increased contribution to the total ALP in serum was most likely of bone origin (bone-ALP isoenzyme), since there is no rise in GGT activity in association with bone pathology (31); electrophoretic analysis of serum samples from four animals confirmed, indeed, that the increase in the total ALP level was due to bone-ALP isoenzyme. There were no significant increases in serum creatinine levels. The levels of other serum markers (such as liver enzymes, triglycerides, and cholesterol) were generally within reference intervals, with no biologically consistent alterations; occasional changes were considered secondary to opportunistic infections in SIV-infected animals. None of the animals had increases in parathyroid hormone levels.
For 12 of these 13 animals which had received daily treatment with PMPA at 30 mg/kg subcutaneously for at least 8 months, urine samples were collected by cystocentesis at least once: 11 animals had glucosuria (urine glucose level range, 250 to > 2,000 mg/dl); a single urine sample from the 12th animal (animal 29283) had a glucose-negative measurement at the time of euthanasia (due to simian AIDS). Urine samples from 9 of the 10 animals tested demonstrated generalized aminoaciduria. When urine samples were tested with urine strips, the majority of samples from these animals had undetectable or trace levels of protein, similar to what is found in healthy rhesus macaques; when proteinuria (urine protein concentration, >30 mg/dl) was detected, it was considered a consequence of the simultaneous detection of blood contamination associated with the cystocentesis sampling procedure. In contrast, when the urine samples available from 10 animals were tested with the more sensitive Microprotein-PR reagent, 9 had elevated protein concentration/creatinine concentration ratios (range, 2.9 to 10; reference values for untreated animals, ≤1). The urinary pH values for the treated animals were within the same range as those for the untreated animals. Pharmacokinetic data obtained for four of these PMPA-treated animals revealed that chronic administration of PMPA (30 mg/kg per day subcutaneously) was associated with an approximately three- to sevenfold reduction in PMPA clearance and an increase in the area under the plasma concentration-versus-time curve (AUC) in comparison to that obtained after subcutaneous administration of a single dose of 30 mg/kg to 1-year-old rhesus macaques (Table 3).
TABLE 3.
Relationship between PMPA pharmacokinetics and bone disease in rhesus macaques on long-term therapy with PMPA at 30 mg/kga
| Animal no. or group size | Age (mo) | Dose of PMPA (mg/kg) | Duration (mo) of PMPA treatment or no. of dosesb | Cmax (μg/ml) | AUC (μg · h/ml) | t1/2 (h) | CL/F (ml/h/kg) | Bone disease |
|---|---|---|---|---|---|---|---|---|
| 27999c | 39 | 30 | 11 | 61 | 222 | 8.4 | 135 | Severe |
| 29008c | 25 | 30 | 24 | 59 | 101 | 7.5 | 297 | Moderate |
| 29045c | 24 | 30 | 23 | 66 | 124 | 9.6 | 241 | Moderate |
| 63 | 10 | 38 | 29 | 94 | 7.5 | 106 | Improved | |
| 83 | 2.5 | 9 | 9.1 | 31 | 7.7 | 80 | Improved | |
| 29046 | 24 | 30 | 24 | 66 | 114 | 10.5 | 262 | Moderate |
| 34 | 10 | 9 | 22 | 50 | 6.5 | 201 | Improved | |
| 66 | 10 | 41 | 24 | 58 | 6.7 | 174 | Improved | |
| 77 | 10 | 52 | 36 | 82 | 6.6 | 122 | Improved | |
| 83 | 5 | 5 | 13 | 31 | 6.8 | 161 | Improved | |
| 29276c,d | 55 | 5 | 32 | 14 | 52 | 7.4 | 97 | Improved |
| 73 | 2.5 | 8 | 11 | 26 | 9.3 | 98 | Improved | |
| n = 2 | 0 | 30 | Single | 45-59 | 153-174 | 3.2-3.5 | 172-196 | NA |
| n = 2 | 1 | 30 | Single | 26-35 | 45-72 | 4.3-4.4 | 416-672 | NA |
| n = 2 | 3 | 30 | Single | 26-43 | 62-92 | 3.5-3.7 | 325-487 | NA |
| n = 2 | 12 | 30 | Single | 17-21 | 25-35 | 3.0-3.3 | 858-1,190 | NA |
| n = 2e | 62-133 | 30 | Single | 18-30 | 56-69 | 2.8-4.0 | 435-534 | NA |
Abbreviations: Cmax, maximum concentration of drug in serum; t1/2, terminal half-life; CL/F, apparent clearance; NA, not applicable.
The duration of daily PMPA treatment at the specified dosage at the time that the 24-h pharmacokinetic study was performed. For more details on the timing of and the changes to the dosage regimens, see Table 2. Samples were collected at 0, 0.5, 1, 2, 4, 6, 8, and 24 h after subcutaneous PMPA administration. The maximum concentration in serum was achieved 0.5 h after PMPA administration.
The animal was SIV infected.
Animal 29276 first received 30 mg/kg, but the dosage was then reduced to 10 and 5 mg/kg (Table 2). No pharmacokinetic data were available for animal 29276 while it was on the 30-mg/kg dosage regimen.
Two adult females (body weight, 7.2 to 8.8 kg).
As discussed next, these animals were euthanized (n = 6), PMPA treatment was stopped (n = 1), or the PMPA dosage regimen was reduced (n = 6).
(ii) Euthanasia and histopathology following prolonged high-dose PMPA administration.
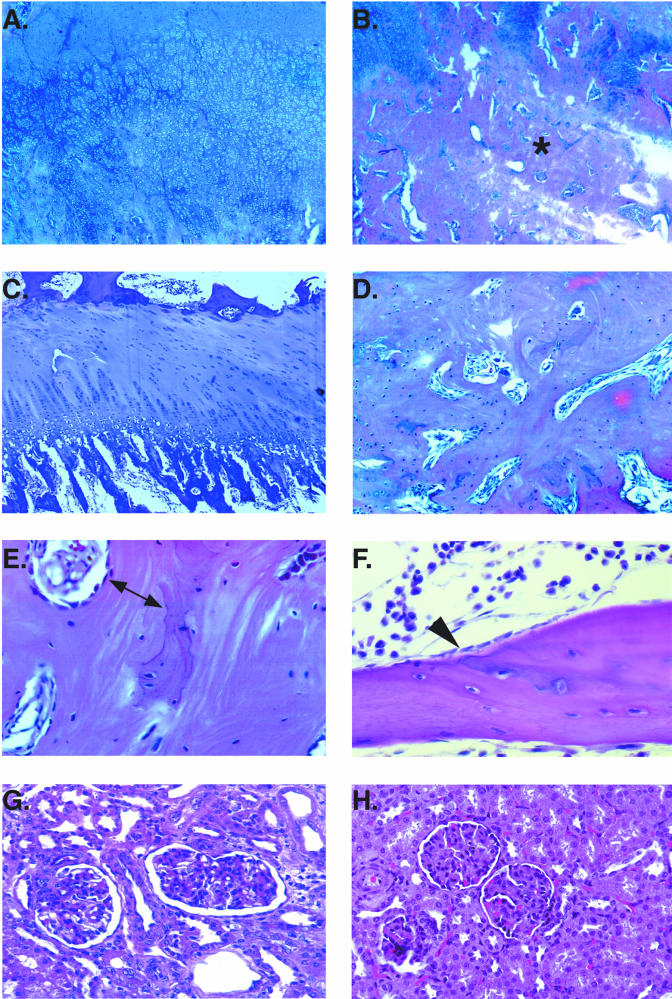
Three animals (animals 27999, 28214, and 29049) which had been receiving daily subcutaneous treatment with PMPA at 30 mg/kg for 12 to 16 months had developed severe skeletal lesions and were euthanized. Bone histology revealed irregular hyperplastic growth plates and trabecular hyperplasia with widened osteoid seams (Fig. 2). For SIV-infected animals 28214 and 27999, renal lesions consisted of tubular atrophy, interstitial nephritis, and fibrosis. However, because these two SIV-infected animals had clinical symptoms of AIDS at the time that PMPA treatment was started, it is impossible to distinguish potential PMPA-induced changes from the renal pathology associated with chronic SIV infection (1). Animal 29049, which was not SIV infected, also had renal tubular atrophy and interstitial nephritis, but in addition, the animal also demonstrated membranoproliferative glomerulonephritis (Fig. 2); it is not clear whether the glomerular changes in this animal represent a primary insult to the glomeruli or were caused by the chronic tubular and interstitial changes.
FIG.2.
Histopathology of bone and renal tissue of macaques following chronic high-dose PMPA administration. (A, B, D, and E) Distal femur of animal 28214 after 11 months of treatment with PMPA at 30 mg/kg per day subcutaneously. The distal femur growth plate shows diffuse hyperplasia and dysplasia (irregular, thickened, and undulating) (A) with widened trabeculae (B; asterisk). (C) Growth plate of an untreated animal for comparison, with the normal palisades of cartilage and normal process of endochondral bone growth. (D and E) Widened trabeculae of animal 28214 at higher magnifications; notice the wide area of poorly mineralized osteoid (E; double arrow). (F) For comparison, the normal, thin unmineralized osteoid seam adjacent to a layer of osteoblasts (arrowhead) of an untreated animal. (G) Kidney of PMPA-treated animal 29049 (after 16 months of daily subcutaneous administration of PMPA at 30 mg/kg). Notice the severe tubular atrophy and degeneration (indicated by the widened lumen). This animal also had membranoproliferative glomerulonephritis. (H) Kidney of untreated animal for comparison.
Three other SIV-infected animals which had received prolonged PMPA therapy for 8 to 20 months (animals 29283, 28006, and 29055) had radiological evidence of moderate bone changes, but the animals had to be euthanized due to the development of life-threatening AIDS (in association with the presence of viral mutants with reduced in vitro susceptibility to PMPA). Animal 28006 also had histological evidence of abnormal bone mineralization. In contrast, animal 29055, which had glucosuria and hypophosphatemia (serum phosphate concentration, <2 mg/dl) but which had ALP activities within reference values for the last 8 months of its life, did not have microscopic bone alterations.
(iii) Reversibility of high-dose PMPA toxicity following drug withdrawal.
Animal 29003 had growth restriction starting at approximately 12 months of age (Fig. 3A). After 21 months of daily administration of PMPA at 30 mg/kg subcutaneously, animal 29003 developed severe bone disease, including mineralization defects of the limbs and a rapidly progressive rib cage collapse which induced respiratory distress over the course of a few weeks (Fig. 1). The animal showed reduced activity. At this time, this animal had glucosuria (≥2,000 mg of glucose/dl of urine), hypophosphatemia (serum phosphate concentration, 1.4 mg/dl), and markedly increased ALP activity (5,343 U/ml). Virus levels in this SIV-infected animal were below the limit of detection. At this time, PMPA treatment was discontinued. Over the course of the next 6 months, the serum phosphorus concentration returned to the normal reference interval, the glucosuria resolved, and the animal gained weight rapidly (Fig. 3A). Five months after PMPA treatment was stopped, bone opacity in the metaphyseal regions was increased but still slightly less than that seen in diaphyseal and epiphyseal bones. The physes were normal in appearance (Fig. 1). By 18 months, the metaphyseal osteopenia had resolved. Although the rib cage deformation resolved completely, some of the limb deformations (such as patella luxation and valgus deviation) did not resolve completely, but there were no clinical abnormalities and the animal's physical activity was similar to that of other macaques. The serum ALP values remained increased during the recovery period (presumably caused by the accelerated bone remodeling), but then returned to the age-matched reference interval 8 months after the discontinuation of PMPA administration. These findings suggest that even in cases of renal toxicity and severe bone mineralization defects, these lesions appear to be largely or completely reversible. The discontinuation of PMPA treatment led to a gradual increase in the level of viremia in this SIV-infected animal. This animal developed immunodeficiency, as evidenced by many opportunistic infections, and had to be euthanized because of rapidly deteriorating health 19 months after the withdrawal of PMPA treatment.
FIG. 3.
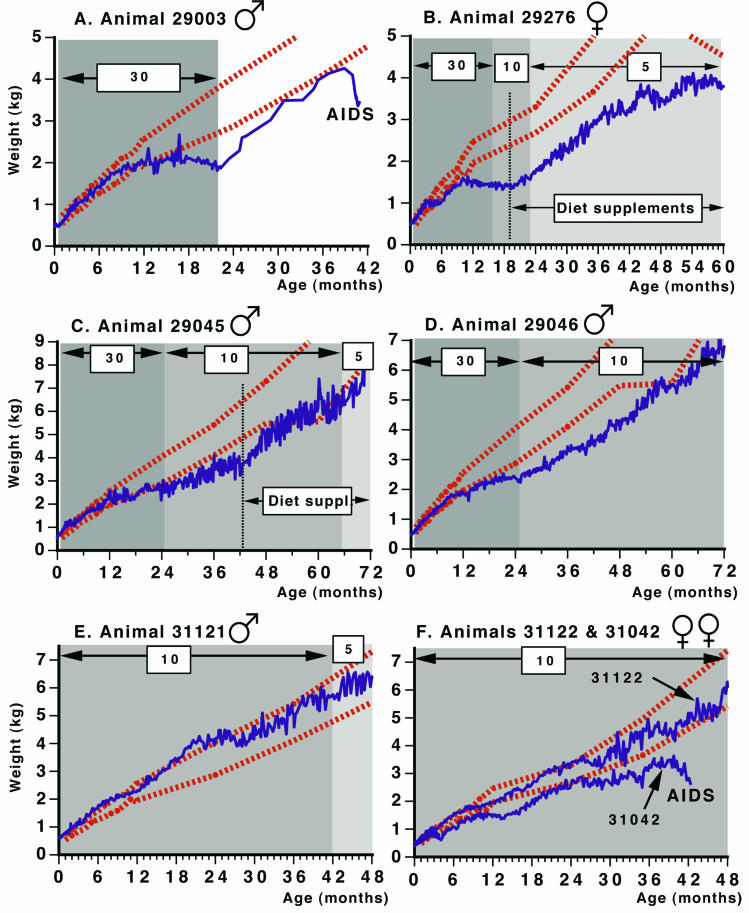
Effects of chronic PMPA treatment on weight gain of infant rhesus macaques. PMPA-treated animals (solid lines) were started on prolonged treatment within the first few weeks after birth, as indicated in Tables 2 and 4. The daily PMPA dosage regimen per kilogram of body weight (administered subcutaneously) is indicated. Two of these animals (animals 29045 and 29276) also received dietary supplements (Diet suppl) that included phosphate, multivitamins, and amino acids, as discussed in the text. Dashed lines indicate the mean ± 1 standard deviation weight of untreated, uninfected sex-matched control animals at the California National Primate Research Center.
(iv) Management of toxicity through PMPA dosage reduction.
For six other animals (Table 2, animals 29276, 29278, 29279, 29008, 29045, and 29046), the PMPA dosage regimen was reduced from daily subcutaneous treatment with 30 mg/kg to either 30 mg/kg twice weekly (animal 29278) or 10 mg/kg once daily (five animals). Although glucosuria was still detected intermittently, this PMPA dosage reduction led to an increase in serum phosphorus levels (>2 mg/dl) in all animals, an improvement in radiological bone opacity, and a resumption of growth (Fig. 3B to D). The dosage reduction did not lead to any significant changes in the levels of viral RNA in plasma. The low-dosage regimen was effective as maintenance therapy, presumably because the three- to sevenfold reduced PMPA clearance (see above; Table 3) resulted in sufficient blood PMPA levels. Three animals (animals 29008, 29278, and 29279) already had moderate to high virus levels prior to the dosage reduction due to the presence of viral mutants with reduced susceptibility to PMPA (the viral RNA levels for these three animals ranged from 0.2 × 106 to 26 × 106 copies per ml of plasma); these three animals were subsequently lost to the study 6 to 15 months after the PMPA dosage reduction due to the development of AIDS (51). Animal 29278 still had a bone mineralization defect at the time of death.
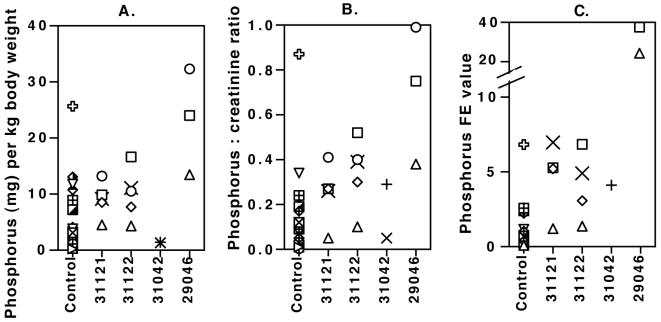
Three animals were still alive at 7 to 8 years of age, approximately 6 years after the PMPA dosage regimen was reduced from 30 to 10 mg/kg per day subcutaneously; as these animals grew older, their daily dosages were further reduced to 2.5 or 5 mg/kg subcutaneously (Table 2). Animal 29046, which was not infected with SIV, has continued to show improvement of bone density, has gained weight without receiving any additional dietary supplements (Fig. 3D), and is healthy at 8 years of age. Twenty-four-hour urine collections for this animal at 66 and 75 months of age (which were conducted while the animal was receiving the 10-mg/kg subcutaneous dosage regimen) and at 90 months (which were conducted after a dosage reduction to 5 mg/kg per day at 78 months) revealed that this animal still had increased loss of protein and phosphorus in urine (Fig. 4) and generalized aminoaciduria, while the urinary excretion of other elements (including calcium, magnesium, potassium, and sodium) was within the range observed for untreated animals or marginally increased. The other two animals (animals 29276 and 29045) were both SIV infected and continued to show a progressive decline in virus levels even after the gradual dosage reduction to 2.5 mg/kg per day subcutaneously (<125 viral RNA copies per ml of plasma at 7 years of age). The clearance of PMPA remained reduced in these animals (Table 3). Both animals had plasma lactate and pyruvate levels and lactate concentration:pyruvate concentration ratios that were in the same range as those for the untreated animals. Analysis of multiple urine samples (collected by cystocentesis or 24-h urine collections) demonstrated that these two animals continued to have elevated urinary protein concentration/creatinine concentration ratios (ratio, >1), hyperphosphaturia, and generalized aminoaciduria; glucosuria was detected at the majority of the time points. Both animals had moderate elevations in serum creatinine levels (∼1.3 to 2.5 mg/dl) but normal BUN values. Although the renal dysfunction did not resolve completely, all other clinical parameters indicate that these animals made dramatic improvements following the PMPA dosage reduction. The PMPA dosage reduction resulted in partial improvement of the bone density and growth of these two animals, but their weight gains remained below normal values (Fig. 3). Their serum phosphorus levels, albeit improved, remained low and variable (between 2 and 4 mg/dl). These two animals showed signs of muscle atrophy, and their fur was of poor quality. We suspected that these animals might have suffered from nutritional deficiencies due to the continued increased loss of nutrients in urine, which was not adequately compensated for by their standard diet. Accordingly, we started to provide both SIV-infected animals 29276 and 29045 with commercially available dietary supplements containing phosphate (Neutraphos), multivitamins (CentrumKids), and amino acids (Amino Fuel) (see Materials and Methods for a more detailed description). Administration of these supplements was associated with improved growth (Fig. 3B and C) and improved serum phosphorus levels. Despite the improvement in radiological bone opacity, some of the long bones remained slightly bent, which was probably due to the mechanical forces that were present during the period of defective mineralization of newly formed bone and during the recovery process. Animal 29276 started menstrual cycles at 3 years of age, indicating normal reproductive development. Animals 29045 and 29276 were clinically stable at ≥7 years of age. Their long survival due to PMPA treatment is remarkable, since both animals were infected with highly virulent SIV isolates at birth and came from studies in which the majority of untreated infants survived for less than 3 months (50, 51, 57b).
FIG. 4.
Urinary excretion of phosphorus. Urine was collected over 24 h to estimate the total daily urinary excretion of phosphorus per kilogram of body weight (A), the phosphorus concentration/creatinine concentration ratio (B), and the fractional excretion (FE) value for phosphorus relative to that for creatinine (C). Control data for total daily urinary excretion and the phosphorus concentration/creatinine concentration ratio were obtained from a total of 17 urine samples collected from 11 untreated age-matched animals (age range, 29 to 57 months; three animals were sampled multiple times); fractional excretion values were based on 14 observations for 8 of these untreated animals. Two animals (animals 31121 and 31122) receiving low-dose PMPA (10 mg/kg per day subcutaneously; Table 4) were sampled at 30, 39, 41, 45, and 54 months of age (circles, diamonds, triangles, squares, and multiplications signs, respectively), but fractional excretion values were available only for the 39-, 41-, 45-, and 54-month time points. Animal 31042, which was SIV infected and which received low-dose PMPA (10 mg/kg per day subcutaneously), was sampled at 37 and 39 months of age, with fractional excretion values available only for the 39-month time point. Animal 29046 had been on a high-dose PMPA regimen (30 mg/kg per day subcutaneously) since birth, but the daily dose was reduced to 10 mg/kg at 25 months of age and to 5 mg/kg at 78 months of age (Table 2); 24-h urine collections were performed for animal 29046 at 66 (circle), 75 (square), and 90 (triangle) months of age.
Safety of prolonged low-dose PMPA treatment (10 mg/kg).
To evaluate further the safety of long-term treatment with a daily low dose of PMPA, three newborn macaques were started on a 10-mg/kg dosage regimen administered subcutaneously once daily (Table 4). These animals received the regular macaque diet at our research center, without any extra mineral or vitamin supplements. Two of these newborn macaques (animals 31121 and 31122) were not inoculated with SIV, and PMPA treatment was initiated within the first 2 days after birth; these animals have now received daily PMPA treatment for more than 5 years and have been tested regularly during this observation period (every 2 to 6 months). Serum phosphorus levels in both PMPA-treated animals remained ≥4.9 mg/dl during the first 3 years of PMPA treatment and were within the normal range of the values for untreated animals (data not shown). The available data suggest a mild increase in total ALP levels in the plasma of these two PMPA-treated animals, because levels were in the high range or were occasionally slightly above the reference interval for age-matched untreated animals. Determination of ALP isoenzyme levels in serum at several time points (6, 38, 41, and 45 months of age) revealed that small increases in total ALP levels were caused by mild increases in bone-specific ALP (which makes up 90 to 100% of total ALP in healthy animals). The levels of all other serum markers (including liver enzymes, creatinine, and BUN) showed no detectable differences between PMPA-treated and untreated infants. Both animals had plasma lactate and pyruvate levels and lactate concentration:pyruvate concentration ratios that were in the same range as those for the untreated animals. No glucosuria or proteinuria has been observed in either of these two PMPA-treated animals during the first 3 years of daily subcutaneous treatment with PMPA at 10 mg/kg. Analysis of urine collected by cystocentesis at regular time intervals demonstrated that there were no differences in the urinary protein concentration/creatinine concentration ratios between these animals and age-matched untreated controls (P = 0.7; two-tailed t test). At five time points (30, 39, 41 45, and 54 months of age), urine was collected over a 24-h period from these two animals treated with a low dose and analyzed, and the results were compared to those for untreated age-matched animals. The different parameters related to urinary excretion of phosphorus, calcium, and potassium in the two PMPA-treated animals were in the upper portion of the range or were slightly above the range for the untreated animals at some of the time points, but there was considerable variability within each animal and over time; there is no evidence to suggest that this loss of elements by urinary excretion in the PMPA-treated animals increased over time (Fig. 4). The creatinine clearance rate and the urinary excretion of the other constituents (protein, magnesium, chloride) in these two PMPA-treated animals were within the ranges observed for the untreated animals.
TABLE 4.
Prolonged daily administration of a low dose of PMPAa to infant macaques
| Animal no. | Sexb | SIV infection status | Age (days) at start of treatment | Duration of treatment |
|---|---|---|---|---|
| 31121 | M | Uninfected | 2 | >5 yr |
| 31122 | F | Uninfected | 1 | >5 yr |
| 31042c | F | Infected | 23 | 41 mo |
The low dose was 10 mg of PMPA per kg administered subcutaneously. The dosage for animal 31121 was reduced to 5 mg/kg subcutaneously once daily at 42 months of age and to 2.5 mg/kg at 63 months of age.
M, male; F, female.
Animal 31042 was euthanized due to terminal SIV infection at 41 months of age.
Pharmacokinetic analysis of these two animals after 10 and 30 months of daily subcutaneous dosing with PMPA at 10 mg/kg revealed that the values of the pharmacokinetic parameters for these animals (including PMPA clearance) were quite similar to those observed for juvenile and adult animals following administration of a single subcutaneous dose of PMPA at 10 mg/kg (Table 5). Animal 31121 had an approximately twofold lower PMPA clearance than animal 31122 at 10 and 30 months of age. A similar approximately twofold range in clearance was also seen in animals which received a single dose of PMPA (Table 5). As mentioned above, glucosuria was never detected in these two animals during the first 3 years of daily subcutaneous dosing with PMPA at 10 mg/kg. However, starting at 39 months of age, animal 31121 showed mild glucosuria (urine glucose concentration, 250 mg/dl) at three consecutive time points, and concomitantly, serum phosphorus levels were mildly reduced (4 to 5.5 mg/dl). This was accompanied by a mild increase in the levels of total ALP (to maximum levels of 1,380 U/liter; reference range for age-matched untreated animals, 244 to 857 U/liter), which consisted mainly of bone-specific ALP. Creatinine levels during this time were 1 to 1.2 mg/dl, which is within the range seen in untreated adult animals (0.8 to 1.4 mg/dl) at the California National Primate Research Center. Because this male macaque was growing to adult size (Fig. 3) and pharmacokinetic studies indicated that adult macaques have approximately twofold reduced clearance of PMPA compared to that for 1-year-old juvenile macaques (Tables 3 and 5), we believed that this animal was gradually being overdosed when his age and weight increased. Pharmacokinetic analysis at 40 months of age revealed increased AUC and decreased clearance compared to those for animal 31122 and age-matched control animals receiving a single dose (Table 5). Accordingly, at 42 months (3.5 years), when the animal weighed approximately 5.6 kg, the daily subcutaneous PMPA dosage was reduced from 10 to 5 mg/kg; the subsequent reduction in AUC (Table 5) was associated with an improvement of the glucosuria (which became intermittent), while the serum phosphorus level increased again (5.9 mg/dl). At 5 years of age, while this male animal continued to grow (body weight, ∼8.5 kg), the glucosuria again became more frequent and serum phosphorus levels decreased slowly to ∼3 to 4 mg/dl (mean ± standard deviation phosphorus levels in healthy adult males, 4.7± 0.9 mg/dl). At that time, a dosage reduction to 2.5 mg/kg per day subcutaneously again resulted in improvements in these markers. In contrast, female animal 31122 was maintained on the daily subcutaneous 10-mg/kg regimen dosage for more than 5 years; glucosuria was never detected, while serum phosphorus levels were normal, with the exception of a transient decrease to levels of ∼3 to 4 mg/dl during pregnancy (see below), which is also seen in untreated pregnant animals and which was therefore considered physiologic (45); serum creatinine levels for animal 31122 remained low (<0.8 mg/dl).
TABLE 5.
PMPA pharmacokinetics in rhesus macaques receiving prolonged therapy with PMPA at 10 mg/kga
| Animal no. or group size | Age (mo) | Wt (kg) | PMPA dose (mg/kg) | Duration (mo) of PMPA treatment or no. of dosesb | Cmax (μg/ml) | AUC (μg · h/ml) | CL/F (ml/h/kg) |
|---|---|---|---|---|---|---|---|
| 31121 | 10 | 2.1 | 10 | 10 | 16.2 | 17.3 | 578 |
| 30 | 4.3 | 10 | 30 | 17.4 | 18.0 | 556 | |
| 40 | 6.0 | 10 | 40 | 26.8 | 48.4 | 206 | |
| 46 | 6.4 | 5 | 4 | 10.3 | 16.7 | 300 | |
| 31122 | 10 | 1.8 | 10 | 10 | 10.2 | 9.6 | 1,045 |
| 30 | 3.8 | 10 | 30 | 10.0 | 9.9 | 1,015 | |
| 40 | 4.8 | 10 | 40 | 16.0 | 12.2 | 821 | |
| 46 | 5.4 | 10 | 46 | 14.9 | 15.7 | 635 | |
| 31042c | 28 | 2.6 | 10 | 27 | 10.4 | 13.1 | 765 |
| n = 3 | 48 (46-52)d | 5.1 (4.3-6.6) | 10 | Single | 13.9 (9.9-15.8) | 14.3 (10.0-17.1) | 738 (582-993) |
| n = 5 | 89 (71-109) | 9.4 (7.1-10.7) | 10 | Single | 12.7 (8.3-23.0) | 17.0 (13.2-20.3) | 602 (493-753) |
Abbreviations: Cmax, maximum concentration of PMPA in serum; CL/F, apparent clearance.
The duration of PMPA treatment at the specified dosage at the time that the 24-h pharmacokinetic study was performed. PMPA was administered subcutaneously. For more details on the timing of and the changes to the dosage regimens, see Table 4. For animal 31121, the dosage regimen was changed from 10 to 5 mg/kg daily at 42 months of age.
The animal was SIV infected.
The values are means (ranges).
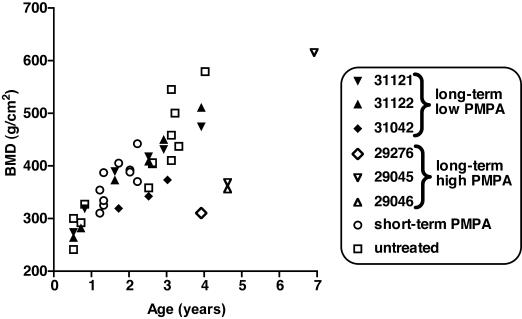
Radiographs of these two PMPA-treated animals were collected regularly from birth to 5 years of age, and there was never radiological evidence of decreased bone opacity or changes in growth plates in these two animals compared to the findings for untreated age-matched animals (Fig. 5). DXA scans were performed on these two animals at 6 and 9 months and at 1.5, 2.5, and 4 years of age; BMC and BMD values were also within the range for age-matched control animals (Fig. 6).
FIG. 5.
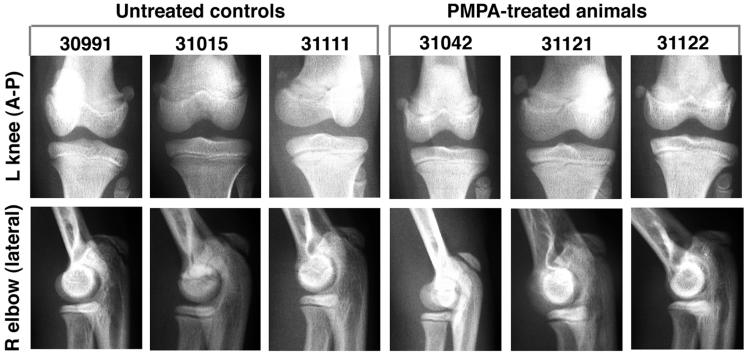
Radiographic evaluation of animals receiving chronic low-dose PMPA treatment. Three macaques, animals 31042 (SIV infected), 31121 (uninfected), and 31122 (uninfected), received daily subcutaneous treatment with PMPA (10 mg/kg) from the first few weeks of life (Table 4). Radiographs taken at 2.5 years of age were compared to those of three untreated, uninfected age-matched control animals. Animals 30991, 31042, and 31122 are females, while animals 31015, 31111, and 31121 are males. The anterior-posterior (A-P) view of the left (L) knee and the lateral view of the right (R) elbow are given as representative samples. No differences in bone opacity or growth plates were observed between treated and untreated animals.
FIG. 6.
DXA scan evaluation of bones in PMPA-treated animals. DXA scans of the femur neck, global proximal femur, distal radius and ulna, and lumbar vertebrae (L2 to L4) were performed. Because each location gave similar results, the bone mineral density of lumbar vertebrae is given as a representative sample. Data for untreated animals are 13 measurements performed for 11 animals (including the two untreated animals whose data are presented in Table 6). Three macaques, animals 31042 (SIV infected), 31121 (uninfected), and 31122 (uninfected), received daily subcutaneous treatment with PMPA (10 mg/kg) from the first few weeks of life (Table 4). For comparison, animals 29276, 29045, and 29046 were started on prolonged high-dose PMPA treatment (30 mg/kg per day subcutaneously) within the first few weeks of life, with dosage reductions starting at 15 to 25 months of age (Table 2). The histories of the 10 animals which received short-term PMPA treatment (animals 30576, 30577, 30581, 30842, 30845, 30339, 30338, 30478, 30007, and 30162) are summarized in Table 1.
At 3.5 years of age (immediately before the PMPA dosage reduction from 10 to 5 mg/kg per day subcutaneously for animal 31121), calcein was administered to both of these PMPA-treated animals and two age-matched control animals (one male and one female), and an iliac bone biopsy specimen was collected. Histomorphometric analysis of the bone biopsy specimen with calculation of the different variables revealed that there were no detectable differences in bone structural measures, with the exception of a lower bone volume (BV/TV) in animal 31121 compared to those in the two untreated animals due to a lower trabecular thickness and number (Tables 6 and 7). However, the iliac morphology was highly variable, and in the absence of decreased bone mass by DXA or of any other abnormal values by histomorphometry, this structural variation is probably not significant. There were also no detectable differences in the parameters of the dynamics of bone formation (including mineralization apposition rate, mineralizing surface, and adjusted apposition rate) and bone resorption (including eroded and osteoclast surfaces). In particular, osteoid thickness and volume, which would be expected to increase during mineralization defects, were indistinguishable between the PMPA-treated and untreated animals (Tables 6 and 7).
TABLE 6.
Histomorphometric evaluation of iliac bone biopsy specimens from animals receiving chronic low-dose PMPA treatmenta
| Animal no. | Sexb | PMPA treatment | Bone structure measures
|
Bone formation dynamics
|
Osteoid and bone formation
|
Bone resorption
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BV/TV | BS/BV | Tb.Th | Tb.N | Tb.Sp | W.Th | It.Th | MS/BS | MAR | BFR/BS | BFR/BV | BFR/TV | Aj.AR | Ac.f | OS/BS | Ob.S/BS | O.Th | OV/BV | FP | ES/BS | Oc.S/BS | Rs.P + Rv.P | |||
| 31007 | M | − | 45.61 | 15.86 | 126.09 | 3.62 | 150.34 | 19.08 | 87.93 | 45.47 | 1.33 | 220.33 | 349.49 | 159.41 | 1.74 | 11.55 | 34.75 | 5.50 | 8.77 | 4.75 | 10.98 | 8.33 | 3.68 | 2.63 |
| 31456 | F | − | 41.82 | 16.87 | 118.59 | 3.53 | 164.95 | 19.18 | 80.22 | 45.64 | 1.52 | 253.56 | 427.63 | 178.85 | 1.74 | 13.22 | 39.98 | 10.65 | 12.80 | 8.46 | 11.04 | 8.40 | 4.20 | 2.32 |
| 31121 | M | + | 28.52 | 21.52 | 92.93 | 3.07 | 232.92 | 19.38 | 54.18 | 42.05 | 1.14 | 174.99 | 376.59 | 107.40 | 1.60 | 9.03 | 29.99 | 13.20 | 8.43 | 5.45 | 12.12 | 14.83 | 10.00 | 6.00 |
| 31122 | F | + | 40.13 | 17.81 | 112.29 | 3.57 | 167.56 | 18.33 | 75.63 | 42.10 | 1.20 | 184.04 | 327.79 | 131.53 | 1.51 | 10.04 | 33.32 | 7.68 | 8.24 | 4.76 | 12.11 | 7.60 | 3.65 | 2.76 |
Animals 31121 and 31122 were started on prolonged low-dose PMPA administration (10 mg/kg subcutaneously once daily) shortly after birth (Table 4). Animals 31007 and 31456 are age-matched control animals. Following calcein labeling, iliac bone biopsies were performed at approximately 3.5 years of age. Units and formulas are explained in Table 7.
M, male; F, female.
TABLE 7.
Units and formulas for histomorphometric measurements and derived variables in
| Measurement or derived variable | Units | Name | Formula |
|---|---|---|---|
| Measurements | |||
| Structural | |||
| T.Ar | mm2 | Tissue area = bone area + marrow area (Ma.Ar) | |
| B.Ar | mm2 | Bone area | |
| B.Pm | mm | Bone perimeter | |
| Labels | |||
| L.Le | mm | Label length | |
| Ir.L.Wi | μm | Interlabel width | |
| Ir.L.t | Days | Interlabel time interval | |
| Histological | |||
| O.Pm | mm | Osteoid perimeter | |
| O.Ar | mm2 | Osteoid area | |
| Ob.Pm | mm | Osteoblastic perimeter | |
| E.Pm | mm | Eroded perimeter | |
| Oc.Pm | mm | Osteoclastic perimeter | |
| O.Wi | μm | Osteoid width | |
| W.Wi | μm | Wall width | |
| Derived variables | |||
| Structural | |||
| BV/TV | % | Bone volume | 100 · B.Ar/T.Ar |
| BS/BV | mm/mm2 | Bone surface/bone volume | (4/π) · (B.Pm/B.Ar) |
| Tb.Th | μm | Trabecular thickness | 2000/(BS/BV) |
| Tb.N | mm−1 | Trabecular number | (2/π) · (B.Pm/T.Ar) |
| Tb.Sp | μm | Trabecular separation | 1000/Tb.N − Tb.Th |
| Labels | |||
| MS/BS | % | Mineralizing surface | (L.Le/2)/B.Pm |
| MAR | μm/day | Mineral apposition rate | (Ir.L.Wi · π/4)/Ir.L.t |
| BFR/BS | μm3/μm2/year | Bone formation rate, surface referent | 3.65 · MAR · (MS/BS) |
| BFR/BV | %/year | Bone formation rate, bone referent | 0.1 · (BFR/BS) · (BS/BV) |
| BFR/TV | %/year | Bone formation rate, bone referent | 0.01 · (BFR/BV) · (BV/TV) |
| Histological | |||
| OS/BS | % | Osteoid surface | O.Pm/B.Pm |
| Ob.S/BS | % | Osteoblast surface | Ob.Pm/B.Pm |
| OV/BV | % | Osteoid volume | O.Ar/B.Ar |
| ES/BS | % | Eroded surface | (E.Pm + Oc.Pm)/B.Pm |
| Oc.S/BS | % | Osteoclast surface | Oc.Pm/B.Pm |
| O.Th | μm | Osteoid thickness | O.Wi · (π/4) |
| W.Th | μm | Wall thickness | W.Wi · (π/4) |
| Combined | |||
| Ac.f | yr−1 | Activation frequency | 3.65 · (OS/BS)/FP |
| Aj.AR | μm/day | Adjusted apposition rate | MAR · (MS/BS)/(OS/BS) |
| FP | Days | Formation period | W.Th/Aj.AR |
| It.Th | μm | Interstitial thickness | Tb.Th − (2 · W.Th) |
| Rs.P + Rv.P | Days | Resorption + reversal period | FP · (ES/BS)/(OS/BS) |
Both of these PMPA-treated uninfected animals continued to have rapid growth that was indistinguishable from that for untreated animals (Fig. 3E and F). The values of their hematology parameters (including complete blood counts and lymphocyte subpopulations, as determined by flow cytometry) were also within the range of those for untreated age-matched animals throughout the observation period. Both animals, which were of different sexes, were housed together. At approximately 4 years of age, animal 31122 delivered a male infant of normal birth weight (0.5 kg) and without any detectable bone lesions; however, the infant died 2 days later of neonatal septicemia (with severe bacterial cerebral and spinal meningitis), which was considered to be unrelated to the PMPA treatment. At 5 years and 4 months of age, animal 31122 delivered another male infant, which is healthy and which has normal development at 7 months of age, with normal serum chemistry values (including serum phosphorus levels) and no glucosuria. PMPA treatment (10 mg/kg per day subcutaneously) of the mother was continued throughout the whole time period.
The third PMPA-treated animal (animal 31042) was inoculated with strain SIVmac251 at birth and 3 weeks later was started on PMPA at a daily subcutaneous dosage of 10 mg/kg. This animal had splenomegaly and a slightly reduced growth rate (Fig. 3F); both of these findings can be attributed to the persistently high virus levels in this animal (107 to 108 copies of viral RNA per ml of plasma during the first 2 years of life) due to the presence of SIV mutants with fivefold reduced in vitro susceptibility to PMPA. Serum phosphorus levels (>5 mg/dl) and serum ALP levels (481 to 1,054 U/liter) were within the normal ranges throughout the 3-year period. The values of the pharmacokinetic parameters were also in the expected range (Table 5). At 3 years of age, this animal had no glucosuria and no proteinuria, and the urinary excretion of elements was similar to that for the untreated control animals (Fig. 4). Radiographs did not detect any bone abnormalities in this animal at 3 years of age (Fig. 5). DXA scans performed at 20, 30, and 36 months of age demonstrated that BMC and BMD values were also within the ranges for age-matched control animals (Fig. 6). After 3 years of age, the health of this animal declined due to immunosuppression (including chronic Cryptosporidium-positive enteritis), and the animal had to be euthanized at 3.5 years of age. Our previous experiments suggest that without PMPA treatment, perinatally SIVmac251-infected macaques with such high virus levels would likely have developed fatal immunodeficiency within the first few months of life (51, 56, 57a).
DISCUSSION
Although the data presented here were compiled from many different studies, most of which involved SIV infection, they provide important information regarding the safety and toxicity of short-term and long-term administration of PMPA to newborn, infant, and juvenile macaques. Toxicity data from studies with nonhuman primates are highly relevant to humans, because of the close similarities in many aspects of physiology, including infant development, metabolism, and kidney and bone physiology (21, 24, 38, 39, 42, 62).
Our studies demonstrated that short-term subcutaneous PMPA administration to newborn or infant macaques, even at a relatively high daily dose of 30 mg/kg, did not lead to any detectable adverse effects. Even those animals which were monitored for more than 3 years had normal development and were reaching adulthood. Our previous studies demonstrated that short-term regimens of PMPA, even one or two doses, can be highly effective in protecting newborn macaques against SIV infection (49, 53, 55, 56). In addition, PMPA is, unlike nonnucleoside reverse transcriptase inhibitors, also active against HIV type 2. Thus, all these data suggest that the short-term administration of PMPA can be a safe and highly effective strategy to reduce the rate of mother-to-infant transmission of HIV types 1 and 2, especially in developing countries. Unlike the rapid development of drug-resistant mutants during nevirapine treatment in such a setting (17), the emergence of PMPA-resistant mutants during short-term perinatal PMPA administration would be very unlikely.
We demonstrated that prolonged administration of a relatively high dose of PMPA (30 mg/kg per day subcutaneously) resulted in renal tubular dysfunction. The observation of glucosuria (in the absence of hyperglycemia), hyperphosphaturia, and aminoaciduria strongly suggests that a prolonged high-dose PMPA treatment course induced a proximal renal tubular disorder, similar to Fanconi syndrome, which is characterized by increased losses of glucose, phosphate, amino acids, and other nutrients in urine due to decreased reabsorption at the proximal renal tubuli. The histopathologies of the three animals that were euthanized after chronic dosing with PMPA at 30 mg/kg revealed renal tubular atrophy and degeneration as common lesions. This toxicity of PMPA is probably related to its renal clearance; studies with animals and humans indicate that PMPA and its related nucleotide analogs are excreted in unchanged form in urine through a combination of renal filtration and active tubular secretion (10, 14, 15, 16). Thus, accumulation of drug in tubular epithelial cells (due to more drug uptake from plasma than secretion into the tubular lumen) may be the direct cause of these renal effects. Evidence for the direct renal toxicity of very high PMPA levels comes from the observation that daily subcutaneous treatment of cynomolgus macaques with PMPA at 75 mg/kg led to a reversible decrease in serum phosphorus levels to <2 mg/dl within 4 weeks (48), which suggests that higher plasma PMPA levels lead directly to the enhanced accumulation of PMPA in the renal tubular cells and toxicity.
This renal toxicity observed with high-dose PMPA treatment is similar to the renal toxicity observed in humans receiving a daily dose of 120 mg of 9-[2-(phosphonomethoxy)ethyl]adenine (adefovir). In these adefovir-treated HIV-infected adults, hypophosphatemia (serum phosphate levels, <2 mg/dl) and elevations in serum creatinine levels (>0.5 mg/dl above the baseline level) developed in 17 to 60% of patients between 24 and 48 weeks of treatment; the changes in both creatinine and phosphate levels resolved in ∼90% of patients within 24 weeks of drug discontinuation (18, 25).
Prolonged daily subcutaneous administration of the high 30-mg/kg dosage of PMPA resulted in bone lesions, consisting of decreased mineralization, osteopenia, and abnormally widened growth plates. The pathogenesis of the bone disease in our animals is most likely due to the increased urinary loss of phosphate and chronic hypophosphatemia. The histopathological finding of impaired osteoid mineralization provides further evidence that the lesions are similar to hypophosphatemic rickets and osteomalacia. The development of hypophosphatemic rickets and osteomalacia has also been observed in children and adults with congenital or acquired Fanconi syndrome and is often accompanied by muscle atrophy (11, 28, 41; S. Dalmak, E. Erek, K. Serengecti, I. Okar, U. Ulku, and M. Basaran, Letter, Nephron 72:121-122, 1996).
Pharmacokinetic analysis demonstrated that prolonged subcutaneous high-dose (30 mg/kg) PMPA treatment of macaques led to an approximately three- to sevenfold reduced clearance of PMPA and higher AUC values. This increased systemic exposure to PMPA was probably the result of the renal toxicity, because damage to the renal tubular epithelial cells presumably resulted in decreased secretion of PMPA. Thus, it is plausible that once the process of renal toxicity had started during prolonged high-dose PMPA treatment, a vicious circle was initiated in which increased exposure to PMPA induced further renal toxicity and a further decrease in the renal clearance of PMPA. A small (∼27%) reduction in PMPA clearance was also observed in the phase I human trials with PMPA after 7 days of daily intravenous dosing at 3 mg/kg, although the mechanism and biological significance of this are unclear (16). No statistically significant changes in PMPA clearance were observed in HIV-infected patients following 35 days of daily treatment with oral doses of 300 or 600 mg (2).
No glucosuria or hypophosphatemia was observed in two infant macaques after 6 weeks of daily subcutaneous treatment with PMPA at 30 mg/kg that had started shortly after birth (Table 1). On first sight, this absence of toxicity may look surprising because the clearance of PMPA in newborn macaques is reduced approximately fivefold compared to that in juvenile macaques, resulting in a fivefold increased systemic exposure (Table 3). However, because transport mechanisms in the proximal renal tubuli are poorly developed in primates and humans at birth (6, 29, 61), one can postulate that even in the presence of relatively high levels of PMPA in plasma, an impaired uptake of PMPA by these tubular epithelial cells in the kidney prevented accumulation to toxic intracellular levels.
Others have reported that chronic high-dose PMPA treatment (30 mg/kg per day subcutaneously) of pregnant macaques starting during the first or second trimester led to hypophosphatemia and increased ALP levels for the maternal macaques and the fetal and infant macaques and reduced birth weights for some infants (45, 46). Some of these infants, when they were continued on the high-dose PMPA regimen after birth, developed severe growth restriction and bone lesions within 2 to 7 months of age (46). Those investigators (46) proposed that the transplacental transfer of PMPA (with fetal plasma PMPA levels being approximately 10-fold less than those achieved in the mother) had a direct toxic effect on the development of the offspring. An alternative explanation is, however, also possible. Because adult macaques have lower PMPA clearance than juvenile macaques (Table 3), a high-dose regimen of PMPA that was based on body weight (including the weight gain associated with pregnancy) explains the very high plasma PMPA levels (AUCs, 87 to 117 μg · h/ml) that were reported in these pregnant mothers (46). Our data suggest that such high plasma PMPA levels were likely to rapidly induce renal toxicity in the mothers, which would explain the rapid development of severe hypophosphatemia that was observed in some of these pregnant animals within several weeks of treatment (45, 46). Because the fetus is totally dependent on the mother for its nutrients, this maternal renal toxicity and severe hypophosphatemia rather than a direct toxic effect of the transplacental transfer of PMPA on the fetus may have been the main cause for the hypophosphatemia and growth restriction that were observed in their respective offspring in utero and at birth (45, 46). In this context, the severity of the hypophosphatemia in these pregnant macaques correlated with that in their offspring (45). The phosphate deficiency and relative growth restriction at birth was also likely to predispose some of these infants to have an accelerated bone lesion development when they were continued on a high-dose PMPA regimen after birth (46).
Our study demonstrates that the bone changes associated with chronic high-dose PMPA treatment improve or resolve following dose reduction or discontinuation. For one animal (animal 29003), which had developed severe bone lesions, we stopped PMPA treatment completely. The disappearance of glucosuria, the normalization of serum phosphorus levels, the radiological normalization of the bone opacity and growth plates, and the rapid weight gain suggest that even in such severe cases, the renal toxicity and the associated bone loss may be largely or completely reversible. The other animals, for which the PMPA dosage regimen was reduced, showed a marked improvement, even though the renal toxicity, as indicated by the presence of glucosuria, proteinuria, aminoaciduria, and hyperphosphaturia, did not resolve completely.
Following a reduction of the PMPA dosage, two of these animals which had developed bone disease seemed to benefit from the receipt of dietary supplements containing phosphate, multivitamins, and calcium. Human patients with rickets or osteomalacia and muscle atrophy due to Fanconi syndrome generally also respond well to such dietary supplementation, which compensates for the urinary loss of these nutrients (11).
The prolonged high-dose PMPA treatment did not lead to any detectable abnormalities in the levels of triglycerides and cholesterol in serum. This observation suggests that unlike some of the other currently used drugs (8, 30, 40), PMPA does not have any adverse effects on lipid metabolism. Our limited data did not find any effect of long-term PMPA treatment on lactate and pyruvate levels in blood; this is consistent with the observation of low mitochondrial toxicity of tenofovir in vitro compared to those of other nucleoside reverse transcriptase inhibitors (4). No changes in mitochondrial DNA content or enzyme levels were detected in the tissues of rhesus monkeys after 8 weeks of oral treatment with tenofovir disoproxil fumarate (TDF) at 30 or 250 mg/kg (3). Recent observations from clinical trials also demonstrate that tenofovir has a favorable safety profile in terms of lipid metabolism and mitochondrial activity in humans (19). There are conflicting reports whether HIV-infected patients receiving other nucleoside reverse transcriptase inhibitors and/or protease inhibitors are at risk for the development of osteopenia or osteoporosis in association with lactic acidemia (7, 27, 32-34, 47).
Following the observation of the renal toxicity and the bone loss resulting from the high 30-mg/kg PMPA dosage regimen, we investigated the long-term effects of a daily subcutaneous 10-mg/kg regimen. Three animals (of which one was SIV infected) were started on this daily subcutaneous 10-mg/kg dosage regimen shortly after birth. These PMPA-treated animals did not receive any extra dietary phosphate supplements. Most importantly, these animals did not develop any significant bone abnormalities, as determined by regular radiographs and DXA scans throughout the observation period of more than 5 years. In addition, there were no changes in bone histomorphometry parameters in an iliac bone biopsy specimen that was collected from the two uninfected animals at 3.5 years of age. While these three animals did not have glucosuria or proteinuria during the first 3 years of treatment, 24-h urine collections suggested that for two of these animals, the levels of excretion of phosphorus, calcium, and potassium in urine were in the high range or were slightly above the range for untreated animals, but there was considerable variability within each animal over time. Thus, even if this low-dosage regimen has some inhibitory effect on tubular reabsorption, the biological significance may be minor. The urinary loss of phosphorus in these animals treated with a low dose of PMPA (estimated to be ∼4 to 13 mg of phosphorus per kg of body weight per day; Fig. 4) is probably compensated for sufficiently by the supply of phosphorus through their regular diet (estimated to be ∼90 mg/kg/day [5, 35]). Pharmacokinetic analysis of these animals after 2.5 years of daily subcutaneous dosing with 10 mg/kg suggested that the renal clearance of PMPA differed approximately twofold among individuals, similar to the variability observed for control animals of the same age and weight. The largest of these animals, animal 31121, which had a lower PMPA clearance, developed mild glucosuria and reduced PMPA clearance after 3.5 years of daily subcutaneous treatment with PMPA at 10 mg/kg, but the glucosuria and clearance improved when the dosage regimen was reduced. A gradual decrease in PMPA clearance following administration of a single dose occurs when juvenile animals mature into adulthood (Tables 3 and 5) and is probably caused by the finding that for adult macaques, the kidney volume is independent of the sex, total body size, and weight (23). In other words, for a PMPA dosage regimen that is calculated on the basis of total body weight, large adult animals receive a relatively larger amount of PMPA per amount of kidney tissue. Chronic exposure to PMPA at AUC values above a certain threshold is likely to trigger the renal toxicity. The second animal (animal 31122), which had higher levels of drug clearance even at a young age, also showed a slow increase in AUC values with age (∼10 to 16 μg · h/ml) but did not develop glucosuria after 5 years of daily subcutaneous treatment with PMPA at the 10-mg/kg dosage; this animal had PMPA clearance indistinguishable from those for age-matched control animals receiving a single dose. Long-term studies of the efficacy and safety of PMPA in macaques should take into account these individual and age-related physiological differences in pharmacokinetics so that appropriate dosage adjustments can be made to reduce the risk for renal toxicity.
Following the demonstration of a very favorable antiviral efficacy and safety profile in clinical trials with HIV-infected adults (2), the orally bioavailable PMPA prodrug TDF has received approval for use as an oral regimen of 300 mg of TDF per day. This oral 300-mg TDF dosage regimen results in an AUC value of ∼3 μg · h/ml for human adults. Thus, the subcutaneous 10-mg/kg dosage that we started administering to macaques at birth and that we continued for more than 3 years resulted in an exposure to PMPA (on the basis of AUC values) which is approximately 18-fold (at birth) and 3-fold (in juvenile macaques) higher than that from the 300-mg TDF regimen used at present for HIV-infected adults. Although so far the majority of TDF-treated patients have not developed any signs of renal toxicity with the 300-mg TDF regimen (44), a few cases of renal tubular dysfunction (which was reversible upon withdrawal) have recently been described, especially in individuals with low body weight or preexisting renal disease (e.g., decreased creatinine clearance) or individuals receiving concomitant therapy with other drugs that affect tenofovir clearance (12, 13, 26, 37a, 58).
In conclusion, the findings from the studies with macaques suggest that the renal tubular dysfunction and bone changes associated with PMPA are restricted to chronic high-dose treatment regimens, while prolonged low-dose regimens are expected to be safe for the majority of patients. This safety, coupled with the high degree of antiviral activity and favorable resistance profile (22, 59), suggests that PMPA will be a very useful drug in prophylactic and therapeutic regimens that are used to combat pediatric and adult HIV infection.
Acknowledgments
We thank E. Agatep, N. Aguirre, K. Anderson, S. Au, V. Bakula, I. Bolton, D. Brandt, B. Capuano, L. Carruthers, I. Cazares, K. Christe, S. Davis, Z. Dehqanzada, A. Enriquez, D. Florence, K. Gilardi, C. Johnson, R. Jorgensen, J. Lawson, D. Robb, J. Roberts, B. Rodello, R. Singh, L. Summers, G. Vicino, R. Walgenbach, J. Watanabe, C. Young, and the staff of the Veterinary & Colony Services of the California National Primate Research Center for expert technical assistance and sharing data; Q. Rogers and D. Wong (University of California, Davis) for amino acid analysis of urine samples; and K. Cundy and B. Kearney (Gilead Sciences) for assistance with the pharmacokinetic studies.
This research was supported by Gilead Sciences; PHS grant RR-00169 to the California National Primate Research Center; and Elizabeth Glaser Pediatric AIDS Foundation grants PG-50609, PG-50757, and PG-50853 (to K. K. A. Van Rompay). M. L. Marthas is an Elizabeth Glaser Scientist.
REFERENCES
- 1.Alpers, C. E., C. C. Tsai, K. L. Hudkins, Y. Cui, L. Kuller, R. E. Benveniste, J. M. Ward, and W. R. Morton. 1997. Focal segmental glomerulosclerosis in primates infected with a simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 13:413-424. [DOI] [PubMed] [Google Scholar]
- 2.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus type 1-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biesecker, G., S. Karimi, J. Desjardins, D. Meyer, B. Abbott, R. Bendele, and F. Richardson. 2003. Evaluation of mitochondrial DNA content and enzyme levels in tenofovir DF-treatment rats, rhesus monkeys and woodchucks. Antivir. Res. 58:217-225. [DOI] [PubMed] [Google Scholar]
- 4.Birkus, G., M. J. M. Hitchcock, and T. Cihlar. 2002. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 46:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourne, G. H. 1975. Nutrition of the rhesus monkey, p. 97-116. In G. H. Bourne (ed.), The rhesus monkey, vol. II. Management, reproduction, and pathology. Academic Press, Inc., New York, N.Y.
- 6.Calcagno, P. L., and M. I. Rubin. 1963. Renal extraction of para-aminohippurate in infants and children. J. Clin. Investig. 42:1632-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr, A., J. Miller, J. A. Eisman, and D. A. Cooper. 2001. Osteopenia in HIV-infected men: association with asymptomatic lactic acidemia and lower weight pre-antiretroviral therapy. AIDS 15:703-709. [DOI] [PubMed] [Google Scholar]
- 8.Carr, A., K. Samaras, S. Burton, M. Law, J. Freund, D. J. Chisholm, and D. A. Cooper. 1998. A syndrome of peripheral lipodystrophy, hyperlipidemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12:F51-F58. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1997. Update: trends in AIDS incidence—United States, 1996. Morbid. Mortal. Wkly. Rep. 46:862-867. [PubMed] [Google Scholar]
- 10.Cihlar, T., E. S. Ho, D. C. Lin, and A. S. Mulato. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20:641-648. [DOI] [PubMed] [Google Scholar]
- 11.Clarke, B. L., A. G. Wynne, D. M. Wilson, and L. A. Fitzpatrick. 1995. Osteomalacia associated with adult Fanconi's syndrome: clinical and diagnostic features. Clin. Endocrinol. 43:479-490. [DOI] [PubMed] [Google Scholar]
- 12.Coca, S., and M. A. Perazella. 2002. Rapid communication: acute renal failure associated with tenofovir: evidence of drug-induced nephrotoxicity. Am. J. Med. Sci. 324:342-344. [DOI] [PubMed] [Google Scholar]
- 13.Créput, C., G. Gonzalez-Canali, G. Hill, C. Piketty, M. Kazatchkine, and D. Nochy. 2003. Renal lesions in HIV-1 positive patient treated with tenofovir. AIDS 17:935-937. [DOI] [PubMed] [Google Scholar]
- 14.Cundy, K. C., P. Barditch-Crovo, R. E. Walker, A. C. Collier, D. Ebeling, J. Toole, and H. S. Jaffe. 1995. Clinical pharmacokinetics of adefovir in human immunodeficiency virus type 1-infected patients. Antimicrob. Agents Chemother. 39:2401-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cundy, K. C., C. Sueoka, G. R. Lynch, L. Griffin, W. A. Lee, and J.-P. Shaw. 1998. Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Antimicrob. Agents Chemother. 42:687-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks, S. G., P. Barditch-Crovo, P. S. Lietman, F. Hwang, K. C. Cundy, J. F. Rooney, N. S. Hellmann, S. Safrin, and J. Kahn. 1998. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob. Agents Chemother. 42:2380-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshleman, S. H., M. Mracna, L. A. Guay, M. Deseyve, S. Cunningham, M. Mirochnick, P. Musoke, T. Fleming, M. G. Fowler, L. M. Mofenson, F. Mmiro, and J. B. Jackson. 2001. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET012). AIDS 15:1951-1957. [DOI] [PubMed] [Google Scholar]
- 18.Fisher, E. J., K. Chaloner, D. L. Cohn, L. Bjorling Grant, B. Alston, C. L. Brosgart, B. Schmetter, W. El-Sadr, and J. Sampson for the Terry Beirn Community Programs for Clinical Research on AIDS. 2001. The safety and efficacy of adefovir dipivoxil in patients with advanced HIV disease: a randomized, placebo-controlled trial. AIDS 15:1695-1700. [DOI] [PubMed] [Google Scholar]
- 19.Gallant, J., and S. Deresinski. 2003. Tenofovir disoproxil fumarate. Clin. Infect. Dis. 37:944-950. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin, B. T., and C. P. Jerome. 1987. Iliac biopsy for histomorphometric analysis of trabecular bone in cynomolgus monkeys and baboons. Lab. Anim. Sci. 37:213-216. [PubMed] [Google Scholar]
- 21.Gribnau, A. A. M., and L. G. M. Geijsberts. 1981. Developmental stages in the rhesus monkey (Macaca mulatta). Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 22.Harrigan, P. R., M. D. Miller, P. McKenna, Z. L. Brumme, and B. Larder. 2002. Phenotypic susceptibilities to tenofovir in a large panel of clinically derived human immunodeficiency virus type 1 isolates. Antimicrob. Agents Chemother. 46:1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill, L. R., K. R. Hess, L. C. Stephens, R. E. Price, and K. N. Gray. 2001. Correlation of kidney weight and volume and selected skeletal parameters to sex in the adult rhesus monkey (Macaca mulatta). J. Med. Primatol. 30:56-60. [DOI] [PubMed] [Google Scholar]
- 24.Jerome, C. P., and P. E. Peterson. 2001. Nonhuman primate models in skeletal research. Bone 29:1-6. [DOI] [PubMed] [Google Scholar]
- 25.Kahn, J., S. Lagakos, M. Wulfsohn, D. Cherng, M. Miller, J. Cherrington, D. Hardy, G. Beall, R. Cooper, R. Murphy, N. Basgoz, E. Ng, S. Deeks, D. Winslow, J. J. Toole, and D. Coakley for the GS-408 Study Team. 1999. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy. A randomized controlled trial. JAMA 282:2305-2312. [DOI] [PubMed] [Google Scholar]
- 26.Karras, A., M. Lafaurie, A. Furco, A. Bourgarit, D. Droz, D. Sereni, C. Legendre, F. Martinez, and J.-M. Molina. 2003. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin. Infect. Dis. 36:1070-1073. [DOI] [PubMed] [Google Scholar]
- 27.Lawal, A., E. S. Engelson, J. Wang, S. B. Heymsfield, and D. P. Kotler. 2001. Equivalent osteopenia in HIV-infected individuals studied before and during the era of highly active antiretroviral therapy. AIDS 15:278-280. [DOI] [PubMed] [Google Scholar]
- 28.Lian, L.-M., Y.-C. Chang, C.-C. Yang, J.-C. Yang, K.-P. Kao, and M.-Y. Chung. 1994. Adult Fanconi syndrome with proximal muscle weakness and hypophosphatemic osteomalacia: report of a case. J. Formos. Med. Assoc. 93:709-714. [PubMed] [Google Scholar]
- 29.Lopez-Anaya, A., J. D. Unadkat, L. A. Schumann, and A. L. Smith. 1990. Pharmacokinetics of zidovudine (azidothymidine). II. Development of metabolic and renal clearance pathways in the neonate. J. Acquir. Immune Defic. Syndr. 3:1052-1058. [PubMed] [Google Scholar]
- 30.Madge, S., S. Kinloch-de-Loes, D. Mercey, M. A. Johnson, and I. V. D. Weller. 1999. Lipodystrophy in patients naive to HIV protease inhibitors. AIDS 13:735-737. [DOI] [PubMed] [Google Scholar]
- 31.Meyer, D. J., and J. W. Harvey. 1998. Veterinary laboratory medicine. Interpretation and diagnosis, p. 157-186. The W. B. Saunders Co., Philadelphia, Pa.
- 32.Mondy, K., K. Yarasheski, W. G. Powderly, M. Whyte, S. Claxton, D. DeMarco, M. Hofffmann, and P. Tebas. 2003. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin. Infect. Dis. 36:482-490. [DOI] [PubMed] [Google Scholar]
- 33.Moore, A., A. Vashisht, C. A. Sabin, A. Mocroft, S. Madge, A. N. Phillips, J. W. W. Studd, and M. A. Johnson. 2001. Reduced bone mineral density in HIV-positive individuals. AIDS 15:1731-1733. [DOI] [PubMed] [Google Scholar]
- 34.Mora, S., N. Sala, D. Bricalli, G. Zuin, G. Chiumello, and A. Vigano. 2001. Bone mineral loss through increased bone turnover in HIV-infected children treated with highly active antiretroviral therapy. AIDS 15:1823-1829. [DOI] [PubMed] [Google Scholar]
- 35.National Research Council. 1978. Nutrient requirements of nonhuman primates. National Academy of Sciences, Washington, D.C.
- 36.National Research Council. 1996. Guide for the care and use of laboratory animals. Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources. National Academy Press, Washington, D.C.
- 37.Parfitt, A. M., M. K. Drezner, F. H. Glorieux, J. A. Kanis, H. Malluche, P. J. Meunier, S. M. Ott, and R. R. Recker. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2:595-610. [DOI] [PubMed] [Google Scholar]
- 37a.Peyrière, H., J. Reynes, I. Rouanet, N. Daniel, C. Merle de Boever, J.-M. Mauboussin, H. Leray, L. Moachon, D. Vincent, and D. Salmon-Céron. 2004. Renal tubular dysfunction associated with tenofovir therapy. Report of 7 cases. J. Acquir. Immune Defic. Syndr. 35:269-273. [DOI] [PubMed]
- 38.Pope, N. S., K. G. Gould, D. C. Anderson, and D. R. Mann. 1989. Effects of age and sex on bone density in the rhesus monkey. Bone 10:109-112. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, J., E. W. Ford, and J. L. Southers. 1998. Part A. Urinary system, p. 312-323. In B. T. Bennett, C. R. Abee, and R. Henrickson (ed.), Nonhuman primates in biomedical research diseases. Academic Press, Inc., San Diego, Calif.
- 40.Saint-Marc, T., M. Partisani, I. Poizot-Martin, F. Bruno, O. Rouviere, J.-M. Lang, J.-A. Gastaut, and J.-L. Touraine. 1999. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS 13:1659-1667. [DOI] [PubMed] [Google Scholar]
- 41.Segal, L. S., R. C. Palumbo, and W. W. Robertson. 1995. The development of rickets as a complication of chemotherapy for the treatment of Wilm's tumor. Orthopedics 18:261-264. [DOI] [PubMed] [Google Scholar]
- 42.Sharer, J. E., L. A. Shipley, M. R. Vandenbranden, S. N. Binkley, and S. A. Wrighton. 1995. Comparisons of phase I and phase II in vitro hepatic enzyme activities of human, dog, rhesus monkey, and cynomolgus monkey. Drug Metab. Dispos. 23:1231-1241. [PubMed] [Google Scholar]
- 43.Shaw, J. P., C. M. Sueoka, R. Oliyai, W. A. Lee, M. N. Arimilli, C. Kim, and K. C. Cundy. 1997. Metabolism and pharmacokinetics of a novel oral prodrug of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm. Res. 14:1824-1829. [DOI] [PubMed] [Google Scholar]
- 44.Squires, K., A. Pozniak, G. Pierone, C. R. Steinhart, D. Berger, N. C. Bellos, S. L. Becker, M. Wulfsohn, M. D. Miller, J. J. Toole, D. F. Coakley, and A. Cheng for the Study 907 Team. 2003. Tenofovir disoproxil fumarate in nucleoside-resistant HIV-1 infection. A randomized trial. Ann. Intern. Med. 139:313-320. [DOI] [PubMed] [Google Scholar]
- 45.Tarantal, A. F., A. Castillo, J. E. Ekert, N. Bischofberger, and R. B. Martin. 2002. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta). J. Acquir. Immune Defic. Syndr. 29:207-220. [DOI] [PubMed] [Google Scholar]
- 46.Tarantal, A. F., M. L. Marthas, J.-P. Shaw, K. Cundy, and N. Bischofberger. 1999. Administration of 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) to gravid and infant rhesus macaques (Macaca mulatta): safety and efficacy studies. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:323-333. [DOI] [PubMed] [Google Scholar]
- 47.Tebas, P., W. G. Powderly, S. Claxton, D. Marin, S. Tantisiriwat, L. Teitelbaum, and K. E. Yarasheski. 2000. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS 14:F63-F67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai, C.-C., K. E. Follis, T. W. Beck, A. Sabo, N. Bischofberger, and P. J. Dailey. 1997. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection. AIDS Res. Hum. Retrovir. 13:707-712. [DOI] [PubMed] [Google Scholar]
- 49.Van Rompay, K. K. A., C. J. Berardi, N. L. Aguirre, N. Bischofberger, P. S. Lietman, N. C. Pedersen, and M. L. Marthas. 1998. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS 12:F79-F83. [DOI] [PubMed] [Google Scholar]
- 50.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, C. J. Berardi, A. S. Mulato, A. Spinner, R. P. Tarara, D. R. Canfield, S. Telm, N. Bischofberger, and N. C. Pedersen. 1996. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob. Agents Chemother. 40:2586-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, P. D. Lamy, P. J. Dailey, D. R. Canfield, R. P. Tarara, N. Bischofberger, and N. C. Pedersen. 1999. 9-[2-(Phosphonomethoxy)propyl]adenine (PMPA) therapy prolongs survival of infant macaques inoculated with simian immunodeficiency virus with reduced susceptibility to PMPA. Antimicrob. Agents Chemother. 43:802-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Rompay, K. K. A., P. J. Dailey, R. P. Tarara, D. R. Canfield, N. L. Aguirre, J. M. Cherrington, P. D. Lamy, N. Bischofberger, N. C. Pedersen, and M. L. Marthas. 1999. Early short-term 9-[2-(phosphonomethoxy)propyl]adenine treatment favorably alters subsequent disease course in simian immunodeficiency virus-infected newborn rhesus macaques. J. Virol. 73:2947-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Rompay, K. K. A., M. L. Marthas, J. D. Lifson, C. J. Berardi, G. M. Vasquez, E. Agatep, Z. A. Dehqanzada, K. C. Cundy, N. Bischofberger, and N. C. Pedersen. 1998. Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res. Hum. Retrovir. 14:761-773. [DOI] [PubMed] [Google Scholar]
- 54.Van Rompay, K. K. A., M. L. Marthas, R. A. Ramos, C. P. Mandell, E. K. McGowan, S. M. Joye, and N. C. Pedersen. 1992. Simian immunodeficiency virus (SIV) infection of infant rhesus macaques as a model to test antiretroviral drug prophylaxis and therapy: oral 3′-azido-3′-deoxythymidine prevents SIV infection. Antimicrob. Agents Chemother. 36:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Rompay, K. K. A., M. B. McChesney, N. L. Aguirre, K. A. Schmidt, N. Bischofberger, and M. L. Marthas. 2001. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J. Infect. Dis. 184:429-438. [DOI] [PubMed] [Google Scholar]
- 56.Van Rompay, K. K. A., M. D. Miller, M. L. Marthas, N. A. Margot, P. J. Dailey, R. P. Tarara, D. R. Canfield, J. M. Cherrington, N. L. Aguirre, N. Bischofberger, and N. C. Pedersen. 2000. Prophylactic and therapeutic benefits of short-term 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J. Virol. 74:1767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Rompay, K. K. A., M. G. Otsyula, M. L. Marthas, C. J. Miller, M. B. McChesney, and N. C. Pedersen. 1995. Immediate zidovudine treatment protects simian immunodeficiency virus-infected newborn macaques against rapid onset of AIDS. Antimicrob. Agents Chemother. 39:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a.Van Rompay, K. K. A., R. Singh, L. Brignolo, J. R. Lawson, K. A. Schmidt, B. Pahar, D. R. Canfield, R. P. Tarara, N. Bischofberger, and M. Marthas. The clinical benefits of tenofovir for simian immunodeficiency virus-infected macaques are larger than predicted by its effects on standard viral and immunological parameters. J. Acquir. Immune Defic. Syndr., in press. [DOI] [PubMed]
- 57b.Van Rompay, K. K. A., R. P. Singh, B. Pahar, D. L. Sodora, C. Wingfield, J. R. Lawson, M. L. Marthas, and N. Bischofberger. CD8+-cell-mediated suppression of virulent simian immunodeficiency virus during tenofovir treatment. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 58.Verhelst, D., M. Monge, J.-L. Meynard, B. Fouqueray, B. Mougenot, P.-M. Girard, P. Ronco, and J. Rossert. 2002. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am. J. Kidney Dis. 40:1331-1333. [DOI] [PubMed] [Google Scholar]
- 59.Wainberg, M. A., M. D. Miller, Y. Quan, H. Salomon, A. S. Mulato, P. D. Lamy, N. A. Margot, K. E. Anton, and J. M. Cherrington. 1999. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir. Ther. 4:87-94. [DOI] [PubMed] [Google Scholar]
- 60.Watson, D. C., and J. J. Farley. 1999. Efficacy of and adherence to highly active antiretroviral therapy in children infected with human immunodeficiency virus type 1. Pediatr. Infect. Dis. J. 18:682-689. [DOI] [PubMed] [Google Scholar]
- 61.West, J. R., H. W. Smith, and H. Chasis. 1948. Glomerular filtration rate, effective renal blood flow, and maximal tubular excretory capacity in infancy. J. Pediatr. 32:10-18. [DOI] [PubMed] [Google Scholar]
- 62.Zoetis, T., and M. Hurtt. 2003. Species comparison of anatomical and functional renal development. Birth Defects Res. Part B Dev. Reprod. Toxicol. 68:111-120. [DOI] [PubMed] [Google Scholar]