Abstract
Anthropogenic litter is present in all marine habitats, from beaches to the most remote points in the oceans. On the seafloor, marine litter, particularly plastic, can accumulate in high densities with deleterious consequences for its inhabitants. Yet, because of the high cost involved with sampling the seafloor, no large-scale assessment of distribution patterns was available to date. Here, we present data on litter distribution and density collected during 588 video and trawl surveys across 32 sites in European waters. We found litter to be present in the deepest areas and at locations as remote from land as the Charlie-Gibbs Fracture Zone across the Mid-Atlantic Ridge. The highest litter density occurs in submarine canyons, whilst the lowest density can be found on continental shelves and on ocean ridges. Plastic was the most prevalent litter item found on the seafloor. Litter from fishing activities (derelict fishing lines and nets) was particularly common on seamounts, banks, mounds and ocean ridges. Our results highlight the extent of the problem and the need for action to prevent increasing accumulation of litter in marine environments.
Introduction
Litter disposal and accumulation in the marine environment is one of the fastest growing threats for the world's oceans health. Marine litter is defined as ‘‘any persistent, manufactured or processed solid material discarded, disposed of or abandoned in the marine and coastal environment”[1]. The issue has been highlighted by the United Nations Environment Program [1] and was included in the 11 Descriptors set by Europe's Marine Strategy Framework directive (2008/56/EC) (MSFD) [2]. The MSFD requires each Descriptor in all European marine waters not to deviate from the undisturbed state and reach Good Environmental Status (GES) by 2020.
With an estimated 6.4 million tonnes of litter entering the oceans each year [1], the adverse impacts of litter on the marine environment are not negligible. Besides the unquestionable aesthetic issue, litter can be mistaken for food items and be ingested by a wide variety of marine organisms [3]–[8]. Entanglement in derelict fishing gear is also a serious threat, particularly for mammals [9]–[11], turtles [12] and birds [13] but also for benthic biota such as corals [14], [15]. High mortality of fish through “ghost fishing” is another consequence of derelict fishing gear in the marine environment [16]. Moreover, floating litter facilitates the transfer of non-native marine species (e.g. bryozoans, barnacles) to new habitats [17], [18]. Barnes et al. [19] estimated that the dispersal of alien species through marine litter more than doubles the rate of natural dispersal processes, especially during an era of global change.
Although the type of litter found in the world's oceans is highly diverse, plastics are by far the most abundant material recorded [20]–[22]. Because of their persistence and hydrophobic nature, their impact on marine ecosystems is of great concern. Plastics are a source of toxic chemicals such as polychlorinated biphenyls (PCBs) and dioxins that can be lethal to marine fauna [23]. Furthermore, the degradation of plastics generates microplastics which, when ingested by organisms, can deliver contaminants across trophic levels [24]–[27].
Litter type, composition and density vary greatly among locations and litter has been found in all marine habitats, from surface water convergence in the pelagic realm (fronts) down to the deep sea where litter degradation is a much slower process [21]. The spatial distribution and accumulation of litter in the ocean is influenced by hydrography, geomorphological factors [21], [28], prevailing winds and anthropogenic activities [29]. Hotspots of litter accumulation include shores close to populated areas, particularly beaches [30], but also submarine canyons, where litter originating from land accumulates in large quantities [28], [31].
In Europe, much has been written on the abundance and distribution of litter on the coastline and in surface waters [32]–[41]. As more areas of Europe's seafloor are being explored, benthic litter is progressively being revealed to be more widespread than previously assumed [15], [28], [29], [31], [42]–[52]. The sources of litter accumulating on the seafloor are variable, depending upon interactions between distances from shore [31], [45], oceanographic and hydrographic processes [47] and human activities such as commercial shipping [29] and leisure craft [43].
Early studies used trawling to quantify litter abundance on the seafloor [53], whilst more recent studies have demonstrated the potential of remotely operated vehicles (ROV), manned submersibles or towed cameras to study litter in the deep sea [15], [31], [43], [47], [54], [55]. However, understanding spatial patterns in litter abundance and distribution in the deep sea is challenging, owing to the lack of standardization in the sampling and analytical methodologies used. Furthermore, the high cost of sampling in the deep sea has limited our ability to perform standardized surveys across large areas to understand fully the extent of this pollution issue.
The problem of marine litter on the deep seafloor was addressed by the EU-FP7 project HERMIONE, recognising the need to use the surveys conducted by all partners (although designed for other purposes) to gather data on litter in the deep sea. This paper presents the results on the distribution and densities of marine litter obtained during these surveys, with additional data provided by the UK's Mapping the Deep project as well as other previous projects. It provides a unique large-scale analysis of litter on the seafloor across different physiographic settings and depths.
Materials and Methods
Study areas
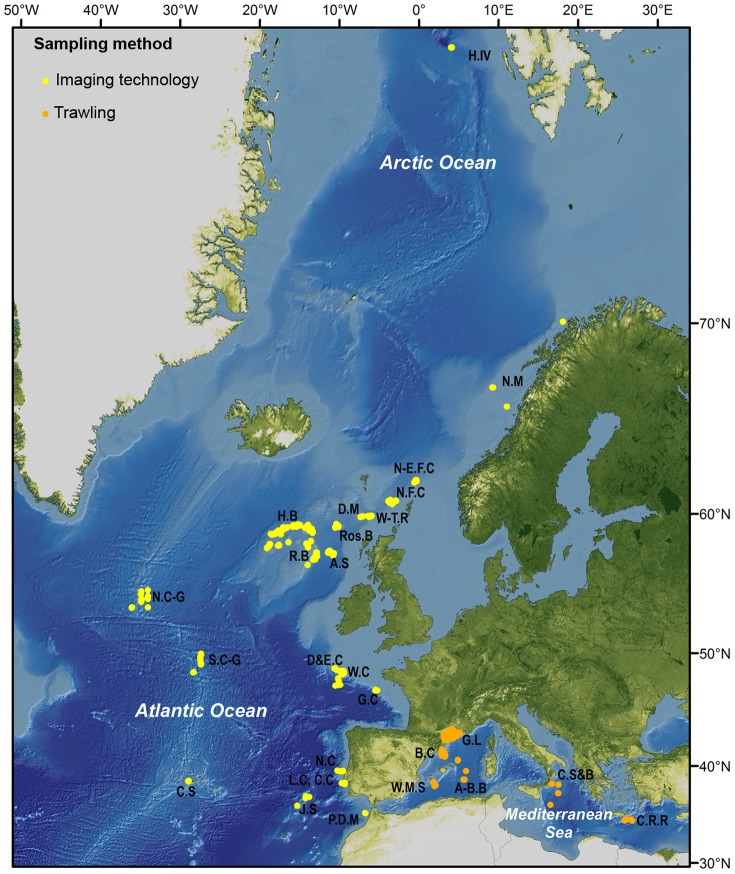
Data were gathered from surveys conducted during research cruises led by various European institutions between 1999 and 2011. A total of 32 sites in the northeastern Atlantic Ocean, Arctic Ocean and Mediterranean Sea were surveyed (Table 1; Figure 1). Surveyed sites were located on continental shelves and slopes, submarine canyons, seamounts, banks, mounds, ocean ridges and deep basins, at depths ranging from 35 to 4500 meters (Table 1).
Table 1. Sampling locations, date and methods for the collection of data on litter along with litter densities (mean number of items ha−1 and kg ha−1 ± standard errors).
| Location | Year | Method | N° of samples | Mean depth (m) | Density (n ha−1) | Density (kg ha−1) | Area covered (ha) |
| ATLANTIC | |||||||
| Continental slopes | |||||||
| North Faroe-Shetland Channel | 2006 | TC | 19 | 657 | 0.3±0.2 | - | 2.3 |
| North-East Faroe-Shetland Channel | 2006 | TC | 11 | 501 | 1.9±1.0 | - | 1.2 |
| Continental shelf | |||||||
| Norwegian Margin | 2007 | SUB | 9 | 304 | 9.7±3.8 | - | 0.6 |
| Submarine canyons | |||||||
| Dangeard & Explorer Canyons | 2007 | TC | 44 | 578 | 7.2±2.7 | - | 3 |
| Nazaré Canyon | 2007 | ROV | 13 | 3144 | 4.2±1.6 | - | 9 |
| Lisbon Canyon | 2007 | ROV | 1 | 1602 | 66.2 | - | 1 |
| Setúbal Canyon | 2007 | ROV | 1 | 2194 | 24.6 | - | 0.9 |
| Cascais Canyon | 2007 | ROV | 1 | 4574 | 10.6 | - | 1 |
| Guilvinec Canyon | 2008–2010 | ROV | 8 | 661 | 31.9±28.1 | - | 4.1 |
| Whittard Canyon | 2010 | ROV-TC | 11 | 2668 | 1.4±0.4 | - | 12.4 |
| Seamounts, banks and mounds | |||||||
| Anton Dohrn Seamount | 2005–2009 | TC | 24 | 992 | 1.9±1.0 | - | 2.2 |
| Condor Seamount | 2010–2011 | ROV | 48 | 258 | 14.6±3.0 | - | 5.6 |
| Josephine Seamount | 2012 | ROV | 4 | 1455 | 5.7±3.3 | - | 0.9 |
| Hatton Bank | 2005–2011 | ROV-TC | 52 | 706 | 1.9±0.8 | - | 4 |
| Rockall Bank | 2005–2011 | ROV-TC | 29 | 702 | 0.7±0.5 | - | 2.4 |
| Rosemary Bank | 2006 | TC | 14 | 577 | 3.3±2.3 | - | 1.1 |
| Pen Duick Alpha/Beta Mound | 2009 | ROV | 7 | 534 | 2.5±1.7 | - | 1.1 |
| Darwin Mounds | 2011 | ROV | 7 | 1007 | 9.7±2.9 | - | 1.8 |
| Ocean ridges | |||||||
| North Charlie Gibbs Fracture Zone | - | ROV | 24 | 2300 | 0.4±0.3 | - | 2.4 |
| South Charlie Gibbs Fracture Zone | - | ROV | 24 | 2600 | 2.9±1.4 | - | 2.4 |
| Wyville-Thomson Ridge | 2006 | TC | 15 | 670 | 10.9±4.3 | - | 1.2 |
| MEDITERANEAN | |||||||
| Continental slopes | |||||||
| Calabrian Slope (Central Med.) | 2009 | Trawl | 4 | 1400 | - | 0.6±0.4 | 18.9 |
| Western Mediterranean Slope | 2009 | Trawl | 8 | 1500 | - | 4±1.8 | 56 |
| Crete-Rhodes Ridge (E. Med.) | 2009 | Trawl | 8 | 1500 | - | 1.1±0.3 | 37.9 |
| Blanes slope (NW Med.) | 2009 | Trawl | 94 | 1387 | - | 1.2±0.4 | 407 |
| Continental shelf | |||||||
| Gulf of Lion (NW Med.) | 2009 | Trawl | 52 | 85 | 0.4±0.1 | - | 276.4 |
| Submarine canyons | |||||||
| Blanes Canyon (NW Med.) | 2009–2011* | ROV-Trawl | 4 (13) | 1496(1431) | 32.1±11.9 | 0.7±0.2 | 2(33.9) |
| Gulf of Lion Canyons (NW Med.) | 2009 | Trawl | 11 | 510 | 0.4±0.1 | - | 126.5 |
| Deep basins | |||||||
| Algero-Balearic Basin (W. Med.) | 2009 | Trawl | 3 | 2883 | - | 1.8±1.5 | 16 |
| Crete-Rhodes Ridge (E. Med.) | 2009 | Trawl | 2 | 3000 | - | 1.2±0.3 | 2.8 |
| Calabrian Basin (Central Med.) | 2009 | Trawl | 3 | 2967 | - | 1.7±0.6 | 12.5 |
| ARCTIC | |||||||
| Continental slope | |||||||
| HAUSGARTEN, station IV | 1999–2011 | TC-ROV | 10 | 2450 | 13.6±7.9 | - | 72.2 |
*Numbers in parentheses refer to trawl surveys. ROV = remotely operated vehicle; TC = towed camera system; TRAWL = Otter Trawl or Maireta System; SUB = manned submersible.
Figure 1. Locations of the study sites sampled with imaging technology (ROVs, manned submersible, towed camera systems) and trawling.
A-B.B = Algero-Balearic Basin (W. Med.), A.S = Anton Dohrn Seamount, B.C = Blanes Canyon (NW Med.), C.C = Cascais Canyon, C.S = Condor Seamount, Calabrian Slope & Basin = C.S&B, Crete-Rhodes Ridge = C.R.R, D&E.C = Dangeard & Explorer Canyons, D.M = Darwin Mounds, G.L.C = Gulf of Lion canyons (NW Med.), G.L = Gulf of Lion, G.C = Guilvinec Canyon, H.B = Hatton Bank, H.IV = HAUSGARTEN, station IV, J.S = Josephine Seamount, L.C = Lisbon Canyon, N.C = Nazaré Canyon, N.C-G = North Charlie Gibbs Fracture Zone, N-E.F.C = North-East Faroe-Shetland Channel, N.F.C = North Faroe-Shetland Channel, N.W = Norwegian margin, P.D.M = Pen Duick Alpha/Beta Mound, R.B = Rockall Bank, Ros.B = Rosemary Bank, S.C = Setúbal Canyon, S.C-G = South Charlie Gibbs Fracture Zone, W.C = Whittard Canyon, W.M.S = Western Mediterranean slope, W-T.R = Wyville-Thomson Ridge.
Sampling methods
Sampling methods included both imaging technology (still photograph and video) and fishing trawls (Figure 1; Table 2). The Atlantic sites were surveyed uniquely using imaging technology, whilst sites located in the Mediterranean Sea were primarily investigated by trawling (except for some ROV transects in the Blanes submarine canyon). Video footage was collected by different ROVs (Genesis, Isis, Liropus, Luso, Lynx, SP and Victor 6000), manned submersible (JAGO, GEOMAR) and towed camera systems (Seatronics and the HD-video hopper video system). Still photographs were taken with the Ocean Floor Observation System (OFOS) at the HAUSGARTEN observatory, station IV. Technical details about each platform can be found elsewhere (see Table 2). Trawl samples were collected using two different gears: a net (GOC 73) with a 20 mm-diamond stretched mesh size at the cod-end [56] and an otter trawl Maireta System (OTMS), with a cod-end mesh size of 40 mm and an outer cover of 12 mm [29], [57].
Table 2. Information on each platform used to collect video and photographs for the collection of data on litter densities and distribution on the seafloor of European waters.
| Sampling platform | Name | Format | N° of samples | Total area surveyed (m2) | Field of view (m) | References |
| Manned submersible | Jago | video | 13 | 5561 | 1.5 | [95] |
| ROVs | Luso | video | 8 | 35587 | 3.6–4.4 | [15] |
| Sp | video | 44 | 29749 | 2.3 | [15] | |
| Isis | video | 64 | 167308 | 2.0 | [31] | |
| Genesis | video | 20 | 86700 | 2.6 | [96] | |
| Liropus | video | 4 | 19867 | 3.0 | [97] | |
| Lynx | video | 19 | 3750 | 1.0 | [98] | |
| Victor 6000 | video | 6 | 421840 | 10.0 | [46] | |
| Towed camera systems | Seatronics | video | 194 | 158528 | 1.5 | [99] |
| HD video hopper system | video | 6 | 21490 | 3.0 | [100] | |
| Ocean Floor Observation System | photographs | 2882 | 8570 | 0.8–11.6 | [43] |
Further technical information about each platform can be found in the indicated references.
Analysis of image data
Protocols for video analysis varied slightly according to the platform used, but followed the same general outline. The entire footage was visualised and the number of litter items and depth recorded. Each litter item was classified into six different categories: plastic (all plastic with exception of fishing line and net), derelict fishing gear (fishing line or net), metal, glass, clinker (residue of burnt coal). Because of the low densities found at all sites, paper and cardboard, fabric, wood and unidentified items were grouped in the same category (other items). Although fishing lines and nets are mostly made of plastic, fishing gear was considered as a separate litter category because of our knowledge on its source and social implications and the particular impacts of this type of litter, such as ghost fishing and entanglement.
For each dive (sample), the area covered was calculated by multiplying the linear distance on the seafloor (off bottom footage were excluded from the analysis) by the average width of view of each of the platforms (Table 2).
For data derived from still photographs (OFOS), all images along each transect (taken at 30 s to 50 s-intervals) were analysed for the presence of litter items. Parallel laser points on the images allowed calculations of the area for each image; ranging between 0.8 and 11.6 m2. For OFOS, each image was considered to be a separate sample, while for video data, each dive was considered a single sample.
Trawl data
Hauls in the Gulf of Lion (shelf and submarine canyons) were performed with a bottom trawl equipped with a GOC 73 net [56]. After trawling, litter items were counted and classified into the different categories (see above).
Trawling at the other Mediterranean sites was performed using an otter trawl Mareita System (OTMS). All litter items were separated and classified into different categories (see above) and weighed, after excess water and mud had been removed. The use of weight rather than number to quantify litter was based on the high abundance of broken plastics (from whole plastic bags to very small (<0.5 cm) pieces of plastics) and broken glass, which impeded the quantification of single items without overestimating abundances of certain categories over others [29].
Data analysis
For each sample (video and still photographs), litter density was estimated as items of litter hectare−1 (ha; 10,000 m2) of seafloor surveyed. For trawl data where litter was measured in weight, litter density was estimated as kg of litter ha−1. Sites were grouped into 6 different groups according to physiographic characteristics (Table 1); (1) continental shelves; (2) continental slopes (excluding submarine canyons); (3) submarine canyons; (4) seamounts, banks and mounds; (5) ocean ridges and (6) deep basins. Tests for investigating differences among litter densities across physiographic settings were done separately according to the unit in which litter density was estimated (number ha−1 or weight ha−1). For both cases, the data were not normally distributed but variances were equal, therefore, the non-parametric Kruskal-Wallis rank sum test followed by a multiple comparison test (Dunn's pairwise comparison) were performed using the statistical package R. Variation in litter composition between physiographic settings were tested for significance using ANOSIM (Analysis of similarity) in PRIMER v6 software [58]. Bray-Curtis similarity [59] was calculated on log(x+1) transformation of the percentage contribution of litter type for each of the physiographic settings, across the entire data set. A similarity percentage analysis (SIMPER) was applied to identify the discriminating feature of the dissimilarities and similarities between physiographic settings.
Results
Litter density
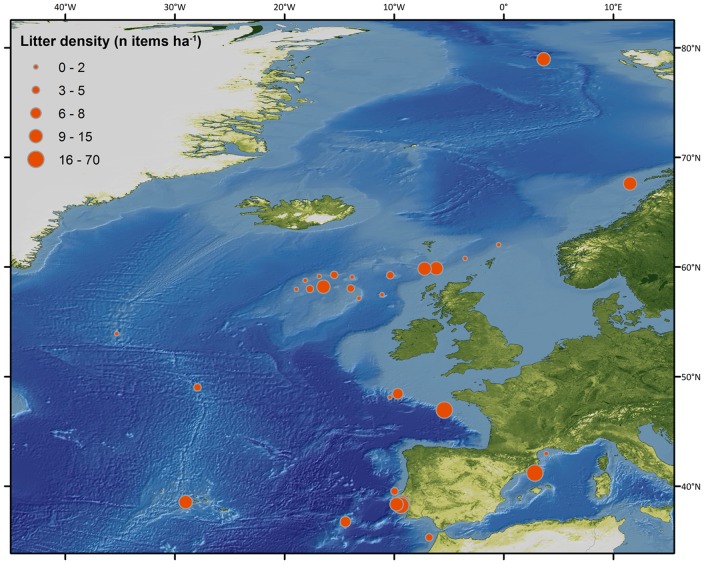
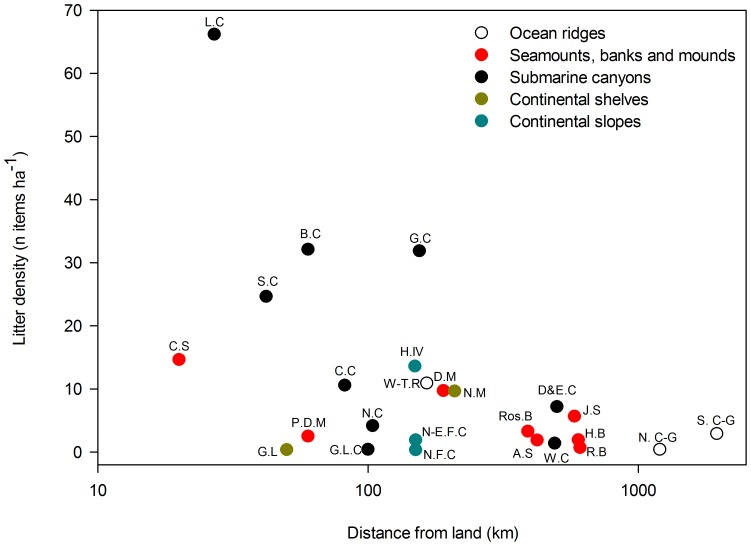
Litter was found at all sites and all depths (from 35 m down to 4500 m) sampled. Most common litter items included plastic bags, glass bottles and derelict fishing lines and nets (Figure 2). Locations with highest litter densities (>20 items ha−1) included the Lisbon Canyon, the Blanes Canyon, the Guilvinec Canyon, and the Setúbal Canyon (Table 1; Figure 3). Sites with intermediate litter density (between 10 and 20 items ha−1) were found on the Condor Seamount, the Wyville-Thomson Ridge, the continental slope of the HAUSGARTEN observatory and the Cascais Canyon (Figure 3). Low densities (between 2 and 10 items ha−1) were recorded on the Darwin Mounds, off the Norwegian margin, in Dangeard and Explorer Canyons, on the Josephine Seamount, in the Nazaré Canyon, on the Rosemary Bank, south of the Charlie-Gibbs Fracture Zone and on the Pen Duick Alpha and Beta Mounds (Figure 3). The lowest litter density (<2 items ha−1) was found on the Hatton Bank, the continental slope on the northern side of the Faroe-Shetland Channel, on the Anton Dohrn Seamount, in the Whittard Canyon, on the Rockall Bank, north of the Charlie-Gibbs Fracture Zone, and in the Gulf of Lion (in both the continental shelf and submarine canyons). Sites with higher litter density were found principally closer to shore (Figure 4), but there were exceptions, such as the samples from the Gulf of Lion where litter densities were low (Table 1).
Figure 2. Litter items on the seafloor of European waters.

A = Plastic bag entrapped by a small drop stone harbouring sponges (Cladorhiza gelida, Caulophacus arcticus), shrimps (Bythocaris sp.) and a crinoid (Bathycrinus carpenterii) recorded by an OFOS at the HAUSGARTEN observatory (Arctic) at 2500 m; B = Litter recovered within the net of a trawl in Blanes open slope at 1500 m during the PROMETO V cruise on board the R/V “García del Cid”; C = “Heineken” beer can in the upper Whittard canyon at 950 m water depth with the ROV Genesis; D = Plastic bag in Blanes Canyon at 896 m with the ROV “Liropus”; E = “Uncle Benn's Express Rice” packet at 967 m in Darwin Mound with the ROV “Lynx” (National Oceanography Centre, UK); F = Cargo net entangled in a cold-water coral colony at 950 m in Darwin Mound with the ROV “Lynx” (National Oceanography Centre, UK).
Figure 3. Litter densities (number of items ha−1) in different locations across European waters obtained with ROVs, towed camera systems, manned submersible and trawls.
Figure 4. Litter densities (number of items ha−1) in different locations across European waters according to their closest distances from land.
x axis is in a Log10 scale. A.S = Anton Dohrn Seamount, B.C = Blanes Canyon (NW Med.), C.C = Cascais Canyon, C.S = Condor Seamount, D&E.C = Dangeard & Explorer Canyons, D.M = Darwin Mounds, G.L.C = Gulf of Lion canyons (NW Med.), G.L = Gulf of Lion, G.C = Guilvinec Canyon, H.B = Hatton Bank, H.IV = HAUSGARTEN, station IV, J.S = Josephine Seamount, L.C = Lisbon Canyon, N.C = Nazaré Canyon, N.C-G = North Charlie Gibbs Fracture Zone, N-E.F.C = North-East Faroe-Shetland Channel, N.F.C = North Faroe-Shetland Channel, N.W = Norwegian margin, P.D.M = Pen Duick Alpha/Beta Mound, R.B = Rockall Bank, Ros.B = Rosemary Bank, S.C = Setúbal Canyon, S.C-G = South Charlie Gibbs Fracture Zone, W.C = Whittard Canyon, W-T.R = Wyville-Thomson Ridge.
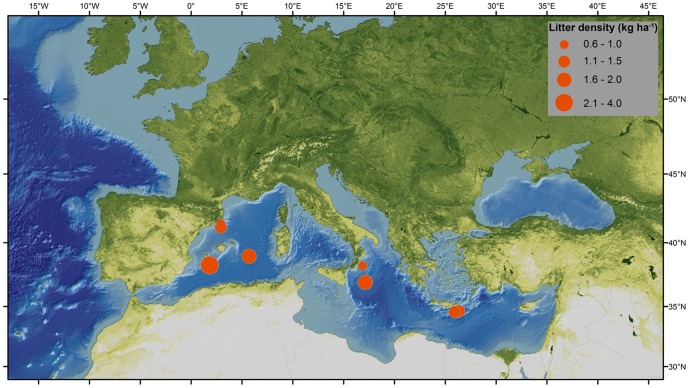
The sites sampled by trawling in the Mediterranean revealed a relatively even distribution of litter but with a higher density on the continental slope, south of Palma de Mallorca (western Mediterranean) with a mean (±SE) of 4.0±1.8 kg of litter ha−1 as opposed to densities ranging between 0.7 and 1.8 kg of litter ha−1 at the other sites (Figure 5).
Figure 5. Litter densities (kg ha−1) in different locations across the Mediterranean Sea obtained from trawl surveys.
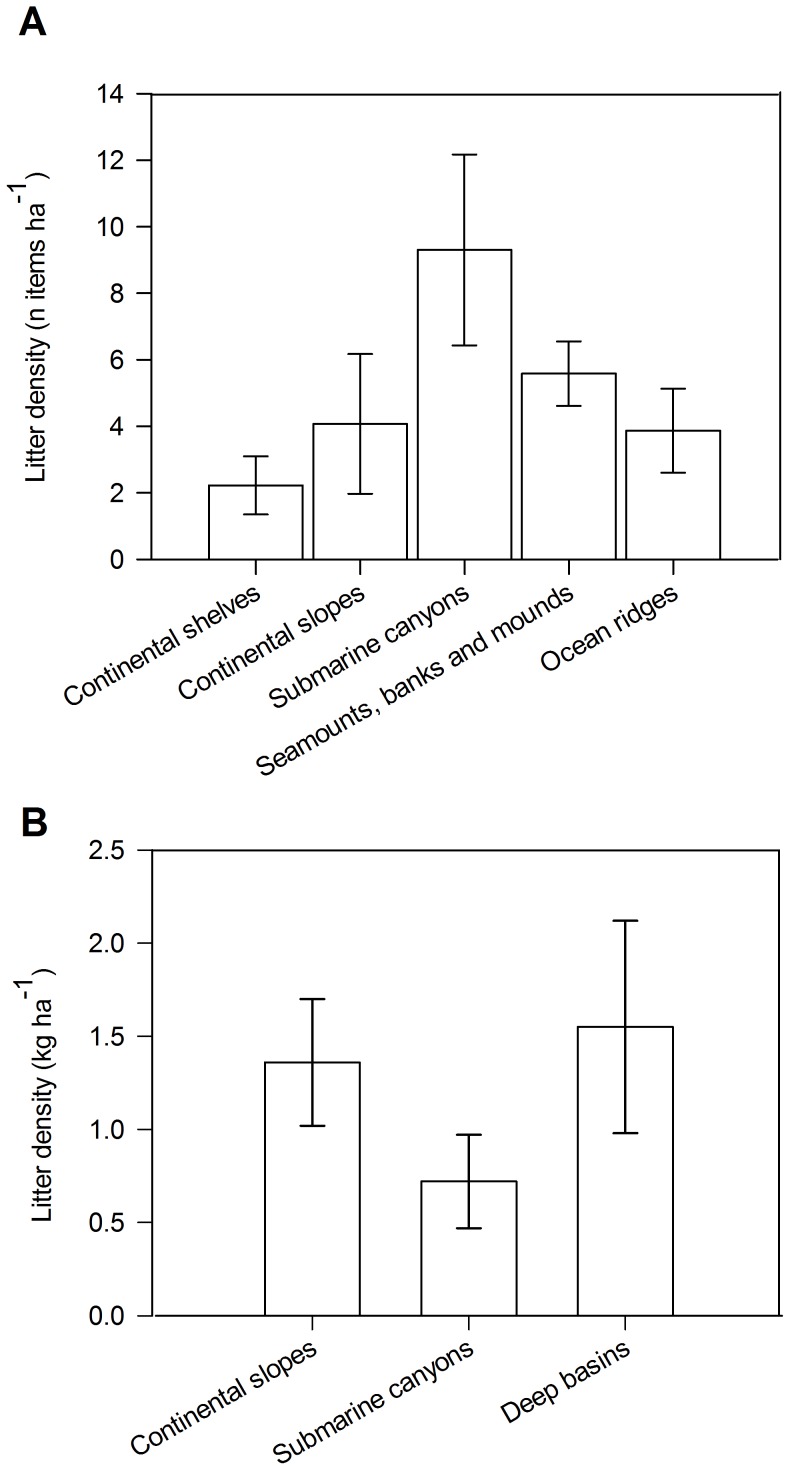
When grouping all sites into physiographic settings, there were significant differences in litter density (items ha−1) between the various groups (Kruskal-Wallis χ2 = 26.68; p<0.01; DF = 4). Multiple comparisons tests indicated that litter density in submarine canyons was significantly higher than those from all other physiographic settings, reaching an average (± SE) of 9.3±2.9 items ha−1 (Figure 6a). Litter density on seamounts, mounds and banks was similar to the densities found on the continental slopes with mean (± SE) densities of 5.6±1.0 and 4.1±2.1 items ha−1, respectively (Figure 6a). Mean (± SE) litter density for continental shelves and ocean ridges was 2.2±0.8 and 3.9±1.3 items ha−1, respectively (Figure 6a). For Mediterranean sites, where litter density was quantified by weight rather than number of items, no significant differences were found in litter density between the three different physiographic settings (Kruskal-Wallis χ2 = 3.88; p = 0.144; DF = 2). However, litter density in deep basins was slightly higher (1.55±0.57 kg ha−1) compared to continental slopes (1.36±0.34 kg ha−1) and submarine canyons (0.71±0.25 kg ha−1) (Figure 6b).
Figure 6. Mean litter density (± standard error) in A = number of items ha−1 and B = in kg of items ha−1, across different physiographic settings in European waters.
Litter composition
There was a high variability in the composition of litter across the different sites (Table 3). A total of 546 litter items were encountered throughout all sites surveyed with imaging technology. Plastic and derelict fishing gear were the most abundant litter items. Plastic represented 41% of the litter items, whilst derelict fishing gear accounted for 34% of the total. Clinker, glass and metal were least common (1, 4 and 7%, respectively). Items classified as “other items” accounted for 13% of the litter items encountered in sites surveyed by imaging technology and included wood, paper/cardboard, clothing, pottery, and unidentified material. Analysis of litter density from trawl surveys found plastic to be the most common litter type to be recovered (found in 98% of the trawls), followed by clinker (73%), fabric (48%), derelict fishing gear (33%), metal (31%) and glass (28%).
Table 3. Composition of litter (%) in different locations on the seafloor of European waters.
| Location | Derelict fishing gear | Glass | Metal | Plastic | Other items | Clinker |
| ATLANTIC | ||||||
| Continental slopes | ||||||
| North Faroe-Shetland Channel | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| North-East Faroe-Shetland Channel | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Continental shelf | ||||||
| Norwegian Margin | 80.0 | 0.0 | 0.0 | 20.0 | 0.0 | 0.0 |
| Submarine canyons | ||||||
| Dangeard & Explorer Canyons | 72.2 | 0.0 | 0.0 | 16.7 | 11.1 | 0.0 |
| Nazaré Canyon | 37.1 | 0.0 | 17.1 | 25.7 | 20.0 | 0.0 |
| Lisbon Canyon | 9.2 | 0.0 | 1.5 | 86.2 | 3.1 | 0.0 |
| Setúbal Canyon | 8.7 | 4.3 | 4.3 | 30.4 | 52.2 | 0.0 |
| Cascais Canyon | 9.1 | 0.0 | 0.0 | 54.5 | 36.4 | 0.0 |
| Guilvinec Canyon | 43.8 | 0.0 | 0.0 | 43.8 | 6.3 | 6.3 |
| Whittard Canyon | 28.6 | 7.1 | 14.3 | 42.9 | 0.0 | 7.1 |
| Seamounts, banks and mounds | ||||||
| Anton Dohrn Seamount | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| Condor Seamount | 85.5 | 14.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Josephine Seamount | 42.9 | 28.6 | 14.3 | 0.0 | 14.3 | 0.0 |
| Hatton Bank | 87.5 | 0.0 | 12.5 | 0.0 | 0.0 | 0.0 |
| Rockall Bank | 33.3 | 0.0 | 66.7 | 0.0 | 0.0 | 0.0 |
| Rosemary Bank | 66.7 | 0.0 | 33.3 | 0.0 | 0.0 | 0.0 |
| Pen Duick Alpha/Beta Mound | 75.0 | 0.0 | 25.0 | 0.0 | 0.0 | 0.0 |
| Darwin Mounds | 10.0 | 0.0 | 15.0 | 60.0 | 15.0 | 0.0 |
| Ocean ridges | ||||||
| North Charlie Gibbs Fracture Zone | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| South Charlie Gibbs Fracture Zone | 0.0 | 28.6 | 28.6 | 28.6 | 14.3 | 0.0 |
| Wyville-Thomson Ridge | 85.7 | 0.0 | 14.3 | 0.0 | 0.0 | 0.0 |
| MEDITERANEAN | ||||||
| Continental slopes | ||||||
| Calabrian Slope (Central Med.) | 13.2 | 0.0 | 8.4 | 36.2 | 26.6 | 15.5 |
| Western Mediterranean Slope | 21.6 | 0.6 | 0.2 | 12.1 | 0.6 | 64.9 |
| Crete-Rhodes Ridge (E. Med.) | 1.6 | 9.3 | 6.0 | 17.0 | 20.5 | 45.5 |
| Blanes slope (NW Med.) | 2.3 | 7.9 | 8.4 | 12.6 | 11.6 | 57.1 |
| Continental shelf | ||||||
| Gulf of Lion (NW Med.) | 0.0 | 0.0 | 0.0 | 88.9 | 11.1 | 0.0 |
| Submarine canyons | ||||||
| Blanes Canyon (NW Med.) | 3 (0.2) | 3 (4.9) | 6 (2.2) | 78 (76.3) | 9 (1.7) | 0 (14.7) |
| Gulf of Lion Canyons (NW Med.) | 0.0 | 0.0 | 0.0 | 67.3 | 32.7 | 0.0 |
| Deep basins | ||||||
| Algero-Balearic Basin (W. Med.) | 16.5 | 0.8 | 29.6 | 14.0 | 2.1 | 37.0 |
| Crete-Rhodes Ridge (E. Med.) | 0.0 | 9.7 | 25.0 | 19.5 | 7.2 | 38.5 |
| Calabrian Basin (Central Med.) | 0.5 | 6.7 | 0.7 | 5.9 | 36.1 | 50.1 |
| ARCTIC | ||||||
| Continental slope | ||||||
| HAUSGARTEN, station IV | 2.5 | 2.5 | 2.5 | 60 | 32.5 | 0 |
*Numbers in parentheses refer to trawl surveys.
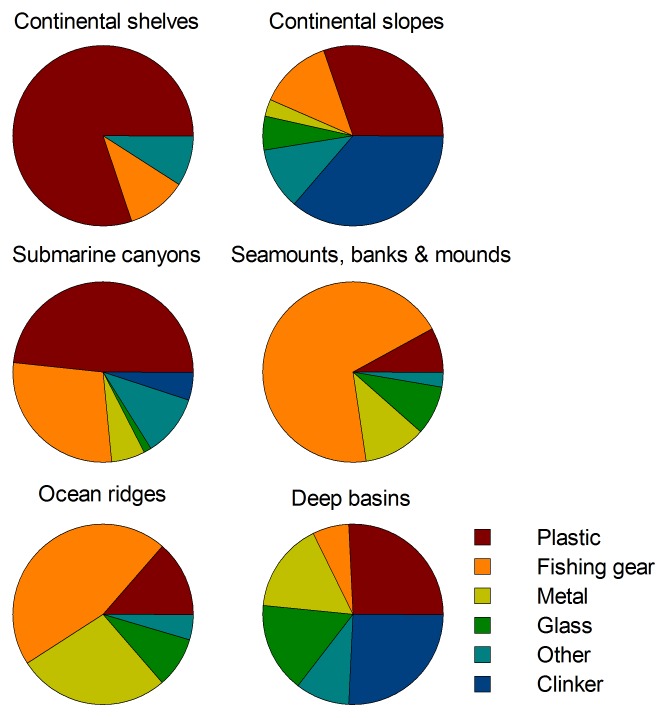
Results from ANOSIM showed that there were significant differences in litter composition between physiographic settings (1-way ANOSIM; Global R = 0.32; p<0.001), the analysis also showed some settings to be similar (Table S1). There were no significant differences between litter composition in submarine canyons and continental shelves (R = 0.01; p = 0.58). According to SIMPER analysis (Table S2), the similarity in composition between submarine canyons and continental shelves was mostly driven by plastic. Plastic was the dominant litter category for both settings (Figure 7). Litter composition on ocean ridges and on seamounts, banks and mounds did not show significant differences in litter composition (R = 0.17; p = 0.06), due to a predominance of derelict fishing gear (Figure 7). Finally, litter composition found on continental slopes was similar to deep basins (R = −0.11; p = 0.87). Clinker and plastic were the categories contributing most to the similarities between these two physiographic settings.
Figure 7. Litter composition in different physiographic settings across European waters.
Discussion
The occurrence of litter on the seafloor has been far less investigated than in surface waters or on beaches, principally because of the high cost and the technical difficulties involved in sampling the seafloor at bathyal and abyssal depths [21], [60]. Considering such limitations and poor knowledge on litter accumulation in deep waters, every survey is of great value for obtaining information on litter density and distribution. In the present study, we integrated data collected during numerous cruises over a large regional scale into a single analysis, providing insight on the density and composition of litter across a wide variety of seafloor settings and over a large geographical area in European waters. Although standardisation of the data permitted comparisons between sites, dissimilarities in the sampling equipment implies that the results should be treated with caution. Furthermore, differences in the areas of the seafloor surveyed between locations may lead to overestimations or underestimations of the litter density. Also, studying litter from trawls introduces the issue of quantification units (number vs. weight), with no correct solution. When using number of items, certain litter categories may be overestimated such as plastic or glass that can break into many small pieces. As a counterpart, if weight is used, the abundance of litter type with different weights (e.g. heavy clinker vs. light plastic) cannot be compared. Ideally, both units for litter quantification will help to understand better trends, but the EU Marine Strategy Framework Directive stresses that for monitoring litter in the marine environment, number is mandatory whilst weight is only recommended [2].
Litter was found at all the locations surveyed, from sites close to population centres such as the Gulf of Lion or the Lisbon Canyon to as far as the South Charlie-Gibbs Fracture Zone on the Mid-Atlantic Ridge, located at about 2000 km from land. Litter was found from shallow waters (35 meters in Gulf of Lion) down to 4500 meters (Cascais Canyon). Such records were not surprising, as litter is known to be present in all seas and oceans of the planet, as remote as the Southern Ocean [21] and at depths as deep as 7216 m in the Ryuku trench, south of Japan [61]. The range of litter densities found on our study sites was within the same order of magnitude to the ones found on the seabed in other parts of the globe (North America [55], [62], [63], China [54], Japan [64], [65]) and for other locations in Europe [28], [44], [45], [47], [48]. On the other hand, macro litter densities on the seabed were higher than reported for surface waters [32], [66]–[69]. At the surface, floating litter tends to accumulate in frontal areas but eventually reaches the seabed when heavily covered by fouling organisms [70] or loaded with sediments. Contrary to a common notion that most plastic items float at the sea surface it has been estimated that 70% of the plastic sinks to the seafloor [23]. This results in macro litter accumulation on the seabed rather than in the open sea [21]. For example, on the seafloor of the Mediterranean Sea, our data showed much higher litter densities (0.4 to 48 litter items ha−1) than that estimated to float at the surface (0.021 items ha−1; [1]). Alternatively, floating litter may be transported for considerable distances and get washed ashore [71], [72]. Litter density on the coastline is typically higher than on the seafloor given that there is an additional input of waste coming from inland sources (e.g. man-made drainage systems, recreational usage, rivers, winds, etc.) [71], [73]. On European coasts, litter densities can exceed 30,000 litter items per linear km [1], [41], [74], while much higher densities have been reported for beaches in Indonesia [75] or on the beaches along Armaçao dos Buzios, Rio de Janeiro, Brazil [76]. However, comparisons between studies are challenging considering differences in the size of the litter items sampled and the sampling methodology used [77].
Our data showed a general increase in litter density in locations closer to the shore, a pattern previously reported for the French Mediterranean coast [47] and off California [55]. Nevertheless, low litter densities in some near-shore sites (e.g. Gulf of Lion or Faroe-Shetland channel) suggest that many other factors (such as geomorphology, hydrography and human activity) affect litter distribution and accumulation rates [29]. In the Gulf of Lion, Galgani et al. [47] suggested that low litter density on the shelf was caused by strong water flow from the Rhone River, transporting litter down south to deeper waters. A similar situation occurs in Monterey Bay where sediment and litter are being swept off the continental shelf down into Monterey Canyon [78]. Such phenomena may explain why continental shelves were the settings with overall lowest litter density, whilst submarine canyons had the highest litter concentration. Litter levels on seamounts, banks, mounds and ocean ridges were characterised by intermediate levels when compared to other physiographic settings. They are typically located far away from coastal areas where the main anthropogenic activities include fishing [79] and seabed mining [80], [81]. The presence of litter on these settings is of concern because they harbor Vulnerable Marine Ecosystems (VMEs) (such as cold-water corals and hydrothermal vents) that have reduced capacity to recover from disturbance events and for which conservation is a global priority [82].
The types of accumulated litter can provide an indication on the human activities impacting a particular location. However, one must be cautious and consider the differences in the buoyancy and longevity of the different types of litter. For example, while some plastics sink to the seafloor, others float on the surface and are able to travel great distances before eventually sinking far from their initial dumping locations, following biofouling and degradation [23]. On the other hand, glass, metal and clinker will sink rapidly and are expected to be recovered from the seafloor close to sites where they were initially released. Cardboard and fabrics (of organic origin) will break down quickly, implying that such items will not reach the deep ocean with the frequency of more resistant materials such as plastic and negatively buoyant items such as glass, metal and clinker. Although it is difficult to determine the exact source of the litter observed on the seafloor, the dominant litter category can be used as an indicator to separate ocean and terrestrial sources [15], [29], [31], [78]. Plastic (other than derelict fishing gear) was the most abundant litter category in submarine canyons, continental shelves and continental slopes. The predominance of plastics in submarine canyons reaffirms that litter accumulation in these habitats comes from coastal and land sources and that submarine canyons act as conduits for litter transport from continental shelves into deeper waters [21], [28], [29], [31], [47], [78]. Therefore, submarine canyons can be considered to be accumulation zones of land-based marine litter in the deep sea. In fact, submarine canyons are areas where macrophyte detritus that originates from coastal areas accumulates in high quantities. This results in a localised increase of organic matter and high abundances of associated fauna, dominated by deposit and suspension-feeding invertebrates [83]–[85]. Since some deposit-feeders (e.g. holothurians) have been shown to select plastic fragments over sediment grains under laboratory conditions [7], the accumulation of plastics in submarine canyons could have detrimental effects for these ecologically important deep-sea organisms. Furthermore, plastic fragments contain a wide variety of persistent organic pollutants (POPs) that may accumulate in the consumer's tissues and can be transferred upwards in the trophic webs to predators, including humans [86].
Derelict fishing gear was the main litter item found on seamounts, banks, mounds and ocean ridges implying that, unlike submarine canyons, fishing activities are the major source of litter at those settings. Seamounts and banks are targeted by commercial fishing activities as they are often highly productive areas supporting dense aggregations of commercially valuable fish and shellfish [87]. At other locations where recreational [55], [88] and commercial [28], [54], [62], [89] fishing activities are intense, derelict fishing gear dominated the litter on the seabed. It was beyond the scope of this study to evaluate the impacts caused by derelict fishing gear, but numerous studies have shown diverse impacts including ghost fishing [16], [90] and entanglement by sessile invertebrates such as corals [15], as well as causing damage to fishing equipment [91]. Discarded trawl gear can also have a compounding effect by trapping more mobile litter resulting in a litter ‘depot’ that has a greater impact than single pieces of litter [31]. Since most fishing equipment (lines and nets) is made mostly of highly resistant plastics, such negative effects will likely persist for a long time. Sites located in deep basins and continental slopes were dominated by clinker. Clinker, the residue of burnt coal, was commonly dumped from steam ships from the late 18th century and well into the 20th century. In the Mediterranean Sea, its occurrence on the deep seafloor has been shown to coincide with such shipping routes [29]. However, it is important to acknowledge that in this study, deep basins and continental slopes were principally sampled by trawling and it is difficult to determine if the differences in litter composition with other physiographic settings are the results of differences in the sampling methodology, particularly since clinker is difficult to identify from underwater footage. Indeed, clinker was present in non-quantitative trawls undertaken at HAUSGARTEN (Bergmann, unpublished data), but could not be detected on images from the seafloor. Similarly, a high abundance of clinker was recovered from trawl surveys in Blanes Canyon that could not be identified in analysis of ROV footage from the same area (Table 3). Given that most of the clinker present on the seafloor was dumped over 100 years ago, sedimentation will have buried it, which would explain the differences in clinker quantification between images and trawl data. The deep seafloor is a passive accumulation area for litter, integrating information over long-time periods. If trawls are able to recover heavy clinker deposited on the seafloor over a century ago, these gears must be retrieving at the same time all of the lighter and most recent litter items, such as plastic for example, that have been accumulating only in the last 50 years. Overall, the composition of litter found on the seafloor showed some dissimilarity with the composition found on the coasts or in surface waters. Although plastics are dominant in all settings [70], some areas of the seafloor investigated here and elsewhere [28], [44], [45], [54], [78] harbour significant quantities of non-buoyant litter such as glass, metal and clinker, directly dumped from ships but that are seldom found in surface waters [41], [68] or on the coasts [41], [72]. The coasts and surface waters are a source of litter items for the open seas and all this litter, sooner or later, will sink to the seafloor where it accumulates.
The most common method used to provide data on benthic marine litter has been trawling, typically as a parallel objective to surveys directed to fish or benthic organism sampling [53]. With the recent development of optical methods fitted to platforms such as submersibles, ROV and drop-down systems, the use of underwater imaging technology has greatly increased our ability to quantify deep-sea litter. Both methods (imaging technology and trawling) have distinct assets for studying benthic litter that should be used in conjunction to best understand the dynamics of pollution on the seafloor. Video surveys can provide data for areas where topography is complex (e.g seamounts or canyon walls), habitats made by structure-building organisms (e.g. cold-water corals), or dynamic systems (e.g. hydrothermal vents and cold seeps), that cannot be accessed with a trawl [53]. Furthermore, imaging is a non-intrusive method that does not remove benthic organisms or damage the environment. On the other hand, a trawl has the advantages of recovering litter items of very small size (e.g. small plastic fragments) or that are buried in the sediments (e.g. clinker), which otherwise would not be detected through imaging technology. In addition, litter items collected with a trawl can be analysed in the laboratory to obtain further important information, such as state of degradation or colonisation by fouling organisms [92]. Such data will help understand sinking processes of plastic, facilitate the identification of their location of arrival into the ocean and provide information on the impacts of litter on marine organisms.
The large quantities of litter reaching the deep ocean floor is a major issue worldwide, yet little is known about its sources, patterns of distribution, abundance and, particularly, impacts on the habitats and associated fauna [1]. At present, density of litter in the deep sea is lower than found on some heavily polluted beaches [33], [93], but unlike the coastal zone, only a tiny fraction of the (deep) seafloor has been surveyed to date. Furthermore, microplastic accumulation may become an important component of pollution in deep-sea ecosystems [94] that urgently needs to be evaluated. Our results for European waters show that litter sources are distinct across different physiographic settings and that their abundance is variable, most probably guided by a complex set of interactions between physiography, anthropogenic activities and hydrography. It is important that in the future, large-scale assessments are done in a standardised manner to understand fully the scale of the problem and set the necessary actions to prevent the accumulation of litter in the marine environment.
Supporting Information
Results of analyses of similarity (ANOSIM) evaluating variation in the composition of litter among physiographic settings. RIDGE: ocean ridges; CANY: submarine canyons; SHELF: continental shelves; SLOPE: continental slopes; SBM: seamounts, banks and mounds; BASIN: deep basins.
(DOCX)
Similarity percentage analysis (SIMPER) of litter composition for each pooled physiographic settings (based on similarities revealed by ANOSIM) and the contribution of litter category to group similarity.
(DOCX)
Acknowledgments
The authors would like to thank the captains, crews and scientific parties of all cruises for their help and support during the data collection. PT would like to thank Gideon Mordecai for analytical work and Doug Masson. Finally, the authors would like to thank Martin Thiel and two other anonymous reviewers, whose suggestions and comments greatly improved the manuscript. This is publication number 33575 of the Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung.
Funding Statement
This research was supported by the European Community's Seventh Framework Programme (FP7/2007∧2013) under the HERMIONE project, Grant agreement (GA) no. 226354. The authors would like to acknowledge further funds from the Condor project (supported by a grant from Iceland, Liechtenstein, Norway through the EEA Financial Mechanism (PT0040/2008)), Corazon (FCT/PTDC/MAR/72169/2006; COMPETE/QREN), CoralFISH (FP7 ENV/2007/1/21314 4), EC funded PERSEUS project (GA no. 287600), the ESF project BIOFUN (CTM2007-28739-E), the Spanish projects PROMETEO (CTM2007-66316-C02/MAR) and DOS MARES (CTM2010-21810-C03-01), la Caixa grant "Oasis del Mar", the Generalitat de Catalunya grant to excellence research group number 2009 SGR 1305, UK's Natural Environment Research Council (NERC) as part of the Ecosystems of the Mid-Atlantic Ridge at the Sub-Polar Front and Charlie-Gibbs Fracture Zone (ECOMAR) project, the Marine Environmental Mapping Programme (MAREMAP), the ERC (Starting Grant project CODEMAP, no 258482), the Joint Nature Conservation Committee (JNCC), the Lenfest Ocean Program (PEW Foundation), the Department for Business, Enterprise and Regulatory Reform through Strategic Environmental Assessment 7 (formerly the Department for Trade and Industry) and the Department for Environment, Food and Rural Affairs through their advisors, the Joint Nature Conservation Committee, the offshore Special Areas for Conservation programme, BELSPO and RBINS-OD Nature (Belgian Federal Government) for R/V Belgica shiptime. The footage from the HAUSGARTEN observatory was taken during expeditions ARK XVIII/1, ARK XX/1, ARK XXII/1, ARK XXIII/2 and ARK XXVI/2 of the German research icebreaker “Polarstern”. The authors also acknowledge funds provided by FCT-IP/MEC to LARSyS Associated Laboratory and IMAR-University of the Azores (R&DU #531), Thematic Area E, through the Strategic Project (PEst-OE/EEI/LA0009/2011∧2014, COMPETE, QREN) and by the Government of Azores FRCT multiannual funding. CKP was supported by the doctoral grant from the Portuguese Science Foundation (SFRH/BD/66404/2009; COMPETE/QREN). AP was supported by Statoil as part of the CORAMM project. MB would like to thank Antje Boetius for financial support through the DFG Leibniz programme. JNGP was supported by the doctoral grant (M3.1.2/F/062/2011) from the Regional Directorate for Science, Technology and Communications (DRCTC) of the Regional Government of the Azores. ERLL was supported by a CSIC-JAE-postdocotral grant with co-funding from the European Social Fund. Publication fees for this open access publication were supported by IFREMER. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNEP (2009) Marine Litter: A Global Challenge. Nairobi. 232 p. [Google Scholar]
- 2. Galgani F, Hanke G, Werner S, De Vrees L (2013) Marine litter within the European Marine Strategy Framework Directive. ICES J. Mar. Sci 70: 1055–1064. [Google Scholar]
- 3. Moser ML, Lee DS (1992) A 14-year survey of plastic ingestion by western north-atlantic seabirds. Colon. Waterbirds 15: 83–94. [Google Scholar]
- 4. Ryan PG (1988) Effects of ingested plastic on seabird feeding: Evidence from chickens. Mar. Pollut. Bull 19: 125–128. [Google Scholar]
- 5. Bjorndal KA, Bolten AB, Lagueux CJ (1994) Ingestion of marine debris by juvenile sea turtles in coastal Florida habitats. Mar. Pollut. Bull 28: 154–158. [Google Scholar]
- 6. Tomás J, Guitart R, Mateo R, Raga JA (2002) Marine debris ingestion in loggerhead sea turtles, Caretta caretta, from the Western Mediterranean. Mar. Pollut. Bull 44: 211–216. [DOI] [PubMed] [Google Scholar]
- 7. Graham ER, Thompson JT (2009) Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Biol. Ecol 368: 22–29. [Google Scholar]
- 8. Carson HS (2013) The incidence of plastic ingestion by fishes: From the prey's perspective. Mar. Pollut. Bull 74: 170–174. [DOI] [PubMed] [Google Scholar]
- 9. Neilson JL, Straley JM, Gabriele CM, Hills S (2009) Non-lethal entanglement of humpback whales (Megaptera novaeangliae) in fishing gear in northern Southeast Alaska. J. Biogeogr 36: 452–464. [Google Scholar]
- 10. Williams R, Ashe E, O′Hara PD (2011) Marine mammals and debris in coastal waters of British Columbia, Canada. Mar. Pollut. Bull 62: 1303–1316. [DOI] [PubMed] [Google Scholar]
- 11. Allen R, Jarvis D, Sayer S, Mills C (2012) Entanglement of grey seals Halichoerus grypus at a haul out site in Cornwall, UK. Mar. Pollut. Bull 64: 2815–2819. [DOI] [PubMed] [Google Scholar]
- 12. Carr A (1987) Impact of nondegradable marine debris on the ecology and survival outlook of sea turtles. Mar. Pollut. Bull 18: 352–356. [Google Scholar]
- 13. Schrey E, Vauk GJM (1987) Records of entangled gannets (Sula bassana) at Helgoland, German Bight. Mar. Pollut. Bull 18: 350–352. [Google Scholar]
- 14. Chiappone M, Dienes H, Swanson DW, Miller SL (2005) Impacts of lost fishing gear on coral reef sessile invertebrates in the Florida Keys National Marine Sanctuary. Biol. Conserv 121: 221–230. [Google Scholar]
- 15. Pham CK, Gomes-Pereira JN, Isidro EJ, Santos RS, Morato T (2013) Abundance of litter on Condor seamount (Azores, Portugal, Northeast Atlantic). Deep-Sea Res. Part II-Top. Stud. Oceanogr 98: 204–208. [Google Scholar]
- 16. Brown J, Macfadyen G (2007) Ghost fishing in European waters: Impacts and management responses. Mar. Pol. 31: 488–504. [Google Scholar]
- 17. Winston JE (1982) Drift plastic—An expanding niche for a marine invertebrate? Mar. Pollut. Bull 13: 348–351. [Google Scholar]
- 18. Barnes DKA, Milner P (2005) Drifting plastic and its consequences for sessile organism dispersal in the Atlantic Ocean. Mar.Biol 146: 815–825. [Google Scholar]
- 19. Barnes DKA (2002) Biodiversity: Invasions by marine life on plastic debris. Nature 416: 808–809. [DOI] [PubMed] [Google Scholar]
- 20. Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull 44: 842–852. [DOI] [PubMed] [Google Scholar]
- 21. Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci 364: 1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheavly SB, Register KM (2007) Marine debris & plastics: environmental concerns, sources, impacts and solutions. J. Polym. Environ 15: 301–305. [Google Scholar]
- 23. Engler RE (2012) The complex interaction between marine debris and toxic chemicals in the ocean. Environ. Sci. Technol 46: 12302–12315. [DOI] [PubMed] [Google Scholar]
- 24. Andrady AL (2011) Microplastics in the marine environment. Mar. Pollut. Bull 62: 1596–1605. [DOI] [PubMed] [Google Scholar]
- 25. Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, et al. (2013) Microplastic ingestion by zooplankton. Environ. Sci. Technol 47: 6646–6655. [DOI] [PubMed] [Google Scholar]
- 26. Farrell P, Nelson K (2013) Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut 177: 1–3. [DOI] [PubMed] [Google Scholar]
- 27. Murray F, Cowie PR (2011) Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull 62: 1207–1217. [DOI] [PubMed] [Google Scholar]
- 28. Galgani F, Leaute JP, Moguedet P, Souplet A, Verin Y, et al. (2000) Litter on the sea floor along European coasts. Mar. Pollut. Bull 40: 516–527. [Google Scholar]
- 29. Ramirez-Llodra E, Company JB, Sard F, De Mol B, Coll M, et al. (2013) Effects of natural and anthropogenic processes in the distribution of marine litter in the deep Mediterranean Sea. Prog. Oceanogr 118: 273–287. [Google Scholar]
- 30. Corcoran PL, Biesinger MC, Grifi M (2009) Plastics and beaches: A degrading relationship. Mar. Pollut. Bull 58: 80–84. [DOI] [PubMed] [Google Scholar]
- 31. Mordecai G, Tyler PA, Masson DG, Huvenne VAI (2011) Litter in submarine canyons off the west coast of Portugal. Deep Sea Res. Part II Top. Stud. Oceanogr 58: 2489–2496. [Google Scholar]
- 32. Aliani S, Griffa A, Molcard A (2003) Floating debris in the Ligurian Sea, north-western Mediterranean. Mar. Pollut. Bull 46: 1142–1149. [DOI] [PubMed] [Google Scholar]
- 33. Ariza E, Jiménez JA, Sardá R (2008) Seasonal evolution of beach waste and litter during the bathing season on the Catalan coast. Waste Manag 28: 2604–2613. [DOI] [PubMed] [Google Scholar]
- 34. Gabrielides GP, Golik A, Loizides L, Marino MG, Bingel F, et al. (1991) Man-made garbage pollution on the Mediterranean coastline. Mar. Pollut. Bull 23: 437–441. [Google Scholar]
- 35. Kornilios S, Drakopoulos PG, Dounas C (1998) Pelagic tar, dissolved/dispersed petroleum hydrocarbons and plastic distribution in the Cretan Sea, Greece. Mar. Pollut. Bull 36: 989–993. [Google Scholar]
- 36. McCoy FW (1988) Floating megalitter in the eastern Mediterranean. Mar. Pollut. Bull 19: 25–28. [Google Scholar]
- 37. Morris RJ (1980) Floating plastic debris in the Mediterranean. Mar. Pollut. Bull 11: 125. [Google Scholar]
- 38. Shiber JG (1982) Plastic pellets on Spain's ‘Costa del Sol’ beaches. Mar. Pollut. Bull 13: 409–412. [Google Scholar]
- 39. Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, et al. (2004) Lost at sea: Where is all the plastic? Science 304: 838. [DOI] [PubMed] [Google Scholar]
- 40. Vauk GJM, Schrey E (1987) Litter pollution from ships in the German Bight. Mar. Pollut. Bull 18: 316–319. [Google Scholar]
- 41. Van Cauwenberghe L, Claessens M, Vandegehuchte MB, Mees J, Janssen CR (2013) Assessment of marine debris on the Belgian Continental Shelf. Mar. Pollut. Bull 73: 161–169. [DOI] [PubMed] [Google Scholar]
- 42. Anastasopoulou A, Mytilineou C, Smith CJ, Papadopoulou KN (2013) Plastic debris ingested by deep-water fish of the Ionian Sea (Eastern Mediterranean). Deep-Sea Res. Part I Oceanogr. Res. Pap 74: 11–13. [Google Scholar]
- 43. Bergmann M, Klages M (2012) Increase of litter at the Arctic deep-sea observatory HAUSGARTEN. Mar. Pollut. Bull 64: 2734–2741. [DOI] [PubMed] [Google Scholar]
- 44. Galgani F, Burgeot T, Bocquene G, Vincent F, Leaute JP, et al. (1995b) Distribution and abundance of debris on the continental shelf of the Bay of Biscay and in Seine Bay. Mar. Pollut. Bull 30: 58–62. [Google Scholar]
- 45. Galgani F, Jaunet S, Campillo A, Guenegen X, His E (1995a) Distribution and abundance of debris on the continental shelf of the north-western Mediterranean Sea. Mar. Pollut. Bull 30: 713–717. [Google Scholar]
- 46. Galgani F, Lecornu F (2004) Debris on the seafloor at “Hausgarten”. Reports on Polar and Marine Research 488: 260–262. [Google Scholar]
- 47. Galgani F, Souplet A, Cadiou Y (1996) Accumulation of debris on the deep sea floor off the French Mediterranean coast. Mar. Ecol. Prog. Ser 142: 225–234. [Google Scholar]
- 48. Galil BS, Golik A, Tuerkay M (1995) Litter at the bottom of the sea: A sea bed survey in the eastern Mediterranean. Mar. Pollut. Bull 30: 22–24. [Google Scholar]
- 49. Katsanevakis S, Katsarou A (2004) Influences on the distribution of marine debris on the seafloor of shallow coastal areas in Greece (Eastern Mediterranean). Water, Air, & Soil Pollution 159: 325–337. [Google Scholar]
- 50. Kidd RB, Huggett QJ (1981) Rock debris on abyssal plains in the northeast Atlantic - a comparison of epibenthic sledge hauls and photographic surveys. Oceanol. Acta 4: 99–104. [Google Scholar]
- 51. Revill AS, Dunlin G (2003) The fishing capacity of gillnets lost on wrecks and on open ground in UK coastal waters. Fish Res 64: 107–113. [Google Scholar]
- 52. Stefatos A, Charalampakis M, Papatheodorou G, Ferentinos G (1999) Marine debris on the seafloor of the Mediterranean Sea: examples from two enclosed gulfs in Western Greece. Mar. Pollut. Bull 38: 389–393. [Google Scholar]
- 53. Spengler A, Costa MF (2008) Methods applied in studies of benthic marine debris. Mar. Pollut. Bull 56: 226–230. [DOI] [PubMed] [Google Scholar]
- 54. Lee D-I, Cho H-S, Jeong S-B (2006) Distribution characteristics of marine litter on the sea bed of the East China Sea and the South Sea of Korea. Est. Coast. Shelf Sci 70: 187–194. [Google Scholar]
- 55. Watters DL, Yoklavich MM, Love MS, Schroeder DM (2010) Assessing marine debris in deep seafloor habitats off California. Mar. Pollut. Bull 60: 131–138. [DOI] [PubMed] [Google Scholar]
- 56. Fiorentini L, Dremière P-Y, Leonori I, Sala A, Palumbo V (1999) Efficiency of the bottom trawl used for the Mediterranean international trawl survey (MEDITS). Aquat. Living Resour 12: 187–205. [Google Scholar]
- 57. Sardà F, Cartes JE, Company JB, Albiol A (1998) A Modified Commercial Trawl Used to Sample Deep-Sea Megabenthos. Fish. Sci 64: 492–493. [Google Scholar]
- 58.Clarke KR, Clarke RK, Gorley RN (2006) Primer V6: User Manual - Tutorial: Plymouth Marine Laboratory.
- 59. Bray JR, Curtis JT (1957) An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr 27: 325–349. [Google Scholar]
- 60. Ramirez-Llodra E, Tyler PA, Baker MC, Bergstad OA, Clark MR, et al. (2011) Man and the last great wilderness: Human impact on the deep sea. PLoS ONE 6: e22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyake H, Shibata H, Furushima Y (2011) Deep-sea litter study using deep-sea observation tools. In: Omori K, Guo X, Yoshie N, Fujii N, Handoh IC et al.., editors. Interdisciplinary Studies on Environmental Chemistry-Marine Environmental Modeling and Analysis: Terrapub. pp. 261–269.
- 62. Hess NA, Ribic CA, Vining I (1999) Benthic marine debris, with an emphasis on fishery-related items, surrounding Kodiak Island, Alaska, 1994–1996. Mar. Pollut. Bull 38: 885–890. [Google Scholar]
- 63. Keller AA, Fruh EL, Johnson MM, Simon V, McGourty C (2010) Distribution and abundance of anthropogenic marine debris along the shelf and slope of the US West Coast. Mar. Pollut. Bull 60: 692–700. [DOI] [PubMed] [Google Scholar]
- 64. Kanehiro H, Tokai T, Matuda K (1996) The distribution of litter in fishing ground of Tokyo Gulf. Fish. Eng 32: 211–217. [Google Scholar]
- 65. Kuriyama Y, Tokai T, Tabata K, Kanehiro H (2003) Distribution and composition of litter of Tokyo Gulf and its age analysis. Nippon Suisan Gakkaishi 69: 770–781. [Google Scholar]
- 66. Hinojosa IA, Thiel M (2009) Floating marine debris in fjords, gulfs and channels of southern Chile. Mar. Pollut. Bull 58: 341–350. [DOI] [PubMed] [Google Scholar]
- 67. Thiel M, Hinojosa I, Vasquez N, Macaya E (2003) Floating marine debris in coastal waters of the SE-Pacific (Chile). Mar. Pollut. Bull 46: 224–231. [DOI] [PubMed] [Google Scholar]
- 68. Thiel M, Hinojosa IA, Joschko T, Gutow L (2011) Spatio-temporal distribution of floating objects in the German Bight (North Sea). J. Sea Res 65: 368–379. [Google Scholar]
- 69. Zhou P, Huang CG, Fang HD, Cai WX, Li DM, et al. (2011) The abundance, composition and sources of marine debris in coastal seawaters or beaches around the northern South China Sea (China). Mar. Pollut. Bull 62: 1998–2007. [DOI] [PubMed] [Google Scholar]
- 70. Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull 44: 842–852. [DOI] [PubMed] [Google Scholar]
- 71. Moore SL, Gregorio D, Carreon M, Weisberg SB, Leecaster MK (2001) Composition and distribution of beach debris in Orange County, California. Mar. Pollut. Bull 42: 241–245. [DOI] [PubMed] [Google Scholar]
- 72. Topcu EN, Tonay AM, Dede A, Ozturk AA, Ozturk B (2013) Origin and abundance of marine litter along sandy beaches of the Turkish Western Black Sea Coast. Mar. Environ. Res 85: 21–28. [DOI] [PubMed] [Google Scholar]
- 73. Silva-Iniguez L, Fischer DW (2003) Quantification and classification of marine litter on the municipal beach of Ensenada, Baja California, Mexico. Mar. Pollut. Bull 46: 132–138. [DOI] [PubMed] [Google Scholar]
- 74. Martinez-Ribes L, Basterretxea G, Palmer M, Tintore J (2007) Origin and abundance of beach debris in the Balearic Islands. Sci. Mar 71: 305–314. [Google Scholar]
- 75. Willoughby NG, Sangkoyo H, Lakaseru BO (1997) Beach litter: an increasing and changing problem for Indonesia. Mar. Pollut. Bull 34: 469–478. [Google Scholar]
- 76. Oigman-Pszczol SS, Creed JC (2007) Quantification and classification of marine litter on beaches along Armacao dos Buzios, Rio de Janeiro, Brazil. J. Coast. Res 23: 421–428. [Google Scholar]
- 77. Ryan PG, Moore CJ, van Franeker JA, Moloney CL (2009) Monitoring the abundance of plastic debris in the marine environment. Philosophical Transactions of the Royal Society B-Biological Sciences 364: 1999–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schlining K, von Thun S, Kuhnz L, Schlining B, Lundsten L, et al. (2013) Debris in the deep: Using a 22-year video annotation database to survey marine litter in Monterey Canyon, central California, USA. Deep-Sea Res. Part I Oceanogr. Res. Pap 79: 96–105. [Google Scholar]
- 79. Halpern BS, Selkoe KA, Micheli F, Kappel CV (2007) Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv. Biol 21: 1301–1315. [DOI] [PubMed] [Google Scholar]
- 80. Halfar J, Fujita RM (2007) Danger of deep-sea mining. Science 316: 987. [DOI] [PubMed] [Google Scholar]
- 81. He G, Ma W, Song C, Yang S, Zhu B, et al. (2011) Distribution characteristics of seamount cobalt-rich ferromanganese crusts and the determination of the size of areas for exploration and exploitation. Acta Oceanol. Sin 30: 63–75. [Google Scholar]
- 82. Davies AJ, Roberts JM, Hall-Spencer J (2007) Preserving deep-sea natural heritage: Emerging issues in offshore conservation and management. Biol. Conserv 138: 299–312. [Google Scholar]
- 83. Amaro T, Bianchelli S, Billett DSM, Cunha MR, Pusceddu A, et al. (2010) The trophic biology of the holothurian Molpadia musculus: implications for organic matter cycling and ecosystem functioning in a deep submarine canyon. Biogeosciences 7: 2419–2432. [Google Scholar]
- 84. Pagès F, Martín J, Palanques A, Puig P, Gili JM (2007) High occurrence of the elasipodid holothurian Penilpidia ludwigi (von Marenzeller, 1893) in bathyal sediment traps moored in a western Mediterranean submarine canyon. Deep-Sea Res. Part I Oceanogr. Res. Pap 54: 2170–2180. [Google Scholar]
- 85. Vetter EW, Dayton PK (1998) Macrofaunal communities within and adjacent to a detritus-rich submarine canyon system. Deep Sea Res. Part II Top. Stud. Oceanogr 45: 25–54. [Google Scholar]
- 86. Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: A review. Environ. Pollut 178: 783–492. [DOI] [PubMed] [Google Scholar]
- 87.Clark MR, Koslow JA (2008) Impacts of fisheries on seamounts. In: Pitcher TJ, Morato T, Hart PJB, Clark MR, Haggan N, et al.., editors. Seamounts: Ecology, Fisheries & Conservation: Blackwell Publishing Ltd. pp. 413–441.
- 88. Moore SL, Allen MJ (2000) Distribution of anthropogenic and natural debris on the mainland shelf of the southern California Bight. Mar. Pollut. Bull 40: 83–88. [Google Scholar]
- 89. Cho D-O (2011) Removing derelict fishing gear from the deep seabed of the East Sea. Mar. Pol 35: 610–614. [Google Scholar]
- 90.Carr HA, Harris J (1997) Ghost-fishing gear: have fishing practices during the past few years reduced the impact? In: Coe J, Rogers D, editors. Marine Debris: Springer New York. pp. 141–151.
- 91. Nash AD (1992) Impacts of marine debris on subsistence fishermen: An exploratory study. Mar. Pollut. Bull 24: 150–156. [Google Scholar]
- 92. Sanchez P, Maso M, Saez R, De Juan S, Muntadas A, et al. (2013) Baseline study of the distribution of marine debris on soft-bottom habitats associated with trawling grounds in the northern Mediterranean. Sci. Mar 77: 247–255. [Google Scholar]
- 93. Martins J, Sobral P (2011) Plastic marine debris on the Portuguese coastline: A matter of size? Mar. Pollut. Bull 62: 2649–2653. [DOI] [PubMed] [Google Scholar]
- 94. Van Cauwenberghe L, Vanreusel A, Mees J, Janssen CR (2013) Microplastic pollution in deep-sea sediments. Environ. Pollut 182: 495–499. [DOI] [PubMed] [Google Scholar]
- 95. Purser A, Orejas C, Gori A, Tong RJ, Unnithan V, et al. (2013) Local variation in the distribution of benthic megafauna species associated with cold-water coral reefs on the Norwegian margin. Cont. Shelf Res 54: 37–51. [Google Scholar]
- 96. Van Rooij D, Blamart D, De Mol L, Mienis F, Pirlet H, et al. (2011) Cold-water coral mounds on the Pen Duick Escarpment, Gulf of Cadiz: The MiCROSYSTEMS project approach. Mar. Geol 282: 102–117. [Google Scholar]
- 97.Tubau X, Canals M, Lastras G,Company JB, Rayo X (2012) The PROMARES-OASIS DEL MAR shipboard party; Marine litter in the deep sections of the North Catalan submarine canyons from ROV video-inspection. 3rd Annual Hermione Meeting, Faro (Portugal), Abstr. Vol ., p. 21. [Google Scholar]
- 98.Huvenne VAI (2011) Benthic habitats and the impact of human activities in Rockall Trough, on Rockall Bank and in Hatton Basin. National Oceanography Centre, Cruise Report No. 04, RRS James Cook Cruise 60: , 133 pp. [Google Scholar]
- 99. Bullimore RD, Foster NL, Howell KL (2013) Coral-characterized benthic assemblages of the deep Northeast Atlantic: defining "Coral Gardens" to support future habitat mapping efforts. ICES J. Mar. Sci 70: 511–522. [Google Scholar]
- 100.Lavaleye MSS (2011) CoralFISH-HERMIONE cruise report of Cruise 64PE345 with RV Pelagia Texel-Vigo, 28 Sept – 14 Oct 2011 to Belgica Mound Province (CoralFISH & HERMIONE) and Whittard Canyon (HERMIONE). NIOZ-cruise report. pp. 47.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of analyses of similarity (ANOSIM) evaluating variation in the composition of litter among physiographic settings. RIDGE: ocean ridges; CANY: submarine canyons; SHELF: continental shelves; SLOPE: continental slopes; SBM: seamounts, banks and mounds; BASIN: deep basins.
(DOCX)
Similarity percentage analysis (SIMPER) of litter composition for each pooled physiographic settings (based on similarities revealed by ANOSIM) and the contribution of litter category to group similarity.
(DOCX)