Abstract
Our goal was to gain a better understanding of the contribution of the burial of polar groups and their hydrogen bonds to the conformational stability of proteins. We measured the change in stability, Δ(ΔG), for a series of hydrogen bonding mutants in four proteins: villin headpiece subdomain (VHP) containing 36 residues, a surface protein from Borrelia burgdorferi (VlsE) containing 341 residues, and two proteins previously studied in our laboratory, ribonucleases Sa (RNase Sa) and T1 (RNase T1). Crystal structures were determined for three of the hydrogen bonding mutants of RNase Sa: S24A, Y51F, and T95A. The structures are very similar to wild type RNase Sa and the hydrogen bonding partners form intermolecular hydrogen bonds to water in all three mutants. We compare our results with previous studies of similar mutants in other proteins and reach the following conclusions. (1) Hydrogen bonds contribute favorably to protein stability. (2) The contribution of hydrogen bonds to protein stability is strongly context dependent. (3) Hydrogen bonds by side chains and peptide groups make similar contributions to protein stability. (4) Polar group burial can make a favorable contribution to protein stability even if the polar groups are not hydrogen bonded. (5) The contribution of hydrogen bonds to protein stability is similar for VHP, a small protein, and VlsE, a large protein.
Keywords: hydrogen bonds, hydrophobic bonds, conformational entropy, protein stability, large proteins, small proteins
Introduction
In 1936, when the structure of globular proteins was beginning to be understood, Pauling and Mirsky concluded “… this chain is folded into a uniquely defined configuration, in which it is held by hydrogen bonds between the peptide nitrogen and oxygen atoms …. The importance of the hydrogen bond in protein structure can hardly be overemphasized”; and they suggested that each hydrogen bond would contribute 5 kcal mol−1 to the stability of the uniquely defined configuration.1 Fifteen years later, Pauling's group made use of constraints derived from structural studies of model compounds and their ideas about hydrogen bonds to discover the most important of these configurations: the α-helix and the β-pleated sheet.2,3 In the paper describing the α-helix, they noted2: “The energy of an N—H ··· O=C hydrogen bond is on the order of 8 kcal mol−1, and such great instability would result from the failure to form these bonds that we may be confident of their presence.” Later the same year in the article describing the β-pleated sheet, they had reached a better understanding and suggested3: “With proteins in an aqueous environment the effective energy of hydrogen bonds is not so great, in as much as the difference between the energy of the system with N—H ··· O hydrogen bonds surrounded by water and a system with the N—H group and the O atom forming hydrogen bonds with water molecules may be no more than about 2 kcal mol−1.” Sixty years later, there is still uncertainty about the contribution of hydrogen bonds to protein stability.
The reaction of interest is:
Based on model compound data, it was not clear whether the free energy change for this reaction was favorable or unfavorable. For example, Klotz4 suggested −750 cal mol−1 and Schellman5 suggested + 400 cal mol−1 as the free energy change for this reaction. In Kauzmann's important 1959 review, he made a strong case for the importance of hydrophobic bonds but concluded6: “··· it is likely that hydrogen bonds between peptide links and hydrophobic bonds are by far the most important in determining the over-all configuration of the protein molecule. Furthermore, it could well be that peptide hydrogen bonds and hydrophobic bonds are barely sufficient to stabilize the native configuration of some proteins, and that a relatively few of the less abundant bonds contribute the decisive increment that stabilizes the native configuration. Therefore, it is not safe to say that any of the bonds are “less important” than others.” Little progress was made over the next 30 years and in an excellent review published in 1990, Dill could only conclude7: “There is evidence that hydrogen bonding or van der Waals interactions among the polar amino acids may be important but their magnitude remains poorly understood.”
In the late 1980s, the Fersht group provided the first experimental evidence that hydrogen bonds might contribute favorably to protein stability.8 He concluded,9 “Recent experiments on engineered enzymes, modified inhibitors and synthetic DNA duplexes indicate that an individual hydrogen bond contributes 0.5–1.8 kcal mol−1 to binding energies.” Since then, several studies of hydrogen bonding groups in individual proteins using site directed mutagenesis have reached similar conclusions: RNase T1,10–12 Barnase,13 Arc Repressor,14 BPTI,15 RNase Sa,11,16–18 Staphylococcal nuclease,19,20 human lysozyme,21,22 and in the membrane protein bacteriorhodopsin.23
In contrast, most theoretical studies reached the opposite conclusion.24,25 For example, “H-bonds do not contribute to the thermodynamic stability of native folds, because the energy balance of H-bond formation is close to zero.”26 “The polar groups contribute little or not at all to protein stability.”27 “The analysis presented here, when combined with the mutation results suggests that, to a first approximation, hydrogen bonding groups make no contribution to protein stability.”28 A more recent study combining a new experimental method and theory concluded: “… the hydrogen-bonded groups in fact destabilize the native conformation.”29
It seems likely that some of the stabilizing and destabilizing forces that contribute to protein stability will depend on protein size. As globular proteins become smaller, a smaller fraction of the side chains and peptide groups will be buried and their environment may differ in small and large proteins. In addition, the denatured state ensembles may depend on the size of the protein and this could influence protein stability. Previous results suggested that hydrophobic interactions were substantially greater in a large protein than in a small protein.30 However, it is not clear whether the contribution of hydrogen bonds will depend on protein size, and that is one of the questions studied in this article.
We have long had an interest in protein stability,31 and have previously studied the contribution of hydrogen bonds to proteins stability using various approaches.10–12,16–18,32–34 Here, we report additional studies of hydrogen bonding mutants of RNase T1 and Sa. In addition, we examine the contribution of hydrogen bonds to the stability of a small protein, villin headpiece subdomain (VHP) with 36 residues35–37 and of a large protein, Borrelia burgdorferi protein (VlsE) with 341 residues.38,39 These new data along with our previous results allow us to gain an improved understanding of the contribution of hydrogen bonds to protein stability.
Results
VlsE
Jones and Wittung-Stafshede have shown that the denaturation of VlsE by urea and guanidine hydrochloride (GuHCl) is completely reversible and closely approaches a two-state folding mechanism.38 Based on their results, we have assumed a two-state folding mechanism for VlsE for the analysis of our results. To study the contribution of hydrogen bonds to the stability of VlsE, we prepared the seven mutants shown in Table I. Urea denaturation curves were determined by measuring the circular dichroism at 220 nm as a function of urea concentration. Typical experimental results were shown in a previous article.30 These curves were analyzed using the linear extrapolation method and the results are shown in Table I.
Table I.
Parameters Characterizing the Urea Unfolding of VlsE and Hydrogen Bonding Variants in 5 mM Sodium Phosphate, pH 7.0, 25°C
| Variant | Urea 1/2a (M) | ΔUrea1/2b (M) | mc (cal mol−1 M−1) | ΔG (H2O)d (kcal mol−1) | ΔΔGe (kcal mol−1) |
|---|---|---|---|---|---|
| WT | 1.19 | – | 3860 | 4.6 | – |
| S122A | 1.02 | −0.17 | 3070 | 3.1 | −0.6 |
| S123A | 0.99 | −0.20 | 3360 | 3.3 | −0.7 |
| S213A | 1.02 | −0.17 | 3350 | 3.4 | −0.6 |
| S296A | 0.99 | −0.20 | 3770 | 3.7 | −0.7 |
| T66V | 1.25 | 0.06 | 2960 | 3.7 | 0.2 |
| T57V | 0.99 | −0.20 | 2720 | 2.7 | −0.7 |
| Y55F | 1.14 | −0.05 | 3250 | 3.7 | −0.2 |
Midpoint of the unfolding curve. The error is ± 2%.
ΔUrea1/2 = Urea1/2 (variant) − Urea1/2 (WT).
The slope of plots of ΔG versus [Urea]. The error is ± 10%.
ΔG (H2O) = the intercept of plots of ΔG versus [Urea] at 0M Urea.
From ΔUrea1/2 × the average m value of WT and the variants (3360 cal mol−1 M−1). The negative values indicate a decrease in stability.
Villin headpiece subdomain
The Kim group was the first to study the thermal and GuHCl denaturation of the VHP subdomain and they showed that the folding of VHP is reversible and approaches a two-state folding mechanism.35 Many other studies of the stability and folding of VHP and related variants have since been published. (For recent examples, see Ref.40.) In this and our previous article, we will assume a two-state folding mechanism for VHP to analyze the data.
Only two of the polar, uncharged side chains in VHP are hydrogen bonded: S43 and T54. To study the contribution of hydrogen bonds to the stability of VHP, we prepared the S43A and T54V mutants and measured their stability using both urea and thermal denaturation curves by measuring the circular dichroism at 222 nm. The results from an analysis of these curves are given in Tables II and III.
Table II.
Parameters Characterizing the Urea Unfolding of VHP and Hydrogen Bonding Variants in 50 mM Sodium Phosphate, pH 7.0, 25°C
| Variant | Urea1/2a (M) | ΔUrea1/2b (M) | mc (cal mol−1M−1) | ΔG (H2O)d (kcal mol−1) | ΔΔGe (kcal mol−1) |
|---|---|---|---|---|---|
| WT | 6.35 | – | 432 | 2.7 | – |
| S43A | 4.69 | −1.66 | 448 | 2.1 | −0.7 |
| T54V | 3.41 | −2.94 | 452 | 1.5 | −1.3 |
Midpoint of the unfolding curve. The error is ± 1%.
ΔUrea1/2 = Urea1/2 (variant) − Urea1/2 (WT).
The slope of plots of ΔG versus [Urea]. The error is ± 10%.
ΔG (H2O) = the intercept of plots of ΔG versus [Urea] at 0M Urea.
From ΔUrea1/2 × the average m value of WT and the variants (444 cal mol−1M−1). The negative values indicate a decrease in stability.
Table III.
Parameters Characterizing the Thermal Unfolding of VHP and Hydrogen Bonding Variants in 50 mM Sodium Phosphate, pH 7.0
| Variant | ΔHma (kcal mol−1) | ΔSmb (cal mol−1 K−1) | Tmc (°C) | ΔTmd (°C) | Δ(ΔG)e (kcal mol−1) | Δ(ΔG)f (kcal mol−1) |
|---|---|---|---|---|---|---|
| WT | 31 | 89 | 74.4 | – | – | – |
| S43A | 28 | 86 | 65.0 | −9.4 | −0.8 | −0.6 |
| T54V | 26 | 78 | 61.6 | −12.8 | −1.1 | −1.1 |
Enthalpy of unfolding at Tm. The error is ± 2 kcal mol−1.
ΔHm/Tm. The error is ± 5 cal mol−1 K−1.
Midpoint of the unfolding curve. The error is ± 0.5°C.
ΔTm = Tm (variant) − Tm (WT).
Δ(ΔG) = ΔTm × the average ΔSm value of WT and the variants (84 cal mol−1 K−1).
Calculated using the Gibbs-Helmholtz equation at a reference temperature = 67.0°C. The value for ΔCp was 0.374 kcal mol−1 K−1 (see text), and the value for ΔHm was 23 kcal mol−1 at 67.0°C. The negative values indicate a decrease in stability.
In related experiments,41 values of the change in heat capacity, ΔCp, were determined to be 0.374 ± 0.003 kcal mol−1 K−1 for wild type and 0.380 ± 0.004 kcal mol−1 K−1 for L75A using the method of Pace and Laurents.42 The value of ΔCp from a Kirchoff analysis (a plot of ΔHm determined by a van't Hoff plot vs. Tm) for all of the mutants gave ΔCp = 0.349 kcal mol−1 K−1.41 These values are in good agreement with a ΔCp = 0.38 kcal mol−1 K−1 based on a Kirchoff analysis by Xiao et al.37 and ΔCp = 0.37 kcal mol−1 K−1 based on the equation relating ΔCp to the change in accessible surface area, Δ(ASA), given by Myers et al.43 and using Δ(ASA) = 2465 Å2.
RNase Sa
We have studied many different aspects of protein stability and folding using RNase Sa.16,17,30,44 To study the contribution of hydrogen bonds to the stability of RNase Sa, we prepared the mutants listed in Table IV. Three thermal denaturation curves were determined for each mutant, and the average results from an analysis of these curves are summarized in Table IV. Crystal structures were determined for three of the hydrogen bonding mutants of RNase Sa: S24A, Y51F, and T95A, and the methods and a summary of these results are given in the Supporting Information online.
Table IV.
Parameters Characterizing the Thermal Unfolding of RNase Sa and Hydrogen Bonding Variants in 30 mM MOPS, pH 7.0
| Variant | ΔHma (kcal mol−1) | ΔSmb (cal mol−1 K−1) | ΔTmc (°C) | Δ(ΔG)d (kcal mol−1) |
|---|---|---|---|---|
| WT | 92 | 286 | – | – |
| N20A | 89 | 276 | −0.1 | 0.0 |
| N39A | 66 | 212 | −7.3 | −2.0 |
| S3A | 86 | 269 | −1.1 | −0.3 |
| S9A | 91 | 289 | −4.5 | −1.3 |
| S24A | 77 | 240 | −0.7 | −0.2 |
| S31A | 91 | 281 | 1.5 | 0.4 |
| S42A | 92 | 286 | 0.4 | 0.1 |
| S48A | 92 | 285 | 1.7 | 0.5 |
| S90A | 86 | 265 | 0.7 | 0.2 |
| T95A | 97 | 303 | −2.1 | −0.6 |
Enthalpy of unfolding at Tm. The error is ± 5 kcal mol−1.
ΔHm/Tm. The error is ± 15 cal mol−1 K−1.
ΔTm = Tm (variant) − Tm (WT). The Tm for WT is 48.4°C.
Δ(ΔG) = ΔTm × the average ΔSm value of WT and the variants (272 cal mol−1 K−1). The negative values indicate a decrease in stability.
RNase T1
We have used RNase T1 in several previous studies, including our first studies of the contribution of hydrogen bonds to protein stability.10–12 To continue our study of the contribution of hydrogen bonds to protein stability, we prepared 8 single and double mutants of RNase T1 and studied their stability using both urea denaturation curves and differential scanning calorimetry (DSC). The results of these studies are summarized in Tables V and VI.
Table V.
Parameters Characterizing the Urea Unfolding of RNase T1 and Hydrogen Bonding Variants in 30 mM MOPs, pH 7.0, 25°C
| Variant | Urea1/2a (M) | ΔUrea1/2b (M) | mc (cal mol−1 M−1) | ΔG (H2O)d (kcal mol−1) | ΔΔGe (kcal mol−1) |
|---|---|---|---|---|---|
| WT | 5.20 | – | 1172 | 6.1 | – |
| T91A | 2.32 | −2.88 | 1250 | 2.9 | −3.4 |
| T91V | 2.07 | −3.13 | 1243 | 2.6 | −3.7 |
| T93A | 4.46 | −0.74 | 911 | 4.1 | −0.9 |
| T93V | 4.16 | −1.04 | 1201 | 5.0 | −1.2 |
| T91A, T93A | 1.90 | −3.30 | 1210 | 2.3 | −3.9 |
| T91A, T93V | 2.35 | −2.85 | 1215 | 2.9 | −3.4 |
| T91V, T93A | 1.90 | −3.30 | 1335 | 2.5 | −3.9 |
| T91V, T93V | 2.19 | −3.01 | 1324 | 2.9 | −3.6 |
Midpoint of the unfolding curve. The error is ± 2%.
ΔUrea1/2 = Urea1/2 (variant) − Urea1/2 (WT).
The slope of plots of ΔG versus [Urea]. The error is ± 10%.
ΔG (H2O) = the intercept of plots of ΔG versus [Urea] at 0M Urea.
From ΔUrea1/2 × the average m value of WT and the variants (1192 cal mol−1 M−1). The negative values indicate a decrease in stability.
Table VI.
Parameters Characterizing the Thermal Unfolding of RNase T1 and Hydrogen Bonding Variants in 30 mM MOPS, pH 7.0
| Variant | ΔHma (kcal mol−1) | ΔSmb (cal mol−1 K−1) | Tmc (°C) | ΔTmd (°C) | Δ(ΔG)e (kcal mol−1) |
|---|---|---|---|---|---|
| WT | 107 | 329 | 52.5 | – | – |
| T91A | 98 | 311 | 41.9 | −10.6 | −3.2 |
| T91V | 95 | 303 | 40.7 | −11.8 | −3.6 |
| T93A | 104 | 322 | 50.2 | −2.3 | −0.7 |
| T93V | 97 | 301 | 49.3 | −3.2 | −1.0 |
| T91A, T93A | 96 | 306 | 40.5 | −12.0 | −3.7 |
| T91A, T93V | 90 | 286 | 41.4 | −11.1 | −3.4 |
| T91V, T93A | 92 | 295 | 38.5 | −14.0 | −4.3 |
| T91V, T93V | 94 | 306 | 41.2 | −11.3 | −3.5 |
Enthalpy of unfolding at Tm. The error is ± 5 kcal mol−1.
ΔHm/Tm. The error is ± 15 cal mol−1 K−1.
Midpoint of the unfolding curve. The error is ± 0.5°C.
ΔTm = Tm (variant) − Tm (WT).
Δ(ΔG) = ΔTm × the average ΔSm value of WT and the variants (306 cal mol−1 K−1). The negative values indicate a decrease in stability.
Discussion
When a proteins folds, 81% of the nonpolar side chains, 70% of the peptide groups, 63% of the polar side chains, and 54% of the charged side chains are buried in the interior of the protein out of contact with water.45 The burial of nonpolar side chains makes a large favorable contribution to protein stability in two ways: the removal of the nonpolar side chains from water, and, equally or more important, the enhanced London dispersion forces that result from the tight packing in the protein interior.30 The burial of uncharged polar groups is more complicated because now hydrogen bonds and longer range Coulombic interactions also contribute. Whether the burial of polar groups makes a favorable contribution to protein stability is still contentious. The experimental results are more difficult to interpret and several of the theoretical results are not in agreement with the experimental results.
Hydrogen bonding mutants have structures similar to the wild type structure
The structures of four hydrogen bonding mutants of RNase Sa are superimposed on the wild type structure in Figure 1. For S24A [Fig. 1(A)], the conformational changes are small and the hydrogen bond of the —OH group of Ser 24 to the O of Gly 26 (2.8 Å) is replaced by hydrogen bonds to water molecules at 2.7 and 2.9 Å. Thus, even the hydrogen bond of a partially exposed —OH group (10% exposed) can make a favorable contribution to the stability of 0.2 kcal mol−1. For T95A [Fig. 1(B)], the situation is similar and the hydrogen bond (2.7 Å) of the 54% exposed —OH group makes an even larger contribution to the stability of 0.6 kcal mol−1. For Y51F and Y80F [Fig. 1(C,D)], both —OH groups form 2.6 Å hydrogen bonds to OE1 and OE2 of Glu 78. The conformational changes are again small and OE1 and OE2 of Glu 78 both hydrogen bond to water molecules in the mutant structures. The —OH groups of these two Tyr residues form almost identical hydrogen bonds to Glu 78 (2.6 Å) and both make large contributions to the stability of 2.3 and 1.5 kcal mol−1. (The structure of Y80F was reported in an earlier article.16)
Figure 1.
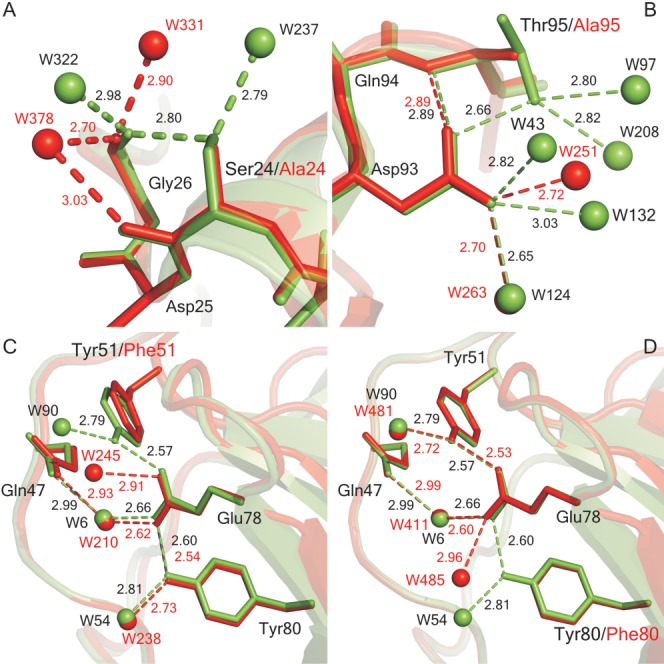
Superposition of mutant and wild-type (PDB ID: 1RGG) RNase Sa structures at the mutation sites: (A) S24A (4GHO), (B) T95A (4J5G), (C) Y51F (4J5K), and (D) Y80F (1I8V). The wild-type structures are in green and the mutant structures are in red. The broken lines represent hydrogen bonds, and the spheres represent water molecules. The numbers shown are the hydrogen bond distances determined using the structures and pfis. The figure was prepared using Pymol (The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC).
Hydrogen bonds make a favorable contribution to protein stability
In a recent review, Bowie analyzed two different approaches for estimating the contribution of individual hydrogen bonds to protein stability.46 In the first approach, double mutant cycles are used to isolate the contribution of a hydrogen bond to the stability. In the article where this approach was used, the contribution estimated using double mutant cycles was the same, within experimental error, as the estimate obtained by just removing the side chain involved in the hydrogen bond, as we did in this article.23 In the second approach, the estimate is based on comparing the Δ(ΔG) values for the same mutation, for example, Ser to Ala, when the Ser —OH is or is not hydrogen bonded.46 This is the approach we will use below. The assumptions used in both of these approaches are discussed in the Bowie review.46
The results for the 25 single mutants from Tables I to VI are summarized in Table VII. For the 20 of these groups that were hydrogen bonded in the folded protein, the average Δ(ΔG) was −1.1 ± 1.0 kcal mol−1, with a range from −3.6 to + 0.2 kcal mol−1. For the 5 groups that were not hydrogen bonded, the average Δ(ΔG) was +0.1 ± 0.3 kcal mol−1, with a range from −0.3 to +0.5 kcal mol−1. The majority of the mutations in Table VII were Ser to Ala mutations and they are compared to similar results for Tyr to Phe and Thr to Val mutations for a large number of different proteins in Table VIII.
Table VII.
Hydrogen Bonding and Accessibility of the Polar Groups Studied in VlsE, VHP, RNase Sa, and RNase T1
| Protein | Residue | % Buried S.C./P.G.a | D···A (Å)b | H-Bond and Partnerb | Δ(ΔG) (kcal mol−1)c |
|---|---|---|---|---|---|
| VlsE | S122A | 100/100 | 2.9 | OH···O A118 | −0.6 |
| S123A | 67/96 | 3.0 | OH···O A121 | −0.7 | |
| S213A | 79/81 | 2.8 | OH···O L164 | −0.6 | |
| S296A | 61/44 | 3.0 | OH···O G292 | −0.7 | |
| T57V | 46/86 | 2.5 | OH···OE2 D119 | −0.7 | |
| T66V | 68/5 | 3.2 | OH···O K62 | +0.2 | |
| Y55F | 87/100 | 2.6 | OH···OG1 T306 | −0.2 | |
| VHP | S43A | 42/90 | 2.9 | OH···N D46 | −0.7 |
| T54A | 43/78 | 3.2 | OH···N A57 | −1.2 | |
| RNase T1 | T91A | 99/100 | 2.6 | OH···OD1 D76 | −3.3 |
| T93A | 54/61 | 3.3 | OH···OG1 T91 | −0.8 | |
| T91V | 99/100 | 2.6 | OH···OD1 D76 | −3.6 | |
| T93V | 54/61 | 3.3 | OH···OG1 T91 | −1.1 | |
| RNase Sa | S3A | 42/53 | – | – | −0.3 |
| S9A | 41/62 | 2.6 | OH···O C96 | −1.3 | |
| S24A | 48/90 | 2.8 | OH···O G26 | −0.2 | |
| S31A | 1/11 | – | – | +0.4 | |
| S42A | 52/72 | – | – | +0.1 | |
| S48A | 2/0 | – | – | +0.5 | |
| S90A | 67/32 | 3.2 | OH···O S3 | +0.2 | |
| T95A | 29/46 | 2.7 | OH···OD1 D93 | −0.6 | |
| Y51Fd | 85/82 | 2.6 | OH···OE2 E78 | −2.3 | |
| Y80Fd | 94/80 | 2.6 | OH···OE1 E78 | −1.5 | |
| N20A | 94/80 | – | OH···OE1 E78 | −1.50 | |
| 31/23 | – | ||||
| N39A | 93/97 | 2.9 | ND2···O L44 | −2.0 |
The percent buried for the side chain (S.C.) and polar group (P.G.) was calculated using pfis.16
The hydrogen bond distance and the hydrogen bonding partner were determined using pfis.16 In cases where a group forms more than one hydrogen bond, only the shortest hydrogen bond is listed.
Data from Ref.16.
Table VIII.
Δ(ΔG) values for Tyr → Phe, Thr → Val, and Ser → Ala mutants
| Mutation | Hydrogen-bonded |
Not hydrogen-bonded |
||
|---|---|---|---|---|
| Number | Δ(ΔG) (kcal mol−1) | Number | Δ(ΔG) (kcal mol−1) | |
| Tyr → Phe a | 35 | −1.4 ± 0.9 | 17 | −0.2 ± 0.4 |
| (−3.6 to 1.2) | (− 1.2 to 0.5) | |||
| Thr → Val b | 25 | −1.0 ± 1.0 | 15 | 0.0 ± 0.5 |
| (−3.5 to 1.9) | (− 1.7 to 1.0) | |||
| Ser → Alac | 44 | −0.8 ± 0.9 | 15 | 0.1 ± 0.4 |
| (−3.8 to 1.3) | (− 0.8 to 0.5) | |||
For each of the three types of mutants in Table VIII, an —OH group is removed that is either hydrogen bonded or not. (The results for Tyr to Phe and Thr to Val mutants were discussed previously.16,17 The analysis that led to the results for the Ser to Ala mutants is described in the Supporting Information. Over 20 different proteins from many different research groups were included.) For each type of mutation, the stability decrease is substantially larger when the groups are hydrogen bonded than when they are not and this is convincing evidence that hydrogen bonds make a favorable contribution to protein stability. Note that the —OH groups of Tyr residues that are not hydrogen bonded also make a favorable contribution to protein stability, and that the —OH group of Thr residues that are not hydrogen bonded make a contribution to the stability that is as large as that of the methyl groups that replace them in Thr to Val mutants. In contrast, removing the non hydrogen bonded Ser —OH groups results in a slight increase in stability. For a large sample of proteins, the —OH groups are more buried for Tyr residues (67%) than for Ser residues (61%).45 Consequently, the —OH groups of Tyr residues generally have more favorable van der Waals interactions than the —OH groups of Ser residues and this may account in part for this difference.
These results show that hydrogen bonds by side chain —OH groups make a favorable contribution to protein stability. They also show that the hydrogen bonding and other interactions of —OH groups in folded proteins can be more favorable than interactions with water in the unfolded protein. We have shown previously that hydrogen bonding increases the packing density in the interior of proteins.47 In addition, the results show that buried polar groups that are not hydrogen bonded can make a favorable contribution to protein stability. It may be surprising that the van der Waals and longer range electrostatic interactions of a buried polar group can be as favorable as the hydrogen bonding interactions of the polar group with water molecules in the unfolded protein, but the data support such a conclusion.
Hydrogen bonds by side chains and peptide groups make similar contributions to protein stability
In Table IX, we compare the results from Table VIII with previous studies of Asn to Ala mutations in which the Asn side chains were hydrogen bonded to peptide groups11,32 and to studies by the Kelly group in which the peptide group is converted to an ester to estimate the contribution of hydrogen bonds by peptide groups to protein stability.48,49 The hydrogen bonds by peptide groups make a contribution to protein stability that is similar to those of the —OH groups of Tyr, Ser, and Thr residues. This is important because the average number of hydrogen bonds formed in a folded protein is 1.1 per residue and 65% of these are between peptide groups, 23% are between peptide groups and side chains, and just 12% between side chains.50 Thus, the hydrogen bonds formed by peptide groups make a much larger contribution to protein stability than side chain hydrogen bonds.
Table IX.
Contribution of Hydrogen Bonds to Protein Stability
| Mutation | ΔGHB (kcal mol−1)a |
|---|---|
| Ser → Alab | −0.9 ± 0.9 |
| Thr → Valb | −1.0 ± 0.5 |
| Tyr → Pheb | −1.2 ± 0.6 |
| Asn → Alac | −1.1 ± 0.6 |
| Peptide → Esterd | −1.1 ± 1.6 |
From TableTable VIII.
From Ref.32.
From Ref.52 and a personal communication from Evan Powers and Jeff Kelly.
The contribution of hydrogen bonds to protein stability is context dependent
The errors are large for the ΔGHB estimates in Table VIII. This reflects in part the fact that the contributions of individual hydrogen bonds depend on their distance and geometry.51 Equally important, we think, is the environment of the individual hydrogen bonds. Studies by Kelly's group have shown that hydrogen bonds can be more than 1 kcal mol−1 stronger in a nonpolar environment as compared to a polar environment.52,53 Since hydrogen bonds are mainly electrostatic interactions, they will be stronger in an environment with a lower dielectric constant.54 This idea is supported by a variety of different studies.55–60
Concluding Remarks
Until about 1990, the prevailing view was that intramolecular hydrogen bonds were necessary for maintaining the structure of proteins but made, at most, a small net contribution to protein stability. The experimental results from many groups accumulated over the last few decades indicate otherwise; strong evidence exists that the contribution of hydrogen bonds to protein stability is significant and averages about 1 kcal mol−1 per hydrogen bond.
Materials and Methods
All buffers and chemicals were of reagent grade. Urea was from Amresco or Nacalai Tesque (Kyoto, Japan), and was used without further purification. The plasmids for VHP, VlsE, and their variants were derived from pET vectors (Novagen) and have been described previously.41 The plasmids for RNase Sa, RNase T1, and their variants were derived from the pEH100 plasmid as described previously.44,61 The expression hosts for all proteins and variants were either E. coli strains RY1988 (MQ), DS2000, or C41(DE3).41,62 Oligonucleotide primers for mutagenesis were from Integrated DNA Technologies (Coralville, IA). Site-directed mutagenesis was performed using a QuikChange™ Site-Directed Mutagenesis Kit from Stratagene (La Jolla, CA). Mutant plasmids were sequenced by the Gene Technologies Laboratory, Texas A&M University.
VHP, VlsE, and their variants were expressed and purified as described previously.41,63 RNases Sa, T1, and their variants were expressed and purified as described previously.61,62,64 The purity of all proteins was confirmed by SDS PAGE and MALDI-TOF mass spectrometry.
Urea and thermal denaturation curves were determined using either an AVIV 62DS or 202SF spectropolarimeter (Aviv Instruments, Lakewood, NJ) to follow unfolding. The methods for VHP and VlsE have been described previously,63 as have the methods for RNases Sa, T1, and their variants.65,66 The analysis of urea and thermal denaturation curves assumed a two-state unfolding model and was performed as described elsewhere.65,66 The DSC experiments were performed as previously described.67 For Tables I, II, III, IX, and VI, two independent denaturation curves were determined and the results in the tables are the average of the two. For Tables I, II, III, V, and VI, three independent thermal denaturation curves were determined for each mutant and the results in the table are the average of the three.
Acknowledgments
The authors thank Evan Powers and Jeff Kelly for providing important information. JS and LU acknowledge the EMBL c/o DESY, Hamburg for providing synchrotron source facilities, and thank A.Popov (EMBL Hamburg) for his help with data processing.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Mirsky AE, Pauling L. On the structure of native, denatured, and coagulated proteins. Proc Natl Acad Sci USA. 1936;22:439–447. doi: 10.1073/pnas.22.7.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pauling L, Corey RB, Branson HR. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci USA. 1951;37:205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pauling L, Corey RB. Configurations of polypeptide chains With favored orientations around single bonds: two new pleated sheets. Proc Natl Acad Sci USA. 1951;37:729–740. doi: 10.1073/pnas.37.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klotz IM, Farnham SB. Stability of an amide-hydrogen bond in an apolar environment. Biochemistry. 1968;7:3879–3882. doi: 10.1021/bi00851a013. [DOI] [PubMed] [Google Scholar]
- 5.Schellman JA. The stability of hydrogen-bonded peptide structures in aqueous solution. C R Trav Lab Carlsberg Chim. 1955;29:230–259. [PubMed] [Google Scholar]
- 6.Kauzmann W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- 7.Dill KA. Dominant forces in protein folding. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 8.Fersht AR, Shi JP, Knill-Jones J, Lowe DM, Wilkinson AJ, Blow DM, Brick P, Carter P, Waye MM, Winter G. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 1985;314:235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
- 9.Fersht AR. The hydrogen bond in molecular recognition. Trends Biochem Sci. 1987;12:301–304. [Google Scholar]
- 10.Shirley BA, Stanssens P, Hahn U, Pace CN. Contribution of hydrogen bonding to the conformational stability of ribonuclease T1. Biochemistry. 1992;31:725–732. doi: 10.1021/bi00118a013. [DOI] [PubMed] [Google Scholar]
- 11.Hebert EJ, Giletto A, Sevcik J, Urbanikova L, Wilson KS, Dauter Z, Pace CN. Contribution of a conserved asparagine to the conformational stability of ribonucleases Sa, Ba, and T1. Biochemistry. 1998;37:16192–16200. doi: 10.1021/bi9815243. [DOI] [PubMed] [Google Scholar]
- 12.Giletto A, Pace CN. Buried, charged, non-ion-paired aspartic acid 76 contributes favorably to the conformational stability of ribonuclease T1. Biochemistry. 1999;38:13379–13384. doi: 10.1021/bi991422s. [DOI] [PubMed] [Google Scholar]
- 13.Serrano L, Kellis JTJ, Cann P, Matouschek A, Fersht AR. The folding of an enzyme. II. Substructure of barnase and the contribution of different interactions to protein stability. J Mol Biol. 1992;224:783–804. doi: 10.1016/0022-2836(92)90562-x. [DOI] [PubMed] [Google Scholar]
- 14.Milla ME, Brown BM, Sauer RT. Protein stability effects of a complete set of alanine substitutions in Arc repressor. Nat Struct Biol. 1994;1:518–523. doi: 10.1038/nsb0894-518. [DOI] [PubMed] [Google Scholar]
- 15.Mendoza JA, Jarstfer MB, Goldenberg DP. Effects of amino acid replacements on the reductive unfolding kinetics of pancreatic trypsin inhibitor. Biochemistry. 1994;33:1143–1148. doi: 10.1021/bi00171a013. [DOI] [PubMed] [Google Scholar]
- 16.Pace CN, Horn G, Hebert EJ, Bechert J, Shaw K, Urbanikova L, Scholtz JM, Sevcik J. Tyrosine hydrogen bonds make a large contribution to protein stability. J Mol Biol. 2001;312:393–404. doi: 10.1006/jmbi.2001.4956. [DOI] [PubMed] [Google Scholar]
- 17.Takano K, Scholtz JM, Sacchettini JC, Pace CN. The contribution of polar group burial to protein stability is strongly context-dependent. J Biol Chem. 2003;278:31790–31795. doi: 10.1074/jbc.M304177200. [DOI] [PubMed] [Google Scholar]
- 18.Pace CN, Trevino S, Prabhakaran E, Scholtz JM. Protein structure, stability and solubility in water and other solvents. Philos Trans R Soc Lond B Biol Sci. 2004;359:1225–1234; discussion 1234–1235. doi: 10.1098/rstb.2004.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green SM, Meeker AK, Shortle D. Contributions of the polar, uncharged amino acids to the stability of staphylococcal nuclease: evidence for mutational effects on the free energy of the denatured state. Biochemistry. 1992;31:5717–5728. doi: 10.1021/bi00140a005. [DOI] [PubMed] [Google Scholar]
- 20.Byrne MP, Manuel RL, Lowe LG, Stites WE. Energetic contribution of side chain hydrogen bonding to the stability of staphylococcal nuclease. Biochemistry. 1995;34:13949–13960. doi: 10.1021/bi00042a029. [DOI] [PubMed] [Google Scholar]
- 21.Yamagata Y, Kubota M, Sumikawa Y, Funahashi J, Takano K, Fujii S, Yutani K. Contribution of hydrogen bonds to the conformational stability of human lysozyme: calorimetry and X-ray analysis of six tyrosine –> phenylalanine mutants. Biochemistry. 1998;37:9355–9362. doi: 10.1021/bi980431i. [DOI] [PubMed] [Google Scholar]
- 22.Takano K, Yamagata Y, Kubota M, Funahashi J, Fujii S, Yutani K. Contribution of hydrogen bonds to the conformational stability of human lysozyme: calorimetry and X-ray analysis of six Ser –> Ala mutants. Biochemistry. 1999;38:6623–6629. doi: 10.1021/bi9901228. [DOI] [PubMed] [Google Scholar]
- 23.Joh NH, Min A, Faham S, Whitelegge JP, Yang D, Woods VL, Bowie JU. Modest stabilization by most hydrogen-bonded side-chain interactions in membrane proteins. Nature. 2008;453:1266–1270. doi: 10.1038/nature06977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honig B, Cohen FE. Adding backbone to protein folding: why proteins are polypeptides. Fold Des. 1966;1:R17–20. [PubMed] [Google Scholar]
- 25.Honig B. Protein folding: from the levinthal paradox to structure prediction. J Mol Biol. 1999;293:283–293. doi: 10.1006/jmbi.1999.3006. [DOI] [PubMed] [Google Scholar]
- 26.Sippl MJ, Ortner M, Jaritz M, Lackner P, Flockner H. Helmholtz free energies of atom pair interactions in proteins. Fold Des. 1996;1:289–298. doi: 10.1016/S1359-0278(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 27.Lazaridis T, Archontis G, Karplus M. Enthalpic contribution to protein stability: insights from atom-based calculations and statistical mechanics. Adv Protein Chem. 1995;47:231–306. doi: 10.1016/s0065-3233(08)60547-1. [DOI] [PubMed] [Google Scholar]
- 28.Honig B, Yang AS. Free energy balance in protein folding. Adv Protein Chem. 1995;46:27–58. doi: 10.1016/s0065-3233(08)60331-9. [DOI] [PubMed] [Google Scholar]
- 29.Campos LA, Cuesta-Lopez S, Lopez-Llano J, Falo F, Sancho J. A double-deletion method to quantifying incremental binding energies in proteins from experiment: example of a destabilizing hydrogen bonding pair. Biophys J. 2005;88:1311–1121. doi: 10.1529/biophysj.104.050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pace CN, Fu H, Fryar KL, Landua J, Trevino SR, Shirley BA, Hendricks MM, Iimura S, Gajiwala K, Scholtz JM, Grimsley GR. Contribution of hydrophobic interactions to protein stability. J Mol Biol. 2011;408:514–528. doi: 10.1016/j.jmb.2011.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pace NC, Tanford C. Thermodynamics of the unfolding of beta-lactoglobulin A in aqueous urea solutions between 5 and 55 degrees. Biochemistry. 1968;7:198–208. doi: 10.1021/bi00841a025. [DOI] [PubMed] [Google Scholar]
- 32.Pace CN. Evaluating contribution of hydrogen bonding and hydrophobic bonding to protein folding. Methods Enzymol. 1995;259:538–554. doi: 10.1016/0076-6879(95)59060-9. [DOI] [PubMed] [Google Scholar]
- 33.Myers JK, Pace CN. Hydrogen bonding stabilizes globular proteins. Biophys J. 1996;71:2033–2039. doi: 10.1016/S0006-3495(96)79401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pace CN. Polar group burial contributes more to protein stability than nonpolar group burial. Biochemistry. 2001;40:310–313. doi: 10.1021/bi001574j. [DOI] [PubMed] [Google Scholar]
- 35.McKnight CJ, Doering DS, Matsudaira PT, Kim PS. A thermostable 35-residue subdomain within villin headpiece. J Mol Biol. 1996;260:126–134. doi: 10.1006/jmbi.1996.0387. [DOI] [PubMed] [Google Scholar]
- 36.Chiu TK, Kubelka J, Herbst-Irmer R, Eaton WA, Hofrichter J, Davies DR. High-resolution x-ray crystal structures of the villin headpiece subdomain, an ultrafast folding protein. Proc Natl Acad Sci USA. 2005;102:7517–7522. doi: 10.1073/pnas.0502495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao S, Bi Y, Shan B, Raleigh DP. Analysis of core packing in a cooperatively folded miniature protein: the ultrafast folding villin headpiece helical subdomain. Biochemistry. 2009;48:4607–4616. doi: 10.1021/bi8021763. [DOI] [PubMed] [Google Scholar]
- 38.Jones K, Wittung-Stafshede P. The largest protein observed to fold by two-state kinetic mechanism does not obey contact-order correlation. J Am Chem Soc. 2003;125:9606–9607. [Google Scholar]
- 39.Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem. 2002;277:21691–21696. doi: 10.1074/jbc.M201547200. [DOI] [PubMed] [Google Scholar]
- 40.Xiao S, Patsalo V, Shan B, Bi Y, Green DF, Raleigh DP. Rational modification of protein stability by targeting surface sites leads to complicated results. Proc Natl Acad Sci USA. 2013;110:11337–11342. doi: 10.1073/pnas.1222245110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu H. Understanding forces that contribute to protein stability: applications for increasing protein stability, Ph.D. Dissertation. College Station. TX: Texas A&M University. 2009 [Google Scholar]
- 42.Pace CN, Laurents DV. A new method for determining the heat capacity change for protein folding. Biochemistry. 1989;28:2520–2525. doi: 10.1021/bi00432a026. [DOI] [PubMed] [Google Scholar]
- 43.Myers JK, Pace CN, Scholtz JM. Denaturant m values and heat capacity changes: relation to changes in accessible surface areas of protein unfolding. Protein Sci. 1995;4:2138–2148. doi: 10.1002/pro.5560041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pace CN, Hebert EJ, Shaw KL, Schell D, Both V, Krajcikova D, Sevcik J, Wilson KS, Dauter Z, Hartley RW, Grimsley GR. Conformational stability and thermodynamics of folding of ribonucleases Sa, Sa2 and Sa3. J Mol Biol. 1998;279:271–286. doi: 10.1006/jmbi.1998.1760. [DOI] [PubMed] [Google Scholar]
- 45.Lesser GJ, Rose GD. Hydrophobicity of amino acid subgroups in proteins. Proteins. 1990;8:6–13. doi: 10.1002/prot.340080104. [DOI] [PubMed] [Google Scholar]
- 46.Bowie JU. Membrane protein folding: how important are hydrogen bonds? Curr Opin Struct Biol. 2011;21:42–49. doi: 10.1016/j.sbi.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schell D, Tsai J, Scholtz JM, Pace CN. Hydrogen bonding increases packing density in the protein interior. Proteins. 2006;63:278–282. doi: 10.1002/prot.20826. [DOI] [PubMed] [Google Scholar]
- 48.Bunagan MR, Gao J, Kelly JW, Gai F. Probing the folding transition state structure of the villin headpiece subdomain via side chain and backbone mutagenesis. J Am Chem Soc. 2009;131:7470–7476. doi: 10.1021/ja901860f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powers ET, Deechongkit S, Kelly JW. Backbone-backbone H-bonds make context-dependent contributions to protein folding kinetics and thermodynamics: lessons from amide-to-ester mutations. Adv Protein Chem. 2005;72:39–78. doi: 10.1016/S0065-3233(05)72002-7. [DOI] [PubMed] [Google Scholar]
- 50.Stickle DF, Presta LG, Dill KA, Rose GD. Hydrogen bonding in globular proteins. J Mol Biol. 1992;226:1143–1159. doi: 10.1016/0022-2836(92)91058-w. [DOI] [PubMed] [Google Scholar]
- 51.Mills JE, Dean PM. Three-dimensional hydrogen-bond geometry and probability information from a crystal survey. J Comput Aided Mol Des. 1996;10:607–622. doi: 10.1007/BF00134183. [DOI] [PubMed] [Google Scholar]
- 52.Gao J, Bosco DA, Powers ET, Kelly JW. Localized thermodynamic coupling between hydrogen bonding and microenvironment polarity substantially stabilizes proteins. Nat Struct Mol Biol. 2009;16:684–690. doi: 10.1038/nsmb.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pace CN. Energetics of protein hydrogen bonds. Nat Struct Mol Biol. 2009;16:681–682. doi: 10.1038/nsmb0709-681. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell JBO, Price SL. On the relative strengths of amide.amide and amide.water hydrogen bonds. Chem Phys Lett. 1991;180:517–523. [Google Scholar]
- 55.Baldwin RL. Desolvation penalty for burying hydrogen-bonded peptide groups in protein folding. J Phys Chem B. 2010;114:16223–16227. doi: 10.1021/jp107111f. [DOI] [PubMed] [Google Scholar]
- 56.Worth CL, Blundell TL. Satisfaction of hydrogen-bonding potential influences the conservation of polar sidechains. Proteins. 2009;75:413–429. doi: 10.1002/prot.22248. [DOI] [PubMed] [Google Scholar]
- 57.Tsemekhman K, Goldschmidt L, Eisenberg D, Baker D. Cooperative hydrogen bonding in amyloid formation. Protein Sci. 2007;16:761–764. doi: 10.1110/ps.062609607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loladze VV, Ermolenko DN, Makhatadze GI. Thermodynamic consequences of burial of polar and non-ponar amino acid residues in the protein interior. J Mol Biol. 2002;320:343–357. doi: 10.1016/S0022-2836(02)00465-5. [DOI] [PubMed] [Google Scholar]
- 59.Makhatadze GI, Privalov PL. Energetics of protein structure. Adv Protein Chem. 1995;47:307–425. doi: 10.1016/s0065-3233(08)60548-3. [DOI] [PubMed] [Google Scholar]
- 60.Brandts JF. The thermodynamics of protein denaturation. II. a model of reversible denaturation and interpretations regarding the stability of chymotrypsinogen. J Am Chem Soc. 1964;86:4302–4314. [Google Scholar]
- 61.Hebert EJ, Grimsley GR, Hartley RW, Horn G, Schell D, Garcia S, Both V, Sevcik J, Pace CN. Purification of ribonucleases Sa, Sa2, and Sa3 after expression in Escherichia coli. Protein Expr Purif. 1997;11:162–168. doi: 10.1006/prep.1997.0776. [DOI] [PubMed] [Google Scholar]
- 62.Pace CN, Shaw KL. Linear extrapolation method of analyzing solvent denaturation curves. Proteins Suppl. 2000;4:1–7. doi: 10.1002/1097-0134(2000)41:4+<1::aid-prot10>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- 63.Pace CN, Huyghues-Despointes BM, Fu H, Takano K, Scholtz JM, Grimsley GR. Urea denatured state ensembles contain extensive secondary structure that is increased in hydrophobic proteins. Protein Sci. 2010;19:929–943. doi: 10.1002/pro.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thurlkill RL, Grimsley GR, Scholtz JM, Pace CN. Hydrogen bonding markedly reduces the pK of buried carboxyl groups in proteins. J Mol Biol. 2006;362:594–604. doi: 10.1016/j.jmb.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 65.Pace CN. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 1986;131:266–280. doi: 10.1016/0076-6879(86)31045-0. [DOI] [PubMed] [Google Scholar]
- 66.Grimsley GR, Huyghues-Despointes BMP, Pace CN, Scholtz JM. Measuring the conformational stability of a protein. In: Simpson R, editor. Protein Purification: a laboratory manual. Cold Spring Harbor. Cold Spring Harbor Laboratory Press; 2004. pp. 535–566. [Google Scholar]
- 67.Gajiwala KS. Structure and stability of ribonuclease T1: a crystallographic and calorimetric study. College Station, TX: Texas A&M University; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


