Summary
Candida albicans and Candida tropicalis are opportunistic fungal pathogens that can transition between white and opaque phenotypic states. White and opaque cells differ both morphologically and in their responses to environmental signals. In C. albicans, opaque cells respond to sexual pheromones by undergoing conjugation, while white cells are induced by pheromones to form sexual biofilms. Here, we show that sexual biofilm formation also occurs in C. tropicalis but, unlike C. albicans, biofilms are formed exclusively by opaque cells. C. tropicalis biofilm formation was dependent on the pheromone receptors Ste2 and Ste3, confirming the role of pheromone signaling in sexual biofilm development. Structural analysis of C. tropicalis sexual biofilms revealed stratified communities consisting of a basal layer of yeast cells and an upper layer of filamentous cells, together with an extracellular matrix. Transcriptional profiling showed that genes involved in pheromone signaling and conjugation were upregulated in sexual biofilms. Furthermore, FGR23, which encodes an agglutinin-like protein, was found to enhance both mating and sexual biofilm formation. Together, these studies reveal that C. tropicalis opaque cells form sexual biofilms with a complex architecture, and suggest a conserved role for sexual agglutinins in mediating mating, cell cohesion and biofilm formation.
Introduction
Candida species are the fourth most common cause of bloodstream infections in hospital patients (Wisplinghoff et al., 2004). While Candida albicans accounts for the majority of such infections, C. tropicalis is also commonly encountered in the clinic, particularly in individuals with hematologic malignancies (Sipsas et al., 2009; Papon et al., 2013). Multiple factors contribute to the ability of Candida species to infect the human host, including the capacity to form biofilms (Hasan et al., 2009). Biofilms are complex structures involving both cell-cell and cell-substrate interactions. C. albicans biofilm organization typically consists of a basal layer of yeast cells upon which a mesh-like layer of hyphal and pseudohyphal cells develops together with an extracellular matrix (Chandra et al., 2001; Bonhomme and d’Enfert, 2013). Biofilms are a growing concern in the medical community; they can be difficult to remove, can impede antifungal drug penetration, and can seed subsequent systemic infections (Donlan and Costerton, 2002; Blankenship and Mitchell, 2006; Finkel and Mitchell, 2011).
Candida species have the ability to colonize multiple clinical devices, including venous and urinary catheters and prosthetics (Febré et al., 1999; Crump and Collignon, 2000; Mohammadi et al., 2013). Similarly, the majority of cases of denture-associated stomatitis, which affects approximately 65% of denture wearers, are caused by Candida species forming biofilms on these devices (Davenport, 1970; Gendreau and Loewy, 2011). Candida species have been observed to colonize other abiotic surfaces important for dental health such as stainless steel and porcelain (Ratnasari et al., 2008; Li et al., 2010). Thus, the factors allowing Candida species to form biofilms on synthetic surfaces are of direct relevance for preventing both mucosal and systemic infections by these pathogens.
C. albicans biofilm formation is strongly influenced by the phenotypic state of the cell. While C. albicans can undergo multiple forms of phenotypic switching, the best-characterized switch is the ‘white-opaque’ transition, which has also been observed in the related species C. tropicalis and Candida dubliniensis (Slutsky et al., 1987; Pujol et al., 2004; Porman et al., 2011; Xie et al., 2012). The white-opaque switch is an epigenetic, heritable transition, in which cells reversibly switch between the white state, where cells appear round and smooth, and the opaque state, where cells are elongated and pimpled (Slutsky et al., 1987). In C. albicans, the two phenotypes are specialized for growth in different in vivo niches and respond differently to environmental stimuli. For example, while white cells are more adept at systemic infection, opaque cells are better suited for colonization of the skin (Kvaal et al., 1997; Kvaal et al., 1999; Xie et al., 2013). In addition, white cells undergo filamentation in response to a variety of environmental cues, including high temperature and elevated CO2 levels, while opaque cells undergo filamentation in response to distinct nutritional cues (Sudbery, 2011; Si et al., 2013). Finally, the two cell types exhibit very different behaviors when challenged with sexual pheromones. While pheromones initiate a mating response in C. albicans opaque cells (Miller and Johnson, 2002), white cells respond to pheromones by becoming cohesive and adherent, forming a ‘sexual’ biofilm (Daniels et al., 2006; Yi et al., 2008; Lin et al., 2013).
The regulation of sexual biofilm formation in C. albicans involves a pheromone-induced MAPK cascade (Daniels et al., 2006; Yi et al., 2008). Pheromones secreted by opaque cells interact with the G-protein coupled receptors Ste2 and Ste3 expressed on a and α cells, respectively. The pheromone signal is transduced via a heterotrimeric G protein complex and conserved MAPK cascade, culminating in activation of the transcription factor Cph1, an ortholog of Saccharomyces cerevisiae Ste12 (Bennett et al., 2003; Yi et al., 2008; Jones Jr. and Bennett, 2011; Lin et al., 2013). Candida mating types are defined by transcription factors encoded at the mating-type-like (MTL) locus (Hull and Johnson, 1999; Butler et al., 2009). Cells containing the MTLa locus mate with cells containing the MTLα locus, while MTLa/α cells are unable to mate (Hull et al., 2000; Magee and Magee, 2000; Tsong et al., 2003). Sexual biofilms in C. albicans are most efficient when formed by a or α white cells responding to pheromones secreted by opaque cells of the opposite mating type. These biofilms contrast with conventional (or asexual) biofilms that are pheromone-independent and formed preferentially by white a/α cells (Baillie and Douglas, 1999; Yi et al., 2011; Bonhomme and d’ Enfert, 2013). Studies comparing the genetic networks involved with sexual and asexual biofilm formation have found both shared and unique transcription factors regulating the two biofilm models. For example, Cph1 and MAPK signaling are essential only for sexual biofilm formation, while a core set of transcription factors (including Bcr1, Brg1, Rob1 and Tec1) are required for both sexual and asexual biofilm formation (Sahni et al., 2010; Nobile et al., 2012; Lin et al., 2013).
Why has the white-opaque switch evolved to regulate mating in a subset of Candida species? One proposal is that sexual biofilms formed by white cells are used to promote mating between rare opaque cells (Soll, 2009). Consistent with this model, experiments have established that sexual biofilms provide an optimal environment for mating to occur. Pheromone gradients accumulate to high concentrations within the sexual biofilm and aid chemotropic growth between opaque cells of opposite mating types (Daniels et al., 2006; Park et al., 2013). Thus, the raison d’etre for the white-opaque switch could be that sexual biofilms formed by white cells provide an appropriate environment for rare opaque cells to undergo successful conjugation in vivo.
Recently, a white-opaque phenotypic switch was discovered in C. tropicalis that shows similarities to that in C. albicans (Porman et al., 2011; Xie et al., 2012; Porman et al., 2013). The master regulator of the white-opaque switch in both Candida species is the transcription factor Wor1 (Huang et al., 2006; Zordan et al., 2006; Srikantha et al., 2006; Porman et al., 2011; Xie et al., 2012). In C. albicans, Wor1 acts as part of a transcriptional network to promote formation of the opaque state (Zordan et al., 2007; Hernday et al., 2013). In C. tropicalis, overexpression of WOR1 was shown to drive switching to the opaque state as well as increase filamentation and biofilm formation (Porman et al., 2013). These results indicate that significant crosstalk exists between the programs regulating the white-opaque switch and biofilm formation.
In this work, we address if mating pheromones can induce sexual biofilm formation in C. tropicalis. Similar to the case in C. albicans, we demonstrate that pheromone signaling in C. tropicalis drives the formation of sexual biofilms on synthetic surfaces. Surprisingly, however, C. tropicalis sexual biofilms are formed exclusively by opaque cells, and pheromone signaling is necessary but not sufficient for biofilm formation. This is in marked contrast to C. albicans, where activation of pheromone signaling in white cells was sufficient for induction of sexual biofilm formation. We also show that C. tropicalis sexual biofilms exhibit a stratified structure composed of a base layer of yeast-like cells, while the upper stratum is composed of highly filamentous cells. This structure contrasts with asexual biofilms induced by WOR1 overexpression in C. tropicalis, which have a homogeneous makeup of filamentous cells surrounded by a more extensive extracellular matrix. Furthermore, our studies led to the identification of Fgr23, an ortholog of S. cerevisiae a-agglutinin, as a mediator of sexual mating and biofilm formation in C. tropicalis. These results are discussed with respect to the role of the white-opaque switch and agglutinin-like proteins in regulating biofilm formation in Candida species.
Results
C. tropicalis Opaque Cells Form Sexual Biofilms
White-opaque phenotypic switching was recently discovered in C. tropicalis, where cells in the opaque phenotypic state were shown to mate a hundred times more efficiently than cells in the white state (Porman et al., 2011; Xie et al., 2012). To determine if C. tropicalis exhibits the same capacity as C. albicans to form sexual biofilms, mixtures of a and α cells from both phenotypic states were tested in a modified biofilm assay. C. tropicalis cells were incubated in Lee’s + Glucose medium on a polystyrene surface at 25°C for 48 hours, then non-adherent cells were removed by washing and the remaining adherent cells quantified. Mixtures of opaque a and α cells formed a robust biofilm under these conditions, while mixtures of white a and α cells did not (Fig. 1A). Analysis of the opaque biofilms revealed the presence of highly filamentous cells (49±1%). In contrast, cells of a single mating type produced very few filamentous cells on the polystyrene surface (Fig. 2D, 4±1%, p = 9.3E-6, Student’s T-test), and no filamentous cells were produced in mixtures of white a and α cells (Fig. 1A).
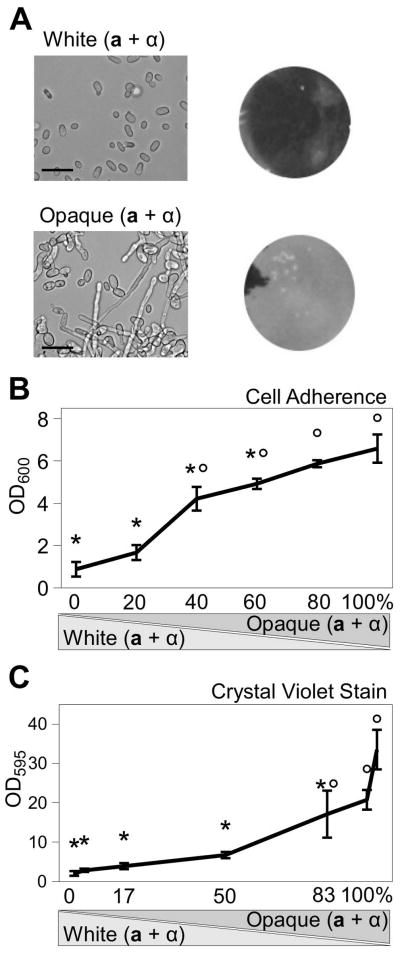
Figure 1.
C. tropicalis opaque cells form sexual biofilms. Mixtures of white and opaque a and α cells were compared for their ability to form biofilms. (A) A 50:50 mix of a and α cells were grown on polystyrene plates for 48 h and non-adherent cells removed by washing. Mixtures of white a and α cells showed little adherence to the plastic while a mixture of opaque a and α cells formed an adherent layer on the plastic. Left side, DIC microscopy of cells scraped from biofilm-inducing conditions, scale bar = 15 μm; right side, images of biofilms following washing of the plate. Black areas indicate unbound polystyrene while lighter-colored areas show adherent Candida cells. (B and C) White and opaque cell populations were mixed together at the indicated ratios. A 50:50 ratio of a and α cells was used for these experiments. Adherent cells were resuspended and quantified by absorbance at 600nm (B) or by crystal violet staining then absorbance at 595nm (C). Mean ± standard deviation (N≥4). * p<0.005 compared to 100% opaque cells. ° p<0.005 compared to 100% white cells (Tukey’s test).
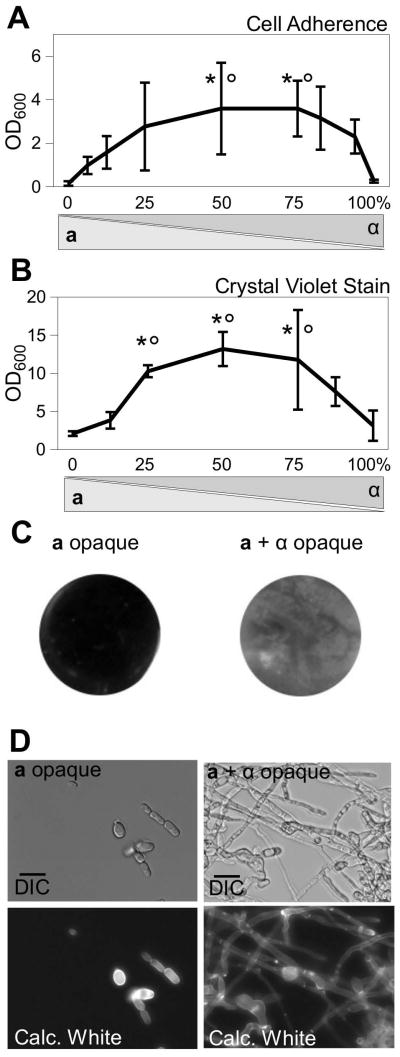
Figure 2.
Equal proportions of a and α cells yield maximum sexual biofilm formation. After growth of opaque cells for 48 h in polystyrene plates, the plates were washed to remove non-adherent cells then (A) adherent cells were resuspended and quantified by absorbance at 600nm, or (B) adherent cells were stained with crystal violet and quantified by absorbance at 595nm. Mean ± standard deviation (N≥3). * p<0.05 compared to 100% a cells. ° p<0.05 compared to 100% α cells. Tukey’s test. (C) Images of biofilms following washing of the plate. Black areas indicate unbound polystyrene while lighter-colored areas show adherent Candida cells. (D) Microscopy of cells scraped from biofilm-inducing conditions. Top panels are DIC images and bottom panels are corresponding calcofluor white-stained cells. Scale bars = 15 μm.
In C. albicans, opaque cells secrete pheromones that can stimulate biofilm formation in responding populations of white cells. In particular, the presence of 2–10% opaque cells mixed with 90–98% white cells was reported to result in optimal sexual biofilm formation (Daniels et al., 2006; Yi et al., 2008). To test if a similar phenomenon occurs in C. tropicalis, white and opaque cells were coincubated at different ratios and the resulting biofilm formation was quantified. As shown by cell density measurements (Fig. 1B) and crystal violet staining (Fig. 1C), C. tropicalis biofilms did not form from mixtures of white a and α cells, whereas robust biofilms formed from mixtures of opaque a and α cells. Overall, the efficiency of biofilm formation increased as white cells were substituted with opaque cells in these assays (Fig. 1B and C). Similar sexual biofilms were formed when opaque cultures were grown at 30 or 37°C (Supp. Fig. 1). The latter result presumably reflects the stability of C. tropicalis opaque cells at 37°C (Porman et al., 2011), whereas C. albicans opaque cells are unstable at this temperature and rapidly revert to the white state (Rikkerink et al., 1988; Lohse and Johnson, 2010).
Next, to determine the role of mating type in C. tropicalis biofilm formation, assays were performed using populations of opaque cells with varying proportions of the two mating types. Both cell adherence (Fig. 2A and C) and crystal violet staining (Fig. 2B) were maximal when both a and α cells were present, whereas biofilms did not form when pure populations of either a or α cells were used. The requirement for both mating types is seen in the efficiency of overall biofilm formation and also at a cell morphology level; experiments involving only a single mating type yielded cells with a predominantly yeast morphology, whereas filamentous cells were prevalent in experiments containing mixtures of both mating types (Fig. 2D).
Given these results, we reasoned that pheromone signaling between opaque a and α cells could be important for C. tropicalis sexual biofilm formation. Pheromone signaling was previously shown to be essential for the formation of sexual biofilms in C. albicans (Daniels et al., 2006; Yi et al., 2008). We therefore tested C. tropicalis strains containing deletions in the pheromone receptor genes STE2 and STE3 in a and α cells, respectively. STE2 encodes the α pheromone receptor expressed on a cells, while STE3 encodes the a pheromone receptor expressed on α cells, and loss of either receptor is sufficient to block mating (Porman et al., 2011). Deletion of either STE2 in a cells or STE3 in α cells effectively abolished sexual biofilm formation in C. tropicalis (Fig. 3A). This result establishes that pheromone signaling is essential for sexual biofilm formation in C. tropicalis.
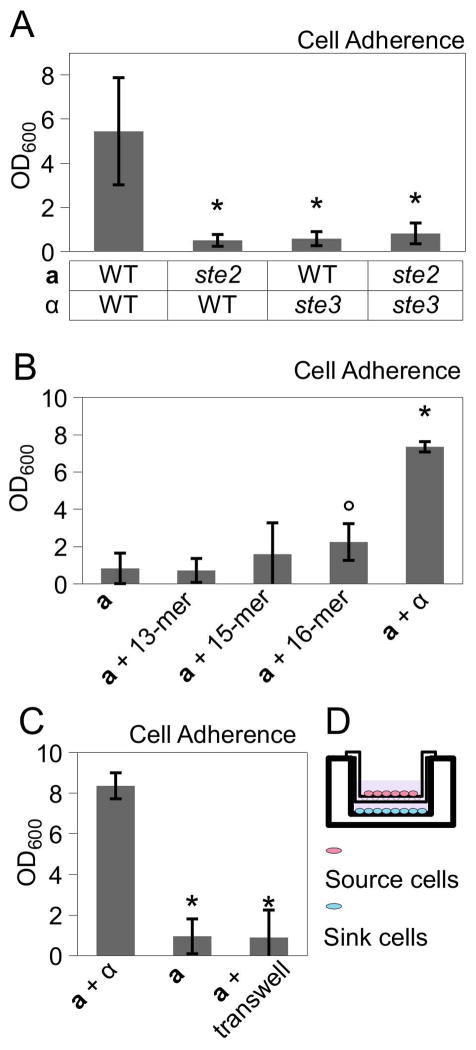
Figure 3.
Pheromone signaling is necessary, but not sufficient, for sexual biofilm formation by C. tropicalis opaque cells. (A) Quantification of biofilm formation by C. tropicalis strains lacking pheromone receptors for α pheromone (Ste2) or for a pheromone (Ste3). * p<0.001. Mann-Whitney test. (B) Biofilm formation by C. tropicalis a cells supplemented with synthetic α pheromone at 100 μg/ml (13-mer, 15-mer or 16-mer) or α cells. ° p<0.05 compared to a cells alone. * p<0.01 compared to a cells alone. Mann-Whitney test. (C) Biofilm formation of a cells (sink cells) exposed to pheromones produced by a mixed culture of opaque a and α cells (source cells), that were separated from sink cells by a semi-permeable transwell. * p<0.001, Tukey’s test. (D) Schematic diagram of transwell setup. Biofilm measurements show mean ± standard deviation (N≥4).
We next tested whether the addition of synthetic C. tropicalis pheromone was sufficient to drive biofilm formation in responding opaque cells. Several synthetic versions of C. tropicalis α pheromone were utilized including 13-mer, 15-mer and 16-mer (A-KF-KFRLTRYGWFSPN) peptides. Each of these peptides activated the C. tropicalis Ste2 receptor when heterologously expressed in C. albicans (Lin et al., 2011). Synthetic pheromones were added to opaque a cells at concentrations up to 100 μg/ml but did not induce efficient biofilm formation (Fig. 3B). This is despite the fact that C. tropicalis a cells effectively formed mating projections in response to synthetic α pheromones (Supp. Fig. 2). We also tested whether biofilm formation was more efficient when cells were responding to natural pheromones secreted by both a and α cells. For this purpose, a transwell system was employed in which a semipermeable membrane separated a mixture of opaque a and α ‘source’ cells from a population of opaque a ‘sink’ cells contacting the polystyrene surface (Fig. 3D). Using this system, biofilms were not formed by ‘sink’ cells, although the induction of mating projections was not as strong as in response to synthetic pheromones or to co-cultures of a and α cells (Fig. 3C and data not shown). Together, these results suggest that pheromone signaling is necessary, but not sufficient, to induce efficient biofilm formation in C. tropicalis opaque cells.
Our results establish that C. tropicalis forms sexual biofilms between opaque a and α cells in a pheromone-dependent manner. However, direct cell-to-cell contact between a and α cells also appears to be necessary for efficient biofilm production. In contrast, pheromone alone is sufficient to stimulate cohesion and biofilm formation amongst responding white cells in C. albicans (Daniels et al., 2006; Lin et al., 2013). It therefore appears that the mechanisms regulating sexual biofilm formation exhibit significant differences between C. tropicalis and C. albicans.
C. tropicalis Sexual Biofilms Exhibit a Complex Structure
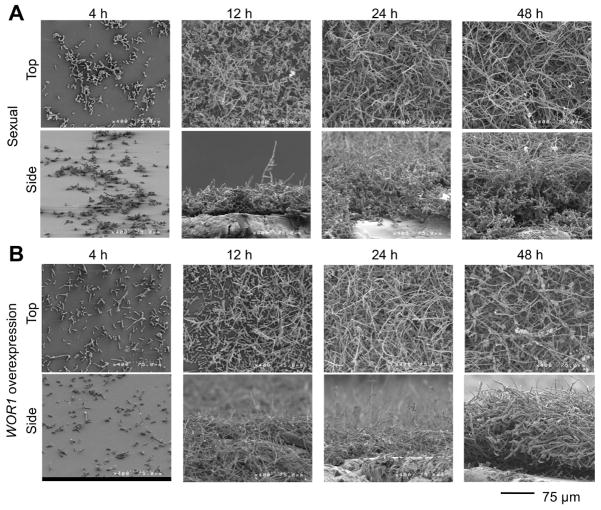
In C. albicans, the hallmarks of a mature biofilm include yeast and hyphal cell layers, as well as the presence of an extracellular matrix (Chandra et al., 2001; Andes et al., 2004; Finkel and Mitchell, 2011). To analyze the structure of C. tropicalis biofilms, scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) were employed. C. tropicalis sexual biofilms examined after co-culture of opaque a and α cells showed a bilayer structure; the cells closest to the polystyrene surface consisted mainly of chains of yeast cells and pseudohyphae, while the upper stratum consisted of much longer, filamentous cells (Fig. 4A, Supp. Fig. 3). Given that sexual biofilms are dependent on pheromone signaling, filamentous cells could include conjugation tubes, true hyphae, or pseudohyphae. Many of the long filaments contained septa with no obvious constrictions between cells, consistent with being true hyphae. However, other elongated cells did not exhibit septation and showed morphologies more consistent with mating projections (Fig. 2D and 4A). We therefore suggest that a combination of filamentous forms contributes to biofilm structure.
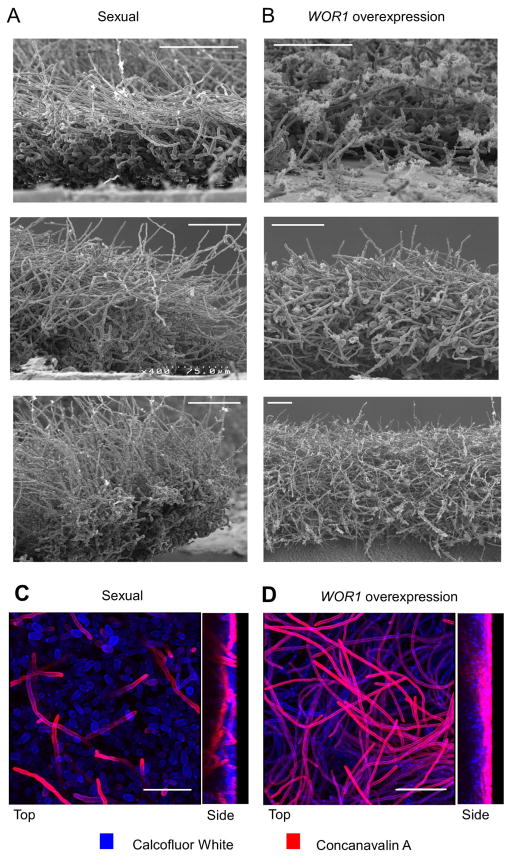
Figure 4.
Microscopic analysis of C. tropicalis sexual and WOR1 overexpression biofilms. Scanning electron micrographs showing side views of biofilms formed after 48 hours of development. (A) C. tropicalis a and α opaque cells (sexual biofilm). (B) WOR1 overexpression biofilm. 600X (top image), 400X (middle and bottom left images) or 200X magnification (bottom right). Scale bar = 60 μm (valid only in foreground). Confocal imaging of (C) sexual and (D) WOR1 overexpression biofilms. Left, top views projected from outer biofilm surface inward. Right, side views of biofilms. Images were taken with the same settings to allow for direct comparison. Scale bar = 36 μm. Blue = Calcoflour White. Red = Rhodamine conjugated Concanavalin A.
To examine the frequency of mating within the sexual biofilm, auxotrophic opaque a and α strains were mixed and grown as a biofilm for 48 hours. Mating frequencies were determined by recovering cells and plating onto selective media (see Experimental Procedures). We observed that productive mating occurred within the sexual biofilm, with a mating frequency of 3.1% (±3.0%). While this indicates relatively efficient mating, a mating frequency of 23.2% (±11.5%) was observed when cells were co-incubated on Lee’s + glucose agar plates under non-biofilm conditions (p= 0.031, Student’s T-test). Thus, under the conditions tested, mating within a biofilm was less efficient than that between opaque cells mixed under standard in vitro conditions.
In order to gain a better understanding for how the sexual biofilm structure develops, SEM was performed on biofilms at different stages of development. At early time points (4 h), a layer of yeast cells was deposited onto the polystyrene surface (Fig. 5A, Supp. Fig. 4A). Adhesion occurred in a subset of cells, as the majority of the cells were washed away post-inoculation. The initial cell distribution was sparse, but by 12 hours of growth the base layer had spread to occupy the majority of the polystyrene surface. In several regions, filamentous cells had also begun to appear. Filamentous growth was extensive by 24 hours, by which time a meshwork of filamentous cells and extracellular matrix (ECM) had grown over the base layer of yeast cells, and by 48 hours the biofilm was completely covered by filamentous cells. Distinct cell morphologies were observed between the two layers of the biofilm; the upper layer was composed mainly of filamentous cells, while the lower layer was composed mainly of yeast cells (Fig 4A and 5A). Biofilm thickness ranged from 30 μm to 100 μm. In addition to extensive filamentous growth, the upper stratum contained a considerable amount of ECM material, as evidenced by clusters of fibrous material throughout this layer (Fig. 5A, Supp. Fig. 4A).
Figure 5.
Time course of C. tropicalis biofilm development. (A) Representative SEM images of sexual biofilms consisting of a 50:50 mixture of opaque a and α cells. (B) Representative SEM images of biofilms from opaque cells overexpressing WOR1. Cells were grown on a polystyrene substrate and then samples were analyzed by scanning electron microscopy at 4, 12, 24, and 48 h following inoculation. Magnification = 400X. Scale bars = 75 μm.
To further determine the distribution pattern of the ECM, cells were stained with the lectin Concanavalin A (ConA). ConA binds mannan and glucan polysaccharides that compose the majority of the cell wall and extracellular matrix (Mandal et al., 1994; Chandra et al., 2001; Gow and Hube, 2012). It appeared that ECM was primarily associated with filamentous cells of the outer layer, although matrix was also evident at appreciable levels throughout the biofilm (Fig. 4C). Matrix production was variable, with some filamentous cells brightly stained for ECM while others were not. An alternative possibility was that differential staining of filamentous cells was due to variable stain penetration, as previously observed in C. albicans (Yi et al., 2011). To address this concern, confocal microscopy was performed on biofilms that were dispersed prior to fixation and staining (Supp. Fig. 5). ConA staining again localized to distinct subsets of cells, suggesting that some cells produce high quantities of ECM material or that ECM is preferentially bound to these cells.
Confocal microscopy was also performed on pure populations of opaque a cells that do not form a sexual biofilm. Although adhesion of some cells to the substrate was seen (Supp. Fig. 5D), dense growth did not occur, and there was a lack of ECM material. Furthermore, filamentous growth was rare in these single-sex experiments, despite the fact that the distribution of adherent cells was similar to that of early biofilms containing both mating types. We conclude that ECM production and filamentous growth occurs during maturation of sexual biofilms, which is dependent on pheromone signaling between the two mating types.
WOR1 Overexpression Biofilms Exhibit Distinct Structures from Sexual Biofilms
In C. tropicalis, overexpression of WOR1, the master transcriptional regulator of the white-opaque switch, was recently shown to induce filamentation on solid media and biofilm formation in cells grown on a polystyrene substrate (Porman et al., 2013). These biofilms involved opaque cells expressing WOR1 under the control of the strong constitutive promoter, TDH3, and were characterized by extensive filamentation, demonstrating links between the white-opaque switch, filamentation, and biofilm formation. In contrast to sexual biofilms, however, WOR1 overexpression biofilms were formed in cultures of a single mating type (opaque a, α, or a/α cells) (Porman et al., 2013). To determine if these biofilms were dependent on pheromone signaling, WOR1 was also overexpressed in cells lacking the Ste2 receptor. Despite the absence of the pheromone receptor, these cells produced biofilms that were indistinguishable from wildtype cells overexpressing WOR1 (data not shown). Thus, in contrast to the sexual biofilms formed by mixed cultures of opaque a and α cells, the biofilms formed by WOR1 overexpression are asexual biofilms.
We directly compared the structures of sexual biofilms with WOR1 overexpression biofilms using SEM and CLSM. Several notable differences were evident between the two models. First, whereas in sexual biofilms filamentous growth was restricted to the upper stratum of the biofilm, in WOR1 overexpression biofilms filamentation occurred throughout the biofilm structure (compare Fig. 4A and B, and Fig. 4C and D). Second, extensive filamentous growth occurred at earlier time points in the WOR1 overexpression biofilm (see 12 h time points in Fig. 5 and Supp. Fig. 4), consistent with high WOR1 expression enhancing filamentation (Porman et al., 2013). Third, ECM was consistently distributed in the WOR1 overexpression biofilms, whereas ECM production was more variable in the sexual biofilms (Fig. 4A and B). Quantification of ECM content by a Glucatell assay confirmed that both biofilm types contained more ECM than wildtype opaque a cells alone (p<0.01, Tukey’s test), and that WOR1 overexpression biofilms contained 36% more ECM than sexual biofilms, although this difference was not significant (Supp. Fig. 6). Despite these structural differences, overall biofilm thicknesses were similar in the two models as determined by SEM (Fig. 4). Together, these results demonstrate that C. tropicalis can form biofilms with different structural features, as exemplified by sexual and WOR1 overexpression biofilms.
Transcriptional Profiling of C. tropicalis Biofilm Formation
Cells within biofilms often exhibit distinct properties compared to cells grown under planktonic conditions. Transcriptional profiling was therefore used to compare non-biofilm populations (white wor1Δ a cells or opaque a cells), sexual biofilm populations (mixtures of opaque a and α cells), and WOR1 overexpression biofilms (opaque a cells overexpressing the WOR1 transcription factor). RNA was recovered from cells grown under each of these conditions, and the resulting cDNA was hybridized to custom C. tropicalis microarrays (see Experimental Procedures).
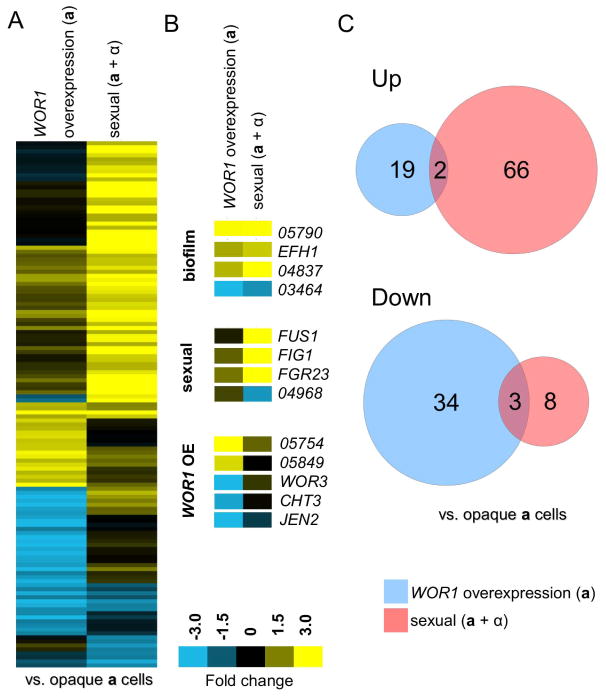
Most informative were the profiles of sexual and WOR1 overexpression biofilms compared to that of planktonic opaque a cells that do not form biofilms under the assay conditions (Fig. 6A). In this comparison, sexual biofilms upregulated 68 genes and downregulated 11 genes (>2 fold change), while WOR1 overexpression biofilms upregulated 21 genes and downregulated 37 genes (Fig. 6C, Supp. Table 3). Significant overlap was found between sets of biofilm-regulated genes with five genes exhibiting similar expression changes in the two biofilm populations (p= 6.9E-4, hypergeometric distribution, Supp. Table 4). Shared upregulated genes included EFH1, whose ortholog regulates filamentation in C. albicans, and CTRG_04837, whose ortholog (orf19.2457) is upregulated in conventional C. albicans biofilms (Doedt et al., 2004; Nobile et al., 2012). In addition, CTRG_03464 was downregulated in both C. tropicalis biofilm types, although its C. albicans ortholog (orf19.5282) is upregulated in rat biofilm models in C. albicans (Nett et al., 2009).
Figure 6.
Transcriptional profiling of C. tropicalis sexual and WOR1 overexpression biofilms. (A) Gene expression profiles comparing a WOR1 overexpression biofilm (pTDH3-WOR1 in opaque a cells) and a sexual biofilm (consisting of a 50:50 mixture of opaque a and α cells) to that of opaque a cells. Genes were filtered for greater than 2-fold expression change and clustered via average linkage (see Experimental Procedures). Upregulated genes are yellow and downregulated genes are blue. (B) Examples of genes regulated by both biofilm conditions, sexual biofilm conditions only, or WOR1 overexpression biofilm conditions only. (C) Venn diagrams illustrate biofilm genes with a 2-fold or more change in expression relative to wild type opaque a cells. Overlaps between transcriptional profiles were significant as determined by the hypergeometric distribution (Supp. Table. 4).
We also directly compared the transcriptional profile of C. tropicalis sexual biofilms to those formed by C. albicans (Supp. Table 5). We found that 13% of the genes upregulated in C. tropicalis sexual biofilms were also induced in C. albicans sexual biofilms (Lin et al., 2013), a significant overlap (p=3.3E-5, hypergeometric distribution, Supp. Table 4 and 5). This is in spite of the fact that sexual biofilm formation in C. albicans occurred in white cells whereas C. tropicalis sexual biofilms occurred in opaque cells. In contrast, a comparison of upregulated genes between C. tropicalis sexual biofilms and conventional C. albicans biofilms (Nobile et al., 2012) revealed no significant overlap (p=0.17, Supp. Table 4 and 5).
GO-Term analysis was performed on C. tropicalis sexual biofilm genes when compared either to the white control strain (Supp. Table 6) or the opaque control strain (Supp. Table 7). In these analyses, significant gene expression changes included those associated with multi-organism processes, pheromone response, conjugation, and biofilm formation. These results indicate that C. tropicalis biofilms undergo significant transcriptional changes in genes involved in specialized growth and communication in order to establish the new community structure. Sexual C. tropicalis biofilms showed specific induction of many genes associated with mating, including FIG1, FUS1, and KAR4. These sex-related genes accounted for 20 of the 55 genes with known orthologs that were upregulated greater than 2-fold in the sexual biofilm assays (Supp. Table 7), and establish a strong connection between the mating response and sexual biofilm formation in C. tropicalis (Fig. 6B).
WOR1 overexpression biofilms also included gene expression changes that were not observed in sexual biofilms. For example, WOR3, CHT3, JEN2, CTRG_05754, and CTRG_05849 were all regulated only in the WOR1-mediated biofilms (Fig. 6B). The transcriptional regulator WOR3 is known to be highly upregulated in both C. albicans opaque cells compared to white cells and in cells grown within biofilms (Nobile et al., 2012; Lohse et al., 2013). This was not the case in C. tropicalis, however, since planktonic opaque cells showed decreased expression of WOR3 relative to white cells (Porman et al., 2013), and WOR3 expression was further decreased in WOR1-overexpressing biofilms compared to control opaque cells (Fig. 6B).
Analysis of the Role of FGR23 and EFH1 in C. tropicalis Biofilm Formation
Cell adhesion and cell fusion are critical steps in the mating process, and it is possible that the physical interactions that facilitate these processes promote biofilm formation. We noted that C. tropicalis FGR23 (CTRG_02409) is strongly induced in sexual biofilms (5.5-fold) as well as weakly induced in WOR1 overexpression biofilms (1.4-fold). The FGR23 gene is an ortholog of S. cerevisiae AGA1, which encodes the anchorage subunit for the a-agglutinin heterodimer (Roy et al., 1991). AGA1 is expressed in both a and α cell types of S. cerevisiae and is also highly induced in response to pheromone (Roberts et al., 2000; Guo et al., 2000; Dranginis et al., 2007). Aga1 is a glycophosphatidylinositol-linked protein that anchors Aga2 to the cell surface of a cells, where it interacts with the complementary α-agglutinin expressed on the surface of α cells. Contacts between agglutinins facilitate the interaction of S. cerevisiae mating partners, and thus loss of Aga1 results in a reduction of mating efficiency in liquid medium (Roy et al., 1991; de Nobel et al., 1995). Deletion of Aga1 also disrupts mating on solid medium if combined with deletion of the related adhesin, Fig2 (Guo et al., 2000).
To determine if Fgr23 facilitates mating or biofilm formation in C. tropicalis, we generated fgr23Δ and FGR23 over-expression (pTDH3-FGR23) strains. Deletion of FGR23 resulted in a decrease in sexual biofilm production, as co-culture of a and α fgr23Δ strains generated biofilms that were significantly reduced compared to those formed by wild type strains (Fig. 7, p= 1.2E-4, Student’s T-test). Interestingly, deletion of FGR23 from either a or α cells alone also resulted in a defect in biofilm formation (data not shown). Reintegration of pTDH3-FGR23 into the deletion strain background significantly increased biofilm formation by 43% compared to deletion strains (p=0.049, Student’s T-test), partially restoring the wildtype phenotype. In contrast, integration of the pTDH3-FGR23 construct into the wildtype background did not significantly alter biofilm formation compared to wildtype (data not shown).
Figure 7.
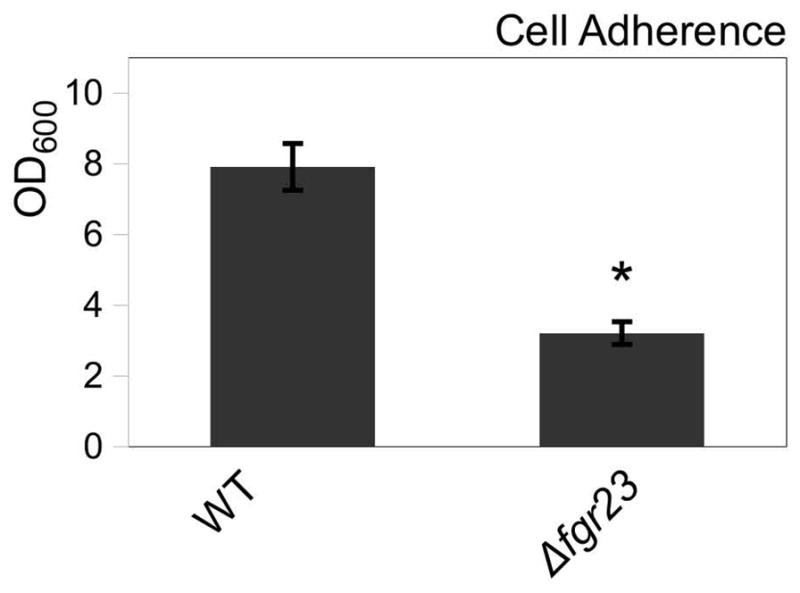
The sexual agglutinin-related protein Fgr23 promotes sexual biofilm development. Deletion of FGR23 decreases biofilm formation. Mean ± standard deviation (N≥4). * p<0.05. Mann-Whitney.
The role of FGR23 in mediating mating in C. tropicalis was also addressed. Wildtype cells and fgr23Δ mutants were compared for mating efficiency when a and α cells were co-incubated on Spider medium. Mutants lacking FGR23 were still able to fuse and mate, although mating efficiency was reduced by an order of magnitude between fgr23Δ cells under standard conditions (Supp. Fig. 7). This indicates that Fgr23 plays an important role in promoting both biofilm formation and conjugation in C. tropicalis.
Filamentation is an integral part of biofilm formation in Candida species, and many mutants defective in filamentation are unable to form biofilms or mount successful infections (Lo et al., 1997; Ramage et al., 2002; Nobile et al., 2006; Laforet et al., 2011). In C. albicans, EFH1 is a regulator of filamentous growth and works in concert with the related APSES transcription factor EFG1 (Doedt et al., 2004). Given that EFH1 was upregulated approximately 2-fold in both sexual and WOR1-overexpression biofilms, the EFH1 gene was deleted to determine its role in biofilm formation. However, deletion of EFH1 had no significant effect on sexual biofilm formation (Supp. Fig. 8). This could be due to functional redundancy with a yet-to-be identified transcription factor, or could simply indicate that EFH1 does not contribute to biofilm efficiency.
Discussion
Here, we report that C. tropicalis forms a sexual biofilm as a result of pheromone signaling between a and α cells. Biofilm formation required that cells be in the opaque phenotypic state, and cells in the white state did not contribute to overall biofilm formation. Structural analysis of the biofilms revealed them to be highly stratified, with the lower layer of the biofilm consisting mostly of yeast cells, while the upper stratum contained primarily filamentous cells. The latter included a mixture of both hyphae and conjugation tubes, and extracellular matrix was associated with sub-populations of filamentous cells. Overall, the structure of C. tropicalis sexual biofilms showed physical similarities to both sexual and asexual biofilms formed by C. albicans. These, too, consist of a basal layer of yeast cells upon which a meshwork of filamentous cells develops together with associated ECM material (Chandra et al., 2001; Daniels et al., 2006; Bonhomme and d’ Enfert, 2013; Daniels et al., 2013).
Previously, it was shown that C. albicans also forms a sexual biofilm (Daniels et al., 2006). In this species, biofilms result from white a or α cells responding to sexual pheromones produced by opaque cells of the opposite sex (Daniels et al., 2006; Park et al., 2013). Thus, the most efficient biofilms are generated by cultures containing a majority of white a and α cells mixed with a minority of opaque cells as a source of pheromone (Daniels et al., 2006). In both white and opaque cells, pheromone signaling occurs via a conserved MAPK cascade that culminates in activation of the Cph1/Ste12 transcription factor (Yi et al., 2008; Sahni et al., 2010; Lin et al., 2013). Additional factors regulating C. albicans sexual biofilm formation include the transcription factors Bcr1, Brg1, Rob1 and Tec1, downstream effectors such as the G1 cyclin-related protein Hgc1, as well as cell surface proteins Eap1, Csh1, and Pga10 (Sahni et al., 2009; Sahni et al., 2010; Lin et al., 2013).
The current studies establish that while both C. albicans and C. tropicalis form sexual biofilms, the regulation of biofilm formation has diverged since these species last shared a common ancestor. In C. albicans, pheromones induce white cells to produce robust biofilms, whereas opaque cells form weak, unstable biofilms (Daniels et al., 2006; Lin et al., 2013). The formation of sexual biofilms was shown to promote C. albicans mating in vitro (Park et al., 2013), as biofilms stabilized pheromone gradients between opaque cells, thereby promoting chemotropism between potential mating partners (Daniels et al., 2006). Given the low frequency of C. albicans mating in natural populations, it was suggested that the white-opaque switch has been retained in this species as a mechanism for promoting biofilm formation rather than for directing rare mating events (Soll, 2009).
In contrast to C. albicans, we demonstrate that sexual biofilms in C. tropicalis are formed exclusively by cells in the opaque state. In addition, whereas substrate adhesion in C. albicans cells can be induced by pheromone alone (Daniels et al., 2006), biofilm formation in C. tropicalis was most efficient when there was physical contact between a and α cells. Taken together, our studies establish that opaque cells of C. tropicalis are both the mating-competent form and the form most capable of generating a sexual biofilm. Furthermore, the thermostability of the opaque state in C. tropicalis allows sexual biofilms to form equally well at both 25°C and 37°C, potentially making sexual biofilm formation relevant for infections in the clinic.
Sexual biofilms in C. tropicalis were also compared to asexual biofilms formed due to overexpression of Wor1, the master regulator of the white-opaque switch. Wor1 forms part of a network of interacting transcriptional feedback loops that promotes stable and heritable formation of the opaque state (Huang et al., 2006; Zordan et al., 2006; Srikantha et al., 2006; Zordan et al., 2007; Hernday et al., 2013). While C. tropicalis white cells and single-sex cultures of opaque cells formed insubstantial biofilms, overexpression of Wor1 in a, α, or a/α cell types induced filamentation and resulted in biofilms that were comparable in size to sexual biofilms. Interestingly, the structures of Wor1-mediated and sexual biofilms were distinct. Whereas sexual biofilms showed clear stratification and variable ECM density, Wor1-mediated biofilms exhibited more homogeneous filamentation throughout the biofilm structure.
Expression profiling of C. tropicalis cells grown in the two biofilm models revealed both similarities and significant differences between these models. For example, only sexual biofilms were associated with the induction of mating genes, including pheromone and pheromone receptor genes, consistent with the key role of mating signaling in generating these biofilms. WOR1 overexpression and sexual biofilms also upregulated a shared set of genes, which included FGR23, EFH1, XOG1 and several uncharacterized genes. XOG1 encodes a glucanase that may potentially affect adhesion of cells to the substrate surface (González et al., 1997), although this possibility was not tested here.
The potential roles of FGR23 and EFH1 in sexual biofilm formation were further assessed by genetic analysis of these two genes. EFH1 is a transcription factor regulating filamentous growth in C. albicans (Doedt et al., 2004), yet deletion of this gene in C. tropicalis did not lead to an obvious colony or cellular phenotype and did not alter sexual biofilm formation. In contrast, deletion of the C. tropicalis FGR23 gene resulted in a significant decrease in sexual biofilm formation. FGR23 is an ortholog of AGA1 in S. cerevisiae, which encodes a component of the mating-type a-agglutinin. S. cerevisiae agglutinins consist of a-agglutinin, formed by a complex of Aga1 and Aga2, and the α-agglutinin, Sag1. These cell surface factors bind one another irreversibly during mating to promote conjugation (Lipke and Kurjan, 1992; Cappellaro et al., 1994; Dranginis et al., 2007). Significantly, the C. albicans Agglutinin-Like Sequence (ALS) gene family shows homology to S. cerevisiae α-agglutinin and this family plays a prominent role in C. albicans biofilm formation (García-Sánchez et al., 2004; Silverman et al., 2010).
We demonstrate that the a-agglutinin-related protein, Fgr23, is necessary for efficient formation of sexual biofilms by C. tropicalis opaque cells. C. tropicalis fgr23Δ mutants also showed a reduced mating efficiency compared to wildtype cells, indicating that the function of FGR23 may be conserved with that of S. cerevisiae AGA1. In S. cerevisiae, aga1 mutants show mating defects that are highly exacerbated by additional loss of the related FIG2 gene (Guo et al., 2000). In fact, S. cerevisiae proteins with adhesive properties are often interchangeable, even if they exhibit considerable differences at the amino acid sequence level. Thus, adhesins that function in flocculation can substitute for adhesins that promote mating and vice versa, if expressed at a high enough level and localized appropriately (Guo et al., 2000). It is therefore apparent that adhesins can play diverse roles in mating, flocculation, and filamentation, and that these functions depend more on their expression pattern than their primary sequence. In the case of C. tropicalis, the function of Fgr23 further supports these conclusions, as this protein promotes both mating and sexual biofilm formation. Additional experiments will be required to address the specific role of Fgr23, and whether this factor acts to promote cell-cell or cell-substrate interactions to enhance biofilm formation by this pathogen.
Experimental Procedures
Media
Preparation of YPD and SCD followed established protocols (Guthrie and Fink, 1991; Liu et al., 1994). Nourseothricin resistant strains (SATR) were selected on yeast extract peptone dextrose (YPD) plates containing 200 μg/ml nourseothricin. Spider medium contained 1% nutrient broth (v/v), 0.4% potassium phosphate (w/v), 2% mannitol (pH 7.2) (w/v), and 1.35% agar (w/v). Lee’s medium was formulated as previously described (Lee et al., 1975) with leucine, arginine and histidine added at 0.13%, 0.01% and 0.01%, respectively.
Strain and Plasmid Construction
Strains used in the current experiments are listed in Supplemental Table 1. WOR1 over-expression and deletion strains were constructed as described (Porman et al., 2013). Opaque strains were obtained by plating white strains on Lee’s + N-acetyl glucosamine medium for 2–7 days, then re-plating on Spider medium for 2–7 days and selecting opaque colonies following visual and microscopic examination of phenotype.
Deletion of FGR23 and EFH1 was performed using a previously described method (Noble and Johnson, 2005). 5′ and 3′ regions flanking the ORF (open reading frame) were PCR amplified (Supp. Table 2). HIS1 and ARG1 auxotrophic markers were PCR amplified from plasmids pSN64 (ARG4) and pSN52 (HIS1). ORF flanks were then combined with the auxotrophic markers using a fusion PCR protocol (Noble and Johnson, 2005). The resulting PCR product was used to transform auxotrophic strains. Correct integration was determined by PCR across the junctions of the integrating construct. The process was repeated to delete the second copy of the ORF, and then ORF PCR was performed to confirm deletion of the gene.
FGR23 complementation strains were constructed by PCR amplification of the FGR23 ORF and the TDH3 promoter, which were subsequently joined in a fusion PCR step. The PCR product was cloned into pSFS2a (Reuss et al., 2004) using KpnI and XhoI restriction sites. The resulting plasmid was used to transform wild type and fgr23 deletion strains. Integration was confirmed by PCR.
Biofilm Adherence Assay and Biofilm Staining
Strains were grown overnight at 25°C in Spider medium. Cells were centrifuged and resuspended in Lee’s + Glucose medium. One ml of Lee’s + Glucose medium was added per well of a 12-well plate. Either a single mating type or combinations of each mating type were added to each well for a total of 4 × 107 cells per well. Plates were incubated at room temperature for two days unless otherwise stated. Each well was then gently washed thrice with 1 ml of Phosphate-Buffered Solution (PBS) to remove non-adherent cells. Biofilms were imaged using a Bio-Rad Chemi-Doc imager. Optical density of biofilms was determined by scraping off adherent cells and resuspending them in PBS, diluting appropriately, and measuring absorbance at 600 nm on a Thermo-Scientific Nanodrop 2000c spectrophotometer.
Crystal violet staining was performed by decanting the wells and drying the adherent cells for 45 minutes. Cells were stained for 45 minutes with 385 μl of 0.4% aqueous crystal violet per well, followed by three 1 ml washes with water and destaining with 700 μl of 95% ethanol. The destain solution was diluted 10-fold into a 96-well plate, and absorbance at 595 nm was read using a BioTek Synergy HT plate reader.
Transwell Assay
Strains were grown overnight at 25°C in Spider medium. Cells were then centrifuged and resuspended in Lee’s + Glucose medium. One ml of Lee’s + Glucose medium was added to the lower region of each well of a transwell plate (Costar Transwell, 0.4 μm polycarbonate membrane,12-well), along with 2 × 107 cells of a single mating type. Then, 0.5 ml of Lee’s + glucose medium was added to the inner chamber, along with 2 × 107 cells of the opposite mating type, or a mix of the two mating types. Plates were incubated at room temperature for two days unless otherwise stated. The transwell chambers were removed, and the wells were washed and treated similarly to the previously described adherence assay.
Mating Assay
Auxotrophic strains were grown overnight at 25°C in Spider medium. Cultures were centrifuged and resuspended in either Lee’s + glucose medium or Spider medium as indicated. Approximately 2*107 cells of each mating type were spotted onto a filter (Millipore Cat. # AAWG01300) on a Lee’s + glucose agar plate, a Spider agar plate (if Spider liquid was used), or cultured in the above-described sexual biofilm assay. After two days, cells were scraped from the filters or biofilms and resuspended in 1 ml of water. Cells were diluted appropriately and plated on selective media to select for parental strains or progeny. CFUs were counted after two days, and mating success was calculated by dividing the number of progeny by the limiting parent.
Zygote staining
Cultures were grown overnight at 25°C in Spider medium, then centrifuged and resuspended in water. FITC-Concanavalin A (a strains) or Rhodamine-Concanavalin A (α strains) was added to each culture at a concentration of 25 μg/ml, and cells stained for one hour at 25°C. Cells were washed and 107 of each mating type were combined and plated onto a filter on Spider medium for one day at 25°C. Cells were recovered from the filters, resuspended in water, and analyzed via fluorescence microscopy using a Zeiss Axio Observer Z1.
Microscopy and Imaging
For scanning electron microscopy, biofilms were prepared similarly to the adherence assay except a 1 cm2 polystyrene square cut from a tissue-culture lid was added to each well prior to the addition of cells so that the majority of the biofilm formed on the square. Each biofilm square was fixed with 2.5% (w/v) glutaraldehyde in buffer (0.1 M Na-cacodylate, pH 7.4) overnight at 4°C. Squares were washed with the buffer, then subjected to 1% aqueous osmium tetroxide in buffer at 25°C for 90 minutes, then washed again. Squares were dehydrated using a 15% gradient ethanol series, dried in a critical point dryer, and finally coated with 20 nm 60:40 gold:palladium in an Emitech K550 sputter coater. Imaging was performed using a Hitachi S-2700 and Quartz PCI software.
For confocal microscopy, biofilms were also prepared on polystyrene squares, however they were fixed overnight in 4% formaldehyde at 4°C. Squares were then washed with water, followed by staining in 1 ml of 25 μg/ml Rhodamine-conjugated Concanavalin A for one hour in the dark at 25°C. Next, they were stained with 50 μg/ml Calcoflour White for one minute and washed with 1 ml water. Squares were then inverted onto coverslips and microscopy was performed on a Zeiss LSM 510 META microscope. Microscope settings were static across samples so that direct comparisons of staining intensities could be made when possible.
To image individual cells, biofilms were prepared similarly to the adherence assay, however, any staining was performed on the wells following washing, and then cells were scraped from the wells and suspended in approximately 10 μl water on a glass slide. Differential Interference Contrast (DIC) and fluorescence images were collected using a Zeiss Axio Observer Z1.
RNA Purification and Microarray Analysis
Biofilms were prepared similarly to the adherence assay, except after the two-day incubation period biofilms were not washed. Rather, adherent cells were resuspended in the medium with non-adherent cells. RNA was extracted from cells using the RiboPure-Yeast Kit (Ambion). DNA was removed using DNAseI (Ambion) followed by phenol/chloroform extraction. Aminoallyl-labeled cDNA was generated from the purified RNA, and then hybridized to a custom C. tropicalis Agilent microarray, as previously described (Porman et al., 2011). The microarrays were read and quantified using a Genepix 4000 scanner with GENEPIX PRO v3.0 (Axon Instruments) software. Data was then normalized using Goulphar web-based software (http://transcriptome.ens.fr/goulphar) with the following conditions: Foreground – Median, Background – Median background subtraction, Normalization – Print-tip group lowess (200), Flags – filter all, Saturating spots – remove if intensity is >50k in a single channel. Data was organized, averaged (two replicates each) and labeled in Microsoft Excel using known C. albicans or S. cerevisiae gene ortholog names for unannotated genes (candidagenome.org, yeastgenome.org, cgob3.ucd.ie, or www.broadinstitute.org/regev/orthogroups). Data was analyzed using Cluster v2.11 and visualized using TreeView v1.60 (http://rana.lbl.gov/EisenSoftware.htm). Cluster was also used for filtering results, where differentially regulated genes were selected by applying a >2-fold change cutoff. Array data is available from GEO (GSE52634 and GSE43267). Gene ontologies were determined using the CGD Gene Ontology Mapper (www.candidagenome.org/cgi-bin/GO/goTermMapper), and p-values for GO terms were calculated using a hypergeometric distribution with Multiple Hypothesis Correction. The p-value for comparing overlap between the sexual and WOR1 overexpression biofilms was generated using a hypergeometric distribution in Microsoft Excel.
Extracellular Matrix Quantification
Extracellular matrix was quantified similarly to previously described methods (Taff et al., 2012) using the Glucatell © assay (Associates of Cape Cod, Inc. Falmouth, MA). Briefly, biofilms formed in the biofilm adherence assay were scraped and resuspended in the supernatant. Next, samples were sonicated for 10 minutes to disassociate cells and matrix material, and then centrifuged three times at 4,500xg for 20 minutes to remove cells. Glucan concentration of the recovered supernatants was then determined per the manufacturer’s supplied protocol.
Statistical Analysis
Statistical analysis was performed using the PAST software package (folk.uio.no/ohammer/past/). All datasets were tested for normality using the Anderson-Darling method, and then the appropriate tests were performed. Tests across sample sets, such as one-way ANOVA or Kruskal-Wallis, were performed first, followed by between-sample tests, including Student’s T-test, Tukey’s test or Mann-Whitney test, as indicated in figure legends.
Supplementary Material
Supplementary Figure 1. Temperature sensitivity of C. tropicalis sexual biofilms. Sexual biofilms were developed at the indicated temperature for 48 h. Mean ± standard deviation (N=3). One-way ANOVA.
Supplementary Figure 2. Opaque a cells respond to synthetic pheromones by forming mating projections. Images of cells from biofilm assay in Fig. 3B. 100 μg/ml of C. tropicalis synthetic pheromone (a 13-mer, 15-mer or 16-mer version) was added to each well containing opaque a cells and incubated at 25°C for two days. Arrows indicate mating projections. Scale bars = 20 μm.
Supplementary Figure 3. Confocal imaging of 48-hour sexual and WOR1 overexpression biofilms stained with Calcofluor White. (A) Horizontal slices through the biofilm structures at indicated heights above the polystyrene substrate. (B) Vertical slices through five equally spaced sections of the biofilms. (C) 3-D projections of biofilm structures from outer biofilm surface inward. Scale bar = 50 μm.
Supplementary Figure 4. Additional time course images of C. tropicalis biofilm development. (A) Representative SEM time course of sexual biofilms consisting of a 50:50 mixture of opaque a and α cells. (B) Biofilms due to overexpression of the Wor1 transcription factor in opaque a cells. Cells were grown on a polystyrene substrate and samples analyzed by scanning electron microscopy at 4, 12, 24, and 48 h following inoculation. Magnification as indicated. Scale bars = (white) 300 μm, (blue) 75 μm, (red) 30 μm.
Supplementary Figure 5. Confocal imaging of dispersed cells from C. tropicalis 48-hour biofilms. Dispersed cells from (A) opaque a cells, (B) sexual biofilms, or (C) WOR1 overexpression biofilms. (D) Top view (left) and side view (right) projections of opaque a cells grown on the polystyrene substrate. Left, top view projected from outer biofilm surface inward. Right, side view. For D, images were taken with the same settings as Figures 4C and 4D. Scale bars = 36 μm. Blue = Calcoflour White. Red = Rhodamine-conjugated Concanavalin A.
Supplementary Figure 6. Extracellular matrix content of sexual and WOR1 overexpression biofilms. Glucan content quantified using the Glucatell © assay. Mean ± standard deviation (N=3). * p<0.01. Tukey’s test.
Supplementary Figure 7. Analysis of the role of FGR23 in mating. (A) Zygote formation is not abolished by deletion of FGR23. Opaque a and α WT and fgr23Δ cells were stained and mated on Spider medium for 24 h. a cells stained with FITC-conjugated Concanavalin A. α cells stained with Rhodamine-conjugated Concanavalin A. Arrows indicate zygotes. Scale bar = 15 μm. (B) FGR23 deletion significantly decreases mating frequency. a and α cells were mixed then mated on Spider medium for 48 h (see Experimental Procedures). Mean ± standard error (N=4). * p<0.05. Student’s T-test (log-transform).
Supplementary Figure 8. Deletion of EFH1 does not significantly affect sexual biofilm formation. Mean ± standard deviation (N=4). One-way ANOVA.
Supplementary Table 1. C. tropicalis strains used in this study.
Supplementary Table 2. Oligonucleotides used in this study. Lowercase letters denote adapter and restriction site sequences.
Supplementary Table 3. Fold-change gene expression of sexual biofilms and WOR1 overexpression biofilms compared to opaque a cells under biofilm-forming conditions. Sheet 1: Expression levels and C. albicans orthologs of all identified C. tropicalis genes. Sheet 2: List of all genes with greater than 2-fold change in either biofilm.
Supplementary Table 4. Statistical analysis of C. tropicalis sexual biofilm and WOR1 overexpression biofilm regulated genes with C. albicans biofilm and C. tropicalis white-opaque regulated genes.
Supplementary Table 5. Comparison of gene expression in C. tropicalis sexual and WOR1 overexpression biofilms to C. tropicalis white- and opaque-specific genes (see Porman et al., 2011), C. albicans sexual biofilm-specific genes (see Lin et al., 2013) and C. albicans conventional (asexual) biofilm-specific genes (see Nobile et al., 2012).
Supplementary Table 6. Gene Ontology term analysis of genes showing a 2-fold or greater change in expression level in either sexual biofilms or WOR1 overexpression biofilms when compared to white (wor1Δ) a cells.
Supplementary Table 7. Gene Ontology term analysis of genes showing a 2-fold or greater change in expression level in sexual biofilms, WOR1 overexpression biofilms, or both biofilms when compared to wild type opaque a cells.
Acknowledgments
We would like to thank Allison Porman and Na Wang for generating several strains used in this study and members of the Bennett lab for comments on the manuscript. S.K.J. is supported by pre-doctoral fellowship F31DE022701 and M.P.H. is supported by a training grant for Graduate Assistance in Areas of National Need (P200A100100). R.J.B. is supported by NIAID (AI081704) and a PATH Award from the Burroughs Wellcome Fund.
References
- Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun. 2004;72:6023–6031. doi: 10.1128/IAI.72.10.6023-6031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie GS, Douglas LJ. Role of dimorphism in the development of Candida albicans biofilms. J Med Microbiol. 1999;48:671–679. doi: 10.1099/00222615-48-7-671. [DOI] [PubMed] [Google Scholar]
- Bennett RJ, Uhl MA, Miller MG, Johnson AD. Identification and characterization of a Candida albicans mating pheromone. Mol Cell Biol. 2003;23:8189–8201. doi: 10.1128/MCB.23.22.8189-8201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9:588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Bonhomme J, Enfert Cd’. Candida albicans biofilms: building a heterogeneous, drug-tolerant environment. Curr Opin Microbiol. 2013 doi: 10.1016/j.mib.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Butler G, Rasmussen MD, Lin MF, Santos MAS, Sakthikumar S, Munro CA, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellaro C, Baldermann C, Rachel R, Tanner W. Mating type-specific cell-cell recognition of Saccharomyces cerevisiae: cell wall attachment and active sites of a- and alpha-agglutinin. EMBO J. 1994;13:4737–4744. doi: 10.1002/j.1460-2075.1994.tb06799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Collignon PJ. Intravascular catheter-associated infections. Eur J Clin Microbiol Infect Dis. 2000;19:1–8. doi: 10.1007/s100960050001. [DOI] [PubMed] [Google Scholar]
- Daniels KJ, Park YN, Srikantha T, Pujol C, Soll DR. Impact of environmental conditions on the form and function of Candida albicans biofilms. Eukaryot Cell. 2013;12:1389–1402. doi: 10.1128/EC.00127-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels KJ, Srikantha T, Lockhart SR, Pujol C, Soll DR. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 2006;25:2240–2252. doi: 10.1038/sj.emboj.7601099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport JC. The oral distribution of Candida in denture stomatitis. Brit Dent J. 1970;129:151–156. doi: 10.1038/sj.bdj.4802540. [DOI] [PubMed] [Google Scholar]
- Doedt T, Krishnamurthy S, Bockmühl DP, Tebarth B, Stempel C, Russell CL, et al. APSES proteins regulate morphogenesis and metabolism in Candida albicans. Mol Biol Cell. 2004;15:3167–3180. doi: 10.1091/10.1091/mbc.E03-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranginis AM, Rauceo JM, Coronado JE, Lipke PN. A biochemical guide to yeast adhesins: glycoproteins for social and antisocial occasions. Microbiol Mol Biol Rev. 2007;71:282–294. doi: 10.1128/MMBR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febré N, Silva V, Medeiros EaS, Wey SB, Colombo AL, Fischman O. Microbiological characteristics of yeasts isolated from urinary tracts of intensive care unit patients undergoing urinary catheterization. J Clin Microbiol. 1999;37:1584–1586. doi: 10.1128/jcm.37.5.1584-1586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol. 2011;9:109–118. doi: 10.1038/nrmicro2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sánchez S, Aubert S, Iraqui I, Janbon G, Ghigo JM, d’Enfert C. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot Cell. 2004;3:536–545. doi: 10.1128/EC.3.2.536-545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau L, Loewy ZG. Epidemiology and etiology of denture stomatitis. J Prosthodont. 2011;20:251–260. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- González MM, Díez-Orejas R, Molero G, Alvarez AM, Pla J, Nombela C, Sánchez-Pérez M. Phenotypic characterization of a Candida albicans strain deficient in its major exoglucanase. Microbiol Read Engl. 1997;143 (Pt 9):3023–3032. doi: 10.1099/00221287-143-9-3023. [DOI] [PubMed] [Google Scholar]
- Gow NAR, Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol. 2012;15:406–412. doi: 10.1016/j.mib.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Guo B, Styles CA, Feng Q, Fink GR. A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc Natl Acad Sci U S A. 2000;97:12158–12163. doi: 10.1073/pnas.220420397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular Biology: Part A–C. Academic Press, Incorporated; 1991. [Google Scholar]
- Hasan F, Xess I, Wang X, Jain N, Fries BC. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009;11:753–761. doi: 10.1016/j.micinf.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernday AD, Lohse MB, Fordyce PM, Nobile CJ, DeRisi JL, Johnson AD. Structure of the transcriptional network controlling white-opaque switching in Candida albicans. Mol Microbiol. 2013;90:22–35. doi: 10.1111/mmi.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Wang H, Chou S, Nie X, Chen J, Liu H. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci U S A. 2006;103:12813–12818. doi: 10.1073/pnas.0605270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CM, Johnson AD. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science. 1999;285:1271–1275. doi: 10.1126/science.285.5431.1271. [DOI] [PubMed] [Google Scholar]
- Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- Jones SK, Jr, Bennett RJ. Fungal mating pheromones: Choreographing the dating game. Fungal Genet Biol. 2011;48:668–676. doi: 10.1016/j.fgb.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaal C, Lachke SA, Srikantha T, Daniels K, McCoy J, Soll DR. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect Immun. 1999;67:6652–6662. doi: 10.1128/iai.67.12.6652-6662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaal CA, Srikantha T, Soll DR. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect Immun. 1997;65:4468–4475. doi: 10.1128/iai.65.11.4468-4475.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforet L, Moreno I, Sánchez-Fresneda R, Martínez-Esparza M, Martínez JP, Argüelles JC, et al. Pga26 mediates filamentation and biofilm formation and is required for virulence in Candida albicans. FEMS Yeast Res. 2011;11:389–397. doi: 10.1111/j.1567-1364.2011.00727.x. [DOI] [PubMed] [Google Scholar]
- Lee KL, Buckley HR, Campbell CC. An amino acid liquid synthetic medium for the development of mycellal and yeast forms of Candida albicans. Med Mycol. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Li L, Finnegan MB, Özkan S, Kim Y, Lillehoj PB, Ho CM, et al. In vitro study of biofilm formation and effectiveness of antimicrobial treatment on various dental material surfaces. Mol Oral Microbiol. 2010;25:384–390. doi: 10.1111/j.2041-1014.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- Lin CH, Choi A, Bennett RJ. Defining pheromone-receptor signaling in Candida albicans and related asexual Candida species. Mol Biol Cell. 2011;22:4918–4930. doi: 10.1091/mbc.E11-09-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Kabrawala S, Fox EP, Nobile CJ, Johnson AD, Bennett RJ. Genetic control of conventional and pheromone-stimulated biofilm formation in Candida albicans. [Accessed May 29, 2013];PLoS Pathog. 2013 9 doi: 10.1371/journal.ppat.1003305. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3630098/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipke PN, Kurjan J. Sexual agglutination in budding yeasts: structure, function, and regulation of adhesion glycoproteins. Microbiol Rev. 1992;56:180–194. doi: 10.1128/mr.56.1.180-194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Lohse MB, Hernday AD, Fordyce PM, Noiman L, Sorrells TR, Hanson-Smith V, et al. Identification and characterization of a previously undescribed family of sequence-specific DNA-binding domains. Proc Natl Acad Sci U S A. 2013;110:7660–7665. doi: 10.1073/pnas.1221734110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MB, Johnson AD. Temporal anatomy of an epigenetic switch in cell programming: the white-opaque transition of C. albicans. Mol Microbiol. 2010;78:331–343. doi: 10.1111/j.1365-2958.2010.07331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- Mandal DK, Bhattacharyya L, Koenig SH, Brown RD, Oscarson S, Brewer CF. Studies of the Binding Specificity of Concanavalin A. Nature of the Extended Binding Site for Asparagine-Linked Carbohydrates. Biochemistry (Mosc) 1994;33:1157–1162. doi: 10.1021/bi00171a015. [DOI] [PubMed] [Google Scholar]
- Miller MG, Johnson AD. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293–302. doi: 10.1016/s0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- Mohammadi S, Mohammadi J, Forrest GN. Epidemiology of Candida Endocarditis. Curr Fungal Infect Rep. 2013:1–5. [Google Scholar]
- Nett JE, Lepak AJ, Marchillo K, Andes DR. Time course global gene expression analysis of an in vivo Candida biofilm. J Infect Dis. 2009;200:307–313. doi: 10.1086/599838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nobel H, Pike J, Lipke PN, Kurjan J. Genetics of a-agglutunin function in Saccharomyces cerevisiae. Mol Gen Genet MGG. 1995;247:409–415. doi: 10.1007/BF00293141. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, et al. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006;2:e63. doi: 10.1371/journal.ppat.0020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, et al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–138. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papon N, Courdavault V, Clastre M, Bennett RJ. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog. 2013;9:e1003550. doi: 10.1371/journal.ppat.1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y-N, Daniels KJ, Pujol C, Srikantha T, Soll DR. Candida albicans Forms a Specialized “Sexual” As Well As “Pathogenic” Biofilm. Eukaryot Cell. 2013 doi: 10.1128/EC.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porman AM, Alby K, Hirakawa MP, Bennett RJ. Discovery of a phenotypic switch regulating sexual mating in the opportunistic fungal pathogen Candida tropicalis. Proc Natl Acad Sci U S A. 2011;108:21158–21163. doi: 10.1073/pnas.1112076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porman AM, Hirakawa MP, Jones SK, Wang N, Bennett RJ. MTL–independent phenotypic switching in Candida tropicalis and a dual role for Wor1 in regulating switching and filamentation. PLoS Genet. 2013;9:e1003369. doi: 10.1371/journal.pgen.1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, VandeWalle K, López-Ribot JL, Wickes BL. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett. 2002;214:95–100. doi: 10.1111/j.1574-6968.2002.tb11330.x. [DOI] [PubMed] [Google Scholar]
- Ratnasari A, Hasegawa K, Yoshihara K, Nagaoka N, Kokeguchi S, Nishigawa G, et al. Deformation of mesh type stainless palatal plate of maxillary complete denture and the growth of microorganisms. Nihon Hotetsu Shika Gakkai Zasshi. 2008;52:555–558. doi: 10.2186/jjps.52.555. [DOI] [PubMed] [Google Scholar]
- Reuss O, Vik A, Kolter R, Morschhäuser J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004;341:119–127. doi: 10.1016/j.gene.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Rikkerink EH, Magee BB, Magee PT. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988;170:895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Lu CF, Marykwas DL, Lipke PN, Kurjan J. The AGA1 product is involved in cell surface attachment of the Saccharomyces cerevisiae cell adhesion glycoprotein a-agglutinin. Mol Cell Biol. 1991;11:4196–4206. doi: 10.1128/mcb.11.8.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni N, Yi S, Daniels KJ, Pujol C, Soll DR. Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in Candida albicans. PLoS Pathog. 2009;5:e1000601. doi: 10.1371/journal.ppat.1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni N, Yi S, Daniels KJ, Huang G, Srikantha T, Soll DR. Tec1 mediates the pheromone response of the white phenotype of Candida albicans: Insights into the evolution of new signal transduction pathways. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Hernday AD, Hirakawa MP, Johnson AD, Bennett RJ. Candida albicans white and opaque cells undergo distinct programs of filamentous growth. PLoS Pathog. 2013;9:e1003210. doi: 10.1371/journal.ppat.1003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RJ, Nobbs AH, Vickerman MM, Barbour ME, Jenkinson HF. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect Immun. 2010;78:4644–4652. doi: 10.1128/IAI.00685-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipsas NV, Lewis RE, Tarrand J, Hachem R, Rolston KV, Raad II, Kontoyiannis DP. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer. 2009;115:4745–4752. doi: 10.1002/cncr.24507. [DOI] [PubMed] [Google Scholar]
- Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll DR. Why does Candida albicans switch? FEMS Yeast Res. 2009;9:973–989. doi: 10.1111/j.1567-1364.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- Srikantha T, Borneman AR, Daniels KJ, Pujol C, Wu W, Seringhaus MR, et al. TOS9 regulates white-opaque switching in Candida albicans. Eukaryot Cell. 2006;5:1674–1687. doi: 10.1128/EC.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, et al. A Candida biofilm-induced pathway for matrix glucan delivery: Implications for drug resistance. PLoS Pathog. 2012;8:e1002848. doi: 10.1371/journal.ppat.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong AE, Miller MG, Raisner RM, Johnson AD. Evolution of a combinatorial transcriptional circuit: a case study in yeasts. Cell. 2003;115:389–399. doi: 10.1016/s0092-8674(03)00885-7. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- Xie J, Du H, Guan G, Tong Y, Kourkoumpetis TK, Zhang L, et al. N-Acetylglucosamine induces white-to-opaque switching and mating in Candida tropicalis, providing new insights into adaptation and fungal sexual evolution. Eukaryot Cell. 2012;11:773–782. doi: 10.1128/EC.00047-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Tao L, Nobile CJ, Tong Y, Guan G, Sun Y, et al. White-opaque switching in natural MTLa/α Isolates of Candida albicans: evolutionary implications for roles in host adaptation, pathogenesis, and sex. PLoS Biol. 2013;11:e1001525. doi: 10.1371/journal.pbio.1001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Sahni N, Daniels KJ, Lu KL, Huang G, Srikantha T, Soll DR. Self-induction of a/a or alpha/alpha biofilms in Candida albicans is a pheromone-based paracrine system requiring switching. Eukaryot Cell. 2011;10:753–760. doi: 10.1128/EC.05055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Sahni N, Daniels KJ, Lu KL, Srikantha T, Huang G, et al. Alternative mating type configurations (a/α versus a/a or α/α) of Candida albicans result in alternative biofilms regulated by different pathways. PLoS Biol. 2011;9:e1001117. doi: 10.1371/journal.pbio.1001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Sahni N, Daniels KJ, Pujol C, Srikantha T, Soll DR. The same receptor, G Protein, and mitogen-activated protein kinase pathway activate different downstream regulators in the alternative white and opaque pheromone responses of Candida albicans. Mol Biol Cell. 2008;19:957–970. doi: 10.1091/mbc.E07-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan RE, Galgoczy DJ, Johnson AD. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci U S A. 2006;103:12807–12812. doi: 10.1073/pnas.0605138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan RE, Miller MG, Galgoczy DJ, Tuch BB, Johnson AD. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 2007;5:e256. doi: 10.1371/journal.pbio.0050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Temperature sensitivity of C. tropicalis sexual biofilms. Sexual biofilms were developed at the indicated temperature for 48 h. Mean ± standard deviation (N=3). One-way ANOVA.
Supplementary Figure 2. Opaque a cells respond to synthetic pheromones by forming mating projections. Images of cells from biofilm assay in Fig. 3B. 100 μg/ml of C. tropicalis synthetic pheromone (a 13-mer, 15-mer or 16-mer version) was added to each well containing opaque a cells and incubated at 25°C for two days. Arrows indicate mating projections. Scale bars = 20 μm.
Supplementary Figure 3. Confocal imaging of 48-hour sexual and WOR1 overexpression biofilms stained with Calcofluor White. (A) Horizontal slices through the biofilm structures at indicated heights above the polystyrene substrate. (B) Vertical slices through five equally spaced sections of the biofilms. (C) 3-D projections of biofilm structures from outer biofilm surface inward. Scale bar = 50 μm.
Supplementary Figure 4. Additional time course images of C. tropicalis biofilm development. (A) Representative SEM time course of sexual biofilms consisting of a 50:50 mixture of opaque a and α cells. (B) Biofilms due to overexpression of the Wor1 transcription factor in opaque a cells. Cells were grown on a polystyrene substrate and samples analyzed by scanning electron microscopy at 4, 12, 24, and 48 h following inoculation. Magnification as indicated. Scale bars = (white) 300 μm, (blue) 75 μm, (red) 30 μm.
Supplementary Figure 5. Confocal imaging of dispersed cells from C. tropicalis 48-hour biofilms. Dispersed cells from (A) opaque a cells, (B) sexual biofilms, or (C) WOR1 overexpression biofilms. (D) Top view (left) and side view (right) projections of opaque a cells grown on the polystyrene substrate. Left, top view projected from outer biofilm surface inward. Right, side view. For D, images were taken with the same settings as Figures 4C and 4D. Scale bars = 36 μm. Blue = Calcoflour White. Red = Rhodamine-conjugated Concanavalin A.
Supplementary Figure 6. Extracellular matrix content of sexual and WOR1 overexpression biofilms. Glucan content quantified using the Glucatell © assay. Mean ± standard deviation (N=3). * p<0.01. Tukey’s test.
Supplementary Figure 7. Analysis of the role of FGR23 in mating. (A) Zygote formation is not abolished by deletion of FGR23. Opaque a and α WT and fgr23Δ cells were stained and mated on Spider medium for 24 h. a cells stained with FITC-conjugated Concanavalin A. α cells stained with Rhodamine-conjugated Concanavalin A. Arrows indicate zygotes. Scale bar = 15 μm. (B) FGR23 deletion significantly decreases mating frequency. a and α cells were mixed then mated on Spider medium for 48 h (see Experimental Procedures). Mean ± standard error (N=4). * p<0.05. Student’s T-test (log-transform).
Supplementary Figure 8. Deletion of EFH1 does not significantly affect sexual biofilm formation. Mean ± standard deviation (N=4). One-way ANOVA.
Supplementary Table 1. C. tropicalis strains used in this study.
Supplementary Table 2. Oligonucleotides used in this study. Lowercase letters denote adapter and restriction site sequences.
Supplementary Table 3. Fold-change gene expression of sexual biofilms and WOR1 overexpression biofilms compared to opaque a cells under biofilm-forming conditions. Sheet 1: Expression levels and C. albicans orthologs of all identified C. tropicalis genes. Sheet 2: List of all genes with greater than 2-fold change in either biofilm.
Supplementary Table 4. Statistical analysis of C. tropicalis sexual biofilm and WOR1 overexpression biofilm regulated genes with C. albicans biofilm and C. tropicalis white-opaque regulated genes.
Supplementary Table 5. Comparison of gene expression in C. tropicalis sexual and WOR1 overexpression biofilms to C. tropicalis white- and opaque-specific genes (see Porman et al., 2011), C. albicans sexual biofilm-specific genes (see Lin et al., 2013) and C. albicans conventional (asexual) biofilm-specific genes (see Nobile et al., 2012).
Supplementary Table 6. Gene Ontology term analysis of genes showing a 2-fold or greater change in expression level in either sexual biofilms or WOR1 overexpression biofilms when compared to white (wor1Δ) a cells.
Supplementary Table 7. Gene Ontology term analysis of genes showing a 2-fold or greater change in expression level in sexual biofilms, WOR1 overexpression biofilms, or both biofilms when compared to wild type opaque a cells.