Abstract
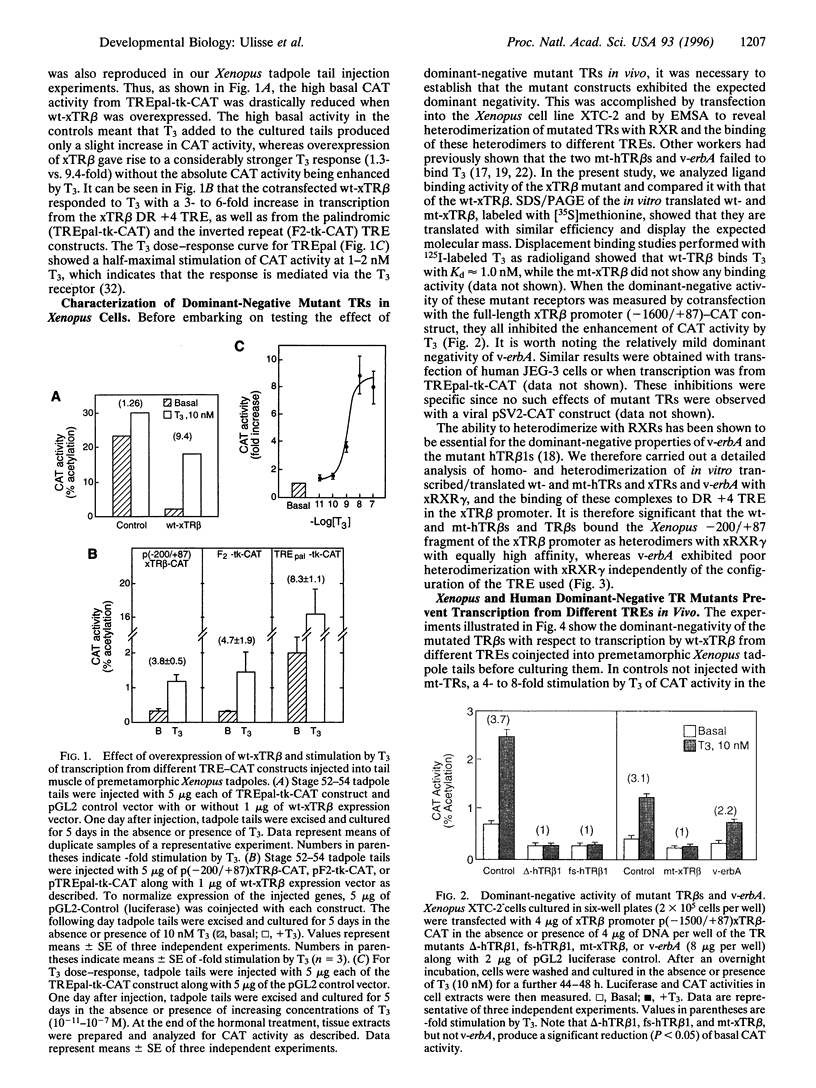
We describe a dominant-negative approach in vivo to assess the strong, early upregulation of thyroid hormone receptor beta (TR beta) gene in response to thyroid hormone, characteristic of the onset of natural and thyroid hormone-induced amphibian metamorphosis, 3,3',5-Triiodo-thyronine (T3) treatment of organ cultures of premetamorphic Xenopus tadpole tails coinjected in vivo with the wild-type Xenopus TR beta (wt-xTR beta) and three different thyroid responsive element chloramphenicol acetyltransferase (TRE-CAT) reporter constructs, including a direct repeat +4 (DR +4) element in the -200/+87 fragment of the xTR beta promoter, resulted in a 4- to 8-fold enhancement of CAT activity. Two human C-terminal TR beta 1 mutants (delta-hTR beta 1 and Ts-hTR beta 1), an artificial Xenopus C-terminal deletion mutant (mt-xTR beta), and the oncogenic viral homology v-erbA, none of which binds T3, inhibited this T3 response of the endogenous wt-xTR in Xenopus XTC-2 cells cotransfected with the -1600/+87 xTR beta promoter-CAT construct, the potency of the dominant-negative effect of these mutant TRs being a function of the strength of their heterodimerization with Xenopus retinoid X receptor gamma. Coinjection of the dominant-negative Xenopus and human mutant TR beta s into Xenopus tadpole tails totally abolished the T3 responsiveness of the wt-xTR beta with different TREs, including the natural DR +4 TRE of the xTR beta promoter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M., Matthews C., Collingwood T. N., Tone Y., Beck-Peccoz P., Chatterjee K. K. Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone. Identification of thirteen novel mutations in the thyroid hormone receptor beta gene. J Clin Invest. 1994 Aug;94(2):506–515. doi: 10.1172/JCI117362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S., Tata J. R. Prolactin prevents the autoinduction of thyroid hormone receptor mRNAs during amphibian metamorphosis. Dev Biol. 1992 Feb;149(2):463–467. doi: 10.1016/0012-1606(92)90301-v. [DOI] [PubMed] [Google Scholar]
- Baniahmad A., Steiner C., Köhne A. C., Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990 May 4;61(3):505–514. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- Chatterjee V. K., Nagaya T., Madison L. D., Datta S., Rentoumis A., Jameson J. L. Thyroid hormone resistance syndrome. Inhibition of normal receptor function by mutant thyroid hormone receptors. J Clin Invest. 1991 Jun;87(6):1977–1984. doi: 10.1172/JCI115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingwood T. N., Adams M., Tone Y., Chatterjee V. K. Spectrum of transcriptional, dimerization, and dominant negative properties of twenty different mutant thyroid hormone beta-receptors in thyroid hormone resistance syndrome. Mol Endocrinol. 1994 Sep;8(9):1262–1277. doi: 10.1210/mend.8.9.7838159. [DOI] [PubMed] [Google Scholar]
- Flug F., Copp R. P., Casanova J., Horowitz Z. D., Janocko L., Plotnick M., Samuels H. H. cis-acting elements of the rat growth hormone gene which mediate basal and regulated expression by thyroid hormone. J Biol Chem. 1987 May 5;262(13):6373–6382. [PubMed] [Google Scholar]
- Hao E., Menke J. B., Smith A. M., Jones C., Geffner M. E., Hershman J. M., Wuerth J. P., Samuels H. H., Ways D. K., Usala S. J. Divergent dimerization properties of mutant beta 1 thyroid hormone receptors are associated with different dominant negative activities. Mol Endocrinol. 1994 Jul;8(7):841–851. doi: 10.1210/mend.8.7.7984146. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yamakawa J., Yukioka M., Morisawa S. Filter-binding assay procedure for thyroid hormone receptors. Anal Biochem. 1983 Oct 1;134(1):176–183. doi: 10.1016/0003-2697(83)90280-4. [DOI] [PubMed] [Google Scholar]
- Iwamuro S., Tata J. R. Contrasting patterns of expression of thyroid hormone and retinoid X receptor genes during hormonal manipulation of Xenopus tadpole tail regression in culture. Mol Cell Endocrinol. 1995 Sep 22;113(2):235–243. doi: 10.1016/0303-7207(95)03634-j. [DOI] [PubMed] [Google Scholar]
- Kanamori A., Brown D. D. The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J Biol Chem. 1992 Jan 15;267(2):739–745. [PubMed] [Google Scholar]
- Kawahara A., Baker B. S., Tata J. R. Developmental and regional expression of thyroid hormone receptor genes during Xenopus metamorphosis. Development. 1991 Aug;112(4):933–943. doi: 10.1242/dev.112.4.933. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X., Tsai S. Y., O'Malley B. W., Tsai M. J. Ligand-dependent conformational changes in thyroid hormone and retinoic acid receptors are potentially enhanced by heterodimerization with retinoic X receptor. J Steroid Biochem Mol Biol. 1993 Dec;46(6):643–661. doi: 10.1016/0960-0760(93)90306-h. [DOI] [PubMed] [Google Scholar]
- Machuca I., Esslemont G., Fairclough L., Tata J. R. Analysis of structure and expression of the Xenopus thyroid hormone receptor-beta gene to explain its autoinduction. Mol Endocrinol. 1995 Jan;9(1):96–107. doi: 10.1210/mend.9.1.7760854. [DOI] [PubMed] [Google Scholar]
- Machuca I., Tata J. R. Autoinduction of thyroid hormone receptor during metamorphosis is reproduced in Xenopus XTC-2 cells. Mol Cell Endocrinol. 1992 Sep;87(1-3):105–113. doi: 10.1016/0303-7207(92)90238-2. [DOI] [PubMed] [Google Scholar]
- Ranjan M., Wong J., Shi Y. B. Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994 Oct 7;269(40):24699–24705. [PubMed] [Google Scholar]
- Refetoff S., Weiss R. E., Usala S. J. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993 Jun;14(3):348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Miyamoto T., Refetoff S., DeGroot L. J. Dominant negative transcriptional regulation by a mutant thyroid hormone receptor-beta in a family with generalized resistance to thyroid hormone. Mol Endocrinol. 1990 Dec;4(12):1988–1994. doi: 10.1210/mend-4-12-1988. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Schmitt J., Stunnenberg H., Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989 Jul 20;340(6230):242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Baker B. S., Machuca I., Rabelo E. M., Yamauchi K. Autoinduction of nuclear receptor genes and its significance. J Steroid Biochem Mol Biol. 1993 Aug;46(2):105–119. doi: 10.1016/0960-0760(93)90286-6. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays. 1993 Apr;15(4):239–248. doi: 10.1002/bies.950150404. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Kawahara A., Baker B. S. Prolactin inhibits both thyroid hormone-induced morphogenesis and cell death in cultured amphibian larval tissues. Dev Biol. 1991 Jul;146(1):72–80. doi: 10.1016/0012-1606(91)90447-b. [DOI] [PubMed] [Google Scholar]
- Ulisse S., Tata J. R. Thyroid hormone and glucocorticoid independently regulate the expression of estrogen receptor in male Xenopus liver cells. Mol Cell Endocrinol. 1994 Oct;105(1):45–53. doi: 10.1016/0303-7207(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Bale A. E., Gesundheit N., Weinberger C., Lash R. W., Wondisford F. E., McBride O. W., Weintraub B. D. Tight linkage between the syndrome of generalized thyroid hormone resistance and the human c-erbA beta gene. Mol Endocrinol. 1988 Dec;2(12):1217–1220. doi: 10.1210/mend-2-12-1217. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Brown D. D. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990 Nov;4(11):1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- Yen P. M., Wilcox E. C., Hayashi Y., Refetoff S., Chin W. W. Studies on the repression of basal transcription (silencing) by artificial and natural human thyroid hormone receptor-beta mutants. Endocrinology. 1995 Jul;136(7):2845–2851. doi: 10.1210/endo.136.7.7789309. [DOI] [PubMed] [Google Scholar]
- de Luze A., Sachs L., Demeneix B. Thyroid hormone-dependent transcriptional regulation of exogenous genes transferred into Xenopus tadpole muscle in vivo. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7322–7326. doi: 10.1073/pnas.90.15.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]