Abstract
The disposition and metabolic profiles of [14C]viramidine and [14C]ribavirin were compared in rat and monkey red blood cells and liver. Our data reveal that the total ribavirin-related components (ribavirin plus its mono-, di-, and triphosphate metabolites) may account for most of the drug in monkey liver following prolonged oral administration of viramidine.
Ribavirin is a purine nucleoside analog (Fig. 1) with broad-spectrum activity against a variety of DNA and RNA viral infections (17, 19). In combination with either alpha interferon or pegylated alpha interferon, the clinical efficacy of ribavirin in the treatment of chronic hepatitis C virus infection, in terms of a sustained virologic response, has been shown to be about 41 to 47% (13, 16) and 54 to 56% (4, 11, 12), respectively. However, ribavirin had a dose-limiting side effect. After entering the circulation, a significant portion of ribavirin is transported into erythrocytes (RBC) (6) and metabolized into various phosphorylated derivatives (15). Owing to the lack of phosphatase activity in RBC, these phosphorylated metabolites of ribavirin are trapped intracellularly and accumulate over time, leading to hemolytic anemia (6, 7, 15). This adverse effect often necessitates dose reduction and discontinuation of ribavirin therapy in a significant proportion of patients. Therefore, a new form of ribavirin that retains ribavirin's clinical efficacy but has less potential for hemolytic anemia would be highly desirable.
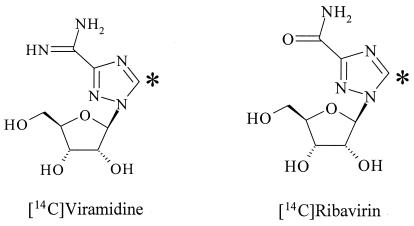
FIG. 1.
Chemical structures of viramidine and ribavirin. The asterisk shows the position of 14C.
Viramidine (Fig. 1) may be converted to ribavirin by adenosine deaminase (21). In rats and monkeys following oral administration, viramidine was extensively converted to ribavirin, followed by further metabolism to ribofuranosyl triazole carboxylic acid and triazole carboxamide (TCONH2) (8). Ribavirin has also been reported to undergo metabolism to ribofuranosyl triazole carboxylic acid and TCONH2 (9). Despite the lower absorption of viramidine compared to that of ribavirin, the plasma ribavirin AUC after viramidine administration in rats was similar to or slightly higher than the plasma ribavirin AUC after ribavirin administration (8, 9). This is in good agreement with the rapid conversion of viramidine to ribavirin in rats. In monkeys, however, the plasma ribavirin AUC following viramidine administration was lower than the plasma ribavirin AUC following ribavirin administration. This is probably related to either lower absorption of viramidine compared to that of ribavirin in monkeys and/or the slower rate of conversion of viramidine to ribavirin (8, 9). [14C]viramidine administration in rats produced a 32% higher liver radioactivity AUC than did [14C]ribavirin administration (10). Cynomolgus monkeys with portal vein cannulas that were given [3H]viramidine retained three times the liver radioactivity of those given [3H]ribavirin (10). However, no metabolic profile in rat and monkey liver has been evaluated. The aim of this study was to compare the disposition and metabolic profiles of viramidine and ribavirin in the RBC and livers of rats and monkeys.
Following an overnight fast, three male Sprague-Dawley rats received an oral dose (30 mg/kg) of [14C]viramidine and three rats received the same oral dose of [14C]ribavirin. At 2 h after administration, RBC and liver samples were collected. Three cynomolgus monkeys received a daily oral dose (10 mg/kg) of [14C]viramidine and three monkeys received the same daily oral dose of [14C]ribavirin for 10 days. At 24 h after administration of a single dose or the 10th daily dose of viramidine or ribavirin, RBC and liver samples were collected.
RBC samples were solubilized with Solvable (Packard Instruments) and then decolorized with hydrogen peroxide. The mixtures were then neutralized with 0.5 N hydrochloric acid and measured for radioactivity by liquid scintillation counting. Liver samples were solubilized in 2 M potassium hydroxide at 50°C for 9 days. After cooling, the samples were decolorized with hydrogen peroxide, followed by 2 h of incubation at room temperature. The mixtures were then neutralized with 0.5 N hydrochloric acid, and the radioactivity was determined by liquid scintillation counting.
EDTA solution (0.5 M) was added to RBC samples, and the mixture was vortexed for 10 s. Deionized water and methanol were added to the sample, and the mixture was vortexed and centrifuged. The supernatant was collected. A portion of liver (1 g) and 3 ml of cold perchloric acid (3.5%) were homogenized and centrifuged. The supernatant was neutralized with 10 N ammonium hydroxide to a pH of 6 to 7. All RBC and liver extracts were stored at −80°C.
The NH2 cartridge was preconditioned with 1.0 ml of water. An aliquot of liver or RBC extracts was premixed with water and loaded onto the cartridge. The eluant was collected, and this fraction contained ribavirin, viramidine, and TCONH2. The fraction containing monophosphates was eluted with 2.0 ml of 100 mM ammonium phosphate-acetonitrile (80/20, vol/vol). The column was then rinsed with 2.0 ml of 300 mM ammonium phosphate-acetonitrile (80/20, vol/vol) to collect diphosphates. Finally, triphosphates were eluted with 2.0 ml of 400 mM potassium chloride in 3% ammonium hydroxide. For fractions containing mono- and diphosphates, the pH was adjusted to 5 by adding 3% ammonium hydroxide. For the fraction containing triphosphates, the pH of the solution was adjusted to 5 by adding 10% formic acid. For each fraction, acid phosphatase (70 μl) was added and the mixtures were incubated at 37°C for 5 h and then evaporated to 200 μl with a TurboVap evaporator.
A Toso Haas DEAE column (4.6 by 250 mm, 5 μm) was used. Solution A consisted of 100 mM ammonium phosphate-acetonitrile (80/20) adjusted to pH 3.0 with phosphoric acid. Solution B consisted of 250 mM ammonium phosphate-acetonitrile (80/20) adjusted to pH 3.5 with phosphoric acid. The mobile phase consisted of 100% solution A from time zero to 30 min. It was switched to 100% solution B at 30 min and returned to 100% solution A at 32 min for at lest 3 min.
Administration of a single oral dose of ribavirin gave radioactivity levels of 3.5 μg/ml and 50.5 μg/g in RBC and liver, respectively. On the other hand, oral administration of viramidine gave radioactivity levels of 1.6 μg/ml and 49.5 μg/g in RBC and liver, respectively. Ribavirin administration gave a similar liver radioactivity level but twice the RBC radioactivity level of viramidine administration (Table 1).
TABLE 1.
Radioactivity concentrations in rat liver and RBC at 2 h following administration of a single oral dose (30 mg/kg) of [14C]ribavirin or [14C]viramidine
| Dosing or parameter | Ribavirin concn in:
|
Viramidine concn in:
|
||
|---|---|---|---|---|
| Liver (μg/g) | RBC (μg/ml) | Liver (μg/g) | RBC (μg/ml) | |
| Single | 43.3 | 3.2 | 53.6 | 1.8 |
| Single | 58.8 | 4.4 | 42.6 | 1.5 |
| Single | 49.3 | 2.9 | 52.2 | 1.4 |
| Mean | 50.5 | 3.5 | 49.5 | 1.6 |
| SD | 7.8 | 0.8 | 6.0 | 0.2 |
| Relative SD | 15.5 | 22.7 | 12.1 | 13.3 |
Ribavirin administration gave RBC radioactivity levels of 8.5 and 73.0 μg/ml after administration of a single dose and multiple doses, respectively (Table 2). It gave liver radioactivity levels of 8.3 and 16.6 μg/g after administration of a single dose and multiple doses, respectively. On the other hand, viramidine administration gave RBC radioactivity levels of 4.4 and 34.3 μg/ml after administration of a single dose and multiple doses, respectively. It gave liver radioactivity levels of 25.9 and 50.7 μg/g after administration of a single dose and multiple doses, respectively. After administration of either a single dose or multiple doses, viramidine gave three times the liver radioactivity level of ribavirin. Both drugs exhibited twofold accumulation in monkey liver after administration of multiple doses. However, after administration of either a single dose or multiple doses, ribavirin gave twice the RBC radioactivity level of viramidine. Both drugs exhibited eightfold accumulation in monkey RBC after administration of multiple doses.
TABLE 2.
Radioactivity concentrations in monkey liver and RBC at 24 h following administration of a single or a 10th oral dose (10 mg/kg) of [14C]ribavirin or [14C]viramidine
| Dosing or parameter | Radioactivity
|
|||
|---|---|---|---|---|
| Ribavirin
|
Virarnidine
|
|||
| Liver (μg/g) | RBC (μg/ml) | Liver (μg/g) | RBC (μg/ml) | |
| Single | 10.3 | 11.1 | 16.8 | 3.8 |
| Single | 7.9 | 7.6 | 26.8 | 4.2 |
| Single | 6.6 | 6.8 | 34.0 | 5.1 |
| Mean | 8.3 | 8.5 | 25.9 | 4.4 |
| SD | 1.9 | 2.3 | 8.6 | 0.7 |
| Relative SD | 22.7 | 26.9 | 33.4 | 15.2 |
| Multiple | 19.2 | 72.2 | 58.0 | 30.7 |
| Multiple | 13.9 | 73.7 | 42.0 | 34.8 |
| Multiple | 52.0 | 37.4 | ||
| Mean | 16.6 | 73.0 | 50.7 | 34.3 |
| SD | NA | NA | 8.1 | 3.4 |
| Relative SD | NA | NA | 16.0 | 9.8 |
NA, not applicable.
At 2 h following administration of a single dose of ribavirin or viramidine, TCONH2, ribavirin triphosphate (RTP), ribavirin monophosphate (RMP), and ribavirin represented the major radioactive components in rat RBC. Following administration of a single oral dose of ribavirin or viramidine, RMP and ribavirin were the predominated components in rat liver rather than RTP (Table 3), probably owing to the instability of RTP at the presence of phosphatases in liver. After ribavirin administration, ribavirin, ribavirin diphosphate (RDP), and RTP were detected. Ribavirin, RDP, RTP, viramidine, viramidine monophosphate (VMP), viramidine diphosphate (VDP), viramidine triphosphate (VTP), and TCONH2 were detected following oral administration of viramidine (Table 3). The total amount of ribavirin-related components after viramidine administration accounted for 68.1% of the total liver radioactivity (Table 3), indicating rapid conversion of viramidine-related components to ribavirin-related components.
TABLE 3.
Metabolic profiles in rat liver and RBC at 2 h following administration of a single oral dose (30 mg/kg) of [14C]ribavirin or [14C]viramidine
| Analyte | Mean % (SD) in:
|
|||
|---|---|---|---|---|
| Liver
|
RBC
|
|||
| Ribavirin | Viramidine | Ribavirin | Viramidine | |
| TCONH2 | 5.2 (1.9) | 8.3 (1.1) | 47.0 (4.2) | 16.0 (5.7) |
| Ribavirin | 27.2 (4.4) | 22.0 (4.7) | 11.0 (3.2) | 17.1 (2.7) |
| RMP | 48.5 (5.5) | 37.2 (4.7) | 13.7 (0.2) | 16.0 (0.7) |
| RDP | 8.7 (4.2) | 3.2 (2.0) | 7.3 (1.0) | 1.5 (0.9) |
| RTP | 6.5 (3.5) | 5.6 (6.0) | 19.9 (4.3) | 24.6 (12.5) |
| Viramidine | 0.0 | 5.2 (0.4) | 0.0 | 9.6 (0.7) |
| VMP | 0.0 | 8.6 (1.6) | 0.0 | 0.0 |
| VDP | 0.0 | 2.9 (2.8) | 0.0 | 0.0 |
| VTP | 0.0 | 3.0 (1.9) | 0.0 | 0.0 |
| Total Ra | 90.9 (2.2) | 68.1 (4.1) | 51.9 (4.2) | 59.6 (11.6) |
| Total Vb | 0.0 | 19.7 (4.8) | 0.0 | 9.6 (0.7) |
Total R, ribavirin plus RMP plus RDP plus RTP.
Total V, viramidine plus VMP plus VDP plus VTP.
RTP represented the largest radioactive component in monkey RBC following administration of a single dose and multiple doses of ribavirin or viramidine (Table 4). TCONH2 was detected after administration of a single dose of ribavirin but not after administration of multiple doses. RDP was detected after administration of either a single dose or multiple doses of ribavirin or viramidine. No viramidine, VMP, VDP, or VTP was detected in monkey RBC after administration of viramidine (Table 4), suggesting that viramidine itself either cannot penetrate monkey RBC or that only a very small fraction of it entered RBC but was quickly converted to ribavirin-related components.
TABLE 4.
Metabolic profiles in monkey liver (n = 3) and RBC at 24 h following administration of single and multiple oral doser (10 mg/kg) of [14C]ribavirin or [14C]viramidine
| Analyte | Mean % (SD) in liver
|
Mean % (SD) in RBC
|
||||||
|---|---|---|---|---|---|---|---|---|
| Ribavirin
|
Viramidine
|
Ribavirin
|
Viramidine
|
|||||
| Single | Multiple | Single | Multiple | Single | Multiple | Single | Multiple | |
| TCONH2 | 4.7 (4.0) | 3.1 (1.2) | 27.6 (11.3) | 17.1 (3.5) | 15.2 (9.9) | 0.0 | 14.6 (3.80) | 0.0 |
| Ribavirin | 32.6 (2.9) | 14.8 (2.2) | 7.9 (3.2) | 5.7 (1.3) | 0.6 (1.0) | 0.4 (0.6) | 0.0 | 0.0 |
| RMP | 54.7 (8.7) | 66.9 (9.7) | 12.2 (1.0) | 29.5 (8.1) | 5.6 (3.70 | 0.0 | 4.5 (5.1) | 0.0 |
| RDP | 5.2 (4.0) | 9.6 (4.9) | 1.1 (0.7) | 5.9 (4.0) | 14.7 (1.2) | 12.0 (1.4) | 7.3 (1.5) | 8.9 (2.1) |
| RTP | 1.7 (1.5) | 5.4 (3.0) | 0.3 (0.3) | 2.7 (2.9) | 63.1 (8.4) | 87.4 (1.7) | 73.7 (6.4) | 91.1 (2.1) |
| Viramidine | 0.0 | 0.0 | 0.5 (0.9) | 0.6 (1.0) | 0.0 | 0.0 | 0.0 | 0.0 |
| VMP | 0.0 | 0.0 | 34.9 (6.7) | 25.3 (7.7) | 0.0 | 0.0 | 0.0 | 0.0 |
| VDP | 0.0 | 0.0 | 6.7 (4.2) | 7.2 (4.5) | 0.0 | 0.0 | 0.0 | 0.0 |
| VTP | 0.0 | 0.0 | 2.5 (2.3) | 3.4 (2.70 | 0.0 | 0.0 | 0.0 | 0.0 |
| Total Ra | 94.2 (5.0) | 96.7 (1.1) | 21.5 (2.1) | 43.8 (5.8) | 84.0 (9.6) | 99.8 (0.5) | 85.5 (3.8) | 100 (0.00) |
| Total Vb | 0.0 | 0.0 | 44.6 (12.1) | 36.5 (12.3) | 0.0 | 0.0 | 0.0 | 0.0 |
Total R, ribavirin plus RMP plus RDP plus RTP
Total V, viramidine plus VMP plus VDP plus VTP.
After administration of a single dose or multiple doses of ribavirin, RMP and ribavirin were the major components in monkey liver, rather than RTP and RDP (Table 4), probably owing to the instability of phosphorylated derivatives of ribavirin in the presence of phosphatases in the liver. After administration of a single dose and multiple doses of viramidine, RMP, VMP, and TCONH2 were the major radioactive components in the monkey liver (Table 4). The total amount of ribavirin-related components (ribavirin plus RMP plus RDP plus RTP) accounted for 21.5% of the total radioactivity in the liver after administration of a single dose and 43.8% after administration of 10 daily oral doses (Table 4), indicating slow conversion of viramidine-related components to ribavirin-related components in monkey liver.
Ribavirin had a very good liver-targeting property. The liver had the highest organ radioactivity concentration in rats and monkeys following intramuscular injection of [14C]ribavirin (4) or in rats following oral administration of [3H]ribavirin (14). The liver also had the highest tissue radioactivity concentration in rats following oral administration of [14C]ribavirin or [14C]viramidine (10). In the present study, very high liver radioactivity concentrations were also obtained in rats and monkeys after oral administration of [14C]ribavirin or [14C]viramidine. Ribavirin and viramidine administration in rats gave similar drug levels in the liver. However, viramidine administration in monkeys gave three times the liver drug levels of ribavirin administration, indicating a better liver-targeting property of viramidine than ribavirin in monkeys.
Ribavirin can be phosphorylated to RMP (20), RDP, and RTP (22). RMP can also be quickly hydrolyzed to ribavirin, whereas this activity was negligible in RBC (15). Miller et al. (14) detected a high level of ribavirin in rat liver following oral administration of [3H]ribavirin, followed by RMP, RDP, and RTP. In the present study, RMP rather than RTP is the predominated component in rat and monkey livers following oral administration of a single dose of ribavirin. This is in good agreement with the findings of Smee and Matthews (18) that upon removal of extracellular ribavirin, intracellular RTP was quickly degraded. After viramidine administration, viramidine was distributed into the rat liver, primarily as RMP and ribavirin with smaller amounts of RDP, RTP, viramidine, VMP, VDP, VTP, and TCONH2. These data indicate good penetration of the liver by viramidine, rapid conversion of viramidine to ribavirin, and phosphorylation of both viramidine and ribavirin in the liver. Viramidine administration and ribavirin administration gave similar levels of RTP (5 to 6%) in the rat liver, whereas ribavirin administration gave a higher level of RTP in the rat liver (5.4%) than in the monkey liver (2.7%). In rats, the total amount of ribavirin-related components (ribavirin plus RMP plus RDP and RTP) reached 68.1% at 2 h after administration, indicating rapid conversion of viramidine-related components (viramidine plus VMP plus VDP plus VTP) to ribavirin-related components after absorption. In monkeys, however, the total amount of ribavirin-related components accounted for 21.5% of the total liver radioactivity after administration of a single dose and 43.8% of the liver radioactivity after administration of 10 daily doses. These data suggest that the total ribavirin-related components may account for most of the drug in the monkey liver following prolonged administration of viramidine.
Canonico et al. (1) reported that monkey RBC accumulated the largest concentration of ribavirin, followed by human and rat RBC. Ribavirin is transported by the nitrobenzylthioinosine-sensitive nucleoside transporter into human RBC (5). Radioactivity levels in RBC were about twice the levels in the plasma of rats and 15 times the levels in the plasma of monkeys at 8 h following administration of [14C]ribavirin (3). In humans following oral administration of [14C]ribavirin, the drug level in RBC was about 100-fold greater than the level in plasma (2). Laskin et al. (6) have shown that ribavirin can be taken up by human RBC and reach a steady-state concentration that exceeds the concentration in plasma by up to 60-fold (7). In the present study, very high RBC radioactivity levels were also obtained in rats after administration of a single oral dose of ribavirin and in monkeys after oral administration of a single dose and multiple doses of ribavirin. Ribavirin administration gave higher RBC radioactivity concentrations than did viramidine administration in both rats and monkeys.
Page and Conners (15) reported that the ratio of mono-, di-, and triphosphates was 1:5:17 in RBC. However, no metabolic profile in the RBC of animals or humans following administration of ribavirin has been reported. In the present study, RTP represented the predominant radioactive component in rat and monkey RBC after ribavirin administration. This is in good agreement with findings in vitro (15). Following administration of viramidine, RTP also was the predominant radioactive component in rat and monkey RBC. Smaller amounts of ribavirin, TCONH2, RMP, and RDP were also detected. However, viramidine, VMP, VDP, and VTP were not detected in RBC, suggesting that viramidine may be converted to ribavirin prior to entering RBC, that viramidine either cannot penetrate RBC, or that only a very small fraction of it entered RBC but was quickly converted to ribavirin-related components.
REFERENCES
- 1.Canonico, P. G., M. D. Kastello, C. T. Spears, J. R. Brown, E. A. Jacjsib, and D. E. Jenkins. 1984. Effect of ribavirin on red blood cells. Toxicol. Appl. Pharmacol. 74:155-162. [DOI] [PubMed] [Google Scholar]
- 2.Catlin, D. H., R. Smith, and A. I. Samuels. 1980.14C-ribavirin: distribution and pharmacokinetic studies in rats, baboon and man, p. 83-98. In R. A. Smith and W. Kirkpatrick (ed.), Ribavirin: a broad spectrum antiviral agent. Academic Press, Inc., New York, N.Y.
- 3.Ferrara, E. A., J. S. Oishi, R. W. Wannemacher, and E. L. Stephen. 1981. Plasma disappearance, urine excretion, and tissue distribution of ribavirin in rats and rhesus monkeys. Antimicrob. Agents Chemother. 19:1042-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis, S. M., J. A. Thorn, and P. Glue. 1998. Ribavirin uptake by human erythrocytes and the involvement of nitrobenzylthioinosine-sensitive (es)-nucleoside transporters. Br. J. Pharmacol. 123:1587-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laskin, O. L., J. A. Longstreth, C. A. Hart, D. Scavuzzo, C. M. Kalman, J. D. Connor, and R. B. Roberts. 1987. Ribavirin disposition in high-risk patients for acquired immunodeficiency syndrome. Clin. Pharmacol. Ther. 47:546-555. [DOI] [PubMed] [Google Scholar]
- 7.Leotora, J. J., A. B. Rege, J. T. Lacour, N. Frenez, W. J. George, R. B. Vandyke, K. C. Agarwal, and N. E. Hyslop. 1991. Pharmacokinetics and long-term tolerance to ribavirin in asymptomatic patients infected with human immunodeficiency virus. Clin. Pharmacol. Ther. 50:442-449. [DOI] [PubMed] [Google Scholar]
- 8.Lin, C., K. Luu, D. Lourenco, and L.T. Yeh. 2003. Pharmacokinetics of [14C]viramidine in rats and cynomolgus monkeys. Antimicrob. Agents Chemother. 47:2458-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin, C., L.-T. Yeh, K. Luu, David Lourenco, and J. Lau. 2003. Pharmacokinetics of [14C]ribavirin in rats and cynomolgus monkeys. Antimicrob. Agents Chemother. 47:1395-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin, C., L. Yeh, D. Vitarella, and Z. Hong. 2003. Viramidine, a prodrug of ribavirin, shows a better liver-targeting property and a better safety profile than ribavirin in animals. Antiviral Chem. Chemother. 14:145-152. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay, K. L., C. Trepo, T. Heintges, M. L. Shiffman, S. C. Gordon, J. C. Hoefs, E. R. Schiff, Z. D. Goodman, M. Laughlin, R. Yao, and J. K. Albrecht. 2001. A randomized double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology 34:395-403. [DOI] [PubMed] [Google Scholar]
- 12.Mann, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, J. K. Albrecht, and The Internatl. Hepatitis Interventional Therapy Group. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C; a randomized trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 13.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. D. Mitchell, M. L. Shiffmann, W.-M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, and J. K. Albrecht. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 14.Miller, J. P., L. J. Kigwana, D. G. Streeter, R. K. Robins, L. N. Simon, and J. Roboz. 1977. The relationship between the metabolism of ribavirin and its proposed mechanism of action. Ann. N. Y. Acad. Sci. 248:211-229. [DOI] [PubMed] [Google Scholar]
- 15.Page, T., and J. D. Conners. 1990. The metabolism of ribavirin in erythrocytes and nucleated cells. Int. J. Biochem. 22:379-383. [DOI] [PubMed] [Google Scholar]
- 16.Poynard, T., P. Marcellin, S. S. Lee, C. Nieerau, G. Minuk, G. Ideo, and V. H. Bain, J. Heathcote, S. Zeuzem, C. Trepo, and J. Albrecht. 1998. Randomized trial of interferon alfa-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alfa-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 17.Sidwell, R. W., J. H. Huffman, G. P. Khare, L. B. Allen, J. T. Witkowski, and R. K. Robins. 1972. Broad-spectrum antiviral activity of virazole: 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177:705-706. [DOI] [PubMed] [Google Scholar]
- 18.Smee, D. F., and T. R. Matthews. 1986. Metabolism of ribavirin in respiratory syncytial virus-infected and uninfected cells. Antimicrob. Agents Chemother. 30:117-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephen, E. L., D. E. Jones, C. J. Peters, G. A. Eddy, P. S. Loizeaux, and P. B. Jahrling. 1980. Ribavirin treatment of toga-, arena-, and bunyavirus infections in subhuman primates and other laboratory animal species, p. 169-183. In R. A. Smith and W. Kirkpatrick (ed.), Ribavirin: a broad spectrum antiviral agent. Academic Press, Inc., New York, N.Y.
- 20.Wills, R. C., D. A. Carson, and J. E. Seegmiller. 1978. Adenosine kinase initiates the major route of ribavirin activation in a cultured human cell line. Proc. Natl. Sci. USA 75:3042-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu, J. Z., H. Walker, J. Y. Lau, and Z. Hong. 2003. Activation and deactivation of a broad-spectrum antiviral drug by a single enzyme: adenosine deaminase catalyzes two consecutive deamination reactions. Antimicrob. Agents Chemother. 47:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerman. T. P., and R. D. Deeprose. 1978. Metabolism of 5-amino-1-β-d-ribofuranosylimidazole-4-carboxamide and related five-membered hetrocycles to 5′-triphosphates in human blood and L517 Y cells. Biochem. Pharmacol. 27:709-716. [DOI] [PubMed] [Google Scholar]