Abstract
Objectives
We evaluated and compared tumor antigen precursor protein (TAPP) profiles in adult and pediatric brain tumors of 31 genes related to tumor associated antigens (TAA) for possible use in immunotherapy. Antigens were selected based on their potential to stimulate T cell responses against tumors of neuroectodermal origin.
Methods
Thirty-seven brain tumor specimens from 11 adult and 26 pediatric patients were analyzed by quantitative real-time PCR for the relative expression of 31 TAPP mRNAs. The age range of adults (4F:7M) was 27–77 years (median 51.5 ± 14.5 years) and for pediatrics (12F:14M) was 0.9–19 years (median 8.3 ± 5.5 years). Histological diagnoses consisted of 16 glioblastomas, 4 low grade astrocytomas, 10 juvenile pilocytic astrocytomas, and 7 ependymomas.
Results
The adult gliomas expressed 94% (29 of 31) of the TAPP mRNAs evaluated compared with pediatric brain tumors that expressed 55–74% of the TAPP mRNAs, dependent on tumor histological subtype. Four types of TAPP expression patterns were observed: (1) equal expression among adult and pediatric cases, (2) greater expression in adult than pediatric cases, (3) expression restricted to adult GBM and (4) a random distribution. The pediatric brain tumors lacked expression of some genes associated with engendering tumor survival, such as hTert and Survivin.
Conclusions
The potential TAA targets identified from the TAPP profiles of 31 genes associated with adult and pediatric brain tumors may help investigators select specific target antigens for developing dendritic cell- or peptide-based vaccines or T cell-based immunotherapeutic approaches against brain tumors.
Keywords: Glioma, Pediatric brain tumors, Tumor associated antigens, Immunotherapy
Introduction
The most common diagnoses among children (ages 0–19) with brain tumors, in decreasing order of incidence, are juvenile pilocytic astrocytoma (JPA) > medulloblastoma > malignant glioma > and ependymoma [1]. Presently, there is no known cure for low-grade astrocytomas, a heterogeneous group of pediatric tumors including: JPA, fibrillary astrocytomas, oligodendrogliomas and mixed oligoastrocytomas [2]. Current treatment options for these tumors rely on surgery, chemotherapy and radiotherapy. All of these approaches have limitations due to resectability (i.e., brain stem, optic pathway glioma), dissemination into the surrounding brain, resistance to cytotoxic therapies and complications arising from the treatments, especially with cognitive and other quality of life issues that are faced by both the children and their parents, since long-term survival is common for pediatric low-grade gliomas. Thus, new and novel less invasive approaches are direly needed for pediatric brain tumor patients. Immunotherapy is a potentially safe therapy compared to surgery, chemotherapy and radiotherapy; it offers the possibility of selectively eliminating brain tumor cells while sparing non-malignant cells.
In general, the immunotherapeutic approaches tested for human gliomas have involved small numbers of patients in Phase I trials where safety has been demonstrated and successes have been anecdotal. Treatments have entailed vaccinations with autologous irradiated glioma cells given with immunomodulatory cytokines or with gene-modified fibroblasts secreting cytokines with hopes of achieving sustained local release. The cytokines have included granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and IL-4 [3–6]. Genetic engineering of autologous glioma cells to produce immunomodulatory factors, such as antisense TGF-β, has been attempted but timeliness issues have arisen for preparing the individualized treatments for patients with gliomas [7].
Cellular therapy with expanded tumor-infiltrating lymphocytes (TILs), suspected to be specifically sensitized in situ to TAA, have been adoptively transferred into the resection cavity of gliomas with promising indications in some patients [8]. Responses have been noted with patients given repeated, intratumoral administrations of allospecific cytotoxic T lymphocytes (CTL) generated to the patient’s histocompatibility antigens [9, 10].
Clinical trials testing combined active and passive immune approaches have been completed. Vaccinations with autologous tumor cells and cytokines were followed by adoptive T cell transfers after ex vivo activation [3, 5]. Importantly, precedence was set for immunotherapy taking a more prominent position and was given to newly diagnosed glioma patients [11].
Groups have developed dendritic cell (DC) vaccine approaches for treatment of gliomas using either crude tumor cell lysates or acid eluted peptides derived from GBM cell cultures or from surgical specimens [12–15]. In several patients a CTL response was elicited, while in others, TILs were observed in the resection specimen obtained at relapse. Although some objective responses were observed with increased tumor-free survival, the current adjunctive approaches are not curative. Thus, movement toward testing several modalities involving combinations of chemotherapy coupled with specific TAA peptide-pulsed DC vaccines has occurred [16]. Despite the fact that factors influencing the host immune response are poorly understood at this time, DC vaccination has emerged as a promising type of active immunotherapy. Safety, feasibility, immunological and clinical responses have been demonstrated in a number of small clinical trials treating a total of 123 adult patients with malignant glioma [17].
One of the limitations for immunotherapy approaches for brain tumors has been the lack of identified TAA that could serve as potential targets. Recently, we profiled 20 human established glioma cell lines and 11 surgical glioblastoma specimens for 16 TAPP mRNAs that could potentially be used for brain tumor immunotherapy [18]. The frequencies of TAPP gene expression correlated well between the cell lines and the tumor specimens. In the present study, we extensively profiled 31 TAPP mRNAs using 11 adult gliomas and 26 pediatric brain tumors in order to identify potential target antigens for use in T-cell based or DC plus peptide-based vaccine immunotherapeutic approaches for four different histological types of human brain tumors.
Materials and methods
Tissue samples
A total of 37 brain tumors were evaluated for tumor associated precursor protein (TAPP) mRNA expression. The tumors were classified according to the World Health Organization (WHO) criteria [19], except the presence of necrosis was required for a diagnosis of glioblastoma. Freshly resected surgical specimens were snap-frozen and kept at −80°C until use. For this study, the adult brain tumor specimens, collected between 1986 and 1998, were obtained at the University of Colorado Health Sciences Center (Denver, CO; provided by C.K.). The pediatric brain tumor specimens, collected between 1993 and 2007, were obtained at New York University School of Medicine (NY, NY; provided by D.Z. and E.W.N.). All tumor samples were collected under IRB-approved protocols. For the analysis, 30 astrocytic samples were used, including 11 adult glioblastoma multiforme (GBM), 5 pediatric GBM, 4 low-grade fibrillary astrocytomas (LGA), and 10 juvenile pilocytic astrocytomas (JPA). Seven non-astrocytic tumors were used in this study consisting of 7 ependymomas (EPEN). Table 1 summarizes the clinicopathological data for tumor subtype, patient age, and gender. For the 11 adult cases, 4 were females and 7 were males, who ranged in age from 27 to 77 years old (median age of 51.1 ± 14.5 years). For the 26 pediatric cases, 12 were females and 14 were males, who ranged in age from 11 months to 19 years old (median age of 8.3 ± 5.5 years).
Table 1.
Brain tumor samples and demographics of study population
| Tissue source | Sample No. | Age/sex | Primary/recurrent | Date of surgery |
|---|---|---|---|---|
| Adult brain tumor samples | ||||
| GBM | 14-23-MG | 51/F | Primary | 08/1986 |
| GBM | 04-10-MG | 60/F | Primary | 08/1986 |
| GBM | 03-02-MG | 27/F | Recurrent | 08/1986 |
| GBM | 16-22-MG | 43/M | Primary | 10/1986 |
| GBM | 23-02-MG | 55/M | Primary | 01/1987 |
| GBM | 08-05-MG | 69/M | Primary | 12/1991 |
| GBM | 23-05-MG | 50/M | Primary | 02/1994 |
| GBM | 05-14-MG | 77/F | Recurrent | 06/1995 |
| GBM | 13-20-MG | 35/M | Primary | 08/1996 |
| GBM | 04-14-MG | 42/M | Recurrent | 10/1997 |
| GBM | 23-20-MG | 57/M | Primary | 02/1998 |
| Pediatric brain tumor samples | ||||
| GBM | 617 | 19/M | Primary | 03/1993 |
| GBM | 847 | 11/F | Primary | 10/1994 |
| GBM | 1292 | 2/M | Primary | 12/1997 |
| GBM | 1476 | 7/M | Recurrent | 10/1998 |
| GBM | 1991 | 14/F | Primary | 02/2003 |
| LGA | 764 | 3/M | Primary | 04/1994 |
| LGA | 1246 | 6/M | Primary | 08/1997 |
| LGA | 1290 | 13/F | Primary | 11/1997 |
| LGA | 1606 | 2/F | Primary | 08/1999 |
| JPA | 2044 | 18/F | Primary | 07/2005 |
| JPA | 2073 | 12/F | Primary | 09/2005 |
| JPA | 2085 | 10/M | Primary | 02/2006 |
| JPA | 2104 | 4/F | Primary | 06/2006 |
| JPA | 2115 | 14/F | Primary | 08/2006 |
| JPA | 2121 | 3/M | Primary | 09/2006 |
| JPA | 2124 | 6/M | Primary | 10/2006 |
| JPA | 2129 | 7/M | Primary | 11/2006 |
| JPA | 2132 | 18/M | Primary | 11/2006 |
| JPA | 2139 | 6/M | Primary | 01/2007 |
| EPEN | 1213 | 6/M | Primary | 05/1997 |
| EPEN | 1879 | 6/M | Primary | 10/2001 |
| EPEN | 1997 | 14/M | Primary | 04/2003 |
| EPEN | 2017 | 11mo/F | Primary | 04/2003 |
| EPEN | 2102 | 2/F | Primary | 05/2006 |
| EPEN | 2082 | 11/F | Recurrent | 11/2005 |
| EPEN | 2110 | 2/F | Primary | 07/2006 |
Tumor antigen selection
We selected the tumor antigens used in this study based on three criteria. First, the TAPPs were originally reported to be expressed by gliomas derived from adult patients. Second, human immune responses against these tumor antigens have been detected either against human gliomas or against other human cancers. Third, quantitative real-time PCR (qRT-PCR) primers could be designed that produced 80–100 base paired amplicons that do not form primer-dimer complexes and specifically detect only the putative antigen. Previously, we reported 16 TAPPs that were grouped as follows: (1) “melanoma/glioma” specific; i.e., melanoma Glycoprotein-100 (GP100 also called Pmel 17), Antigen Isolated from Immunoselected Melanoma-2 (Aim-2), Tyrosinase Related Protein-1 (Trp-1), Tyrosinase Related Protein-2 (Trp-2), Tyrosinase, Melanoma Antigen-1 (Mage-1), Melanoma Antigen Recognized by T cells-1 (Mart-1 also known as Melan-A), Gage-1; (2) growth factor receptors i.e. EphA2, interleukin-13 receptor α2 (IL13Rα2), and Her2/Neu; (3) survival/growth dependent factors; i.e., B-cyclin, Telomerase Reverse Transcriptase (hTert), Survivin or (4) other antigens like β1,6-N-acetylglucosaminyltransferase-V (GnT-V) or Squamous cell carcinoma Antigen Recognized by T cells-1 (Sart-1) [18].
For this study, we have included 15 additional TAPPs for the profiling analysis: Antigen Recognized by T cells-1 and -4 (Art-1, Art-4, respectively), polycomb group protein Enhancer of Zeste Homologue 2 (Ezh2), Fos Related Antigen-1 (Fra-1, also known as Fosl1), UDP-Gal: betaGlcNAc beta1, 3-galactosyltransferase, poly-peptide 3 (Galt-3), Heterogeneous Nuclear Ribonucleoprotein L (HNRPL), Multidrug Resistance Protein-3 (MRP-3), Preferentially expressed Antigen in Melanoma (PRAME), ParaThyroid Hormone- Related Protein (PTH-rP), Squamous cell carcinoma Antigen Recognized by T cells-2 and -3 (Sart-2, Sart-3, respectively), Sox-11, Ubiquitin-conjugated Enzyme 2 Variant kua (Ube2V), YKL-40 and Wolf-Hirschhorn syndrome candidate 2 protein (Whsc2).
Real-time quantitative polymerase chain reaction analysis (qRT-PCR)
Total RNA was isolated from the tissue homogenates using Trizol reagent (Sigma Aldrich, Saint Louis, MO) and passed over an RNeasy Protection column to remove other impurities. Any possible DNA contamination in the sample was eliminated by incubation with RNase free DNase I digestion (Boehringer Mannheim GmbH, Ger-many). cDNA was synthesized using iScriptTM cDNA synthesis Kit (Bio-Rad Laboratories, Hercules, CA) containing 1 μg total RNA/sample. Real-time quantitative PCR reactions were performed on an iCycler iQ detection system (Bio-Rad Laboratories) in conjunction with the SYBR Green kit (Stratagene, San Diego, CA). The thermal profile was 95°C for 15 min, followed by 40 cycles of 95°C for 15 s and 58°C for 30 s, finally holding at 4°C. The primers were synthesized by Operon Biotech (Germantown, MD). The sequences of the primers used for qRT-PCR can be found in the Supplementary Material (Supplementary Table).
Samples were run in duplicate, and a reaction without cDNA established a baseline fluorescence level with 18S RNA. The fluorescent signal was plotted versus cycle number. The threshold cycle (CT) was defined as the cycle number where the fluorescence signal could be reliably detected above background fluorescence. Each PCR run also included non-template controls containing all reagents except cDNA. After cycling, a melting curve was produced by slow denaturation of the PCR end products to validate the specificity of amplification. The relative quantification of expression of any given gene was determined by 2−ΔCT as described [20]. The mRNA expression was considered a strongly positive signal when the ΔCT value was between 6 and 14, a moderately positive signal when the ΔCT score was between 15 and 20, a weakly positive signal when the ΔCT score was between 21 and 24, and a negative signal when the ΔCT score was >24. To statistically analyze the data, an arbitrary ΔCT score of 25 was assigned to specimens where a signal for the TAPP was not detected. Based on our previous experience with qRT-PCR, a cut off point of 24 was set for the ΔCT value as one that was biologically relevant. Cell lines having an mRNA score below this value routinely lacked protein for a given gene detected by antibody-based methods [18]. Data from each tumor sample was grouped according to the tumor types and plotted.
Statistical analysis
Statistical differences between the different groups were evaluated by a Student’s t test. Values less than P < 0.05 were considered statistically significant.
Results
TAPP mRNA expression patterns among the adult and pediatric brain tumors
We evaluated TAPP mRNA profiles for 31 genes in 11 adult GBM and 26 pediatric brain tumors using qRT-PCR. Patient data in Table 1 show the pediatric cases consisted of 19 astrocytic tumors (5 GBM, 4 LGA, 10 JPA) and 7 non-astrocytic tumors (ependymomas). The patient ages ranged from 27 to 77 years for adult GBM, 2–19 years for pediatric GBM, 2–6 years for LGA, 3–18 years for JPA, and 11 months to 11 years for ependymomas. Table 2 summarizes the TAPP mRNA data for each tumor type studied. Figures 1–4 show the relative TAPP mRNA expression for each tumor sample, grouped by tumor type and organized by one of four different expression patterns.
Table 2.
Frequency of TAPP mRNA in adult glioblastoma multiforme and subtypes of pediatric brain tumors
| TAPP Gene | Adult GBM No. positive/total [mean expression] | Pediatric GBM No. positive/total [mean expression] | Pediatric LGA No. positive/total [mean expression] | Pediatric JPA No. positive/total [mean expression] | Pediatric EPEN No. positive/total [mean expression] |
|---|---|---|---|---|---|
| TAPPs expressed in both adult and pediatric brain tumors | |||||
| Art-1 | 8/10 [17.3] | 5/5 [18.5] | 4/4 [17.7] | 10/10 [19.3] | 7/7 [20.6] |
| EphA2 | 10/11 [15.9] | 5/5 [19.8] | 4/4 [18.9] | 10/10 [20.6] | 7/7 [21.4] |
| Fosl1 | 9/9 [16.2] | 4/5 [19.6] | 4/4 [17.2] | 10/10 [16.5] | 7/7 [18.5] |
| HNRPL | 10/10 [12.4] | 5/5 [13.0] | 4/4 [14.0] | 10/10 [14.7] | 7/7 [16.4] |
| IL13Rα2 | 10/11 [20.1] | 3/5 [23.2] | 4/4 [19.1] | 4/10 [24.3] | 4/7 [23.6] |
| Sox11 | 9/9 [16.2] | 5/5 [17.2] | 4/4 [18.5] | 10/10 [19.6] | 7/7 [19.1] |
| Trp-2 | 9/11 [21.0] | 5/5 [20.0] | 3/4 [21.0] | 10/10 [21.4] | 7/7 [20.5] |
| Ube2v | 8/10 [15.6] | 5/5 [18.5] | 4/4 [16.5] | 10/10 [17.6] | 7/7 [19.7] |
| YKL-40 | 10/10 [8.8] | 5/5 [14.2] | 4/4 [12.5] | 10/10 [12.6] | 6/7 [19.8] |
| TAPPs expressed more in adult GBM than in pediatric brain tumors | |||||
| B-cyclin | 10/10 [14.6] | 5/5 [21.1] | 4/4 [19.9] | 9/10 [22.7] | 7/7 [22.3] |
| Ezh2 | 10/10 [12.1] | 3/5 [22.9] | 4/4 [18.1] | 8/10 [22.4] | 7/7 [20.7] |
| GnT-V | 11/11 [16.2] | 3/5 [23.3] | 4/4 [18.5] | 10/10 [21.7] | 6/7 [21.8] |
| Her2/neu | 11/11 [14.4] | 4/5 [22.1] | 4/4 [16.9] | 0/10 [25.5] | 6/7 [20.2] |
| MRP-3 | 10/10 [13.2] | 5/5 [18.7] | 4/4 [20.6] | 10/10 [17.0] | 3/7 [23.3] |
| Sart-1 | 10/11 [17.5] | 1/5 [24.9] | 3/4 [19.5] | 7/10 [23.9] | 3/7 [23.8] |
| Sart-2 | 10/10 [14.2] | 4/5 [21.1] | 2/4 [21.8] | 8/10 [20.7] | 0/7 [27.0] |
| Sart-3 | 10/10 [14.6] | 5/5 [21.8] | 3/4 [19.6] | 9/10 [22.5] | 2/7 [24.6] |
| Whsc2 | 10/10 [13.0] | 3/5 [23.3] | 3/4 [19.3] | 10/10 [20.3] | 2/7 [24.6] |
| TAPPs expressed preferentially in adult GBM | |||||
| Aim-2 | 10/11 [13.4] | 2/5 [25.0] | 3/4 [19.7] | 5/10 [23.9] | 2/7 [25.6] |
| Art-4 | 10/10 [18.3] | 0/5 [26.4] | 1/4 [26.0] | 0/10 [26.6] | 0/7 [27.0] |
| Galt-3 | 10/10 [14.4] | 1/5 [25.7] | 2/4 [23.3] | 0/10 [27.0] | 0/7 [27.0] |
| Mage-1 | 11/11 [16.2] | 1/5 [22.4] | 2/4 [24.6] | 1/10 [26.0] | 0/7 [26.1] |
| PTH-rP | 7/10 [19.3] | 1/5 [26.2] | 2/4 [23.3] | 0/10 [27.0] | 1/7 [25.0] |
| Survivin | 11/11 [16.9] | 1/5 [25.2] | 0/4 [26.6] | 0/10 [27.0] | 0/7 [27.0] |
| hTert | 10/10 [15.7] | 2/5 [24.8] | 1/4 [25.3] | 1/10 [26.3] | 0/7 [26.7] |
| Trp-1 | 10/11 [17.9] | 0/5 [26.9] | 1/4 [25.0] | 0/10 [27.0] | 0/7 [27.0] |
| Tyrosinase | 10/11 [17.5] | 1/5 [25.4] | 0/4 [27.0] | 0/10 [27.0] | 0/7 [27.0] |
| TAPPs with low expression in both adult and pediatric brain tumors | |||||
| GP100 | 9/11 [21.8] | 2/5 [22.7] | 4/4 [20.9] | 3/10 [24.9] | 3/7 [25.1] |
| Gage-1 | 0/11 [26.9] | 1/5 [24.6] | 0/4 [27.0] | 0/10 [27.0] | 0/7 [27.0] |
| Mart-1 | 0/11 [26.0] | 0/5 [26.9] | 0/4 [27.0] | 0/10 [27.0] | 0/7 [27.0] |
| PRAME | 4/9 [23.1] | 3/5 [23.1] | 1/4 [23.5] | 2/10 [25.7] | 1/7 [25.9] |
Numbers in brackets refer to the mean expression value of the threshold cycle (CT), as described in Materials and methods, for the number of cases analyzed for each TAPP gene and corresponds with the value represented by the horizontal bar in each figure
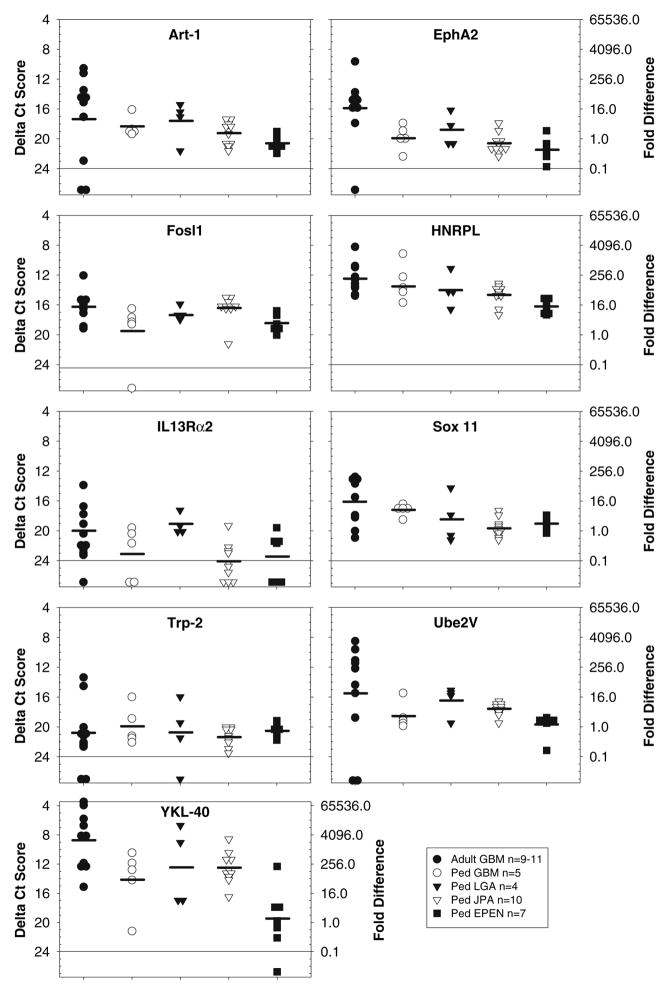
Fig. 1.
TAPP expression patterns common among adult and pediatric brain tumors. The amount of mRNA was quantitated by a ΔCt value on left y-axis in comparison to the 18S RNA. The ΔCt value of 20 was given an arbitrary value of 1 and the fold-difference is presented on right y-axis. The expression of Art-1, EphA2, Fosl1, HNRPL, IL13Rα2, Sox 11, Trp-2, Ube2V and YKL-40 are shown within their respective boxes. The legend box indicates the number of tumors that were analyzed. Due to limited amounts of either RNA or cDNA, only 9 adult GBM were evaluated for Fosl1 and Sox11, 10 adult GBM were evaluated for Art-1, HNRPL, Ube2V, and YKL-40, whereas all other TAPPs were evaluated from 11 adult GBM
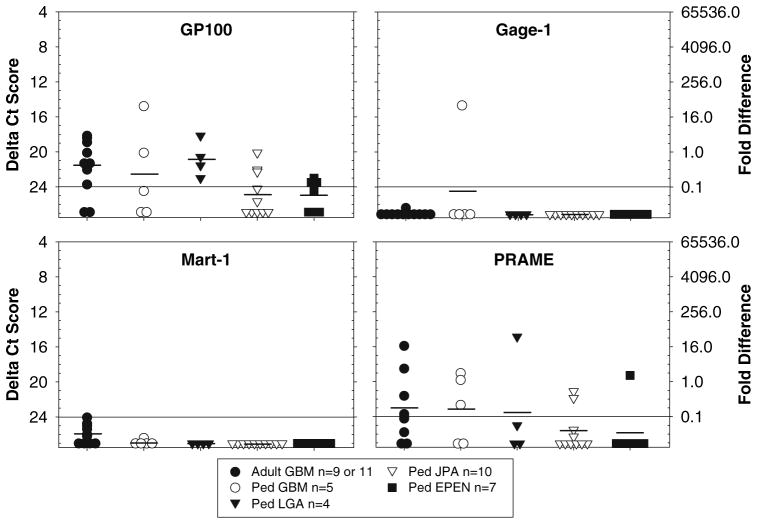
Fig. 4.
TAPP expression with a random pattern among adult and pediatric brain tumors. The amount of GP100, Gage-1, Mart-1 and PRAME mRNA is presented as a ΔCt value (left y-axis) and the fold difference (right y-axis). Due to limited amounts of either RNA or cDNA, only 9 adult GBM were evaluated for PRAME, whereas all other TAPPs were evaluated from 11 adult GBM
Four types of TAPP expression patterns were observed: (1) equal expression among adult and pediatric cases (Fig. 1), (2) greater expression in adult compared with pediatric cases (Fig. 2), (3) expression restricted to adult GBM (Fig. 3), and (4) a random distribution (Fig. 4).
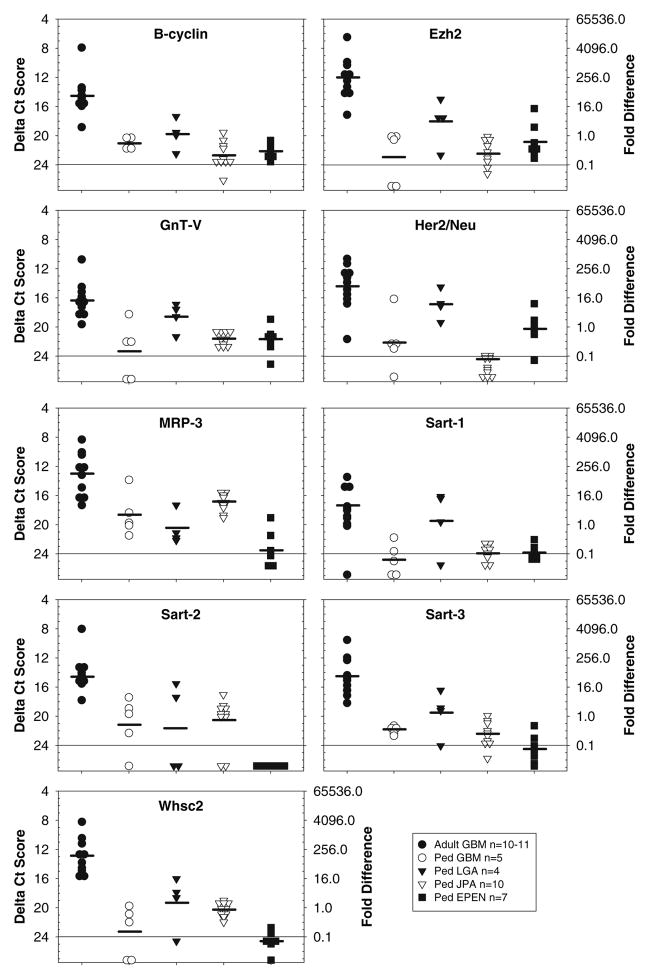
Fig. 2.
TAPP expression that was greater in adult compared with pediatric brain tumors. The amount of mRNA for B-cyclin, Ezh2, GnT-V, Her2/Neu, MRP-3, Sart-1, Sart-2, Sart-3 and Whsc2 is presented as a ΔCt value (left y-axis) and the fold difference (right y-axis). Except for Her2/Neu and Sart-1 expression in the LGA, all the TAPPs expressed in the adult GBM were statistically significant from those expressed in the pediatric brain tumors (P < 0.05). Due to limited amounts of either RNA or cDNA, 10 adult GBM were evaluated for B-cyclin, Ezh2, MRP-3, Sart-2, Sart-3 and Whsc-2, whereas all other TAPPs were evaluated from 11 adult GBM
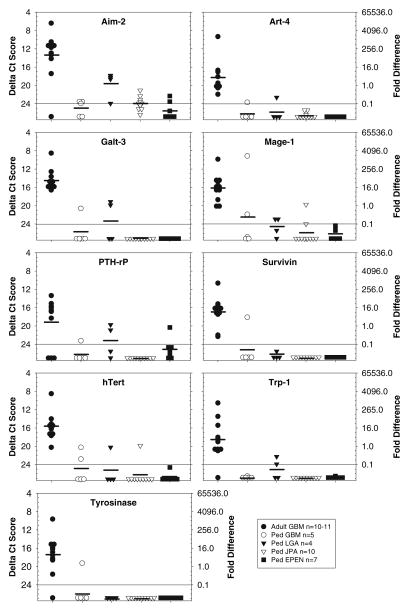
Fig. 3.
TAPP expression patterns restricted to adult GBM. The amount of mRNA for Aim-2, Art-4, Galt-3, Mage-1, PTH-rP, Survivin, hTert, Trp-1 and Tyrosinase is presented as a ΔCt value (left y-axis) and the fold difference (right y-axis). Except for PTH-rP expression in the LGA, all the TAPPs expressed in the adult GBM were statistically significant from those expressed in the pediatric brain tumors (P < 0.05). Due to limited amounts of either RNA or cDNA, 10 adult GBM were evaluated for Art-4, Galt-3, PTH-rP, and hTert, whereas all other TAPPs were evaluated from 11 adult GBM
In group 1 (Fig. 1), the relative amounts of the TAPP mRNAs for nine genes was statistically equal by quantitative real time PCR across all tumor types. The genes HNRPL and YKL-40 showed strong expression in all the brain tumors, except the ependymomas where they were moderately expressed. The genes Art-1, Fosl1, EphA2, Sox 11, Ube2V showed moderate levels of expression across all brain tumor types. The gene IL13Rα2 showed weak expression in all tumors except pediatric LGA, while the Trp-2 gene was weakly expressed across all tumor types.
In group 2 (Fig. 2), the relative amounts of the TAPP mRNAs was significantly greater, in general, in the adult GBM compared with the pediatric brain tumors (P < 0.05). Adult GBM showed strong expression for nine genes including B-cyclin, Ezh2, GnT-V, Her2/Neu, MRP-3, Sart-1, -2, -3 and Whsc2. Expression of the same genes was moderate or weak in the pediatric brain tumors.
In group 3 (Fig. 3), the relative amounts of the TAPP mRNAs showed strong to moderate expression in adult GBM compared with weak to no expression in the majority of the pediatric brain tumors. The difference in the relative amounts of the TAPP mRNAs was signifi-cant (P < 0.05). The nine genes included in this group were Aim-2, Art-4, Mage-1, Galt-3, PTH-rP, Trp-1, hTert, Survivin, and Tyrosinase. Finally, group four (Fig. 4) had a variable gene expression pattern for GP100, PRAME, Gage-1 and Mart-1 that was distinct from the other gene expression patterns.
TAPP mRNA expression in adult GBM
The adult GBM (N = 11) expressed 94% (29 of 31) of the TAPP mRNAs evaluated. The mRNA expression was considered strongly positive when the value was between 6 and 14, moderately positive between 15 and 20, weakly positive between 21 and 24, and negative when the value was >24. Eleven genes showed strong expression (values of 8.8–14.6) including YKL-40, Ezh2, HNRPL, Whsc2, MRP-3, Aim-2, Her2/Neu, Sart-2, Galt-3, Sart-3, and B-cyclin. Fifteen genes showed moderate expression (values of 15.6–20.1) including Ube2V, hTert, EphA2, Fos11, GnT-V, Mage-1, Sox 11, Survivin, Art-1, Sart-1, Tyrosinase, Art-4, Trp-1, PTH-rP, and IL13Rα2. Three genes showed weak expression (values of 21.0–23.1) including Trp-2, GP100, and PRAME. Two genes showed no expression (values >24) including Gage-1 and Mart-1.
TAPP mRNA expression in pediatric GBM
The pediatric GBM (N = 5) expressed 65% (20 of 31) of the TAPP mRNAs evaluated. Two genes showed strong expression (values of 13.0–14.2) including HNRPL and YKL-40. Seven genes showed moderate expression (values of 17.2–20.0) including Sox 11, Art-1, Ube2V, MRP-3, Fosl1, EphA2 and Trp-2. Eleven genes showed weak expression (values of 21.1–23.3) including B-cyclin, Sart-2, Sart-3, Her2/Neu, Mage-1, GP100, Ezh2, PRAME, IL13Rα2, GnT-V and Whsc2. Eleven genes showed no expression (values >24) including Gage-1, hTert, Sart-1, Aim-2, Survivin, Tyrosinase, Galt-3, PTH-rP, Art-4, Mart-1, and Trp-2.
TAPP mRNA expression in pediatric LGA
The pediatric LGA (N = 4) expressed 74% (23 of 31) of the TAPP mRNAs evaluated. Two genes showed strong expression (values of 12.5–14.0) including HNRPL and YKL-40. Fifteen genes showed moderate expression (values of 16.5–20.6) including Ube2V, Her2/Neu, Fosl1, Art-1, Ezh2, GnT-V, Sox 11, EphA2, IL13Rα2, Whsc2, Sart-1, Sart-3, Aim-2, B-cyclin, and MRP-3. Six genes showed weak expression (values of 21.1–23.5) including GP100, Trp-2, Sart-2, Galt-3, PTH-rP, and PRAME. Eight genes showed no expression (values >24) including Mage-1, Trp-1, hTert, Art-4, Survivin, Gage-1, Mart-1, and Tyrosinase.
TAPP mRNA expression in pediatric JPA
The pediatric JPA (N = 10) expressed 58% (18 of 31) of the TAPP mRNAs evaluated. Two genes showed strong expression (values of 12.6–14.7) including HNRPL and YKL-40. Eight genes showed moderate expression (values of 16.5–20.7) including Fosl1, MRP-3, Ube2V, Art-1, Sox 11, Whsc2, EphA2, and Sart-2. Eight genes showed weak expression (values of 21.3–24.3) including Trp-2, GnT-V, Ezh2, Sart-3, B-cyclin, Aim-2, Sart-1, and IL13Rα2. Thirteen genes showed no expression (values >24) including GP100, Her2/Neu, PRAME, Mage-1, hTert, Art-4, Gage-1, Galt-3, Mart-1, PTH-rP, Survivin, Tyrosinase, and Trp-1.
TAPP mRNA expression in pediatric ependymomas
The pediatric ependymomas (N = 7) expressed 55% (17 of 31) of the TAPP mRNAs evaluated. No genes showed strong expression. Nine genes showed moderate expression (values of 16.4–20.7) including HNRPL, Fosl1, Sox 11, Ube2V, YKL-40, Her2/Neu, Trp-2, Art-1, and Ezh2. Eight genes showed weak expression (values of 21.4–24.6) including EphA2, GnT-V, B-cyclin, MRP-3, IL13Rα2, Sart-1, Sart-3, and Whsc2. Fourteen genes showed no expression (values>24) including GP100, PTH-rP, Aim-2, PRAME, Mage-1, Art-4, Gage-1, Galt-3, Mart-1, Sart-2, Survivin, hTert, Tyrosinase, Trp-1.
Discussion
One aim of this study was to conduct comparative TAPP gene profiling between adult and pediatric brain tumors to identify potential new and novel target antigens for developing possible cytotoxic T cell-based therapies or peptide-based dendritic cell vaccine immunotherapeutic approaches against brain tumors. We describe the relative expression of mRNAs encoding 31 TAAs using qRT-PCR on a large series of 37 brain tumor patients because they are expressed in gliomas derived from adult patients [18, 21, 22]. The correlation between mRNA expression detected by qRT-PCR and protein expression detected by immunohistochemistry of formaldehyde-fixed paraffin-embedded tumor samples was high. For example, detection of Trp-2 mRNA above the threshold level was noted in 17% (8 of 47) of adult GBMs where 88% (7 of 8) of the cases demonstrated Trp-2 protein expression [21]. In this study, 96% (25 of 26) of the pediatric brain tumor cases were positive for Trp-2 mRNA expression and 100% (9 of 9) of the formaldehyde-fixed paraffin-embedded tumor samples that could be retrieved stained positive for Trp-2 protein expression (data not shown).
Previously, we reported on 16 TAPP mRNAs expressed in adult GBM grouped according to type/function: (1) “melanoma/glioma” specific antigens (Aim-2, GP100, Gage-1, Mage-1, Mart-1, Trp-1, Trp-2 and Tyrosinase); (2) growth factor receptors (EphA2, Her2/neu, IL13Rα2); (3) survival/growth dependent factors (B-cyclin, hTert and Survivin), and (4) other (GnT-V, Sart-1) [18]. Among the 8 melanoma/glioma antigens surveyed in the 37 adult and pediatric brain tumors, only Trp-2 was expressed on 100% (31 of 31) of the specimens, albeit weakly (values of 21.0–21.4). In general, the adult GBM expressed 75% (6 of 8) of the melanoma/glioma antigens (Aim-2, GP100, Mage-1, Trp-1, Trp-2 and Tyrosinase) with frequencies ranging from 82 to 100%. No expression was detected for Gage-1 or Mart-1. In contrast, the pediatric brain tumors expressed only 38% (3 of 8) of the antigens (Aim-2, GP100, and Mage-1) with frequencies ranging from 35%, 46% and 19%, respectively. The antigens Gage-1, Trp-1 and Tyrosinase were found in 4% (1 of 26) of the cases, and Mart-1 was not detected. The melanoma associated antigen PRAME showed a low frequency of 27% in both the adult (3 of 11) and pediatric (7 of 26) cases and weak levels of expression.
The growth factor receptors EphA2, Her2/Neu, and IL13Rα2 were all expressed in the adult and pediatric brain tumors but with different frequencies and with different levels of mRNA expression. In adult GBM, 91% (10 of 11) of the cases co-expressed all three TAPP mRNAs with strong to moderate expression (values of 15.9, 14.4, 20.1, respectively). In pediatric brain tumors, 54% (14 of 26) co-expressed all three growth factor receptors. The EphA2 receptor showed the highest frequency of 96% (25 of 26), followed by IL13Rα2 with a frequency of 58% (15/26) and finally Her2/Neu with a frequency of 54% (14 of 26). The LGA samples showed moderate expression of the growth factor receptors (values of 18.9, 19, 16.9, respectively) compared with weak expression in pediatric GBM, JPA and ependymomas (values ranging from 20.0 to 23.6).
Another striking difference between adult GBM and pediatric brain tumors was the differential expression of some of the survival/growth dependent factors. B-cyclin is required for progression of the cell through the G2/M stage of the cell cycle; B-cyclin can be viewed as a marker for cell growth and a measure of tumor aggressiveness. Although 97% (36 of 37) of all brain tumors expressed B-cyclin, the levels expressed in the adult GBM were much stronger (value of 14.6) compared with moderate to weak levels of expression observed in the pediatric cases (values of 19.9–22.7).
Telomerase maintains the length of telomeres, repetitive DNA sequences located at the ends of chromosomes [23]. Previous studies of adult gliomas detected high levels of telomerase activity in 50% (21 of 42) of the tumors that correlated well with hTert mRNA levels and a worse prognosis [24]. In this study, 100% (11 of 11) of the adult GBM showed moderate levels of hTert expression (value of 15.7), whereas 15% (4 of 26) of the pediatric samples showed weak to no expression (values of 20.0 to >24.0).
Survivin inhibits apoptosis and is found expressed in a wide variety of cancers, including adult glioma where it has been associated with the grade of malignancy and prognosis [25, 26]. In this study, 100% (11 of 11) of the adult GBM showed moderate levels of Survivin expression (value of 16.9) compared with only 4% (1 of 26) of the pediatric tumors. To our knowledge, this is the first report of Survivin expression in pediatric CNS tumors of GBM, LGA, JPA and ependymoma histologies. Previous studies have documented high expression of Survivin in other pediatric CNS tumors, including neuroblastomas and medulloblastomas that were correlated with poor outcome, similar to adult GBM [27–30].
We also evaluated an additional 15 TAPP mRNAs for the first time in adult and pediatric brain to determine potential therapeutic targets. Among these TAPP mRNAs, HNRPL and YKL-40, showed strong expression in 100% (31 of 31) and 97% (30 of 31), respectively, of the brain tumor cases studied. HNRPL is a member of the heterogeneous nuclear ribonucleoprotein (HNRP) family of abundantly expressed nuclear proteins that regulate pre-mRNA processing and splicing [31]. HNRPL has been shown to regulate the stability of vascular endothelial growth factor (VEGF) mRNA [32]. Under hypoxic conditions, expression of HNRPL was increased in both the nucleus and the cytoplasmic compartments, thereby increasing the stability of the VEGF mRNA and elevating the levels of VEGF expression. VEGF is a well-known angiogenic factor for gliomas driving its malignancy and thus a target for anti-angiogenic therapies [33, 34]. We hypothesize that increased levels of HNRPL mRNA detected in all histological subtypes of brain tumors may be associated with hypoxia and/or hypoxic stress of the tumor cells within the tumor microenvironment.
YKL-40 has been identified as a potential biomarker detectable in the serum from patients with a variety of cancers, including glioma [35, 36]. Although its biological function remains unknown, YKL-40 is thought to play a role in proliferation and differentiation of malignant cells and stimulates angiogenesis and extracellular tissue remodeling. In this study, YKL-40 was consistently detected in all brain tumor types. The adult and pediatric GBMs and pediatric LGA and JPA expressed significantly more mRNA compared with ependymomas (P < 0.05). Our results for JPA differ from a study of pilocytic astrocytoma where Suppression Subtractive Hybridization was used to identify genes differentially expressed between these two types of tumors [37]. Using this method, YKL-40 was found expressed in adult GBM but not in pediatric astrocytoma.
Among the adult GBM an additional 9 TAPP mRNAs were detected with 100% frequency and showed strong (Ezh2, Galt-3, Sart-2, Sart-3, Whsc2) or moderate (Art-4, Fosl1, Sox 11, Ube2V) levels of expression. Similarly among the pediatric brain tumors 5 TAPP mRNAs were detected with 85–100% frequency and moderate levels of expression (Art-1, Ezh2, Fosl1, Sox 11, Ube2V). Table 3 summarizes potential TAA that could be considered for immunotherapy approaches. From these data, one can conclude that adult GBM express a much wider variety of antigens compared with the more restricted profile of antigens detected in the pediatric brain tumors. However, despite this difference it is important to note that there are 8 TAPP genes found in common among the adult and pediatric astrocytic tumors that are expressed at moderate to strong levels, including Art-1, EphA2, Fosl1, HNRPL, MRP-3, Sox 11, Ube2V and YKL-40.
Table 3.
Potential antigens for immunotherapy
| Tumor type | Antigens (mean value of expression 6–20) |
|---|---|
| Adult GBM | Aim-2, Art-1, Art-4, B-cyclin, EphA2, Ezh2, Fosl1, Galt-3, GnT-V, Her2/Neu, HNRPL, IL13Rα2, Mage-1, MRP-3, PTH-rP, Sart-1, Sart-2, Sart-3, Sox 11, Survivin, hTert, Trp-1, Tyrosinase, Ube2V, Whsc2, YKL-40 |
| Pediatric GBM | Art-1, EphA2, Fosl1, HNRPL, MRP-3, Sox 11, Trp-2, Ube2V, YKL-40 |
| LGA | Art-1, B-cyclin, EphA2, Ezh2, Fosl1, GnT-V, Her2/Neu, HNRPL, IL13Rα2, MRP-3, Sart-1, Sart-3, Sox 11, Ube2V, Whsc2, YKL-40 |
| JPA | Art-1, EphA2, Ezh2, Fosl1, HNRPL, MRP-3, Sart-2, Sox 11, Ube2V, Whsc2, YKL-40 |
| EPEN | Ezh2, Fosl1, Her2/Neu, HNRPL, Sox 11, Trp-2, Ube2V, YKL-40 |
In summary, for immunotherapy, the identification of potential TAAs remains a high priority. This study comparing TAPP mRNA profiles of 31 genes between adult and pediatric brain tumors has identified several new potential target antigens for developing T cell-based adoptive immunotherapy or peptide-based dendritic cell vaccination approaches against brain tumors. Additionally, the description of the antigenic makeup of the various histological types of tumors that differ by grade should further our understanding of brain tumorigenesis.
Supplementary Material
Acknowledgments
This work was partially supported by a Veterans Affairs Merit Review grant [MRJ], the Long Island League to Abolish Cancer Fund [EWN] and National Institutes of Health grants NS057829 [EWN], NS046463, CA121258, NS054093, NS056300, and The R. Herbert & Alma S. Manweiler Memorial Research Fund [CAK] and the Making Headway Foundation [DZ].
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-008-9534-4) contains supplementary material, which is available to authorized users.
Note added in proof: After this paper was accepted, Wykosky et al., (Clin. Cancer Res. 14:199, 2008) showed that human GBM express Fra-1 (Fosl1) at the protein level in 85% of their cases.
Contributor Information
Jian Gang Zhang, Department of Pathology, University of California at Irvine, Irvine, CA, USA. Lab Service, Veterans Affairs Medical Center, Long Beach, CA, USA.
Carol A. Kruse, Sidney Kimmel Cancer Center, San Diego, CA, USA
Lara Driggers, Lab Service, Veterans Affairs Medical Center, Long Beach, CA, USA.
Neil Hoa, Department of Pathology, University of California at Irvine, Irvine, CA, USA. Lab Service, Veterans Affairs Medical Center, Long Beach, CA, USA.
Jeffrey Wisoff, Departments of Neurosurgery and Pediatrics, New York University School of Medicine, New York, NY, USA.
Jeffrey C. Allen, Departments of Pediatrics and Neurology, New York University School of Medicine, New York, NY, USA. New York University Cancer Center, New York, NY, USA
David Zagzag, New York University Cancer Center, New York, NY, USA. Departments of Neurosurgery and Neuropathology, New York University School of Medicine, New York, NY, USA. Department of Pathology, New York University School of Medicine, New York, NY, USA.
Elizabeth W. Newcomb, New York University Cancer Center, New York, NY, USA. Department of Pathology, New York University School of Medicine, New York, NY, USA
Martin R. Jadus, Email: martin.jadus@med.va.gov, Department of Pathology, University of California at Irvine, Irvine, CA, USA. Lab Service, Veterans Affairs Medical Center, Long Beach, CA, USA. Division of NeuroOncology, Chao Comprehensive Cancer Center, Orange, CA, USA
References
- 1.CBTRUS. Central Brain Tumor Registry of the United States. CBTRUS; Chicago, IL, USA: 2005. Statistical report: primary brain tumors in the United States, 1998–2002. [Google Scholar]
- 2.Watson GA, Kadota RP, Wisoff JH. Multidisciplinary management of pediatric low-grade gliomas. Semin Radiat Oncol. 2001;11:152–162. doi: 10.1053/srao.2001.21421. [DOI] [PubMed] [Google Scholar]
- 3.Plautz GE, Barnett GH, Miller DW, Cohen BH, Prayson RA, Krauss JC, Luciano M, Kangisser DB, Shu S. Systemic T cell adoptive immunotherapy of malignant gliomas. J Neurosurg. 1998;89:42–51. doi: 10.3171/jns.1998.89.1.0042. [DOI] [PubMed] [Google Scholar]
- 4.Fakhrai H, Shawler DL, Gjerset R, Naviaux RK, Koziol J, Royston I, Sobol RE. Cytokine gene therapy with interleukin-2-transduced fibroblasts: effects of IL-2 dose on anti-tumor immunity. Hum Gene Ther. 1995;6:591–601. doi: 10.1089/hum.1995.6.5-591. [DOI] [PubMed] [Google Scholar]
- 5.Sloan AE, Dansey R, Zamorano L, Barger G, Hamm C, Diaz F, Baynes R, Wood G. Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg Focus. 2000;9(6):e9. doi: 10.3171/foc.2000.9.6.10. [DOI] [PubMed] [Google Scholar]
- 6.Okada H, Lieberman FS, Edington HD, Witham TF, Wargo MJ, Cai Q, Elder EH, Whiteside TL, Schold SC, Pollack IF. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of recurrent glioblastoma: preliminary observations in a patient with a favorable response to therapy. J Neurooncol. 2003;64:13–20. doi: 10.1007/BF02700016. [DOI] [PubMed] [Google Scholar]
- 7.Fakhrai H, Mantil JC, Liu L, Nicholson GL, Murphy-Satter CS, Ruppert J, Shawler DL. Phase I clinical trial of a TGF-β antisense-modified tumor cell vaccine in patients with advanced glioma. Cancer Gene Ther. 2006;13:1052–1060. doi: 10.1038/sj.cgt.7700975. [DOI] [PubMed] [Google Scholar]
- 8.Quattrocchi KB, Miller CH, Cush S, Bernard SA, Dull ST, Smith M, Gudeman S, Varia MA. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol. 1999;45:141–157. doi: 10.1023/a:1006293606710. [DOI] [PubMed] [Google Scholar]
- 9.Kruse CA, Cepeda L, Owens B, Johnson SD, Stears J, Lillehei KO. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol Immunother. 1997;45:77–87. doi: 10.1007/s002620050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruse CA, Rubinstein D. Cytotoxic T-lymphocytes reactive to patient major histocompatibility complex proteins for therapy of brain tumors. In: Liau LM, Becker DP, Cloughesy TF, Bigner DD, editors. Brain tumor immunotherapy. Humana Press; Totowa, NJ: 2001. pp. 149–170. [Google Scholar]
- 11.Plautz GE, Miller DW, Barnett GH, Stevens GHJ, Maffett S, Kim J, Cohen PA, Shu S. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res. 2000;6:2209–2218. [PubMed] [Google Scholar]
- 12.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 13.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovanone AJ, Lin J-W, Chute DJ, Mischel PS, Cloughesy TF, Roth MD. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337–344. doi: 10.1007/s002620100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutowski S, De Vleeschouwer S, Kaempgen E, Wolff JEA, Kuhl J, Demaerel P, Warmuth-Metz M, Flamen P, van Calenbergh F, Plets C, Sorensen N, Opitz A, van Gool SW. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91:1656–1662. doi: 10.1038/sj.bjc.6602195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim CH, Woo SJ, Park JS, Kim HS, Park MY, Park SD, Hong YK, Kim TG. Enhanced antitumour immunity by combined use of temozolomide and TAT-survivin pulsed dendritic cells in a murine glioma. Immunology. 2007;122:615–622. doi: 10.1111/j.1365-2567.2007.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vleeschouwer S, Rapp M, Sorg RV, Steiger H-J, Stummer W, van Gool S, Sabel M. Dendritic cell vaccination in patients with malignant glioma: current status and future directions. Neurosurgery. 2006;59:988–1000. doi: 10.1227/01.NEU.0000245595.38957.3E. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JG, Eguchi J, Kruse CA, Gomez GG, Fakhrai H, Schroter S, Ma W, Hoa N, Minev B, Delgado C, Wepsic HT, Okada H, Jadus MR. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clin Cancer Res. 2007;13:566–575. doi: 10.1158/1078-0432.CCR-06-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleihues P, Cavenee WK, editors. Pathology and Genetics of Tumours of the Nervous System. IARC Press; 2000. World health organization of tumours; pp. 10–30. [Google Scholar]
- 20.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saikali W, Avril T, Collet B, Hamlat A, Bansard J-Y, Drenou B, Guegan Y, Quillien V. Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EG-FRvIII, IL-13Rα2, gp100 and TRP-2 for immunotherapy. J Neurooncol. 2007;81:139–148. doi: 10.1007/s11060-006-9220-3. [DOI] [PubMed] [Google Scholar]
- 22.Okano F, Storkus WJ, Chambers WH. Identification of a novel HLA-A* 0201 restricted cytotoxic T lymphocyte epitope inhuman glioma-associated antigen, interleukin-13 receptor α2 chain. Clin Cancer Res. 2002;8:2851–2855. [PubMed] [Google Scholar]
- 23.Kelland L. Targeting the limitless replicative potential of cancer: the telomerase/telomere pathway. Clin Cancer Res. 2007;13:4960–4963. doi: 10.1158/1078-0432.CCR-07-0422. [DOI] [PubMed] [Google Scholar]
- 24.Boldrini L, Pistolesi S, Gisfredi S, Ursino S, Ali G, Pieracci N, Basolo F, Parenti G, Fontanini G. Telomerase activity and hTERT mRNA expression in glial tumors. Int J Oncol. 2006;28:1555–1560. doi: 10.3892/ijo.28.6.1555. [DOI] [PubMed] [Google Scholar]
- 25.Kajiwara Y, Yamasaki F, Hama S, Yahara K, Yoshioka H, Sugiyama K, Arita K, Kurisu K. Expression of survivin in astrocytic tumors: correlation with malignant grade and prognosis. Cancer. 2003;97:1077–1083. doi: 10.1002/cncr.11122. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Chen N, Wang X, He Y, Chen X, Huang Y, Yin W, Zhou Q. Apoptosis and proliferation markers in diffusely infiltrating astrocytomas: profiling of 17 molecules. J Neuropathol Exp Neurol. 2006;65:905–913. doi: 10.1097/01.jnen.0000235857.79502.c3. [DOI] [PubMed] [Google Scholar]
- 27.Fangusaro JR, Caldas H, Jiang Y, Altura RA. Survivin: an inhibitor of apoptosis in pediatric cancer. Pediatr Blood Cancer. 2006;47:4–13. doi: 10.1002/pbc.20805. [DOI] [PubMed] [Google Scholar]
- 28.Aidida C, Berrebi D, Peucdhmaur M, Reyes-Mugica M, Altieri DC. Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet. 1998;351:882–883. doi: 10.1016/S0140-6736(05)70294-4. [DOI] [PubMed] [Google Scholar]
- 29.Islam A, Kageyama H, Takada N, Kawamoto T, Takayasu H, Isogai E, Ohira M, Hashizume K, Kobayashi H, Kaneko Y, Nakagawara A. High expression of survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19:617–623. doi: 10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- 30.Fangusaro JR, Jian Y, Holloway MP, Caldas H, Singh V, Boue DR, Hayes J, Altura RA. Survivin, Survivin-2B and Survivin-deltaEx32 expression in medulloblastoma: biologic markers of tumour morphology and clinical outcome. Br J Cancer. 2005;92:359–365. doi: 10.1038/sj.bjc.6602317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinol-Roma S, Swanson MS, Gall JG, Dreyfuss G. A novel heterogeneous nuclear RNP protein with a unique distribution on nascent transcripts. J Cell Biol. 1989;109:2575–2587. doi: 10.1083/jcb.109.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih SC, Claffey KP. Regulation of human vascular endothelial growth factor mRNA stability in hypoxia by heterogeneous nuclear ribonucleoprotein L. J Biol Chem. 1999;274:1359–1365. doi: 10.1074/jbc.274.3.1359. [DOI] [PubMed] [Google Scholar]
- 33.Newcomb EW, Ali MA, Schnee T, Lukyanov Y, Fowkes M, Miller DC, Zagzag D. Flavopiridol downregulates hypoxia-mediated HIF-1a expression in human glioma cells by a proteasome-independent pathway: implications for in vivo therapy. Neuro Oncol. 2005;7:225–235. doi: 10.1215/S1152851704000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gagner J-P, Law M, Fischer I, Newcomb EW, Zagzag D. Angiogenesis in gliomas: imaging and experimental therapeutics. Brain Pathol. 2005;15:342–363. doi: 10.1111/j.1750-3639.2005.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanwar MK, Gilbert MR, Holland EC. Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res. 2002;62:4364–4368. [PubMed] [Google Scholar]
- 36.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15:194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 37.Colin C, Baeza N, Bartoli C, Fina F, Eudes N, Nanni I, Martin P-M, Ouafik L, Figarella-Branger D. Identification of genes differentially expressed in glioblastoma versus pilocytic astrocytoma using Suppression Subtractive Hybridization. Oncogene. 2006;25:2818–2826. doi: 10.1038/sj.onc.1209305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.