Abstract
Objective
To determine if metformin improves markers of inflammation, thrombosis, and intrahepatic fat contents in children with uncomplicated obesity.
Methods
Obese children with normal glucose tolerance but elevated highly sensitive C-reactive protein (hsCRP) and/or fibrinogen concentrations (>2 standard deviations) were randomized to structured diet/exercise or diet/exercise and metformin for 6 months. Blood samples, dual energy X-ray absorptiometry data, and liver magnetic resonance images were obtained.
Results
Forty-two of 66 recruited children (7–18 years) completed 6 months. Weight loss was modest but more pronounced in the metformin group (−4.9±1.0 kg) than in the diet/exercise group (−1.7±1.1 kg, p<0.03), whereas hsCRP and fibrinogen decreased more in the diet/exercise pubertal group. Baseline intrahepatic fat was high but decreased only in the diet/exercise (not metformin) pubertal group.
Conclusions
Six months of metformin therapy improved weight loss and reduced abdominal adiposity, but did not enhance the beneficial effect of diet and exercise on markers related to inflammation, thrombosis, or hepatic fat in obese children with normal glucose tolerance.
Keywords: C-reactive protein, fibrinogen, puberty
Introduction
The exponential increase in childhood obesity (~17% of US youth) (1, 2) is associated with increased incidence of dyslipidemia, hypertension, and impaired fasting glucose (components of the metabolic syndrome), which predisposes to cardiovascular disease in adults. We previously showed that children with uncomplicated obesity (those without these co-morbidities) had marked increases in concentrations of highly sensitive C-reactive protein (hsCRP), fibrinogen, interleukin 6 (IL-6), and plasminogen activator inhibitor-1 (PAI-1), reflective of a pro-inflammatory and pro-thrombotic state, even before co-morbidities of the metabolic syndrome are present, and even before the onset of puberty, as compared to lean, age-matched controls (3). These data support more aggressive interventions in very young obese children regardless of the absence of associated co-morbidities. In earlier studies lifestyle modifications improved several cardiovascular risk factors, but failed to restore them to control values in obese children.
Metformin is an insulin-sensitizing biguanide approved for treatment of children with type 2 diabetes (T2D). Its mechanism of action is complex, facilitating insulin-induced suppression of gluconeogenesis and reducing inhibition – and inducing expression of glucose transporters, increasing glucose utilization (4). It does not undergo hepatic metabolism, causes no hypoglycemia, and has an excellent safety record in children (5–14). Studies have shown variable and modest improvements in measures of insulin sensitivity in obese children with normal glucose tolerance when using metformin for 4–6 months (8, 10, 12, 14), but little is known about the effect of metformin, along with diet and exercise, on other measures associated with cardiovascular risk. It is unclear whether the combination of metformin and exercise would have synergistic effects. We therefore designed these studies to determine whether metformin would have a synergistic effect with a program of lifestyle modification with structured diet and exercise, to improve inflammatory status and thrombosis-related markers children with uncomplicated obesity before the establishment of co-morbidities of the metabolic syndrome. Changes in intrahepatic fat contents were assessed as well, as an exploratory aim.
Methods
Study subjects
The institutional review committee at Wolfson Children’s Hospital approved the studies, and informed parental consent and child’s assent were obtained. The study was registered at http://www.clinicaltrials.gov (NCT00139477).
Children with uncomplicated obesity were selected among those that participated in a recent cross-sectional study of pro-inflammatory and pro-thrombosis markers in obese vs. lean, age-matched controls (3) with hsCRP and/or fibrinogen concentrations >2 standard deviations above the mean. Uncomplicated (exogenous) obesity was defined as a body mass index (BMI) >95th percentile for US standards for <5 years, and normal blood pressure (BP), glucose tolerance, and total cholesterol. Recruitment was balanced for gender and prepubertal and late pubertal stage [≥ Tanner IV breasts (females) or genitals (males)]. Menstruating girls were sampled more than 2 weeks after their period. Chronic illness, medications, alcohol use, and smoking were exclusions from participation.
Study procedures
At screening, a physical exam including Tanner staging and sitting BP (×5) was performed; waist circumference was measured at umbilicus; and height and weight measured with a Harpenden stadiometer and digital scale. BMI (kg/m2) and BMI% were determined using Centers for Disease Control standards (1). Blood glucose (BG) was measured (One Touch Ultra, Lifescan Inc, Milpitas, CA, USA) (15), and those with fasting BG> 100 mg/dL, or BP elevated for age-specific standards were excluded from further participation and referred for further medical evaluation. Fasting blood samples were obtained for hormones, cytokines, metabolites and substrates, and lipid and liver panels. If total cholesterol was >200 mg/dL, subjects were dropped from the study.
At baseline, a 3-h oral glucose tolerance test was performed and those with impaired glucose tolerance (fasting ≥5.6<7.0 mmol/L, 2 h≥7.8<11.1 mmol/L) were excluded from further participation (16). Indirect calorimetry (BreezeSuite CPX Ultima; MedGraphics, St. Paul, MN, USA) was performed fasting for resting energy expenditure determination. A 3-day food diary was obtained to analyze caloric intake.
Hepatic magnetic resonance imaging and dual energy X-ray absorptiometry
Intrahepatic fat content was measured using fast magnetic resonance imaging (MRI) as previously described (17, 18). This technique quantitates hepatic fat fraction by replacing the standard spin echo sequence with a fast gradient echo sequence reducing scan time, usually within a single breath for most children. A single radiologist (DM) analyzed all scans using a GE 1.5 Tesla MRI scanner (Signa HDx series model, GE, Fairfield, CT, USA). Dual energy X-ray absorptiometry (DEXA) scan was performed to measure body composition (Hologic Discovery A S/N 45903; Hologic, Waltham, MA, USA).
Randomization
After baseline studies, subjects were randomly assigned to diet and exercise or diet, exercise, and metformin for 6 months. Randomization assignments were balanced for pubertal status.
Interventions
Metformin was started at 250 mg orally, twice daily (bid), before meals titrating up to 500 mg bid in those <12 years old and 1000 mg bid as tolerated in older children. Dietary counseling was provided with a recommended decrease of ~250–500 calories/day, using resting energy expenditure results as a guide. Intense follow-up was provided weekly by the research dietician for 4 weeks, then monthly until 3 months, then again at 6 months. Subjects received a free family membership to their local Young Men’s Christian Association (YMCA), or in those <9 years old to Velocity Sports Performance Gym for the study. Subjects were carefully instructed on equipment use and encouraged to exercise at least three times per week for 30 min per session; children <9 years of age were required to engage in active play activities at home. An activity diary was kept, and pedometers were worn during the day to document physical movement. Physical exam, anthropometry, waist circumference, and blood samples were repeated at 3 and 6 months; body composition determination (DEXA) and liver MRI were repeated at 6 months. Subjects also had biweekly phone calls from the study staff.
Assays
HsCRP and fibrinogen concentrations were measured in our laboratory by immuno-nephelometry (Siemens Healthcare Diagnostics, Deerfield IL, USA) (3), with an hsCRP lower sensitivity of 0.156 mg/L. IL-6 was measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA), PAI-1 by ELISA (American Diagnostica, Stamford, CT, USA), adiponectin by radioimmune assay (RIA) (Linco Research Inc., St. Charles, MO, USA), and insulin-like growth factor-I (IGF-I) levels by RIA (Diagnostic Systems Laboratories Inc., Corporate Webster TX, USA). Insulin levels were determined by RIA using Beckman kits (Beckman Coulter, Brea, CA, USA). The homeostatic model assessment (HOMA) for insulin sensitivity was calculated as fasting glucose (mmol/L)×fasting insulin (μIU/mL)/22.5. Free fatty acids (FFA) were measured by colorimetric assay (Hoffman-La Roche Ltd., Basel, Switzerland) and liver transaminases by standard analyzers.
Statistical analysis
Differences in hsCRP and fibrinogen concentrations at 6 months were the primary outcomes. An n=42 completed subjects provided >90% power to detect significant changes. Mean±SE or medians (Q1, Q3) are shown as appropriate. For each variable of primary and secondary end points, a mixed model repeated measures analysis of variance was used to compare the overall difference in mean changes between two treatment groups. Changes from baseline and at 6 months were used as the model’s dependent variables, each adjusted for gender and/or puberty as appropriate. A p-value <0.30 was considered for these two variables to enter into the model. The least square (LS) mean of overall change, change from baseline to 6 months, were provided along with the p-value or 95% confidence interval. Non-parametric Mann Whitney U and Wilcoxon signed rank tests were used when necessary. Tests were two tailed, significance p<0.05. SAS version 9.1.2 (SAS Institute Inc., Cary, NC, USA) and SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) software were used.
Results
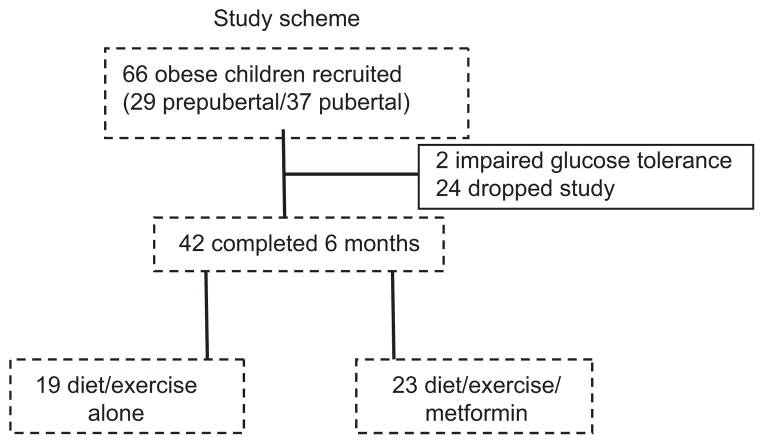
Sixty-six children enrolled (29 prepubertal and 37 pubertal), and their clinical characteristics are summarized in Table 1. Basel, prepubertal children were Tanner stage I genitals (males) or breasts (females). Some early Tanner stage II children were counted as prepubertal when distinction between breast and fat tissue was difficult, yet there was minimal or no pubic hair and estrogen (testosterone in males) was low. We intentionally recruited subjects in late puberty (Tanner stage IV or V). Subjects were well balanced by gender, height, and Tanner stage. Two subjects from the diet/exercise group were excluded owing to impaired glucose tolerance at baseline. Forty-two of 66 subjects completed 6 months and are included in the post-treatment analysis (n=19 diet/exercise, n=23 diet/exercise/metformin) (Figure 1). Subjects that dropped out were mainly lost to follow-up, relocated, or were no longer interested. The average dose of metformin was 1000 mg/day by 6 months. Compliance was monitored by frequent phone calls and subject self-report. The subjects that completed the trial were felt to be compliant with the medication.
Table 1.
Clinical characteristics of the study subjects.
| Diet/exercise | Diet/exercise/metformin | |
|---|---|---|
| n (male/female) | 31 (15/16) | 35 (15/20) |
| Prepubertal/pubertal | 14/17 | 15/20 |
| Age, years | 12.0±0.4 (8–16) | 12.3±0.5 (8–17) |
| Height, cm | 156.7±2.4 | 155.8±2.5 |
| Height Z score | 1.0±0.2 | 0.8±0.2 |
| BMI, kg/m2 | 33.2±0.7 | 32.0±1.0 |
| BMI% | 98.6±0.4 | 98.6±0.2 |
| Waist circumference, cm | 103.0±1.6 | 99.9±2.4 |
| Race | ||
| White | 12 (39%) | 18 (51%) |
| African American | 13 (42%) | 13 (37%) |
| Other | 6 (19%) | 4 (11%) |
Data are mean±SE.
Figure 1.
Study scheme.
Anthropometry and body composition
The metformin group lost 4.9±1.0 kg (p<0.001) vs. 1.7±1.1 kg in the diet/exercise group (p=0.123); p=0.02 between groups. BMI and waist circumference also decreased more in the metformin group compared with the diet/exercise group. Fat mass percentage decreased in both groups. There were no differences in heart rate or BP in either group during treatment (Table 2).
Table 2.
Change in anthropometrics, body composition, and BP.
| Diet/exercise
|
Diet/exercise/metformin
|
|||
|---|---|---|---|---|
| 6 months–0 month | p-Value | 6 months–0 month | p-Value | |
| n | 19 | 23 | ||
| Weight, kga | −1.7±1.1 | 0.123 | −4.9±1.0 | <0.001 |
| BMI, kg/m2 b | −1.1±0.5 | 0.045 | −2.4±0.5 | <0.001 |
| BMI, percentilea | −0.8±0.5 | 0.16 | −2.7±0.5 | <0.0001 |
| Waist circumference, cma | −0.3±2.1 | 0.89 | −6.7±1.8 | 0.001 |
| Waist/height ratioc | −0.3±0.0 | <0.001 | −0.4±0.0 | <0.001 |
| Fat mass% (DEXA) | −2.0±0.8 | 0.02 | −3.9±0.7 | <0.001 |
| Systolic BP, mm Hg | 1.6±2.2 | 0.46 | 4.6±2.0 | 0.03 |
| Diastolic BP, mm Hg | −1.0±2.0 | 0.61 | 2.5±1.8 | 0.16 |
Data are LS mean±SE;
p<0.03,
p=0.086 between groups,
p=0.05.
Carbohydrate, lipid metabolism, and energy expenditure
All subjects who were randomized had normal glucose tolerance with mean fasting BG of 4.9±0.1 mmol/L at baseline and 6.2±0.2 mmol/L at 2 h, and insulin concentration of 132±14 pmol/L at baseline and 535±49 pmol/L at 2 h [to convert glucose (mmol/L to mg/dL), divide by 0.0555; to convert insulin (pmol/L to μIU/mL), divide by 6.945]. Fasting insulin concentrations increased less in the metformin group during the 6-month intervention. HOMA-IR, a measure of insulin resistance, was high in both groups at baseline (diet/exercise: 5.2±0.6, diet/exercise/metformin: 4.8±0.4) and increased significantly after 6 months in both groups (Table 3). Adiponectin concentration, a measure of insulin sensitivity, increased comparably in both groups (Table 3). LS means for total cholesterol and low-density lipoprotein cholesterol showed non-significant changes in both groups, whereas high-density lipoprotein cholesterol increased mostly in the diet/exercise/metformin group (Table 3). FFA concentrations did not change significantly in either group. Resting energy expenditure rates were comparable between groups at baseline (diet/exercise: 36.1±1.8 kcal/kg fat-free mass/day, diet/exercise/metformin: 39.2±2.2 kcal/kg fat-free mass/day, p=0.28 between groups). No significant differences were observed when data were analyzed by pubertal stage.
Table 3.
Change in markers of carbohydrate and lipid metabolism.
| Diet/exercise
|
Diet/exercise/metformin
|
|||
|---|---|---|---|---|
| 6 months–0 month | p-Value | 6 months–0 month | p-Value | |
| Insulin, pmol/La | 67±20 | 0.002 | 2±19 | 0.91 |
| HOMA-IR | 1.6±0.8 | 0.06 | 0.34±0.76 | 0.66 |
| Adiponectin, mg/L | 1.9±0.9 | 0.04 | 2.5±0.8 | 0.004 |
| TC, mmol/L | −0.20±0.11 | 0.1 | 0.03±0.10 | 0.82 |
| LDL-C, mmol/L | −0.14±0.09 | 0.14 | −0.07±0.09 | 0.41 |
| HDL-C, mmol/L | 0.03±0.04 | 0.47 | 0.09±0.03 | 0.008 |
| Triglycerides, mmol/L | −0.002±0.09 | 0.15 | −0.03±0.09 | 0.72 |
| FFA, mmol/L | −0.04±0.05 | 0.38 | −0.01±0.4 | 0.88 |
Data are LS mean±SE.
p=0.02 between groups. To convert insulin to μIU/mL, divide by 6.945; low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and total cholesterol (TC) to mg/dL, divide by 0.0259; triglycerides to mg/dL, divide by 0.0113.
Inflammation and thrombosis-related markers
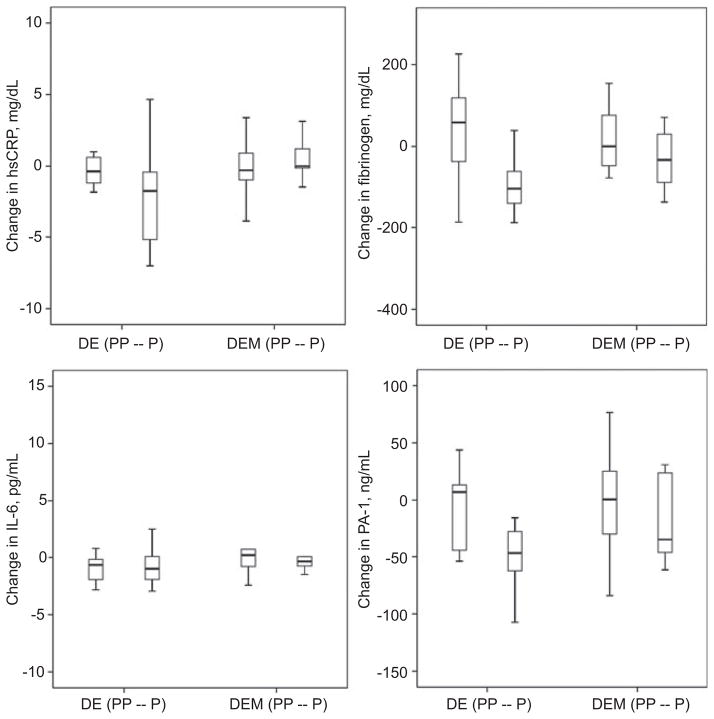
The main outcome measures compared markers related to inflammation and thrombosis in response to metformin in the diet/exercise vs. diet/exercise/metformin groups at 6 months. Many of these concentrations were not normally distributed, hence data are expressed as medians (interquartile range) (Figure 2). Inflammation-related makers showed a trend toward lower hsCRP (p=0.09) and lower IL-6 concentrations (p=0.03) in the diet/exercise group as a whole, not in the metformin group at 6 months. Fibrinogen, a thrombosis-related marker, did not change significantly but PAI-1 concentrations decreased more in the diet/exercise group at 6 months (p=0.01). Subanalysis by prepubertal vs. pubertal stage showed the pubertal diet/exercise group had greater improvements compared with pubertal children in the diet/exercise/metformin group over 6 months in hsCRP (−2.2±1.0 mg/dL vs. 1.0±1.0 mg/dL, p=0.035 between groups) and fibrinogen (−111±28 mg/dL vs. −30±24 mg/dL, p=0.048 between groups). There were no differences in degree of change in hsCRP and fibrinogen in prepubertal cohorts in the two groups. There were no significant differences in prepubertal and pubertal children in the two groups at 6 months for change in PAI-1, IL-6, or adiponectin.
Figure 2.
Boxplots of the changes in pro-inflammatory markers hsCRP and IL-6 concentrations, and changes in pro-thrombotic markers fibrinogen and PAI-1 for the diet/exercise (DE) group and the diet/exercise/metformin (DEM) group at 6 months in the prepubertal (PP) and pubertal (P) groups.
p<0.05 between pubertal DE vs. DEM for hsCRP and fibrinogen.
IGF-I concentrations
IGF-I concentrations were similar and did not change significantly over 6 months in either group regardless of puberty [overall change diet/exercise: −78±60 ng/mL (p=0.04) vs. diet/exercise/metformin: −117±55 ng/mL (p=0.21)].
Intrahepatic fat content
Most children (95%) had increased intrahepatic fat contents at baseline (mean 10.0%±0.6%) compared to reported data that suggest values >5.5% as abnormal (19–21). Overall, neither the diet/exercise group nor the diet/exercise/metformin group had a significant impact on intrahepatic fat contents after 6 months, although there was a trend toward lower percentages of intrahepatic fat over time (diet/exercise baseline: 10.1%±0.8%, 6 months: 8.7%±1.0%, p=0.09; diet/exercise/metformin baseline: 9.3%±0.8%, 6 months: 8.2%±0.4%, p=0.64). Subanalysis by pubertal stage and gender, however, revealed only the pubertal diet/exercise, not the metformin group, had significant decreases in intrahepatic fat content after 6 months intervention (diet/exercise: −2.2%±1.1%, diet/exercise/metformin: 0.7%±0.5%, p=0.038 between groups).
Correlations
Correlation analysis was performed grouping data by treatment (diet/exercise vs. diet/exercise/metformin) for some of the key outcomes and two measures of adiposity, percentage fat mass and waist circumference. There was a positive correlation between fibrinogen concentrations with waist circumference (r=0.488, p=0.047), but no significant correlation between levels of visceral adiposity and hsCRP or IL-6 concentrations (data not shown). There was a positive correlation between intrahepatic fat content and both percentage fat mass (r=0.316, p=0.05) and alanine aminotransferase (ALT) concentrations (r=0.378, p=0.025), as shown in Figure 3. There was no correlation between decreases in BMI and decreases in hsCRP or fibrinogen concentrations.
Figure 3.
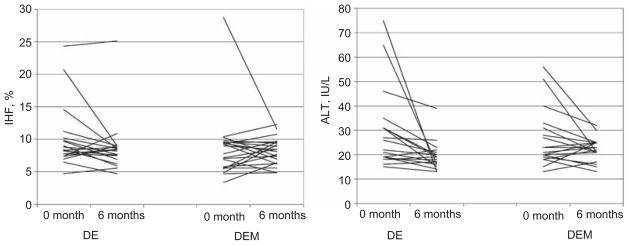
Intrahepatic fat (IHF) contents and ALT concentrations for the diet/exercise (DE) group and the diet/exercise/metformin (DEM) group at baseline and 6 months.
Safety
Metformin was well tolerated and safe. None of the 66 subjects enrolled discontinued study drug because of side effects. Liver function tests remained normal during the studies.
Discussion
As lifestyle modifications are insufficient to normalize cardiovascular risk factors in obese children, we focused on the effect of metformin, in addition to lifestyle interventions, on pro-inflammatory (hsCRP, IL-6) and pro-thrombosis factors (fibrinogen, PAI-1) in a cohort of children with relatively uncomplicated obesity and normal glucose tolerance. We had previously reported these children to have significant elevation of these markers as compared to lean, age- and pubertal-matched controls (3). After 6 months of lifestyle intervention, we observed a modest but significant improvement in weight, BMI, and waist circumference that was more pronounced in the metformin group vs. diet/exercise group. Adiponectin concentrations, a surrogate measure closely related to insulin sensitivity, improved comparably in both groups over 6 months. However, hsCRP and fibrinogen concentrations, the principal study outcomes, were lowered more in the pubertal diet/exercise group as compared with the diet/exercise/metformin group. Growth hormone (GH) deficiency has been shown to be associated with increased markers of cardiovascular risk and GH to have positive effects on inflammatory markers (22–24). As GH production rates are highest in puberty (25), this may in part explain these more positive effects of diet/exercise in the pubertal cohort. The decrease observed in these markers was, however, modest and did not normalize any of these values as compared with our own data in a similar group of age-, puberty-, and gender-matched lean controls (3). These data suggest that the effects of metformin on weight and insulin sensitivity are positive, albeit modest, in obese children without impaired glucose tolerance. It also suggests that metformin does not improve the pro-inflammatory, pro-thrombosis state better than lifestyle intervention alone in youths with uncomplicated obesity.
Adipocytes release IL-6, which stimulates hepatic production of CRP (26, 27), a sensitive marker for systemic inflammation. Fibrin formation, promoted by fibrinogen, is an important risk factor for coronary heart disease (28–30) and its concentrations correlate with adiposity in children (31) through increases in fractional synthesis rates (32, 33). PAI-1, the principal physiological inhibitor of fibrinolysis, is produced by adipocytes and may be causally related to obesity (34, 35). We previously demonstrated significant elevations in fibrinogen and PAI-1 concentrations in the same cohort, even in prepubertal obese children as compared to lean controls (3). In the present study, the level of decrease in concentrations of fibrinogen was more significant in the pubertal children in the diet/exercise-alone arm than in those also taking metformin after 6 months. These observations are intriguing as we had hypothesized that slightly better weight and fat loss associated with metformin (and consequent improvement in insulin sensitivity) would have resulted in better inflammatory- and thrombosis-related profile. These data suggest that metformin per se does not significantly improve the measured markers of cardiovascular risk measured here, at least not at the level of visceral fat change detected. This may be related to the relatively modest weight and fat mass loss observed.
Deposition of liver fat can lead to non-alcoholic steatohepatitis and may be the consequence of both fatty infiltration and oxidative stress, and is common in obese children (18, 36). Metformin decreases ALT after 4 months in adults (37) and decreases biopsy-proven hepatic steatosis in children (38, 39). Beneficial effects were also reported in a large multiethnic group of obese children treated with metformin vs. placebo (13). In our study, the average intrahepatic fat measured by rapid abdominal MRI was 10.0%±0.6% (range 4.7%–28.7%), well above the 5.5% considered normal (19, 21), an increase observed in 95% of baseline scans. These results were remarkable considering that approximately half of the cohort was prepubertal. Although normal, ALT concentrations were highly correlated with intrahepatic fat and they were not significantly improved by either intervention. However, similar to the effects on hsCRP and fibrinogen, intrahepatic fat decreased significantly in the lifestyle-alone pubertal group over 6 months. This lack of change in liver fat with metformin suggests that when the drug is combined with a structured program of diet/exercise, it does not have added benefit on intrahepatic fat contents in the context of children with uncomplicated obesity and modest, not dramatic weight change.
Previous studies on metformin use in obese pre-diabetic children have yielded mixed results. A recent meta-analysis in obese, non-diabetic adults and adolescents suggests that metformin has positive, yet variable results in enhancing weight loss and improving metabolic parameters (11). Srinivasan et al. (8) studied a more heterogeneous group of obese children treated with metformin or placebo for 6 months and observed beneficial effects of metformin on weight, BMI, and fasting insulin, but not on insulin sensitivity or visceral adiposity. Love-Osborne et al. (10) compared weight changes in obese multiethnic adolescents and observed no group differences between metformin and placebo in weight loss measures, whereas other investigators showed a modest decrease in weight and BMI in obese adolescents with normal glucose tolerance treated with metformin vs. placebo (9, 12, 14, 40). However, several recent studies in women with obesity, T2D, and polycystic ovary syndrome, and first-degree relatives of patients with T2D, for example (41–48), have also shown a relative lack of efficacy of metformin in normalizing measures of inflammation (hsCRP) or thrombosis (fibrinogen) similar to our findings here. Even in the diet/exercise group, the changes were modest and the intervention improved, but did not normalize the values as compared to normal children.
In conclusion, although the addition of metformin improved weight loss and reduced abdominal adiposity, a 6-month treatment program with metformin did not enhance the beneficial effect of diet and exercise on markers related to inflammation, thrombosis, or hepatic fat in obese children with normal glucose tolerance. Although the positive effects of lifestyle modifications were more pronounced in puberty, diet and exercise alone, even in a research-supported setting, only provides modest improvement in cardiovascular risk in young children with uncomplicated obesity. There remains a dire need for better pharmacological strategies to curb cardiovascular risk in this population.
Acknowledgments
The authors are grateful to Shiela Smith, RN, and the expert nursing staff of the Clinical Research Center at Wolfson’s Children’s Hospital for the care of our patients; to Shawn Sweeten for laboratory assistance; to Katie Black for research assistance; and to Drs. Karen Oerter Klein, Robert Rapaport, and Donald George for their participation in the Data Safety Management Board. Special thanks to Mr. Ray Purvis, Director of the YMCA in Duval County, for providing membership support to the children and their parents participating in these studies, and to Mr. W.J. Wadsworth for his generous donation to this research program.
Research funding: Thrasher Research Fund and a generous grant from Mr. W.J. Wadsworth and the support of the Nemours Research Programs.
Footnotes
Authors’ conflict of interest disclosure: The authors have nothing to disclose.
References
- 1.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat Series. 2002;11(246):1–190. (DHHS publication PHS2002) [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabek CJ, et al. Prevalence of overweight and obesity in the United States, 1999–2004. J Am Med Assoc. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Mauras N, Delgiorno C, Kollman C, Bird K, Morgan M, et al. Obesity without established comorbidities of the is associated with a proinflammatory and prothrombotic state, even before the onset of puberty in children. J Clin Endocrinol Metab. 2010;95:1060–8. doi: 10.1210/jc.2009-1887. [DOI] [PubMed] [Google Scholar]
- 4.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 5.Sarnblad S, Kroon M, Aman J. Metformin as additional therapy in adolescents with poorly controlled type 1 diabetes: randomised placebo-controlled trial with aspects on insulin sensitivity. Eur J Endocrinol. 2003;149:323–9. doi: 10.1530/eje.0.1490323. [DOI] [PubMed] [Google Scholar]
- 6.Willi SM, Martin K, Datko FM, Brant BP. Treatment of type 2 diabetes in childhood using a very-low-calorie diet. Diabetes Care. 2004;27:348–53. doi: 10.2337/diacare.27.2.348. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman FR. Type 2 diabetes mellitus in children and youth: a new epidemic. J Pediatr Endocrinol Metab. 2002;15(Suppl 2):737–44. doi: 10.1515/JPEM.2002.15.s2.737. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan S, Ambler GR, Baur LA, Garnett SP, Tepsa M, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91:2074–80. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 9.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, et al. The impact of metformin, oral contraceptives and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love-Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J Pediatr. 2008;152:817–22. doi: 10.1016/j.jpeds.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desilets AR, Dhakal-Karki S, Dunican KC. Role of metformin for weight management in patients without type 2 diabetes. Ann Pharmacother. 2008;42:817–26. doi: 10.1345/aph.1K656. [DOI] [PubMed] [Google Scholar]
- 12.Burgert TS, Duran EJ, Goldberg-Gell R, Dziura J, Yeckel CW, et al. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatr Diabetes. 2008;9:567–76. doi: 10.1111/j.1399-5448.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 13.Nadeau KJ, Ehlers LB, Zeitler PS, Love-Osborne K. Treatment of non-alcoholic fatty liver disease with metformin versus lifestyle intervention in insulin-resistant adolescents. Pediatr Diabetes. 2009;10:5–13. doi: 10.1111/j.1399-5448.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilson DM, Abrams SH, Aye T, Lee PD, Lenders C, et al. Glaser Pediatric Research Network Obesity Study Group. Metformin extended release treatment of adolescent obesity: a 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med. 2010;164:116–23. doi: 10.1001/archpediatrics.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabetes Research In Children Network (DirecNet) Study Group. A multicenter study of the accuracy of the One Touch Ultra home glucose meter in children with type 1 diabetes. Diabetes Technol Ther. 2003;5:933–41. doi: 10.1089/152091503322640971. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fishbein MH, Stevens WR. Rapid MRI using a modified Dixon technique: a non-invasive and effective method for detection and monitoring of fatty metamorphosis of the liver. Pediatr Radiol. 2001;31:806–9. doi: 10.1007/s002470100547. [DOI] [PubMed] [Google Scholar]
- 18.Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36:54–61. doi: 10.1097/00005176-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–94. doi: 10.1210/jc.2006-1010. [DOI] [PubMed] [Google Scholar]
- 20.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 21.Cali AM, Zern TL, Taksali SE, de Oliveira AM, Dufour S, et al. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes Care. 2007;30:3093–8. doi: 10.2337/dc07-1088. [DOI] [PubMed] [Google Scholar]
- 22.Russell M, Bredella M, Tsai P, Mendes N, Miller KK, et al. Relative growth hormone deficiency and cortisol excess are associated with increased cardiovascular risk markers in obese adolescent girls. J Clin Endocrinol Metab. 2009;94:2864–71. doi: 10.1210/jc.2009-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setola E, Monti LD, Lanzi R, Lucotti P, Losa M, et al. Effects of growth hormone treatment on arginine to asymmetric dimethylarginine ratio and endothelial function in patients with growth hormone deficiency. Metabolism. 2008;57:1685–90. doi: 10.1016/j.metabol.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Lanes R, Marcano H, Villaroel O, Gunczler P, Morillo E, et al. Circulating levels of high-sensitivity C-reactive protein and soluble markers of vascular endothelial cell activation in growth hormone-deficient adolescents. Horm Res. 2008;70:230–5. doi: 10.1159/000151595. [DOI] [PubMed] [Google Scholar]
- 25.Mauras N, Blizzard RM, Link K, Johnson ML, Rogol AD, et al. Augmentation of growth hormone secretion during puberty: evidence for a pulse amplitude-modulated phenomenon. J Clin Endocrinol Metab. 1987;64:596–601. doi: 10.1210/jcem-64-3-596. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 27.Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, et al. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99:2221–2. [PubMed] [Google Scholar]
- 28.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. J Am Med Assoc. 1987;258:1183–6. [PubMed] [Google Scholar]
- 29.Stec JJ, Silbershatz H, Tofler GH, Matheney TH, Sutherland P, et al. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham Offspring Population. Circulation. 2000;102:1634–8. doi: 10.1161/01.cir.102.14.1634. [DOI] [PubMed] [Google Scholar]
- 30.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. J Am Med Assoc. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 31.Bao W, Srinivasan SR, Berenson GS. Plasma fibrinogen and its correlates in children from a biracial community: the Bogalusa Heart Study. Pediatr Res. 1993;33:323–6. doi: 10.1203/00006450-199304000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Balagopal P, Sweeten S, Mauras N. Increased synthesis rate of fibrinogen as a basis for its elevated plasma levels in obese female adolescents. Am J Physiol Endocrinol Metab. 2002;282:E899–904. doi: 10.1152/ajpendo.00412.2001. [DOI] [PubMed] [Google Scholar]
- 33.Balagopal P, George D, Sweeten S, Mann KJ, Yarandi H, et al. Response of fractional synthesis rate (FSR) of fibrinogen, concentration of D-dimer and fibrinolytic balance to physical activity-based intervention in obese children. J Thromb Haemost. 2008;6:1296–303. doi: 10.1111/j.1538-7836.2008.03037.x. [DOI] [PubMed] [Google Scholar]
- 34.Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–46. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 35.Alessi MC, Poggi M, Juhan-Vague I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr Opin Lipidol. 2007;18:240–5. doi: 10.1097/MOL.0b013e32814e6d29. [DOI] [PubMed] [Google Scholar]
- 36.Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. 2004;8:549–58. doi: 10.1016/j.cld.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, et al. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–4. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 38.Schwimmer JB, Middleton M, Deutsch R, Lavine JE. Metformin as a treatment for non-diabetic NASH [abstract] J Pediatr Gastroenterol Nutr. 2003;37:342. [Google Scholar]
- 39.Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–9. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 40.Yanovski J, Krakoff J, Calaita CG, McDuffie JR, Kozlosky M, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children. A randomized clinical trial. Diabetes. 2011;60:477–85. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sari R, Eray E, Ozdem S, Akbas H, Coban E. Comparison of the effects of sibutramine versus sibutramine plus metformin in obese women. Clin Exp Med. 2010;10:179–84. doi: 10.1007/s10238-009-0080-y. [DOI] [PubMed] [Google Scholar]
- 42.Pradhan AD, Everett BM, Cook NR, Rifai N, Ridker PM. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. J Am Med Assoc. 2009;302:1186–94. doi: 10.1001/jama.2009.1347. [DOI] [PubMed] [Google Scholar]
- 43.Lima LM, Wiernsperger N, Kraemer-Aguiar LG, Bouskela E. Short-term treatment with metformin improves the cardiovascular risk profile in first-degree relatives of subjects with type 2 diabetes mellitus who have a and normal glucose tolerance without changes in C-reactive protein or fibrinogen. Clinics (Sao Paulo) 2009;64:415–20. doi: 10.1590/S1807-59322009000500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakubowska J, Bohdanowicz-Pawlak A, Milewicz A, Szymczak J, Bednarek-Tupikowska G, et al. Plasma cytokines in obese women with polycystic ovary syndrome, before and after metformin treatment. Gynecol Endocrinol. 2008;24:378–84. doi: 10.1080/09513590802128968. [DOI] [PubMed] [Google Scholar]
- 45.Kjotrod SB, Romundstad P, von During V, Sunde A, Carlsen SM. C-reactive protein levels are unaffected by metformin during pretreatment and an IVF cycle in women with polycystic ovary syndrome. Fertil Steril. 2008;89:635–41. doi: 10.1016/j.fertnstert.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 46.Stocker DJ, Taylor AJ, Langley RW, Jezior MR, Vigersky RA. A randomized trial of the effects of rosiglitazone and metformin on inflammation and subclinical atherosclerosis in patients with type 2 diabetes. Am Heart J. 2007;153:445, e1–6. doi: 10.1016/j.ahj.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Kang ES, Kim DJ, Kim SH, Ahn CW, et al. Effects of rosiglitazone and metformin on inflammatory markers and adipokines: decrease in interleukin-18 is an independent factor for the improvement of homeostasis model assessment-β in type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2007;66:282–9. doi: 10.1111/j.1365-2265.2006.02723.x. [DOI] [PubMed] [Google Scholar]
- 48.De Jager J, Kooy A, Lehert P, Bets D, Wulffele MG, et al. Effect of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2005;257:100–9. doi: 10.1111/j.1365-2796.2004.01420.x. [DOI] [PubMed] [Google Scholar]