Abstract
Clinical trials evaluating the efficacy of nonoxynol-9 (N-9) as a topical microbicide concluded that N-9 offers no in vivo protection against human immunodeficiency virus type 1 (HIV-1) infection, despite demonstrated in vitro inactivation of HIV-1 by N-9. These trials emphasize the need for better model systems to determine candidate microbicide effectiveness and safety in a preclinical setting. To that end, time-dependent in vitro cytotoxicity, as well as in vivo toxicity and inflammation, associated with N-9 exposure were characterized with the goal of validating a mouse model of microbicide toxicity. In vitro studies using submerged cell cultures indicated that human cervical epithelial cells were inherently more sensitive to N-9-mediated damage than human vaginal epithelial cells. These results correlated with in vivo findings obtained by using Swiss Webster mice in which intravaginal inoculation of 1% N-9 or Conceptrol gel (containing 4% N-9) resulted in selective and acute disruption of the cervical columnar epithelial cells 2 h postapplication accompanied by intense inflammatory infiltrates within the lamina propria. Although damage to the cervical epithelium was apparent out to 8 h postapplication, these tissues resembled control tissue by 24 h postapplication. In contrast, minimal damage and infiltration were associated with both short- and long-term exposure of the vaginal mucosa to either N-9 or Conceptrol. These analyses were extended to examine the relative toxicity of polyethylene hexamethylene biguanide (PEHMB), a polybiguanide compound under evaluation as a candidate topical microbicide. In similar studies, in vivo exposure to 1% PEHMB caused minimal damage and inflammation of the genital mucosa, a finding consistent with the demonstration that PEHMB was >350-fold less cytotoxic than N-9 in vitro. Collectively, these studies highlight the murine model of toxicity as a valuable tool for the preclinical assessment of toxicity and inflammation associated with exposure to candidate topical microbicides.
Within the cervicovaginal environment, natural barriers to infection by human immunodeficiency virus type 1 (HIV-1), including a low pH (10), the presence of lactobacilli (11), a continuous epithelial surface, and the mucosal immune system of the genital tract, may be compromised by the vaginal application of topical compounds that have less-than-ideal safety profiles. Application of such compounds may disrupt the physical barrier to infection, resulting in a direct portal of entry for HIV-1 to the submucosa, or cause the recruitment of HIV-1-susceptible immune cells to the epithelial surface. The ideal topical microbicide would not only be effective at preventing HIV-1 transmission but would also be safe for cervicovaginal application, preserving the inherent defenses of the genital tract and causing little or no epithelial trauma or genital inflammation.
Nonoxynol-9 (N-9), which was one of the first compounds to be considered for use as a topical microbicide effective against HIV-1, was initially evaluated for safety using a variety of animal models. Experiments to determine the sensitivity of the vaginal mucosa in albino rabbits and rats to N-9 exposure indicated that the levels of irritation after five consecutive daily doses of N-9 (2.5 to 25%) were concentration dependent in both animals (9). Similar studies evaluating the toxicity associated with N-9 application in rabbits and rats also demonstrated that changes in the continuity of the epithelial lining, edema of the submucosal layer, and inflammatory cell infiltrates were proportional to the concentration of N-9 administered (4). The most comprehensive animal study of N-9 evaluated genital toxicity associated with a single application of 5% N-9 in Wistar rats (21). Histopathological analyses indicated that the vaginal mucosa was more sensitive to degenerative epithelial changes and acute inflammation than the cervical mucosa and that maximal severity was observed at 24 h. However, the high dose of unformulated N-9 and use of metallic clips to close the vulval labia may have resulted in aberrant findings, since these results do not correlate with conclusions from human clinical trials. A recent study with mice indicated that a single application of N-9 elicited an intense inflammatory response, with leukocytes infiltrating the vaginal lumen within 4 h (14). The safety of N-9 was also evaluated in pigtailed macaques after daily vaginal applications of Conceptrol (4% N-9) for 3 or 4 days (16). Colposcopic examination indicated that no epithelial disruption of either the ectocervical or the vaginal epithelium was evident 24 h after a single exposure to N-9. However, the endocervical epithelium, which is morphologically distinct from both the vaginal and ectocervical epithelium, was not evaluated in the present study. Furthermore, the effect of N-9 application on the cervicovaginal epithelium prior to 24 h postapplication was not examined. The cumulative results from these studies suggest that vaginal application of N-9 can adversely affect the cervicovaginal epithelium. However, studies regarding the time course and location of N-9-associated epithelial damage and inflammation were limited in scope.
In addition to the animal studies, numerous clinical trials were conducted to evaluate the safety, tolerability, and local toxic effects of several N-9 formulations. A single-dose, phase I, local toxicity study examined the effect of multiple daily applications of N-9 (150 mg) by colposcopy (15). After four daily doses for 14 consecutive days, epithelial disruption of the vagina and cervix occurred in 43% of women. Another N-9 dosing study indicated that the rate of epithelial disruption in women by using 150 mg of N-9 every other day for 2 weeks was essentially the same as that of women by using placebo (18). However, in women using N-9 either once or twice daily for 2 weeks, elevated levels of epithelial disruption were observed and genital irritation was primarily located on the vagina or cervix. In a comparative study of Conceptrol (100 mg of N-9) and Advantage 24 (50 mg of N-9), each formulation was administered once daily for seven consecutive days (17). Cervical abnormalities were observed in 26 and 6% of subjects, respectively. A once-daily application of N-9 (100 mg) for seven consecutive days was also associated with increased irritation and inflammation but was not associated with epithelial disruption (20). However, another study determined that multiple daily applications of COL-1492 (52.5 mg of N-9) resulted in no increase in the local toxicity over that of a placebo (22). In general, these studies indicate that frequent or high dose use of N-9 can result in epithelial disruption and/or inflammation.
The conclusion of clinical trials examining the efficacy of an N-9-based formulation for the prevention of HIV-1 infection (23) ends nearly two decades of preclinical and clinical development of N-9 as a microbicidal agent. The preclinical chronology of N-9 development has suggested that this compound was advanced into human safety and efficacy trials despite animal studies indicating that N-9 safety might have been insufficient to warrant its further consideration as a topical microbicidal agent. The failure of N-9 to achieve clinical success as an anti-HIV-1 microbicidal agent (23) clearly emphasizes the need for better methods to evaluate candidate microbicides during preclinical development. In the following studies, N-9 cytotoxicity was examined in vitro in human genital tract cells and compared to the toxicity associated with in vivo intravaginal application of N-9 in a murine model of cervicovaginal toxicity. Our results indicated a clear correspondence between N-9 cytotoxicity in experiments with submerged cell cultures and cervical epithelial disruption and inflammation associated with application of N-9 as an unformulated compound or as a formulated product (Conceptrol). Similar studies using the polybiguanide compound polyethylene hexamethylene biguanide (PEHMB), which is currently under consideration as a candidate microbicidal agent, demonstrated that the low in vitro cytotoxicity of this compound was reflected in the minimal cervicovaginal disruption and inflammation associated with vaginal application of this compound (at 1% unformulated) in the mouse model. These studies support the validation of the mouse model of toxicity as an extension of in vitro cytotoxicity assessments and as a potential preclinical assay for microbicidal compound safety.
MATERIALS AND METHODS
Chemicals.
N-9 was obtained from Rhone-Poulenc Rorer (now Aventis, Strasbourg, France). Conceptrol, which contains 4% N-9, was obtained as an over-the counter product (Ortho Pharmaceutical Laboratories, Raritan, N.J.). PEHMB was synthesized at Novaflux Biosciences, Inc. (Princeton, N.J.).
Cell lines.
Endocervical and vaginal keratinocyte cell lines were assessed for their sensitivity to N-9 and PEHMB. These cell lines were selected because they are representative of the cell types found in genital tissues that would be exposed to a topical vaginal microbicide. The endocervical End1/E6E7 (End1) and vaginal keratinocyte Vk2/E6E7 (Vk2) cell lines (6) were kindly provided by Raina Fichorova (Harvard Medical School, Boston, Mass.). Vk2 and End1 cells were maintained in keratinocyte serum-free medium (Invitrogen Life Technologies) supplemented with bovine pituitary extract (50 μg/ml), epidermal growth factor (0.1 ng/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml). The medium was further supplemented with CaCl2 to a final concentration of 0.4 mM.
Animals.
Six- to ten-week-old female Swiss Webster mice were purchased from Charles River Laboratories (Wilmington, Mass.). Research with these animals conformed to the Guiding Principles in the Care and Use of Animals approved by the Council of the American Physiological Society and was approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University College of Medicine, where the animal model studies were performed.
In vitro cellular sensitivity to microbicide exposure.
Submerged cultures of cells of genital tract origin were assessed for their sensitivity to N-9 and PEHMB by utilizing a colorimetric cell viability assay. Cells were seeded overnight in a 96-well plate at a density of 3 × 104 to 4 × 104 cells per well. After exposure to compound, the cells were washed twice with Hanks buffered saline solution and assessed for cellular viability using the CellTiter 96 AQueous Non-Radioactive cell proliferation assay (Promega, Madison, Wis.) according to the manufacturer's instructions. Viable cells reduce a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; MTS]into a formazan product that is soluble in tissue culture medium. The absorbance of formazan was measured directly at 490 nm (corrected for background at 690 nm) in 96-well assay plates by using a MRX II Revelation microplate photometer (Thermo Labsystems, Vantaa, Finland). The quantity of the formazan product is directly proportional to the number of viable cells. At least two independent experiments were conducted in which each concentration was examined at least in triplicate for each experiment.
In vivo toxicity after microbicide exposure.
A Swiss Webster mouse model of cervicovaginal toxicity was utilized to assess cellular damage and inflammation within the cervicovaginal mucosa after microbicide exposure. Six- to ten-week-old female Swiss Webster mice were hormonally synchronized 7 and 3 days prior to the start of each experiment with a subcutaneous injection of Depo-Provera (Pharmacia and Upjohn Company, Peapack, N.J.) diluted in lactated Ringer's saline solution to a final dose of 3 mg/mouse (13). After synchronization, anesthetized mice received an intravaginal inoculation (60 μl) of either 1% N-9 (unformulated) or Conceptrol (Ortho). Untreated mice and mice treated with the diluent alone (water) were utilized as controls to document the normal tissue architecture and inflammation status in the cervicovaginal mucosa. Two independent experiments were performed with a total of at least five mice at each time point examined. Mice were sacrificed at 10 min, 2 h, 4 h, 8 h, and 24 h after application, and the entire reproductive tract was surgically excised. Tissues were formalin fixed and embedded in paraffin by standard procedures. Gross morphological analyses were performed on tissues stained with hematoxylin and eosin (H&E). Tissue sections from all animals within a treatment group were visually examined by using an Olympus IX81 microscope to assess the gross morphological condition of the cervicovaginal mucosa. After the overall assessment of the tissue, a representative field from the vaginal and cervical epithelium of each treatment group was photographed by using a high-resolution digital camera.
In vivo genital inflammation following microbicide application.
Tissues harvested from control (water-treated) and microbicide-treated mice were stained with a monoclonal rat anti-mouse antibody specific to CD45, which identifies all cells of hematopoietic origin with the exception of erythrocytes. Tissue sections were baked in a vacuum oven at 60°C for 1 h. Samples were then dehydrated and rehydrated by incubation first in xylene, which further deparaffinizes the tissue and then in a graded alcohol series. Endogenous peroxidases were quenched by treating tissue with 3% hydrogen peroxide in methanol for 10 min and rinsing sections in phosphate-buffered saline (PBS) for 5 min. Antigen retrieval was performed by submersing tissue sections in Retrievagen A solution (pH 6.0; BD Biosciences, Franklin Lakes, N.J.), followed by heating in a microwave according to the manufacturer's instructions. Slides were then cooled to room temperature and rinsed in water. Nonspecific binding was blocked by incubating tissues for 1 h with 10% horse serum (Vector Laboratories, Burlingame, Calif.). After blocking, tissues were incubated with a 1:10 dilution of purified rat anti-mouse CD45 immunoglobulin G2b (IgG2b; BD Biosciences) for 1 h at room temperature and then overnight at 4°C. Primary antibodies were diluted by using antibody diluent (BD Biosciences) according to manufacturer's instructions. The following steps were performed at room temperature. After primary antibody staining, the tissue was washed twice with PBS for 5 min each. Tissue sections were then incubated for 30 min with a 1:20 dilution of biotinylated mouse anti-rat IgG2b secondary antibody (BD Biosciences). Tissue sections were again washed twice with PBS for 5 min. Vectastain ABC reagent (Vector Laboratories), which contains the streptavidin-peroxidase conjugate, was then applied and incubated with tissue sections for 30 min. The tissue was washed one final time, and color visualization was achieved by incubation with diaminobenzidine tetrahydrochloride containing Ni2+ (Pierce Biotechnology, Rockford, Ill.) until the desired stain intensity developed (ca. 2 to 10 min). Tissue sections were then briefly rinsed in water, counterstained with hematoxylin, and mounted for visualization. Tissue sections stained with secondary antibody alone or a purified rat isotype control (BD Biosciences) served as additional staining controls. Histological and immunohistochemical (IHC) staining was visualized by using an Olympus IX81 microscope with a high-resolution digital camera.
Statistical analyses.
Mean and standard deviation of viability index values for each concentration, cell line, and time point were calculated. The concentration values that corresponded to an average viability index just above and below 0.5 CC50 (i.e., the concentration that reduces cellular viability by 50% relative to mock-exposed cells) were identified. A linear regression analysis (concentration versus viability index) was then performed and used to calculate the predicted CC50 value and its 95% confidence interval for each cell line and time point. A pairwise comparison (End1 and Vk2) for each time point was performed to determine statistical significance of the differences observed. The P value for each pairwise comparison was calculated based on the Wald statistic. All analyses were performed by using the SAS PROC REG procedure (3).
RESULTS
Cell lines of human female genital tract origin exhibit differential sensitivity to N-9 in vitro.
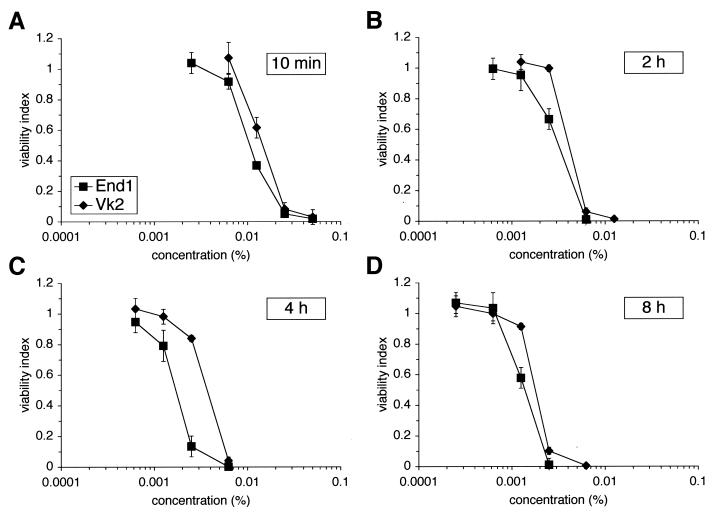
Human female genital tract cell lines were used to determine relative sensitivity to N-9 and to establish correlations between in vitro and in vivo experimental observations. Endocervical cells (End1 cells) appeared to be consistently more sensitive to N-9-mediated cell death than vaginal keratinocytes (Vk2 cells) during short- and long-term exposures (Fig. 1). The CC50 value calculated for each exposure and cell line verified that End1 cells were more susceptible to N-9-induced damage at all time points evaluated (Table 1). Pairwise comparisons (End1 versus Vk2) of the CC50 values at each time point indicated that the observed differences in sensitivity to N-9 between cell lines were statistically significant (P < 0.01). Overall, End1 cells were ca. 1.3 to 2.3 times more sensitive than Vk2 cells to N-9-mediated cytotoxicity. The results of additional studies using the Ect1/E6E7 ectocervical cell line (6) demonstrated that these cells were similar to End1 cells in their sensitivity to N-9 exposure (data not shown). As expected, the sensitivity of all cell lines to N-9 increased with exposure duration and concentration. These results suggested the possibility of regional differences in cervicovaginal toxicity in vivo in response to N-9 exposure.
FIG. 1.
Human endocervical cells are more sensitive to N-9 exposure than human vaginal keratinocytes. Vaginal (Vk2/E6E7, vaginal keratinocytes) and cervical (End1/E6E7, endocervical keratinocytes) cell lines were exposed to N-9 for 10 min (A), 2 h (B), 4 h (C), or 8 h (D). Cells were assayed for viability immediately after N-9 exposure by using an MTS assay. The results are expressed relative to that of mock-exposed cells (viability index). Each graph illustrates the average of at least two independent experiments in which each concentration was examined at least in triplicate for each experiment. Error bars indicate the standard deviations of the calculated mean values.
TABLE 1.
In vitro sensitivity of human vaginal and cervical cell lines to N-9 after short- and long-term exposure
| Cell line | Cytotoxicity (CC50)a at:
|
|||
|---|---|---|---|---|
| 10 min | 2 h | 4 h | 8 h | |
| Vk2/E6E7 | 15.2 | 4.5 | 4.1 | 1.9 |
| End1/E6E7 | 11.0 | 3.4 | 1.8 | 1.4 |
Cytotoxicity is expressed as the CC50 value, which is the N-9 percent concentration that reduced cellular viability by 50% relative to that of mock-exposed cells. CC50 values were calculated from the data presented in Fig. 1 and are expressed as CC50 × 103 for clarity of presentation.
Epithelial disruption and inflammation after application of 1% N-9 in a mouse model of cervicovaginal toxicity.
As suggested by recent clinical trials (23), disruption of the genital epithelium and/or inflammation of the genital tract following microbicide exposure may increase the risk of HIV-1 transmission. To screen candidate topical microbicides for this undesirable characteristic in a preclinical setting, we propose the use of a mouse model of topical microbicide toxicity. In experiments to test this animal model, regions of the lower reproductive tract of Swiss Webster mice were evaluated to determine whether microbicide exposure resulted in differential toxicity and inflammation to the vaginal and cervical mucosa.
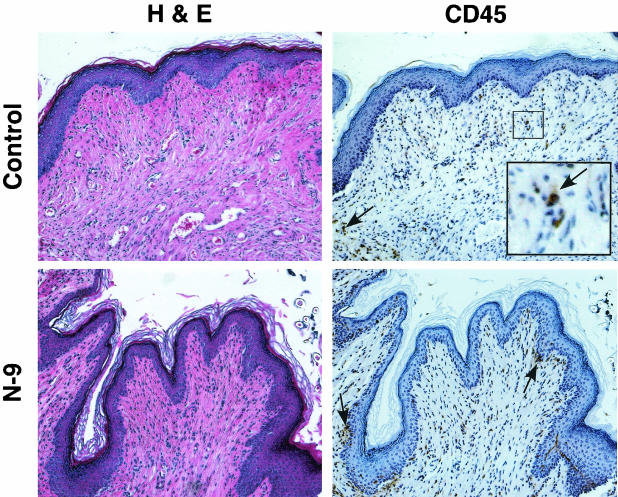
At 10 min after 1% N-9 application, the integrity of the vaginal epithelium and distribution of CD45-positive cells within the vaginal mucosa was similar to that of untreated and water-treated control tissues (data not shown). The number and distribution of CD45-positive cells was examined by using IHC analyses to determine whether microbicide treatment induced an infiltration of immune cells, which is indicative of inflammation, into the cervicovaginal mucosa. The gross morphological appearance and distribution of immune cells within the vaginal mucosa did not change substantially with extended exposure durations (2 to 24 h). A representative tissue section from 2 h postapplication is presented in Fig. 2. The vaginal epithelium remained protected by a covering of keratin and the integrity of the squamous epithelium appeared intact. In addition, the immune cell distribution was similar to that of control tissues, with CD45-positive cells dispersed throughout the submucosa. Some minor sloughing involving only the upper epithelium was observed at 2 and 4 h postapplication. Slightly elevated levels of CD45-positive cells distributed throughout the submucosa were observed in some animals. However, the majority of animals exhibited no evidence of epithelial disruption or elevated levels of immune cells within the vaginal mucosa at all time points evaluated.
FIG. 2.
Application of N-9 results in little or no change in mouse vaginal epithelial integrity and immune cell recruitment. Swiss Webster mice were inoculated with 60 μl of 1% N-9 intravaginally. At 10 min, 2 h, 4 h, and 8 h postapplication, mice were sacrificed, and the genital tracts were harvested. Tissue sections were stained with H&E for morphological analyses and anti-CD45 for IHC analyses. Tissue sections for IHC analyses were counterstained with hematoxylin. Two independent experiments were performed with a total of at least five mice at each time point. Since N-9-treated tissues were similar to control tissues in all cases, only representative water- and N-9-treated tissue sections from 2 h postapplication are presented. Arrows indicate examples of CD45-positive cell staining; the inset picture shows higher magnification of the region outlined by box.
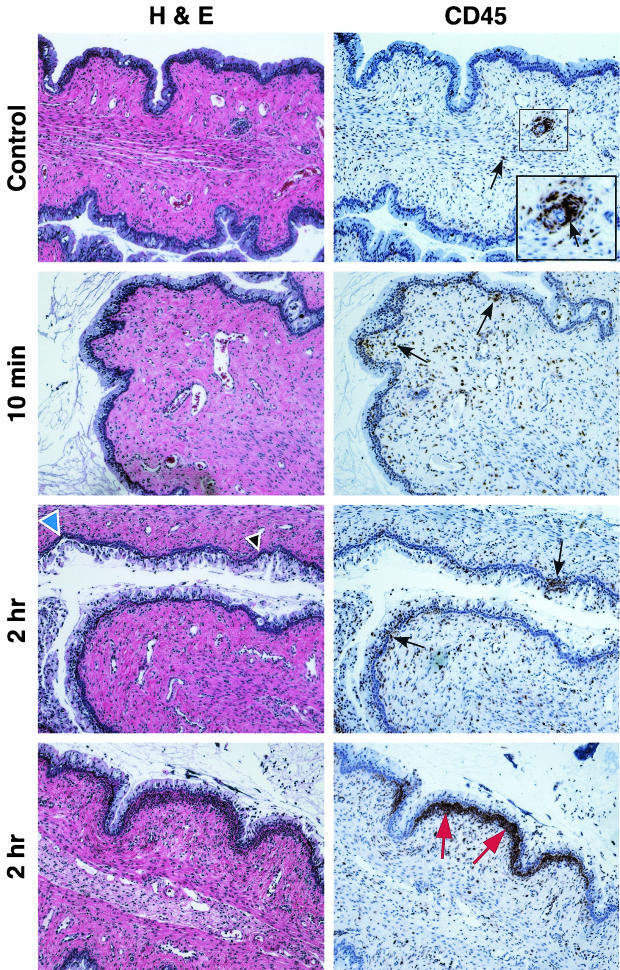
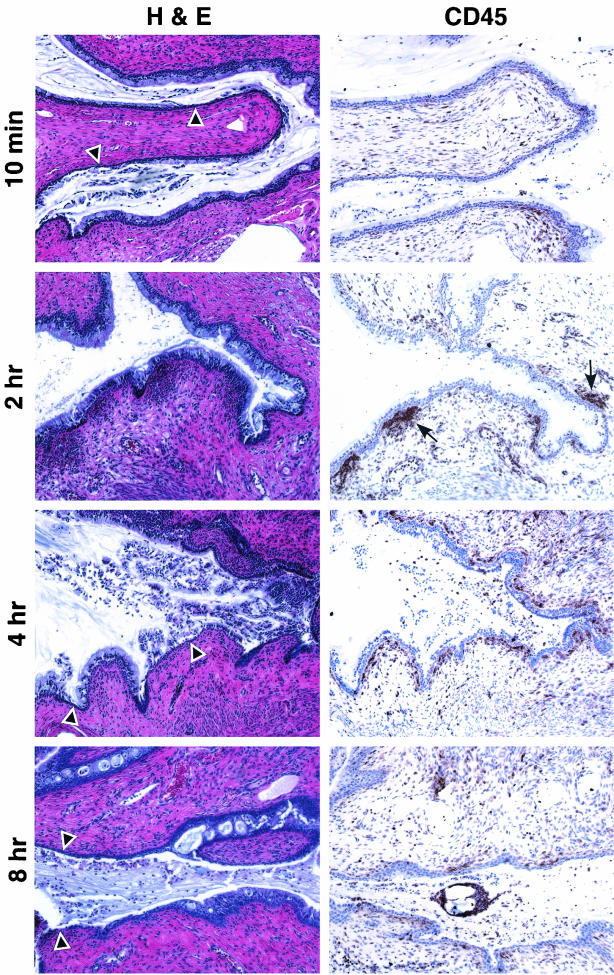
Mice treated with N-9 exhibited minimal cellular damage or inflammation within the cervix at 10 min postapplication (Fig. 3). In contrast, severe disruption of the cervical epithelium was evident at 2 h postapplication. Several areas of damage were observed in which the columnar cells were stripped off the mucosal surface, exposing the basal cell layer. In addition, a massive infiltration of CD45-positive cells was observed directly below the basal cell layer in some regions of the cervix. However, areas of CD45-positive cell infiltration in the cervix did not always coincide with the appearance of epithelial disruption. Some areas were observed to contain only epithelial disruption (Fig. 3, blue arrowhead); other areas exhibited only CD45-positive cell infiltration (Fig. 3, red arrows).
FIG. 3.
N-9 causes severe mouse cervical epithelial disruption and immune cell infiltration at 2 h postexposure. Swiss Webster mice were inoculated with 60 μl of water only or 1% N-9 intravaginally. At 10 min and 2 h postapplication, mice were sacrificed, and the genital tracts were harvested. Tissue sections were stained with H&E for morphological analyses and anti-CD45 for IHC analyses. Tissue sections for IHC analyses were counterstained with hematoxylin. Two independent experiments were performed with a total of at least five mice at each time point. Representative tissue sections are presented. Arrowheads indicate regions of epithelial disruption, and arrows indicate regions of intense CD45-positive cell staining. The inset picture shows a higher magnification of CD45-positive cell aggregates surrounding a lymphatic vessel in control tissues. The blue arrowhead indicates an area epithelial disruption was not accompanied by CD45-positive cell infiltration. The red arrows indicate CD45-positive cell infiltration in the absence of epithelial disruption.
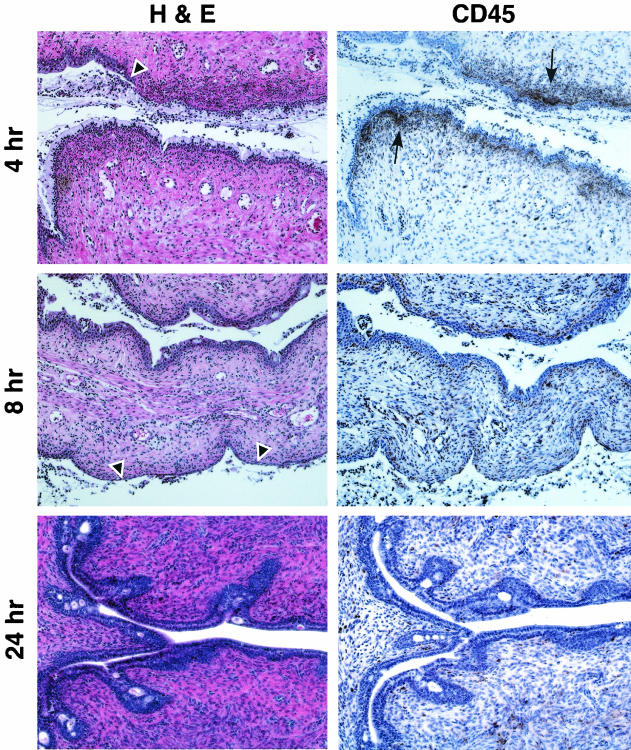
After a 4-h exposure to N-9 (Fig. 4), severe sloughing of the columnar epithelium was observed in several areas of the cervix, exposing the basal cell layer. Elevated levels of CD45-positive cells were also evident. However, the distribution of CD45-positive cells was more dispersed, indicating that the inflammatory response was diminishing. This observation was confirmed at 8 h postapplication, by which time the numbers of CD45-positive cells were decreased and more dispersed throughout the lamina propria. Despite the reduction in inflammatory infiltrate, severe disruption of the cervical epithelium down to and including the basal cell layer was observed at 8 h postapplication. By 24 h postapplication, the cervical mucosa resembled that of control animals. However, the columnar epithelial cells were smaller, suggesting a recent regeneration of the epithelial structure.
FIG. 4.
Mouse cervical epithelial tissues, which are severely disrupted by N-9 at 4 and 8 h postapplication, have undergone regeneration by 24 h postapplication. Swiss Webster mice were inoculated with 60 μl of 1% N-9 intravaginally. At 4, 8, and 24 h postapplication, mice were sacrificed, and the genital tracts were harvested. Tissue sections were stained with H&E for morphological analyses and anti-CD45 for IHC analyses. Two independent experiments were performed with at least two mice at each time point. Representative tissue sections are presented. Arrowheads indicate regions of epithelial disruption; arrows indicate regions of intense CD45-positive cell staining.
Cervicovaginal toxicity and inflammation associated with intravaginal inoculation of Conceptrol (4% N-9).
Studies were also performed to evaluate cervicovaginal integrity and inflammation after exposure to a formulated product (Conceptrol) containing N-9 (4%). As previously observed, the vaginal mucosa was relatively insensitive to N-9-mediated damage. Minimal epithelial disruption or inflammation of the vaginal mucosa was observed throughout the evaluation period after application of Conceptrol (data not shown). Regions of moderate to severe epithelial disruption of the cervix were observed as early as 10 min postapplication of Conceptrol (Fig. 5). The most severe disruption occurred at 2 h postapplication in regions of the cervix containing columnar epithelial cells, paralleling observations made by using unformulated N-9 (1%). Inflammation was also observed at 2 and 4 h postapplication, as indicated by aggregates of CD45-positive cells below the basal cell layer. CD45-positive cells were slightly elevated at 8 h postapplication but were dispersed throughout the submucosa. The appearance of the cervical mucosa at 24 h postapplication was similar to that of control animals, indicating that regeneration of the columnar epithelium had occurred.
FIG. 5.
Intravaginal inoculation of Conceptrol (4% N-9) also results in mouse cervical epithelial disruption and immune cell infiltration. Swiss Webster mice were inoculated with 60 μl of Conceptrol intravaginally. At 10 min and 2, 4, and 8 h postapplication, mice were sacrificed, and the genital tracts were harvested. Tissue sections were stained with H&E for morphological analyses and anti-CD45 for IHC analyses. Tissue sections for IHC analyses were counterstained with hematoxylin. Two independent experiments were performed with a total of at least five mice at each time point. A representative tissue section is presented. Arrowheads indicate regions of epithelial disruption; arrows highlight regions of intense CD45-positive cell staining.
Evaluation of toxicity and inflammation associated with application of the candidate topical microbicide PEHMB.
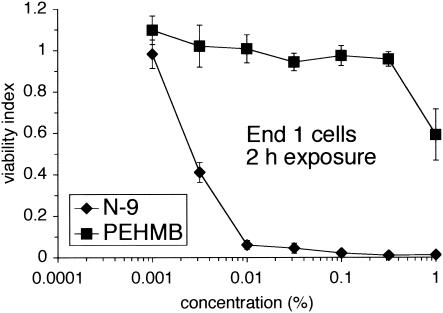
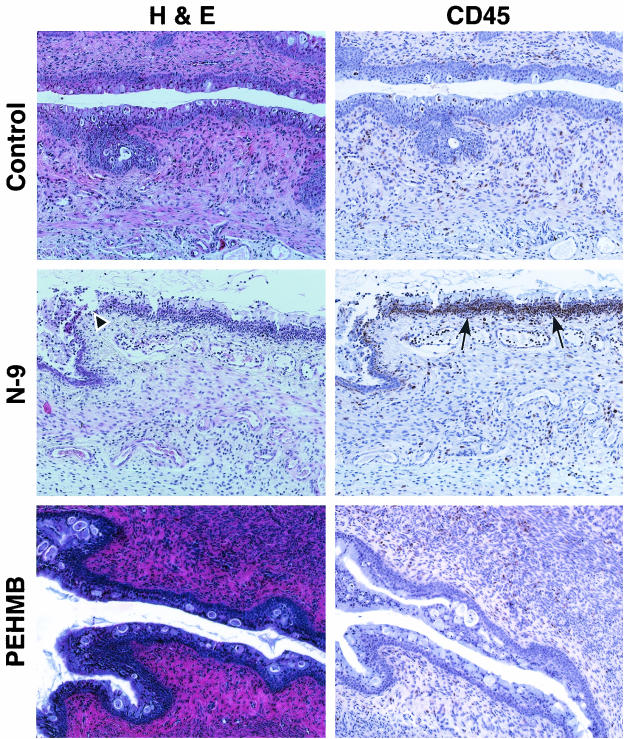
The initial characterization of the Swiss Webster mouse model was extended to examine a new class of topical microbicides, polybiguanides, and to demonstrate the utility of this model for the preclinical evaluation of candidate microbicides. Polybiguanides are polymeric, cationic compounds with activity against cell-free and cell-associated HIV-1 (F. C. Krebs and B. Wigdahl, unpublished data). We recently identified a highly active and minimally cytotoxic polybiguanide, PEHMB. An initial in vitro evaluation indicated that PEHMB was over 350-fold less cytotoxic (CC50 value > 1%) than N-9 (CC50 value 0.0028%) after a 2-h exposure of End1 cells (Fig. 6). This observation was extended by in vivo studies, in which an intravaginal inoculation of 1% PEHMB resulted in minimal epithelial disruption and negligible inflammation of the cervicovaginal mucosa at all time points evaluated (10 min, 2 h, 4 h, 8 h, and 24 h). Similarly, the number and distribution of CD45-positive cells was similar to that of control animals throughout the time course. A representative tissue section at 2 h postapplication is presented in Fig. 7. Tissue sections for control (water-treated) and N-9-treated animals are included for comparative purposes. The concentration of PEHMB utilized in the mouse vaginal model studies provides complete protection in in vitro cell-free and cell-associated HIV-1 inactivation studies and completely prevents HIV-1 transmission in an in vitro HIV-1 viral binding-entry inhibition assay (Krebs and Wigdahl, unpublished). In addition, PEHMB at 1% is ca. 12-, 52-, and 100-fold greater than the respective concentrations required to reduce infectious HIV-1 activity by 50% in in vitro cell-free inactivation, cell-associated inactivation, and viral binding inhibition assays (Krebs and Wigdahl, unpublished). These results have indicated a direct correlation between relative in vitro cytotoxicity and in vivo toxicity as demonstrated in the mouse model.
FIG. 6.
Endocervical cells are much less sensitive to PEHMB compared to N-9 after a 2-h exposure. End1 cells were exposed to N-9 or PEHMB for 2 h and then assayed for cellular viability by using an MTS assay. The results are expressed relative to that of mock-exposed cells (viability index). Each graph illustrates the average of two independent experiments in which each concentration was examined at least in triplicate. Error bars indicate the standard deviations of the calculated mean values.
FIG. 7.
Intravaginal inoculation of 1% PEHMB causes minimal mouse cervical epithelial disruption and immune cell infiltration. Swiss Webster mice were inoculated with 60 μl of 1% PEHMB intravaginally. At 10 min and 2, 4, 8, and 24 h postapplication, mice were sacrificed, and the genital tracts were harvested. Tissue sections were stained with H&E for morphological analyses and anti-CD45 for IHC analyses. Tissue sections for IHC analyses were counterstained with hematoxylin. Two independent experiments were performed with a total of at least five mice at each time point. Minimal epithelial disruption and inflammation of the cervical mucosa was observed after the application of 1% PEHMB at all time points evaluated. A representative tissue section from 2 h postapplication is presented. Control (water-treated) and N-9-treated tissue sections from 2 h postapplication are presented for comparison.
DISCUSSION
During early efforts to develop safe and effective topical microbicides to be used to reduce or eliminate the risk of sexual transmission of HIV-1, N-9 emerged as the lead candidate due to its potent activity against HIV-1 in vitro and widespread commercial use as a contraceptive. However, recent clinical trials evaluating the safety and efficacy of N-9-based products for the prevention of HIV-1 transmission have concluded that N-9 offers no significant protection against HIV-1 infection and may enhance infection in some cases. A randomized, controlled phase II/III trial evaluating the effectiveness of COL-1492 (3.5% N-9) against HIV-1 transmission concluded that no protective effect was observed in high-risk women (23). In addition, a comprehensive systematic review of nine randomized, controlled trials found that N-9 use resulted in no statistically significant reduction in the risk of infection by either HIV-1 or other sexually transmitted disease (STD) pathogens (24).
The apparent lack of N-9 efficacy in these clinical trials can likely be attributed to local toxicity caused by N-9 application. Clinical trials evaluating the contribution of STDs to sexual transmission of HIV-1 indicated that the inflammation that accompanies infection by some STDs may increase susceptibility to HIV-1 by recruiting HIV-1-susceptible target cells to the region of pathogen exposure (7). In addition, disruption of the mucosal barrier by STD-associated lesions may provide a direct portal of entry for HIV-1 to the genital submucosa. Cervicovaginal toxicity and inflammation after application of N-9 may enhance HIV-1 infection by similar mechanisms. These clinical trials have highlighted the need for additional preclinical methodologies to evaluate the genital tract toxicity and inflammation associated with microbicide application.
In the present study, the time course of local toxicity and inflammation mediated by intravaginal inoculation of N-9 was examined. These studies indicated that a single application of either N-9 or Conceptrol resulted in severe damage to the cervical epithelium. The toxic effect of N-9 was manifested as epithelial cell death and sloughing and was accompanied by an acute inflammatory response. The cervical epithelium clearly exhibited the highest sensitivity to N-9-mediated damage and inflammation. This is consistent with a previous study in CF-1 mice which indicated that columnar epithelial cells exhibited high susceptibility to N-9-mediated cell death after a 15-min exposure to 2% N-9 (1). In contrast, minimal vaginal abnormalities were observed after application of N-9. This observation has correlated with colposcopic findings from a human safety and tolerability study in which ulceration and irritation was found to be more severe in the cervical mucosa than in either the vaginal or vulvar mucosa after application of N-9-containing spermicides (17). Histopathological examination of mouse tissue samples indicated that the cervix was especially susceptible to damage with substantial regions of the columnar epithelium stripped from the surface of the tissue, exposing the basal cell layer. This is likely due to the fact that the endocervix consists of only a single layer of continuous columnar epithelium, in contrast to the stratified squamous epithelium of the vagina and ectocervix. Epithelial disruption of the cervix was most severe at 2 h postapplication and continued out to 8 h postapplication. Since exposure to STD pathogens is likely to occur within this time frame, epithelial disruption of the endocervix could potentially provide a portal of entry for HIV-1 and other STD pathogens.
Epithelial disruption of the cervix at 2 h postapplication was accompanied by an intense infiltration of inflammatory cells just below the basal cell layer, primarily in regions without epithelial disruption. This observation has correlated with previous findings in human trials following exposure to N-9 (20). High levels of localized inflammatory cells were also evident at 4 h postapplication in the cervix but were only slightly elevated and more dispersed throughout the submucosa by 8 h postapplication. The localization of this inflammatory response in the cervix may be caused by several factors. First, the columnar epithelium has been shown to be a single cell layer, and therefore may be more sensitive and responsive to toxic insult than the stratified squamous epithelium of the vagina and ectocervix. Second, the human endocervix, which has been distinguished by the presence of columnar epithelium, has been shown to be the primary site of T-cell localization in the lower genital tract (2). Finally, previous studies have suggested that columnar epithelial cells of the endocervix constitutively express RANTES, a T-lymphocyte and monocyte attractant, and interleukin-8, a proinflammatory cytokine (5).
The time course of the appearance and retreat of the acute inflammatory infiltrate associated with N-9 application was also consistent with a finite limit on the retention of a topical agent within the cervicovaginal space. In previous studies of N-9 retention following a single application of an N-9-containing formulation, the concentration of N-9 in the vaginal tract decreased significantly between 4 and 8 h postapplication (12, 25). These results have raised the possibility that a similar time course of compound loss was responsible, in part, for the decline in the murine immune response at 8 h postapplication. However, similar studies regarding compound or formulation retention in the mouse will be necessary before more definitive parallels can be drawn between results obtained in humans and in the mouse model of microbicide toxicity.
These results also highlight a potential limitation of clinical trials designed to evaluate the safety of candidate microbicidal compounds. The results of the N-9 exposure experiments indicated that there was considerable recovery of the cervical epithelium at 24 h postapplication. If we assume a similar course of recovery in the human female genital tract after exposure to N-9 or other compounds with measurable toxicity, significant levels of toxicity may be missed if colposcopic examinations are performed at a time when epithelial recovery has already taken place. Such considerations may also explain why some clinical trials of N-9-containing products reported N-9-associated toxicity, whereas others did not. The timing of examinations with respect to product application should clearly be a consideration for future clinical trials of candidate microbicides.
After the characterization of the cervicovaginal toxicity and inflammation associated with N-9 application, the Swiss Webster mouse model was utilized to evaluate a new candidate microbicide, PEHMB. The relatively low cervicovaginal toxicity and inflammation that resulted from in vivo exposure to PEHMB correlated with the low in vitro cytotoxicity of PEHMB relative to N-9. PEHMB was >350-fold less cytotoxic in culture and minimal damage to the cervicovaginal mucosa was observed after application of 1% PEHMB. These results provide further validation of the mouse model of toxicity as a preclinical screen for the safety of microbicidal candidates. In addition, these observations have suggested that PEHMB may be worthy of further development as a microbicide, since the concentration of PEHMB used in the in vivo studies described above was 10-fold greater than that required for total inhibition of in vitro infection by cell-free HIV-1 (Krebs and Wigdahl, unpublished).
The preclinical Swiss Webster mouse model offers several distinct advantages over other approaches used for the evaluation of cervicovaginal toxicity and inflammation associated with exposure to topical microbicides. The costs of human clinical trials are substantial, whereas the Swiss Webster mouse provides investigators with a relatively inexpensive model that can be used to evaluate cervicovaginal toxicity and inflammation at the cellular and tissue level prior to phase I safety trials. The correlation of human colposcopic findings with observations from the Swiss Webster mouse also supports the use of this model for the preclinical evaluation of candidate topical microbicides. Another advantage of the Swiss Webster mouse model is that the entire genital tract can be harvested and each specific region can be thoroughly evaluated for epithelial disruption and evidence of inflammation. This is critically important because HIV-1 has been shown to infect tissues from both the upper and lower human female reproductive tract (8), and damage or inflammation of any region may promote HIV-1 infection.
In summary, the mouse model of microbicide toxicity may provide an effective and inexpensive method to evaluate the safety of candidate microbicides in a preclinical setting. This assay, which has the added advantage of readily providing information regarding inflammation and immune cell recruitment in response to microbicide application, can be used in addition to other methods, including the traditional rabbit vaginal irritation assay. It may also provide a much-needed bridge between in vitro assays of cytotoxicity and activity, and clinical trials designed to determine safety and efficacy. The murine model has already been utilized extensively to examine the efficacy of potential topical microbicides in the prevention of HSV-2 infection (19). Inclusion of this model system in microbicide development strategies may help prevent unnecessary expenditures of effort and funds on clinical trials of agents such as N-9 that have little or no potential as microbicidal products.
Acknowledgments
These studies were supported by Public Health Service grant PO1 AI37829.
We thank Hung-Mo Lin (Penn State College of Medicine) for assistance with the statistical analyses of differences in cell line sensitivity to N-9. We also thank Cheryl Furtek, Timothy F. Madden, and Lori A. Schlipf for critical reviews of the manuscript.
REFERENCES
- 1.Achilles, S. L., P. B. Shete, K. J. Whaley, T. R. Moench, and R. A. Cone. 2002. Microbicide efficacy and toxicity tests in a mouse model for vaginal transmission of Chlamydia trachomatis. Sex. Transm. Dis. 29:655-664. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. J. 1996. The importance of mucosal immunology to problems in human reproduction. J. Reprod. Immunol. 31:3-19. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 2001. The SAS/STAT user guide, version 8, p. 2875-3026. SAS Institute, Inc., Cary, N.C.
- 4.Chvapil, M., W. Droegemueller, J. A. Owen, C. D. Eskelson, and K. Betts. 1980. Studies of nonoxynol-9. I. The effect on the vaginas of rabbits and rats. Fertil. Steril. 33:445-450. [PubMed] [Google Scholar]
- 5.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508-514. [DOI] [PubMed] [Google Scholar]
- 6.Fichorova, R. N., J. G. Rheinwald, and D. J. Anderson. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847-855. [DOI] [PubMed] [Google Scholar]
- 7.Fleming, D. T., and J. N. Wasserheit. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell, A. L., R. D. Edkins, S. E. Rier, G. R. Yeaman, J. E. Stern, M. W. Fanger, and C. R. Wira. 1997. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J. Virol. 71:3498-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaminsky, M., M. M. Szivos, K. R. Brown, and D. A. Willigan. 1985. Comparison of the sensitivity of the vaginal mucous membranes of the albino rabbit and laboratory rat to nonoxynol-9. Food Chem. Toxicol. 23:705-708. [DOI] [PubMed] [Google Scholar]
- 10.Kempf, C., P. Jentsch, F. B. Barre-Sinoussi, B. Poirier, J. J. Morgenthaler, A. Morell, and D. Germann. 1991. Inactivation of human immunodeficiency virus (HIV) by low pH and pepsin. J. Acquir. Immune Defic. Syndr. 4:828-830. [PubMed] [Google Scholar]
- 11.Klebanoff, S. J., and R. W. Coombs. 1991. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J. Exp. Med. 174:289-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauck, C. K., S. Allen, J. M. Baker, S. P. Barr, T. Abercrombie, and D. F. Archer. 1997. An evaluation of the amount of nonoxynol-9 remaining in the vagina up to 4 h after insertion of a vaginal contraceptive film (VCF) containing 70 mg of nonoxynol-9. Contraception 56:103-110. [DOI] [PubMed] [Google Scholar]
- 13.Milligan, G. N., and D. I. Bernstein. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 14.Milligan, G. N., K. L. Dudley, N. Bourne, A. Reece, and L. R. Stanberry. 2002. Entry of inflammatory cells into the mouse vagina following application of candidate microbicides: comparison of detergent-based and sulfated polymer-based agents. Sex. Transm. Dis. 29:597-605. [DOI] [PubMed] [Google Scholar]
- 15.Niruthisard, S., R. E. Roddy, and S. Chutivongse. 1991. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex. Transm. Dis. 18:176-179. [DOI] [PubMed] [Google Scholar]
- 16.Patton, D. L., G. G. Kidder, Y. C. Sweeney, L. K. Rabe, and S. L. Hillier. 1999. Effects of multiple applications of benzalkonium chloride and nonoxynol 9 on the vaginal epithelium in the pigtailed macaque (Macaca nemestrina). Am. J. Obstet Gynecol. 180:1080-1087. [DOI] [PubMed] [Google Scholar]
- 17.Poindexter, A. N., III, H. Levine, H. Sangi-Haghpeykar, M. L. Frank, A. Grear, and K. O. Reeves. 1996. Comparison of spermicides on vulvar, vaginal, and cervical mucosa. Contraception 53:147-153. [DOI] [PubMed] [Google Scholar]
- 18.Roddy, R. E., M. Cordero, C. Cordero, and J. A. Fortney. 1993. A dosing study of nonoxynol-9 and genital irritation. Int. J. STD AIDS 4:165-170. [DOI] [PubMed] [Google Scholar]
- 19.Roy, S., P. Gourde, J. Piret, A. Desormeaux, J. Lamontagne, C. Haineault, R. F. Omar, and M. G. Bergeron. 2001. Thermoreversible gel formulations containing sodium lauryl sulfate or n-lauroylsarcosine as potential topical microbicides against sexually transmitted diseases. Antimicrob. Agents Chemother. 45:1671-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stafford, M. K., H. Ward, A. Flanagan, I. J. Rosenstein, D. Taylor-Robinson, J. R. Smith, J. Weber, and V. S. Kitchen. 1998. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:327-331. [DOI] [PubMed] [Google Scholar]
- 21.Tryphonas, L., and H. S. Buttar. 1982. Genital tract toxicity of nonoxynol-9 in female rats: temporal development, reversibility and sequelae of the induced lesions. Fundam. Appl. Toxicol. 2:211-219. [DOI] [PubMed] [Google Scholar]
- 22.Van Damme, L., V. Chandeying, G. Ramjee, H. Rees, P. Sirivongrangson, M. Laga, J. Perriens, et al.. 2000. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. AIDS 14:85-88. [DOI] [PubMed] [Google Scholar]
- 23.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, S. S. Karim, B. Masse, J. Perriens, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson, D., M. Tholandi, G. Ramjee, and G. Rutherford. 2002. Nonoxynol-9 spermicide for prevention of vaginally acquired HIV and other sexually transmitted infections: systematic review and meta-analysis of randomised controlled trials including more than 5000 women. Lancet Infect. Dis. 2:613-617. [DOI] [PubMed] [Google Scholar]
- 25.Witter, F. R., P. Barditch-Crovo, L. Rocco, and C. B. Trapnell. 1999. Duration of vaginal retention and potential duration of antiviral activity for five nonoxynol-9 containing intravaginal contraceptives. Int. J. Gynaecol. Obstet. 65:165-170. [DOI] [PubMed] [Google Scholar]