Abstract
The optimal treatment for invasive aspergillosis remains elusive, despite the increased efficacy of newer agents. The immunosuppressants cyclosporine (CY), tacrolimus (FK506), and sirolimus (formerly called rapamycin) exhibit in vitro and in vivo activity against Candida albicans, Cryptococcus neoformans, and Saccharomyces cerevisiae, including fungicidal synergy with azole antifungals. We report here that both FK506 and CY exhibit a clear in vitro positive interaction with caspofungin against Aspergillus fumigatus by disk diffusion, microdilution checkerboard, and gross and microscopic morphological analyses. Microscopic morphological analyses indicate that the calcineurin inhibitors delay filamentation, and in combination with caspofungin there is a positive interaction. Our findings suggest a potential role for combination therapy with calcineurin pathway inhibitors and existing antifungal agents to augment activity against A. fumigatus.
Clinical trials with both caspofungin and voriconazole show significant favorable responses in certain patients with invasive aspergillosis (17; J. Maertens, I. Raad, C. Sable, A. Ngai, R. Berman, T. F. Patterson, D. Denning, and T. Walsh, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1103, 2000), but optimal treatment remains elusive (37) and combination antifungal therapy with mechanistically different agents is common (38). The immunosuppressants cyclosporine (CY), tacrolimus (FK506), and sirolimus (formerly called rapamycin) all suppress T-cell-proliferative responses. CY and FK506 inhibit calcineurin, a Ca2+-calmodulin-dependent protein phosphatase that is important in cell signaling (5, 22), while sirolimus functions through a separate target of the rapamycin (TOR) signaling pathway (33). These immunosuppressants have revolutionized modern transplantation, but their role as potential antifungals is only beginning to be understood (3, 16). We report an in vitro positive interaction between CY or FK506 and caspofungin against Aspergillus fumigatus based on four different assay methods. These results highlight the potential role that inhibition of the calcineurin stress response pathway could play in the treatment of invasive aspergillosis.
MATERIALS AND METHODS
Fungal strains.
Eight strains of Aspergillus fumigatus (AF293, DUMC 119.00, DUMC 153.90, DUMC 165.86, DUMC 182.99, DUMC 168.95, DUMC 131.00, and DUMC 101.01) and four control strains of Candida albicans (ATCC 22019, SC5314, YAG171, and JRB12) were studied. The Aspergillus strains were all clinical isolates from patients with invasive aspergillosis from our institution, except strain DUMC 119.00, which was a clinical isolate from a nearby hospital submitted for antifungal testing, and strain AF293 (NCPF 7367), which was from a patient who died of invasive aspergillosis in the United Kingdom. The Candida strains were previously described: SC5314 is a wild-type C. albicans strain, and JRB12 expresses a dominant sirolimus-resistant Tor1-1 (TOR1-1/TOR1) mutant that fails to bind to FK506-binding protein 12-sirolimus. YAG171 is sirolimus-resistant and FK506-resistant and lacks the sirolimus-binding protein (rbp1Δ/rbp1Δ) (8, 9).
Antifungals and immunosuppressants.
Caspofungin (Cancidas; Merck & Co., Rahway, N.J.) and voriconazole (Vfend; Pfizer, Inc., New York, N.Y.) were obtained as powders and prepared as outlined in the National Committee for Clinical Laboratory Standards (NCCLS) M38-A document (28). Sirolimus was obtained from the National Cancer Institute, and sirolimus analog 3, a nonimmunosuppressive analog with structural differences in the drug effector domain that binds to Tor (8, 12), was supplied by Abbott Laboratories. FK506 was supplied by Fujisawa Healthcare, Inc. (Deerfield, Ill.), and L-685,818, a nonimmunosuppressive analog of FK506 that differs by an allyl→ethyl substitution at C-21 and a hydroxyl group introduced at C-18 (2, 13, 29, 34), was supplied by Merck. CY was purchased from Alexis Corporation.
Disk diffusion assay.
After standardized growth and harvest (28), 100 μl of a 106 conidia/ml suspension of AF293 was spread uniformly onto yeast extract-peptone-dextrose agar plates. Blank 6-mm-diameter paper disks (Becton Dickinson, Sparks, Md.) were impregnated with a 4-log dosage range (0.01, 0.1, 1, or 10 μg) of caspofungin, immunosuppressants, or a combination, and after drying were placed onto inoculated agar plates. Plates were incubated at 37°C, and zones of inhibition diameters were measured at 24 and 48 h; in addition, a description of the degree of growth within the zone of inhibition was recorded. Each disk diffusion assay was performed in duplicate, with the mean diameter value reported.
Broth susceptibility testing.
In vitro MIC testing with voriconazole, caspofungin, and the immunosuppressants was performed according to the M38-A method (28), modified for diluting the five immunosuppressants, with ATCC 22019 Candida parapsilosis as an antifungal control. The final concentrations were 0.0313 to 32 μg/ml for caspofungin, 0.0313 to 16 μg/ml for voriconazole, and 0.012 to 50 μg/ml for each immunosuppressant. For an end point, we used an MIC at which 80% of isolates tested were inhibited, and for caspofungin-treated wells, we also reported the minimum effective concentration (MEC) as determined by the formation of microscopically aberrant hyphal tips (1).
Antifungal and immunosuppressant checkerboard testing.
We tested eight A. fumigatus strains in duplicate by a microdilution checkerboard technique. For each of the first four strains (AF293, DUMC 119.00, DUMC 153.90, and DUMC 165.86), we performed a total of 10 checkerboard tests, one for each immunosuppressant with caspofungin as well as voriconazole. Based on the preliminary checkerboard data, the last four A. fumigatus strains (DUMC 101.01, DUMC 131.00, DUMC 168.95, and DUMC 182.99) were assessed by a microdilution checkerboard test with caspofungin or voriconazole combined with only FK506 or CY.
The fractional inhibitory concentration (FIC) of a drug was defined as the MIC of that drug in combination divided by the MIC of that drug alone. An FIC index (Σ FIC) is the sum of the two FIC values of the individual drugs (15). An FIC index value of <0.5 revealed synergy, a value of 0.5 to 1.0 revealed additivity, a value of 1 to 4 revealed indifference, and a value of >4 represented antagonism.
Fungicidal activity.
Fungicidal activity was considered to be present when ≥99% killing of the inoculum occurred, as demonstrated by <1% of original inoculum growth on subculture. We subcultured 100 μl from each well with no visible growth at 48 h onto yeast extract-peptone-dextrose agar and incubated it for 7 days at 37°C.
Gross and microscopic morphological changes.
We performed an in vitro time-kill study to investigate the activity of the calcineurin inhibitors (FK506 and CY) with and without caspofungin. Five milliliters of a 104-conidia/ml suspension was prepared in Sabouraud's broth and incubated at 35°C with shaking at 200 rpm in the presence of caspofungin (32 μg/ml), CY (50 μg/ml), FK506 (50 μg/ml), or the combination of caspofungin plus FK506 or caspofungin plus CY. At specific time points (0, 3, 6, and 9 h after inoculation), 100-μl aliquots were removed and spread in triplicate on Sabouraud's agar plates. The plates were incubated at 35°C for 48 h, and we took photographs to demonstrate the gross morphological colony differences between the treatment arms.
In a separate experiment using the same six treatment arms detailed above, we placed 10-μl aliquots on a slide for bright-field microscopic morphological examination (Olympus BH-2 microscope; magnification, ×400). Two parallel experiments were performed: either the drugs were added immediately to the inoculum as described above, or the inoculum was grown for 9 h until there was germination visible under microscopy before the drugs were added. Micrographs were taken at 0, 3, 6, 9, 12, 18, 21, 24, and 30 h.
RESULTS
Immunosuppressants and caspofungin have positive interactions based on disk diffusion assay.
All three immunosuppressants and sirolimus analog 3 showed similar or larger zones of inhibition than caspofungin at comparable doses after 48 h of incubation. The mean zones of inhibition ± standard deviations at 10 μg of each drug were 46 ± 3 mm for FK506, 21 ± 1 mm for CY, 41 ± 2 mm for sirolimus, 37 ± 3 mm for sirolimus analog 3, 6 ± 1 mm for L-685,818, and 20 ± 2 mm for caspofungin. At 48 h, FK506 showed the least growth inside the zones of inhibition, followed by sirolimus, caspofungin, and then CsA. These initial disk diffusion results demonstrated the inherent activity of the calcineurin inhibitors against A. fumigatus.
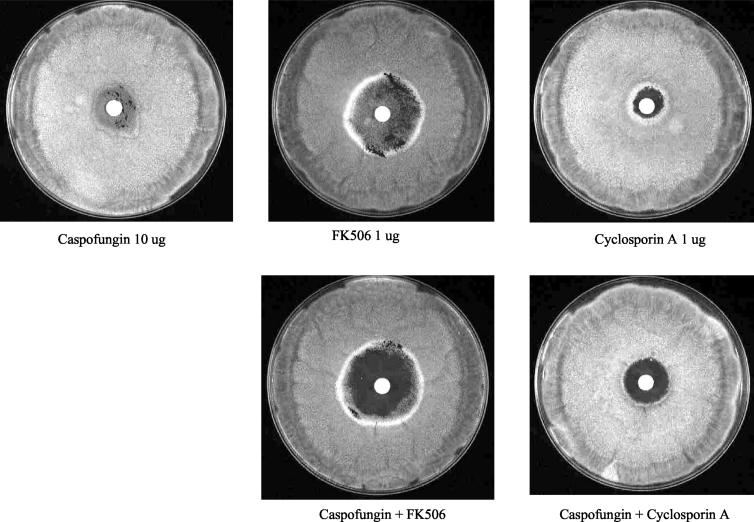
In the experiments with 10 μg of drug in combination, the mean sizes of the zones of inhibition of the drugs in combination were not statistically different from those of the individual drugs. However, all combinations did suggest some degree of a positive interaction at several of the lower dose levels based on an increase in the size of the zone of inhibition over that of the single drugs alone. Growth density analysis for the inside of the zones of inhibition of drugs in combination did show increased antifungal activity (Fig. 1). The combination of caspofungin plus FK506 yielded a clearer zone than either drug alone, while caspofungin plus CY produced a totally clear zone. We concluded from these initial disk diffusion assays that there is a potential positive interaction between the two classes of agents against A. fumigatus, and this conclusion prompted further quantitative testing.
FIG. 1.
Enhanced activity and growth suppression with calcineurin inhibitors and caspofungin by disk diffusion assay.
Immunosuppressants possess activity against A. fumigatus by broth susceptibility testing.
CY and FK506 showed consistent activity in a broth microdilution assay at 24 and 48 h (48-h CY geometric mean MIC, 6.25 μg/ml; 48-h FK506 geometric mean MIC, 1.56 μg/ml). Sirolimus and sirolimus analog 3 showed excellent activity at 24 h (mean geometric MICs, 0.096 and 1.98 μg/ml, respectively) but lost all antifungal activity by 48 h. The L-685,818 analog showed no antifungal effect at 24 or 48 h. These findings confirmed that FK506 and CY have inherent activity against A. fumigatus, unlike previous results with Saccharomyces cerevisiae or C. albicans (9).
In vitro FIC index synergism and additivity between FK506, CsA, and caspofungin.
The geometric mean FIC index of caspofungin plus FK506 for eight clinical A. fumigatus strains was 0.27, and that of caspofungin plus CY was 0.53 (Table 1). When analyzed with an MEC end point instead of an MIC end point with caspofungin, the FIC indices were generally higher, with the mean FIC index of caspofungin plus FK506 increasing to 0.80 and that of caspofungin plus CY increasing to 1.22. The FIC indices of voriconazole and FK506 or CY were generally indifferent or antagonistic (Table 2), with geometric mean FIC indices of 4.20 and 3.20, respectively.
TABLE 1.
In vitro synergy and indifference between caspofungin and immunosuppressants against A. fumigatus by microdilution checkerboard interactiona
| Strain | MIC (μg/ml) after 48 h of incubation
|
FIC indexb
|
|||||
|---|---|---|---|---|---|---|---|
| FK506 | CY | Caspo | Caspo + FK506 | Caspo + CY | Caspo + FK506 | Caspo + CY | |
| 119.00 | 1.56 | 3.125 | >32 (1) | 4 (0.25)/0.19 | 8 (0.25)/1.56 | 0.18 (0.37) | 0.63 (0.75) |
| 153.90 | 1.56 | 6.25 | >32 (1) | 8 (1)/0.39 | 4 (1)/1.56 | 0.38 (1.25) | 0.31 (1.25) |
| 165.86 | 6.25 | 6.25 | >32 (0.5) | 4 (0.25)/0.78 | 4 (0.5)/1.56 | 0.19 (0.63) | 0.31 (1.25) |
| AF293 | 1.56 | 3.125 | >32 (1) | 1 (0.5)/0.39 | 8 (1)/0.78 | 0.27 (0.75) | 0.38 (1.25) |
| 182.99 | 0.39 | 6.25 | >32 (1) | 4 (1)/0.097 | 16 (1)/3.125 | 0.31 (1.25) | 0.75 (1.5) |
| 168.95 | 3.125 | 6.25 | >32 (1) | 8 (0.5)/0.097 | 8 (0.5)/3.125 | 0.16 (0.53) | 0.63 (1.0) |
| 131.00 | 0.78 | 6.25 | >32 (0.5) | 4 (0.5)/0.097 | 16 (0.5)/3.125 | 0.19 (1.12) | 0.75 (1.5) |
| 101.01 | 1.56 | 25 | >32 (1) | 16 (0.5)/0.78 | 16 (1)/12.5 | 0.75 (1.0) | 0.75 (1.5) |
| Geometric mean | 1.56 | 6.25 | >32 (0.84) | 4.76 (0.5)/0.25 | 8.72 (0.65)/2.41 | 0.27 (0.80) | 0.53 (1.22) |
Caspo, caspofungin. Values in parentheses are MECs.
For FIC index calculations with caspofungin, a value of 64 was used for those isolates with an MIC of >32 μg/ml.
TABLE 2.
In vitro antagonism and indifference between voriconazole and immunosuppressants against A. fumigatus by microdilution checkerboard interaction
| Strain | MIC (μg/ml) after 48 h of incubationa
|
FIC index
|
|||||
|---|---|---|---|---|---|---|---|
| FK506 | CY | VCZ | VCZ + FK506 | VCZ + CY | VCZ + FK506 | VCZ + CY | |
| 119.00 | 0.78 | 25 | 0.25 | 0.5/0.78 | 0.5/25 | 3 | 3 |
| 153.90 | 0.78 | 25 | 0.25 | 1/1.56 | 0.5/25 | 5 | 3 |
| 165.86 | 0.78 | 12.5 | 0.5 | 1/3.125 | 1/12.5 | 6 | 3 |
| AF293 | 0.78 | 25 | 0.25 | 0.5/3.125 | 0.5/25 | 6 | 3 |
| 182.99 | 0.78 | 6.25 | 0.5 | 1/1.56 | 1/6.25 | 4 | 3 |
| 168.95 | 3.13 | 12.5 | 0.5 | 1/3.125 | 1/12.5 | 3 | 3 |
| 131.00 | 0.78 | 6.25 | 0.25 | 1/0.78 | 1/6.25 | 5 | 5 |
| 101.01 | 3.13 | 25 | 1 | 2/3.125 | 2/25 | 3 | 3 |
| Geometric mean | 1.10 | 14.87 | 0.39 | 0.92/1.86 | 0.84/14.87 | 4.20 | 3.20 |
VCZ, voriconazole.
FK506 with caspofungin has fungicidal activity.
Voriconazole alone showed fungicidal activity (≥99% killing), while caspofungin alone was fungistatic. FK506 was the only immunosuppressant that alone resulted in fungicidal activity, but only for four of eight A. fumigatus strains (AF293, 165.86, 131.00, and 101.01). Importantly, for the four strains which showed fungistatic activity with both caspofungin and FK506 alone (strains 119.00, 182.99, 168.95, and 153.90), there was fungicidal activity with the combination of caspofungin and FK506.
Calcineurin inhibitors create gross and microscopic morphological changes in A. fumigatus colonies.
At the earliest time points, gross morphology revealed an obvious stunting of growth in FK506-exposed cells, resulting in much smaller colonies, while the CY-exposed cells were indistinguishable from controls in number and size (data not shown). However, after further incubation, there was a clear decrease in CFU in the CY-exposed samples, while the FK506-exposed samples had higher numbers of cells and continued to exhibit smaller colonies.
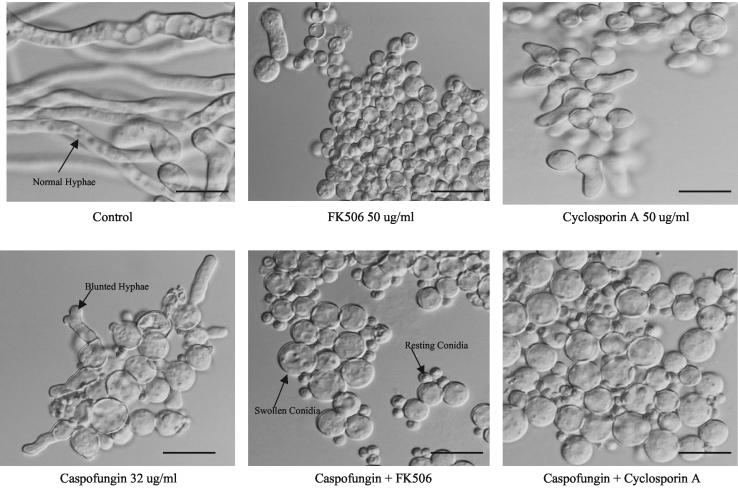
In evaluating microscopic morphologies, when drugs were initially added to the conidial suspension, the caspofungin-treated sample had stunted, swollen, branched hyphae at 12 and 21 h of growth compared to those of the control sample. Treatment with FK506 and CY alone delayed hyphal elongation, as most conidia were swollen, with only a few cells beginning to elongate at 12 h and more hyphal elongation occurring at 21 h. It was noted that FK506 had more of an inhibitory effect on filamentation than CY. After caspofungin was combined with the calcineurin inhibitors, further delays in filamentation were observed, as evidenced by a mixture of swollen (ready-to-germinate) conidia and the continued presence of nonswollen resting conidia (Fig. 2). This phenomenon continued at 21 h, where fewer hyphal elements were present in the combination-treated samples than in the treatment groups receiving caspofungin or calcineurin inhibitor alone.
FIG. 2.
Microscopic colony morphology after 12 h of incubation. Bar, 10 μm.
In the germinated-hypha treatment arm, in which drugs were added after 9 h of incubation and hyphae had already been allowed to form, the well-described blunting of apical hyphal tips by caspofungin (21) was observed at both 18 and 30 h (data not shown). The calcineurin inhibitors appeared to have an effect similar to that of caspofungin on hyphal development, and the combination of the two classes of drugs appeared to generally delay filamentation and produce even smaller hyphae.
DISCUSSION
The immunophilin-targeting drugs CY, FK506, and sirolimus inhibit conserved signal transduction cascades required for T-cell activation and fungal growth, and as a consequence they exhibit activity against a number of fungal species, including the model yeast S. cerevisiae and the pathogenic fungi C. albicans and Cryptococcus neoformans (6, 8, 10, 26, 27, 29-31, 35, 40). There is also an in vitro fungicidal synergism between the calcineurin inhibitors and the normally fungistatic azole drugs against S. cerevisiae (4, 14) and C. albicans (9, 23, 25), which is supported by a rat model of C. albicans endocarditis (24).
There are several clinical reports showing a decrease in invasive aspergillosis (39) and mortality (36) in patients receiving specific calcineurin inhibitors as their immunosuppressants. The only animal model study of A. fumigatus with calcineurin inhibitors (19) found that median survival was shortest in the CY-treated mice and that survival was significantly longer in the controls and the group receiving FK506 at 1 mg/kg of body weight/day, with no survival advantage for the group receiving FK506 at 10 mg/kg/day. Histopathologic evaluation revealed widely disseminated hyphae in the brains and kidneys of CY-treated animals but an almost total absence of hyphae in the brains of FK506-treated mice. This model highlights the complex work needed to decipher the correct balance between the immunosuppressive and antifungal effects of these drugs.
A previous in vitro disk diffusion study showed that FK506 and sirolimus possessed activity against A. fumigatus (18). The only published in vitro combination study of an antifungal and immunosuppressant used with Aspergillus spp. utilized disk diffusion assays and found that 1 μg of caspofungin combined with 1 μg of L-685,818 yielded enhanced activity against 8 of 11 clinical A. fumigatus isolates (20). However, no formal MIC studies were performed, and no other doses of the two drugs were reported.
In our studies, we confirmed and then expanded on those earlier findings by using a wider 4-log dose range and performing broth susceptibility testing to demonstrate clear antifungal activity with the calcineurin inhibitors but no sustainable antifungal activity with the TOR pathway agents. Our results suggest differences in the immunosuppressant susceptibilities of yeasts and A. fumigatus. Whereas sirolimus has consistent activity against C. albicans (40) and C. neoformans (8), we found no consistent activity for it or its analog against A. fumigatus. Similarly, although FK506 had inherent activity against A. fumigatus, the MICs of FK506 were higher than those for C. neoformans (29).
We found zone-of-inhibition size differences for the drug combinations at lower dose ranges, as well as differences in the amount of trailing growth within the zones. In the microdilution checkerboard testing, we observed synergy with the combination of caspofungin plus FK506 in seven of eight clinical isolates, and caspofungin plus CY was synergistic in three of eight isolates, while there was indifference or a negative interaction with the triazole and the calcineurin inhibitors. Here again the differences between the yeasts and A. fumigatus are evident. While there is a fungicidal synergism with an azole antifungal and a calcineurin inhibitor against C. albicans (9, 23, 25), voriconazole combined with the immunosuppressants produced in vitro indifference or antagonism against A. fumigatus. Caspofungin combined with a nonimmunosuppressive analog (L-685,818) previously showed synergy against C. neoformans (11), whereas we found no apparent positive interaction against A. fumigatus.
Finally, we described the gross and microscopic morphological effects of calcineurin inhibition delaying filamentation in A. fumigatus, which extends the range of this observation previously reported for other fungi (7, 32). The findings from microscopic observation clearly show that the combination results in an increased delay in filamentation. Taken together, these in vitro and microscopic findings implicate calcineurin as a potential antifungal target for A. fumigatus, based on the antifungal activities of known calcineurin inhibitors used alone and in combination with caspofungin.
Drawing from other infectious disease examples (e.g., human immunodeficiency virus, tuberculosis, and cryptococcosis), combination antifungal therapy for invasive aspergillosis is hypothesized to be a possibility. Several recently developed antifungals have revolutionized the approach to patients with invasive aspergillosis, and now novel pathways can be utilized for a true combination therapeutic approach. The patients at highest risk for invasive aspergillosis are often already on immunosuppressants, so determining the optimal combination and balance of immunosuppressive and antifungal activities to combat aspergillosis will be crucial. These studies validate further investigations into links between signaling pathways, cell membrane, and cell wall targets in Aspergillus.
Acknowledgments
We thank Merck for donating both caspofungin and L-685,818, Pfizer for providing voriconazole, and Fujisawa for supplying FK506. W.J.S. was supported by grant K12-HD00850, J.H. was supported by grant AI50438, and J.R.P. was supported by grant P01-AI-449175.
REFERENCES
- 1.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, J. W., J. Rotonda, B. M. McKeever, H. K. Chan, A. I. Marcy, G. Wiederrecht, J. D. Hermes, and J. P. Springer. 1993. FK-506-binding protein: three dimensional structure of the complex with the antagonist L-685,818. J. Biol. Chem. 25:11335-11339. [PubMed] [Google Scholar]
- 3.Blankenship, J. R., W. J. Steinbach, J. R. Perfect, and J. Heitman. 2003. Teaching old drugs new tricks: reincarnating immunosuppressants as antifungal drugs. Curr. Opin. Investig. Drugs 4:192-199. [PubMed] [Google Scholar]
- 4.Bonilla, M., K. K. Nastase, and K. W. Cunningham. 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21:2343-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas, M. E., M. C. Cruz, M. Del Poeta, N. Chung, J. R. Perfect, and J. Heitman. 1999. Antifungal activities of antineoplastic agents: Saccharomyces cerevisiae as a model system to study drug action. Clin. Microbiol. Rev. 12:583-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz, M. C., M. Del Poeta, P. Wang, R. Wenger, G. Zenke, V. F. J. Quesniaux, N. R. Movva, J. R. Perfect, M. E. Cardenas, and J. Heitman. 2000. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob. Agents Chemother. 44:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz, M. C., D. S. Fox, and J. Heitman. 2001. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 20:1020-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz, M. C., A. L. Goldstein, J. Blankenship, M. Del Poeta, J. R. Perfect, J. H. McCusker, Y. L. Bennani, M. E. Cardenas, and J. Heitman. 2001. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob. Agents Chemother. 45:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz, M. C., R. A. L. Sia, M. Olson, G. M. Cox, and J. Heitman. 2000. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect. Immun. 68:982-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Poeta, M., M. C. Cruz, M. E. Cardenas, J. R. Perfect, and J. Heitman. 2000. Synergistic antifungal activities of bafilomycin A1, fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickman, D. A., H. Ding, Q. Li, A. M. Nilus, D. J. Balli, S. J. Ballaron, J. M. Trevillyn, M. L. Smith, L. S. Seif, K. Kim, A. Sarthy, R. C. Goldman, J. J. Plattner, and Y. L. Bennani. 2000. Antifungal rapamycin analogues with reduced immunosuppressive activity. Bioorg. Med. Chem. Lett. 10:1405-1408. [DOI] [PubMed] [Google Scholar]
- 13.Dumont, F. J., M. J. Staruch, S. L. Koprack, J. J. Siekierka, C. S. Lin, R. Harrison, T. Sewell, V. M. Kindt, T. R. Beattie, M. Wyvratt, and N. H. Sigal. 1992. The immunosuppressive and toxic effects of FK-506 are mechanistically related: pharmacology of a novel antagonist of FK-506 and rapamycin. J. Exp. Med. 176:751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edlind, T., L. Smith, K. Henry, S. Katiyar, and J. Nickels. 2002. Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signaling. Mol. Microbiol. 46:257-268. [DOI] [PubMed] [Google Scholar]
- 15.Ellopoulos, G. M., and R. C. Molellering. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 16.Fox, D. S., and J. Heitman. 2002. Good fungi gone bad: the corruption of calcineurin. Bioessays 24:894-903. [DOI] [PubMed] [Google Scholar]
- 17.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 18.High, K. P. 1994. The antimicrobial activities of cyclosporine, FK506, and rapamycin. Transplantation 57:1689-1700. [PubMed] [Google Scholar]
- 19.High, K. P., and R. G. Washburn. 1997. Invasive aspergillosis in mice immunosuppressed with cyclosporin A, tacrolimus (FK506), or sirolimus (rapamycin). J. Infect. Dis. 175:222-225. [DOI] [PubMed] [Google Scholar]
- 20.Kontoyiannis, D. P., R. E. Lewis, N. Osherov, N. D. Albert, and G. S. May. 2003. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J. Antimicrob. Chemother. 51:313-316. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, J., J. D. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807-815. [DOI] [PubMed] [Google Scholar]
- 23.Maesaki, S., P. Marichal, M. A. Hossain, D. Sanglard, H. V. Bossche, and S. Kohno. 1998. Synergic effects of tacrolimus and azole antifungal agents against azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 42:747-753. [DOI] [PubMed] [Google Scholar]
- 24.Marchetti, O., J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon. 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44:2932-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchetti, O., P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mody, C. H., G. B. Toews, and M. F. Lipscomb. 1988. Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model. Infect. Immun. 56:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mody, C. H., G. B. Toews, and M. F. Lipscomb. 1989. Treatment of murine cryptococcosis with cyclosporin-A in normal and athymic mice. Am. Rev. Respir. Dis. 139:8-13. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 29.Odom, A., M. Del Poeta, J. Perfect, and J. Heitman. 1997. The immunosuppressant FK506 and its nonimmunosuppresssive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob. Agents Chemother. 41:156-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. R. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perfect, J. R., and D. T. Durack. 1985. Effects of cyclosporine in experimental cryptococcal meningitis. Infect. Immun. 50:22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prokisch, H., O. Yarden, M. Dieminger, M. Tropschug, and I. B. Barthelmess. 1997. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol. Gen. Genet. 256:104-114. [DOI] [PubMed] [Google Scholar]
- 33.Rohde, J., J. Heitman, and M. E. Cardenas. 2001. The TOR kinases link nutrient sensing to cell growth. J. Biol. Chem. 276:9583-9586. [DOI] [PubMed] [Google Scholar]
- 34.Rotonda, J., J. J. Burbaum, H. K. Chan, A. I. Marcy, and J. W. Becker. 1993. Improved calcineurin inhibition by yeast FKBP12-drug complexes. Crystallographic and functional analysis. J. Biol. Chem. 268:7607-7609. [DOI] [PubMed] [Google Scholar]
- 35.Sehgal, S. N., H. Baker, and C. Vezina. 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. (Tokyo) 28:727-732. [DOI] [PubMed] [Google Scholar]
- 36.Singh, N., R. K. Avery, P. Munoz, T. L. Pruett, B. Alexander, R. Jacobs, J. G. Tollemar, E. A. Dominguez, C. M. Yu, D. L. Paterson, S. Husain, S. Kusne, and P. Linden. 2003. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin. Infect. Dis. 36:46-52. [DOI] [PubMed] [Google Scholar]
- 37.Steinbach, W. J., and D. A. Stevens. 2003. Review of newer antifungal and immunomodulatory strategies for invasive aspergillosis. Clin. Infect. Dis. 37(Suppl. 3):S157-S187. [DOI] [PubMed] [Google Scholar]
- 38.Steinbach, W. J., D. A. Stevens, and D. W. Denning. 2003. Combination and sequential antifungal therapy for invasive aspergillosis: review of published in vitro and in vivo interactions and 6281 clinical cases from 1966-2001. Clin. Infect. Dis. 37(Suppl. 3):S188-S224. [DOI] [PubMed] [Google Scholar]
- 39.Torre-Cisneros, J., R. Manex, S. Kusne, M. Allessiani, M. Martin, and T. E. Starzl. 1991. The spectrum of aspergillosis in liver transplant patients: comparison of FK506 and cyclosporine immunosuppression. Transplant. Proc. 23:3040-3041. [PMC free article] [PubMed] [Google Scholar]
- 40.Vezina, C., A. Kudelski, and S. N. Sehgal. 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 28:721-726. [DOI] [PubMed] [Google Scholar]