Abstract
Neonatal infection has enduring effects on the brain, both at the cellular and behavioral levels. We determined the effects of peripheral infection with Escherichia coli at postnatal day (P) 4 in rats on a water maze task in adulthood, and assessed neuronal activation in the dentate gyrus (DG) following the memory test. Rats were trained and tested on one of 3 distinct water maze task paradigms: 1) minimal training (18 trials/ 3 days), 2) extended training (50 trials/ 10 days) or 3) reversal training (extended training followed by 30 trials/ 3 days with a new platform location). Following a 48HR memory test, brains were harvested to assess neuronal activation using activity-regulated cytoskeleton-associated (Arc) protein in the DG. Following minimal training, rats treated neonatally with E. coli had improved performance and paradoxically reduced Arc expression during the memory test compared to control rats treated with PBS early in life. However, neonatally-infected rats did not differ from control rats in behavior or neuronal activation during the memory test following extended training. Furthermore, rats treated neonatally with E. coli were significantly impaired during the 48HR memory test for a reversal platform location, unlike controls. Specifically, whereas neonatally-infected rats were able to acquire the new location at the same rate as controls, they spent significantly less time in the target quadrant for the reversal platform during a memory test. However, neonatally-infected and control rats had similar levels of Arc expression following the 48HR memory test for reversal. Together, these data indicate that neonatal infection may improve the rate of acquisition on hippocampal-dependent tasks while impairing flexibility on the same tasks; in addition, network activation in the DG during learning may be predictive of future cognitive flexibility on a hippocampal-dependent task.
Keywords: Arc, immediate early gene, spatial learning, reversal learning
1. Introduction
Early-life experiences have significant and enduring effects on the health and normal functioning of many organisms. The central nervous system (CNS) develops rapidly during the perinatal period and, thus, is especially vulnerable to disruption. A growing body of research suggests that immune activation during early life has lasting consequences for the immune system, the CNS, and the communication between these two systems [1]. Notably, adult hippocampal function is often especially vulnerable to disruption following immune challenges during critical periods of development [2-6]. For instance, maternal immune activation or neonatal immune challenge with a diverse number of pathogens and immune activators (e.g., polyriboinosinic-polyribocytidylic acid (poly(I:C)), Escherichia coli, lipopolysaccharide (LPS), human immunodeficiency virus (HIV)-1 and interleukin (IL)-6) similarly disrupts spatial learning in adult male rats, suggesting convergence of immune activation onto common plasticity mechanisms [7-12]. Our laboratory has extensively characterized the lifelong effects of early-life infection with E. coli on hippocampal function throughout the lifespan [13-17]. Previously, we have shown that in young adulthood neonatally-infected male rats acquire a platform location more quickly than controls on a Morris Water Maze task. Aged male rats that were treated with E. coli on P4, however, have impaired memory for the platform location 24HR after testing [18]. We have also demonstrated alterations at the cellular level in neonatally-infected rats. E. coli infection on P4 significantly reduced proliferation of neurons in the CA1 and CA3 sub-regions of P6 pups and reduced the maturation and integration of neurons in the CA1, CA3 and dentate gyrus (DG) regions of P33 rats [19]. Nevertheless, our previous work has not examined the effects of E. coli infection on hippocampal neuronal networks. The persistent changes in neurogenesis following early-life infection may be indirect evidence that hippocampal circuitry is enduringly altered in these rats.
Neuronal activation during behavioral tasks can be measured in a variety of ways. Immediate early gene (IEG; e.g. Arc/Arg 3.1, Zif268, cFos) expression is a non-invasive technique for measuring cellular activation in the brain. Protein expression of IEGs has a well-defined time course; thus, assessing protein expression at specific times following behavior reveals the populations of neurons that were activated during a given behavioral task [20-25]. The intracellular kinetics of activity-regulated cytoskeleton-associated (Arc) mRNA and protein are well-characterized [21, 26-27] and extensive work on Arc at both the mRNA and protein levels demonstrates a significant role for its activity-dependent transcription and translation as a mechanism for synapse-specific plasticity [for reviews, see 28, and 29]. Inhibiting Arc protein expression impairs long-term potentiation (LTP) and memory consolidation [30]. Thus, we characterized Arc protein in this study as both a time-sensitive readout of neuronal activation and a representation of possible plasticity during a learning and memory task.
In light of the growing literature on enduring cognitive changes following perinatal infection or inflammation, this study examines the effects of bacterial infection on spatial learning and, indirectly, its underlying neural correlates. We assessed the impact of neonatal E. coli infection on water maze acquisition and memory in adulthood, and measured Arc expression following the memory probe to examine hippocampal activation patterns in rats exposed to bacterial infection early in life, along with age-matched controls. Based on the acquisition and memory behavior that we observed and the potential for neonatal infection to alter many brain regions and not solely the hippocampus, we then examined reversal learning acquisition and memory on the water maze task to assess cognitive flexibility in our neonatally-infected rats.
2. Material and Methods
Specific animal, apparatus and procedural details appear below in the General Methods section.
2.1. Experiment 1
The goal of this experiment was to test the effects of neonatal infection on a challenging paradigm of minimal training on the Morris Water maze (MWM) task in adulthood. We trained the rats with limited, minimal exposure to the apparatus and, thus, increased the difficulty of the task compared to training over a greater number of days or with more trials per day. We trained neonatally-infected and control rats for 3 days, 6 trials per day, to assess memory for a platform location after training. On the third day, half of the trained animals (n=36) were tested on a memory probe trial 2HR following their final training trial. The other half of the group (n=36) was tested on the probe trial 48HR following their last training trial. One hour after their respective probe trials, rats were taken for euthanasia. In addition to the trained rats, we assessed the effect of a single experience in the MWM on Arc expression in the DG, compared to expression after repeated experiences during training and testing. These rats were given a single 60s trial in the pool without a submerged platform. One hour after their single trial, rats were taken for euthanasia.
2.2. Experiment 2
Based on our findings in Experiment 1, we assessed memory performance following extended training on the MWM task, increasing both trials per day and number of days of training. Rats received 5 days of 10 trials per day during training. One hour after a 48HR probe trial (60s), rats were euthanized and brains were collected. We assessed a separate group of rats that was “yoked” by latency to the trained rats to examine the importance of learning on neuronal activation in the DG during a hippocampal-dependent task. Yoked rats were treatment- and latency-matched to rats from the Extensive Training group. All yoked trials were conducted without a submerged platform, allowing for a similar experience in the environment without the act of learning. One hour after a memory probe trial (60s trial 48HR after last training trial), rats were euthanized and brains were collected.
2.3. Experiment 3
We next assessed cognitive flexibility with a reversal task paradigm on the MWM. Once rats were trained to the first platform location (5 days X 10 trials per day, as in Experiment 2) and tested on memory probe trials, the platform was moved to another location in the opposite quadrant (SE) of the pool. Rats were trained for 3 days of 10 trials per day on the new platform location until they reached criterion (less than 10s average escape latency). They were tested 48HR after the last trial for memory for the reversal platform location. Rats were taken 1 hour after the 48HR probe trial for brain collection. The reversal task was assessed in 2 separate groups of rats (total n =16) and their data for acquisition, reversal and the 48HR probe trial for the reversal platform location were pooled.
2.4. General Methods
2.4.1. Animals
Adult male and female Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN) and were pair housed for breeding after a week of acclimation to the facility. Female breeders were visually examined daily for confirmation of pregnancy, and male breeders were removed from cages prior to the birth of pups (P0). All rats were housed in individually ventilated polypropylene cages with ad libitum access to food and filtered water. The colony was maintained at 22°C on a 12:12h light:dark cycle (lights on at 0700 h). Sentinel animals were housed in the colony room and screened periodically for the presence of common rodent diseases; all screens were negative. All experiments were conducted with protocols approved by the Duke University Institutional Animal Care and Use Committee.
2.4.2. Neonatal Manipulations and Bacterial Cultures
All litters were culled on P4 to a maximum of 10 pups/litter, retaining 2 female and as many male pups as possible. The females were retained to prevent single sex litters, but all female pups were euthanized at weaning (P21). All litters were born within 1 week of each other, and all studies were limited to males, limiting the conclusions about the data to males alone. Escherichia coli culture (ATCC 15746; American Type Culture Collection, Manassas, VA) vial contents were hydrated and grown overnight in 30ml of brain-heart infusion (BHI; Difco Labs, Detroit, MI) at 37°C. Cultures were aliquoted into 1ml stock vials supplemented with 10% glycerol and frozen at -20°C. One day before injections, a stock culture was thawed and incubated overnight in 40ml of BHI at 37°C. The number of bacteria in cultures was read using a microplate reader (Bio-Tek Instruments Inc., Winooski, VT) and quantified by extrapolating from previously determined growth curves. Cultures were centrifuged for 15 min at 4000rpm, the supernatants were discarded, and the bacteria were re-suspended in the dose-appropriate volume of sterile Dulbecco's PBS (Invitrogen Corp., Carlsbad, CA). Male pups were injected subcutaneously using a 30G needle on P4 with either 0.1 × 106 colony forming units (CFU) of live bacterial E. coli/g suspended in 0.1 ml PBS or 0.1 ml PBS. All pups were removed from the mother at the same time and placed into a clean cage with bedding, injected individually, and returned to the mother as a group. Elapsed time away from the mother was less than 5 min. All pups from a single litter received the same treatment due to concerns of possible cross-contamination from E. coli. All injections were given between 1300 and 1430h. To control for possible litter effects, a maximum of 2 pups per litter were assigned to a single experimental group. Pups were weaned on P21 into sibling pairs and remained undisturbed until adulthood. In adulthood, all male rats were trained and tested between ages P60 and P90 on the behavioral task.
2.4.3. Behavior
2.4.3.1. Apparatus
The water maze task consisted of a black circular pool approximately 1.8m in diameter and filled with room temperature water. A circular platform 15.5cm in diameter was submerged 2cm below the surface of the water in the center of one of the pool quadrants, and the water was clouded by black non-toxic water-based tempera paint to obscure the platform location. The pool was located in a well-lit room (approximately 5.8m × 2.6m in dimension) with salient extra-maze cues, such as a table with a computer, large black stripes adhered to two walls, shelving that contained large objects and the experimenter who sat in a chair near the computer.
2.4.3.2. Water Maze Training and Testing
For three days prior to behavioral testing, all rats were weighed and handled for approximately 60 seconds each per day. Testing was conducted over several days, depending on the training paradigm. On the first day, rats were habituated to the pool and the platform. On the following days, rats were trained for 6 to 10 trials per day to find the platform in the pool in a constant location (center of NW quadrant). On each training trial, the rat started at a random start location (N, S, E, W, SE, SW, NE) and was allowed to swim until climbing onto the escape platform or until 60 s had elapsed, at which point it was guided to the platform. After 15 s on the platform, the rat was dried with a towel and placed in a holding cage for a 5-min inter-trial interval. After the final training day, rats were given a memory probe test either 2HR or 48HR after the final trial, depending on the paradigm. For the probe trial, the platform was removed from the pool and the rats swam for 60s. One hour after their respective probe trials, rats were taken for euthanasia.
Memory probe tests are reported as difference scores, calculated by assessing the time spent in the Target quadrant (previously containing the platform) and subtracting the time spent in the quadrant with the next highest value. If the rat spent more time in the Target quadrant than any other quadrant, the difference score is positive. If the rat spent more time in a quadrant other than the Target, the difference score is negative. Each group's score is an average of all the difference scores from the rats in the treatment group. The difference score is a conservative measure of performance in the MWM test, as it requires animals to be both accurate and precise in their identification of the target quadrant.
2.4.3.3. Tissue Harvest
Rats were deeply anesthetized with ketamine-xylazine cocktail and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1M PBS. Brains were post-fixed for 24h in 4% paraformaldehyde in 0.1M PBS and cryoprotected in 30% sucrose for at least 3 days and then quickly frozen in -30°C isopentane. 40-μm coronal sections were cut through the dorsal and ventral hippocampus in a -20°C cryostat using the atlas of Paxinos and Watson [31] as a guide. Sections were stored at 4°C in cryoprotectant until immunohistochemistry was performed.
2.4.4. Immunohistochemistry
2.4.4.1. Fluorescence Staining
In Experiment 1, we examined Arc protein expression in one series of brain slices and NeuN (neuronal nuclei) protein in another series double-labeled with a cell proliferation marker not assessed here. Free-floating sections were first rinsed for 3×5 min in 0.01M phosphate buffered saline (PBS) and also rinsed before each subsequent step, except between the blocking and primary antibody steps. Next, sections were washed in 50% methanol for 30 min. Sections were then blocked for another 30 min in 5% normal goat serum and 0.3% Triton-X to block and permeabilize, respectively, in PBS (blocking buffer). Sections were then incubated overnight at room temperature in Arc primary antibody (1:1000, rabbit polyclonal, Wako Pure Chemical Industries, Ltd., Osaka, Japan) or NeuN primary antibody (1:500, mouse monoclonal, Chemicon International, Temecula, CA USA) in blocking buffer. The following day, sections were incubated for 2 h at room temperature in a solution of goat anti-rabbit antibody bound to AlexaFluor 568 (Arc) or goat anti-mouse antibody bound to AlexaFluor 488 (NeuN) (both 1:200, Invitrogen, Grand Island, NY, USA) in blocking buffer. After PBS washes, sections were mounted on gel-coated slides and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). Coverslips were adhered to the slides using nail polish.
2.4.4.2. Diaminobenzidine (DAB) Staining
In Experiments 2 and 3, we did not perform double-label staining. Thus, we performed immunohistochemistry with a chromogen appropriate for a light microscope (DAB) for all subsequent experiments. Free-floating sections were first rinsed for 3×5 min in 0.01M phosphate buffered saline (PBS) and also rinsed before each subsequent step, except between the blocking and primary antibody steps. Next, sections were washed in 50% methanol for 30 min. Sections were then quenched in 0.6% hydrogen peroxide for 30 min and then blocked for another 30 min in 5% normal goat serum and 0.3% Triton-X to block and permeabilize, respectively, in PBS (blocking buffer). Sections were then incubated overnight at room temperature in Arc primary antibody (1:1000, rabbit polyclonal, Wako Pure Chemical Industries, Ltd., Osaka, Japan) in blocking buffer. The following day, sections were incubated for 2 h at room temperature in a solution of biotinylated goat anti-rabbit secondary antibody (1:200, Vector Laboratories, Burlingame, CA USA) in blocking buffer. The Avidin-Biotin Complex (ABC) method was used to bind a complex of streptavidinbiotin peroxidase to the secondary antibody (1 h incubation), which was then developed with diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA, USA) for 15-45 min to produce a colorimetric stain. Sections were mounted on gel-coated slides, dehydrated and coverslipped with Permount.
2.4.5. Cell Quantification
Fluorescent images were visualized using a Zeiss 510 Metaseries inverted laser scanning confocal microscope (representative images shown in Figure 5A and 5B). Slices were continuously scanned from top to bottom (approximately 20μm) exhaustively at 40X through the DG and at two sites (250μm × 250μm) in the CA1 to count Arc-positive cells. Five sections were counted for each rat and each section was at least 200 micrometers from the previously counted section. The counts from each individual section were combined to create a single Arc-positive cell count value per rat.
Figure 5. Representative images from immunohistochemical staining.
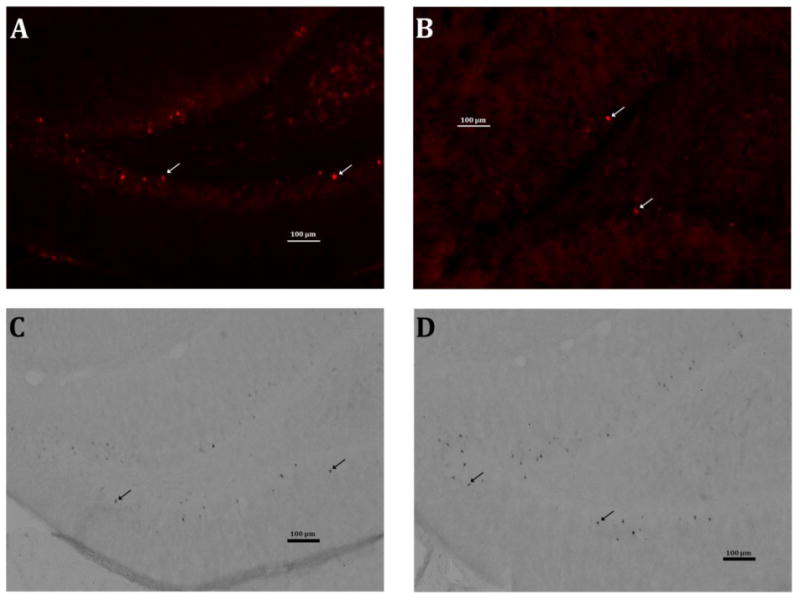
Each image shows the dentate gyrus in one hemisphere at 10X magnification. The scale bars indicate 100 μm and the arrows indicate representative Arc+ cells. (A) DG from a minimally trained control rat after the 48HR probe. (B) DG from a minimally trained neonatally-infected rat after the 48HR probe. (C) DG from an extensively trained control rat after the 48HR probe. (D) DG from an extensively trained neonatally-infected rat after the 48HR probe.
DAB-developed images were visualized with a light microscope (representative images shown in Figure 5C and 5 D). Quantification of Arc-positive cells in the granule cell layer of the dentate gyrus in the hippocampus was performed using a Nikon Eclipse 80i microscope on a Dell PC running StereoInvestigator software (MBF Bioscience, Inc., Williston, VT, USA). The boundaries of the dentate gyrus were traced using this software at a magnification of 10X and Arc-positive cells in the region of interest were counted at 40X. Five sections were counted for each rat and each section was at least 200 micrometers from the previously counted section. The counts from each individual section were combined to create a single Arc-positive cell count value per rat.
2.4.6. Statistical Analyses
Behavioral training was analyzed with a repeated-measures two-way ANOVA to analyze latencies in the water maze between treatment groups over several days of training. Memory probe tests and cell counts were analyzed with t-tests, comparing PBS and E. coli groups. F and t values for each analysis are reported in the Results section.
3. Results
3.1 Experiment 1
3.1.1. Neonatal treatment did not affect platform acquisition on the MWM during minimal training. However, neonatally-infected rats performed better than controls during a 48HR memory test
Minimal training on the MWM tested the effects of neonatal infection on a challenging spatial navigation task. Both neonatally-infected and control rats acquired the platform location during minimal MWM training at the same rate and improved over days of training (F(1,76)=91.3, p<0.001) (Figure 1A). Both groups performed equally well at the 2HR memory probe test (t(38)= −1.28, p=0.207) (data not shown), but neonatally-infected rats had significantly better memory at the 48HR probe (t(30)= −4.83, p=0.026) (Figure 1B).
Figure 1. Neonatally-infected rats have better memory and decreased neuronal activation in the DG following minimal training.
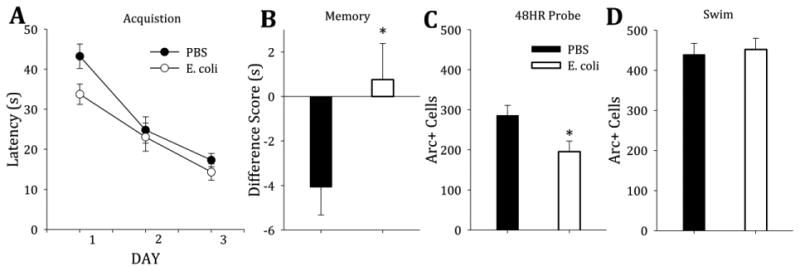
A) Both groups of adult rats (PBS- vs. E. coli-treated on P4) acquired the platform location on the water maze task equally well with minimal training (F(1,76)=91.3, p<0.001). B) Neonatally-infected rats had significantly better performance on the memory probe at 48HR (t(30)= −1.90, p=0.067). C) Arc protein in the DG of neonatally-infected rats was significantly lower at the 48HR probe than in PBS-treated control rats (t(29)=2.47, p=0.020). There were no differences in Arc protein expression in the CA1 (not shown). D) After a single trial (SWIM), both treatment groups had equal Arc expression in the DG (t(25)=−0.32, p=0.75) and greater average expression compared to trained rats.
3.1.2. Neonatally-infected rats had significantly less Arc activation in the DG during the 48HR probe following minimal training
During the 48HR memory probe test after minimal training, neonatally-infected rats had significantly fewer Arc-positive cells in the DG compared to controls (t(29)=2.47, p=0.020) (Figure 1C). During the 2HR memory probe test after minimal training, there were no significant differences in Arc expression between groups (t(37)=1.79, p=0.082; data not shown). In the CA1 region, both groups had equal Arc activation (t(29)=0.394, p=0.696; data not shown). Both groups had equal numbers of Arc-positive cells during a single trial (SWIM; t(25)=−0.32, p=0.75) and had greater Arc expression overall compared to rats that had been trained on the task (Figure 1D). All Arc-positive cells counted in this experiment were also NeuN-positive (data not shown), confirming Arc expression only in mature neurons.
3.2. Experiment 2
3.2.1. Neonatal treatment did not affect acquisition of a platform location on the MWM during extended training. Both treatment groups performed equally on a 48HR memory test
Extensive training improved acquisition latency and memory for the platform. Neonatally-infected and control rats acquired the platform location during extended training at the same rate and improved over days of training (F(1,56)=31.0, p<0.001) (Figure 2A). Both groups of rats performed equally well at the 48HR memory probe test (t(46)= 1.64, p=0.619) (Figure 2B).
Figure 2. All rats exhibit similar acquisition, memory and neuronal activation following extensive training.
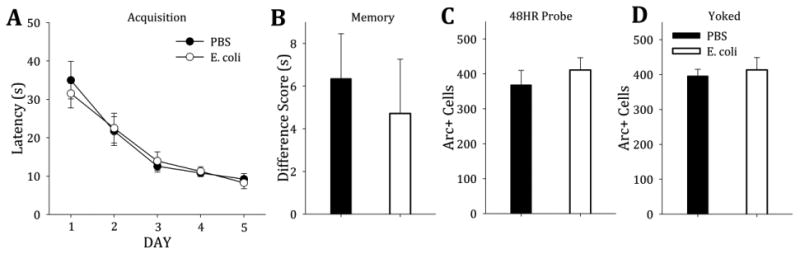
A) Both neonatally-infected rats and controls acquired the platform location at the same rat during extensive training (5 days of training with 10 trials per day) (F(1,56)=31.0, p<0.001). B) When tested 48HR after their last training trial, both groups performed equally on the probe test (t(14)= 1.08, p=0.298). C) When the brains of the trained rats were assessed, both groups had similar numbers of Arc-positive cells in the DG (t(14)=−0.781, p=0.448). D) The “yoked” rats that matched the trained rats in latency in the pool had similar Arc-positive cell counts in the DG, regardless of neonatal treatment (t(14)=−0.44, p=0.67).
3.2.2. Both controls and neonatally-infected rats had similar levels of Arc activation in the DG during the 48HR probe following extended training
Both groups also had equal Arc-positive cells in the DG during the 48HR memory test following extended training (t(14)=−0.781, p=0.448) (Figure 2C) and the rats that were yoked to the extended training rats also showed equal Arc-positive cells in the DG (t(14)=−0.44, p=0.67) (Figure 2D). Interestingly, in contrast to minimal training (Exp. 1), overall Arc expression in yoked rats did not differ between the trained and yoked groups (Figure 2, Panels C and D).
3.3. Experiment 3
3.3.1. Neonatal treatment did not affect platform location acquisition on extended training to one platform location (NW) or a subsequent reversal platform location (SE)
Reversal training after extensive training tested cognitive flexibility. Both groups acquired the platform location during the MWM training to both the original and reversal platform locations and improved over 5 and 3 days of training, respectively (F(4,159)=142.4, p<0.001 and F(2,95)=140.0, p<0.001, respectively) (Figure 3A).
Figure 3. Neonatally-infected rats are significantly impaired on a reversal task, despite acquisition of both the original and reversal platform locations comparable to controls.
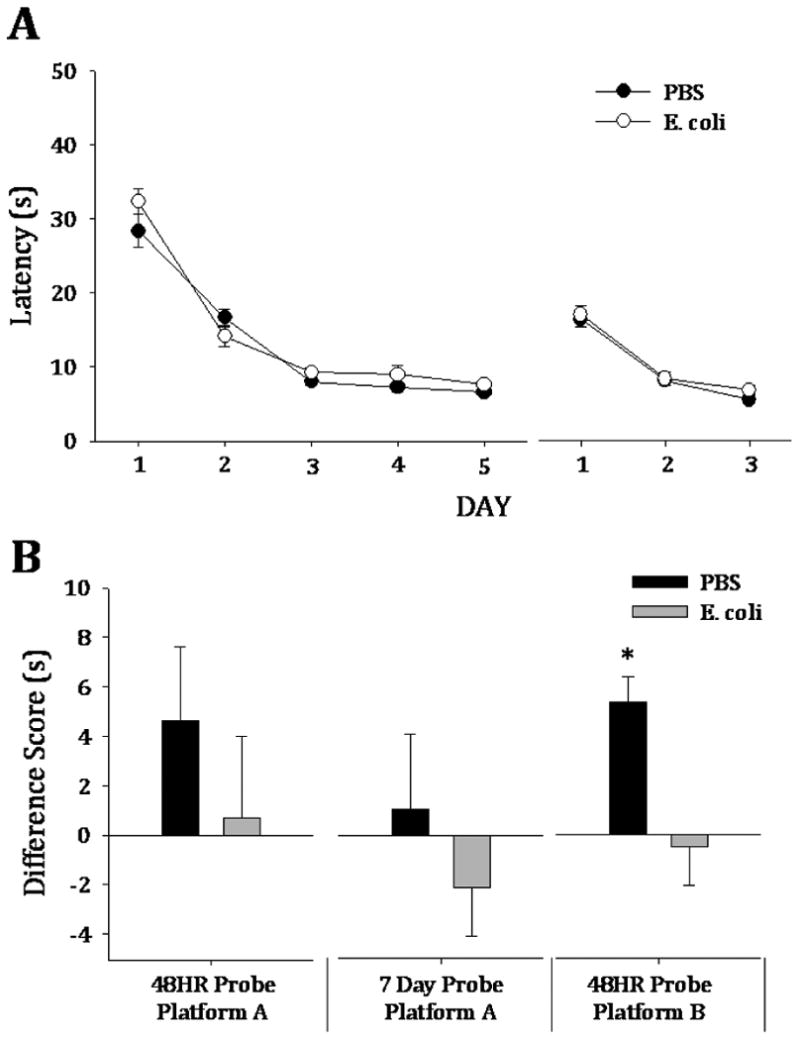
A) Both neonatal treatment groups acquired the platform at the same rate during the 5 days of extensive training (F(4,159)=142.4, p<0.001) and the 3 days of reversal training (F(2,95)=140.0, p<0.001). B) Controls and neonatally-infected rats performed similarly on the 48HR (t(13)= 0.78, p=0.451) and 7 Day (t(13)=0.33, p=0.745) memory probe tests for the original platform. After reversal training, however, neonatally-infected rats had an impaired memory for the reversal platform location (t(28)=2.98, p=0.006).
3.3.2. Both groups performed equally well on memory tests to the original platform location, but neonatally-infected rats demonstrated a memory impairment for the reversal platform location
For the original platform location (NW), both groups of rats performed equally well at the 48HR memory probe test (t(13)= 0.78, p=0.451) (Figure 3B, 48HR Platform A). Each group also performed equally on the 7 day memory probe test (t(13)=0.33, p=0.745) (Figure 3B, 7 Day Platform A). However, the neonatally-infected rats had significantly impaired memory for the reversal platform location during the 48HR probe (t(28)=2.98, p=0.006) (Fig 3B, 48HR Platform B).
3.3.3. Both treatment groups had similar Arc activation in the DG after the 48HR memory probe on the reversal platform location
Both groups had equal counts of Arc-positive cells in the DG during the 48HR memory test for the reversal platform (t(14)=−0.259, p=0.8) (Figure 4).
Figure 4. Neuronal activation does not differ between treatment groups at the 48HR reversal probe.
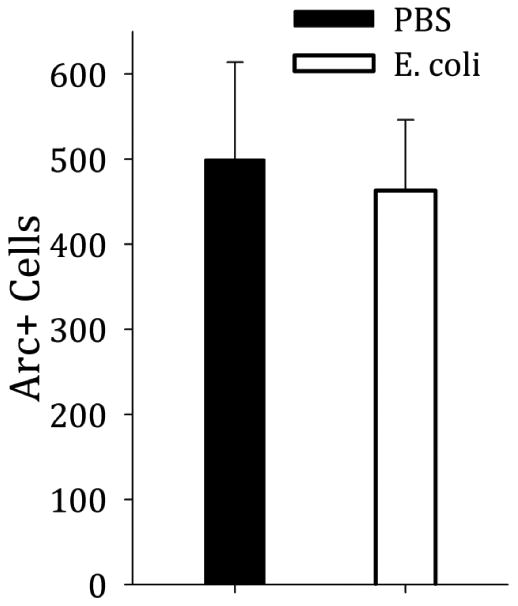
In spite of a behavioral difference in which neonatally-infected rats performed worse on the probe test, both groups had equal numbers of Arc-positive cells in the DG during that probe (t(14)=−0.259, p=0.8).
4. Discussion
We assessed the impact of neonatal infection on spatial learning and memory in adulthood, based on the growing evidence suggesting that early-life immune activation permanently alters the function of the hippocampus. Following minimal training, neonatally-infected adult rats acquired a place memory for the water maze platform more efficiently than controls, whereas no group differences in acquisition or memory probe performance were observed following extensive training. However, even after extensive training on the reversal task, neonatally-infected rats were markedly impaired on the 48HR memory test for the reversal platform. Finally, neuronal activation assessed using Arc protein was paradoxically reduced in neonatally-infected rats in response to the minimally-trained platform memory probe, during which performance was enhanced in this group, suggesting Arc expression within the DG may correlate with accuracy on certain cognitive tasks and perhaps predict flexibility on future performance.
The mRNA and protein level expressions of Arc have been analyzed in detail in the CA1 and CA3 regions of the hippocampus during spatial exploration tasks [30, 32-36]. Arc activation in the CA1 and CA3 is highly correlated with place cell firing in the same regions during the same behavioral tasks [21, 32, 37], and Arc expression has become a valid correlate of cell activation in place of prior invasive in vivo studies or in vitro studies of the CA1 or CA3. In the DG, however, Arc expression has only recently been explored. Arc expression in the DG identified newborn neurons that had matured and were capable of activation [38-39]. In another study, Arc mRNA and protein expression following two exploration bouts did not result in clear network activation in the DG [36]. Recent work by the same group showed that renewed or continuous transcription within the same population of DG granule cells is responsible for sustained Arc expression up to 8H after an exploratory event [40]. Our study is one of the first to examine Arc expression in the DG after a complex hippocampal-dependent task, assessing its correlation with memory retrieval after minimal and extensive water maze training paradigms.
Significantly reduced Arc expression in the DG coupled with improved memory in the neonatally-infected rats after minimal training was initially surprising. However, recent evidence demonstrates that irradiated mice with reduced neurogenesis have increased Arc expression in the DG during impaired performance on a hippocampal-dependent task (e.g. active place avoidance) [41], similar to our PBS controls. Another recent study observed sustained Arc mRNA expression in the DG for hours following exploration and suggested BDNF release as a mechanism for this continuous expression [40]. We have previously reported that neonatally-infected rats have reduced hippocampal BDNF at baseline and following fear conditioning in adulthood [42]; thus, enduring changes in BDNF signaling may contribute to altered Arc expression in the DG of neonatally-infected rats in response to initial spatial learning. Reduced Arc expression during a challenging task, such as the memory test following minimal MWM training, may be correlated to increased accuracy on hippocampal-dependent tasks. The minimally trained accuracy seen in the neonatally-infected rats may, however, interfere with memory flexibility on the reversal task.
Alterations in neuronal circuitry may not be the primary cause for the memory differences we have observed between control and neonatally-infected rats. The MWM task is known to activate the hypothalamic-pituitary-adrenal (HPA) axis and to increase plasma corticosterone (CORT) levels [43]. While we did not measure CORT during or after the MWM task, we have shown that neonatally-infected rats have attenuated serum CORT levels following stressors (e.g., inescapable tail shock or restraint stress), but are not different from controls at baseline [44]. It is possible that attenuated CORT levels during the acquisition of the task could have contributed to improved performance after minimal training or altered neuronal activation in the DG, but we are unable to test CORT levels in the experiments described here. In addition to changes in CORT responses, neonatally-infected rats demonstrate increased microglial reactivity within the hippocampus following fear conditioning [17]. Fear conditioning causes increased microglial reactivity after a single trial, but we have not examined microglial markers or inflammatory cytokines following several days of water maze training. Alterations in microglial signaling could underlie the changes in neuronal activation we observed in the hippocampi of these rats during memory testing. In addition to changes in microglia, there may be changes at the receptor level on neurons (e.g., composition of NMDA receptor subunits on granule cells in the DG) that we are unable to measure here, but we have measured Arc protein in an attempt to assess potential differences at the level of hippocampal circuitry in the neonatally-infected rats.
Neonatal treatment had no effect on 48HR probe performance or DG Arc expression in extensively trained rats that were trained to only one platform location. To our surprise, the improved performance by neonatally-infected rats did not hold during the 48HR probe test for the reversal platform location. As we have seen previously, cognitive deficits in the neonatally-infected rats are often revealed following a second challenge in adulthood, including aging [18] and endotoxin exposure[13-14, 16-17]. The cognitive challenge of reversal learning may have provided a similar “unmasking”. The neonatally-infected rats had significantly worse memory performance than controls on the behavioral task, but both groups had similar Arc-positive cells in the DG. The similarity in Arc expression may be the result of many trials of exposure to the same environment; indeed, as we observed, both minimal and extensive training on the task resulted in lower induced Arc expression on average compared to rats that experience a single trial (SWIM). The equivalent protein expression between neonatal treatment groups in response to reversal memory testing could also be reflective of well-strengthened synapses that do not require the particular cellular mechanisms that Arc provides to maintain LTP or synaptic strength, especially after many exposures to the same environment. Rats in Experiment 2 that were “yoked” by trial latencies to extensively trained rats had similar Arc expression in the DG compared to rats that learned, even without a platform as a goal. Thus, after many trials, the act of learning does not appear to mediate DG Arc expression further and the neural networks of the DG no longer correlate with behavior on spatial learning tasks.
The lack of differences in Arc expression in DG after reversal memory testing may be because the alterations in signaling occur in other brain regions, such as prefrontal cortex and striatum, that are required for reversal task learning and memory. The hippocampus is critical for spatial learning, but the orbitofrontal region of the prefrontal cortex (PFC) has been implicated in reversal learning [45-47]. Furthermore, lesions of the medial prefrontal cortex impair reversal platform location acquisition on a water maze task [48]. In another water maze study, lesions of the medial striatum in adult rats significantly impaired acquisition and memory on a reversal task [49]. We can explore the possibilities of neural signaling changes in these regions in future studies.
Future studies can also examine the specific effects of our neonatal infection model on these neural substrates. Others have shown that cortical circuitry is altered by prenatal and neonatal infection, including studies demonstrating that inflammatory signaling molecules inhibit dendrite development in cortical neurons in vitro [50] and apoptosis occurs in the prefrontal cortex, cerebellum and dentate gyrus for several weeks post-inoculation with Bornavirus [51]. Infection with an AIDS-like murine virus (LP-BM5) impaired reversal acquisition on the water maze as well [52], indicating that altered immune signaling within the brain may underlie deficits on the reversal task.
As we and others have shown, perinatal infection alters spatial learning in adulthood [e.g., 8, 10, 12] and may alter the underlying neural circuitry for spatial learning, but this hypothesis remains to be fully explored. In the hippocampus, a critical neural substrate for spatial learning, immune signaling molecules, such as cytokines and chemokines, have been implicated in synaptic formation and transmission [for review, see 53], offering a potential mechanism by which perinatal infection may have its effects on normal neural and synaptic function. Immune molecules are increasingly implicated in normal neural communication and any enduring changes in the hippocampal neural connectivity in neonatally-infected rats may underlie their distinct spatial learning behavior. While many of the cellular and molecular effects of neonatal infection remain to be elucidated, it seems that hippocampal function, especially on spatial learning tasks, is reliably altered by early-life immune activation. The examination of changes in neural circuitry are beginning to shed more light on the influence of the immune system within the nervous system and its effects on normal learning and memory, and the neonatal infection model provides a foundation with which we can explore the critical homeostatic functions for neuroimmune interactions in cognitive function. The enduring effects of such an infection demonstrate that cognitive disabilities and disruptions may have their origins in the brain's earliest days and the assessment of such difficulties must evaluate the entire lifespan to elucidate underlying causes.
Highlights.
Neonatal infection increases memory accuracy on a challenging water maze task.
Memory is impaired in neonatally infected rats on a reversal water maze task.
Arc expression in the dentate gyrus correlates with memory during the challenging task.
Acknowledgments
The authors would like to acknowledge the technical assistance of Rishi Mistry and Agnes Chao. LLW is supported by a National Science Foundation Graduate Research Fellowship. This work was made possible by NIH (ARRA) R01 MH083698 and the authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–86. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge—effects on behavioural indices of adult rat fear and anxiety. Behavioural brain research. 2005;164:231–8. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Amaral D, Lavenex P. Hippocampal Neuroanatomy. In: Andersen P, Morris RG, Amaral D, Bliss TV, O'Keefe J, editors. The hippocampus book. New York: Oxford University Press; 2007. [Google Scholar]
- 4.Schwarz JM. The immune system and the developing brain. San Rafael, Calif: Morgan & Claypool Life Sciences; 2012. [Google Scholar]
- 5.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–86. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Bilbo SD, Smith SH, Schwarz JM. A lifespan approach to neuroinflammatory and cognitive disorders: a critical role for glia. J Neuroimmune Pharmacol. 2012;7:24–41. doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Li N, Meng Q, Shao F, Wang W. Maternal immune activation impairs reversal learning and increases serum tumor necrosis factor-alpha in offspring. Neuropsychobiology. 2011;64:9–14. doi: 10.1159/000322455. [DOI] [PubMed] [Google Scholar]
- 8.Cronise K, Kelly SJ. Maternal urinary tract infection alters water maze performance in the offspring. Neurotoxicol Teratol. 2001;23:373–9. doi: 10.1016/s0892-0362(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 9.Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–56. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- 10.Chlodzinska N, Gajerska M, Bartkowska K, Turlejski K, Djavadian RL. Lipopolysaccharide injected to pregnant mice affects behavior of their offspring in adulthood. Acta Neurobiol Exp (Wars) 2011;71:519–27. doi: 10.55782/ane-2011-1868. [DOI] [PubMed] [Google Scholar]
- 11.Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal injection of the HIV-1 proteins gp120 and Tat: differential effects on behavior and the relationship to stereological hippocampal measures. Brain Res. 2008;1232:139–54. doi: 10.1016/j.brainres.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace KL, Lopez J, Shaffery JP, Wells A, Paul IA, Bennett WA. Interleukin-10/Ceftriaxone prevents E. coli-induced delays in sensorimotor task learning and spatial memory in neonatal and adult Sprague-Dawley rats. Brain Res Bull. 2010;81:141–8. doi: 10.1016/j.brainresbull.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–9. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- 15.Bilbo SD, Newsum NJ, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain Behav Immun. 2007;21:332–42. doi: 10.1016/j.bbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. J Neurosci. 2011;31:15511–21. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, et al. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329–38. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly MP, Deadwyler SA. Acquisition of a novel behavior induces higher levels of Arc mRNA than does overtrained performance. Neuroscience. 2002;110:617–26. doi: 10.1016/s0306-4522(01)00605-4. [DOI] [PubMed] [Google Scholar]
- 21.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 22.Pinaud R, Penner MR, Robertson HA, Currie RW. Upregulation of the immediate early gene arc in the brains of rats exposed to environmental enrichment: implications for molecular plasticity. Brain Res Mol Brain Res. 2001;91:50–6. doi: 10.1016/s0169-328x(01)00121-8. [DOI] [PubMed] [Google Scholar]
- 23.Nedivi E. Molecular analysis of developmental plasticity in neocortex. J Neurobiol. 1999;41:135–47. [PMC free article] [PubMed] [Google Scholar]
- 24.Worley PF, Christy BA, Nakabeppu Y, Bhat RV, Cole AJ, Baraban JM. Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1991;88:5106–10. doi: 10.1073/pnas.88.12.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada Y, Hada Y, Imamura K, Mataga N, Watanabe Y, Yamamoto M. Differential expression of immediate-early genes, c-fos and zif268, in the visual cortex of young rats: effects of a noradrenergic neurotoxin on their expression. Neuroscience. 1999;92:473–84. doi: 10.1016/s0306-4522(99)00003-2. [DOI] [PubMed] [Google Scholar]
- 26.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–45. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 27.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, et al. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–8. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steward O, Worley P. Local synthesis of proteins at synaptic sites on dendrites: role in synaptic plasticity and memory consolidation? Neurobiol Learn Mem. 2002;78:508–27. doi: 10.1006/nlme.2002.4102. [DOI] [PubMed] [Google Scholar]
- 29.Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci U S A. 2001;98:7062–8. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic press; 2007. [DOI] [PubMed] [Google Scholar]
- 32.Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–4. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Guzowski JF, Miyashita T, Chawla MK, Sanderson J, Maes LI, Houston FP, et al. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc Natl Acad Sci U S A. 2006;103:1077–82. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guzowski JF, Worley PF. Cellular compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH) Curr Protoc Neurosci. 2001;Chapter 1:8. doi: 10.1002/0471142301.ns0108s15. Unit 1. [DOI] [PubMed] [Google Scholar]
- 35.Miyashita T, Kubik S, Haghighi N, Steward O, Guzowski J. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:898–906. doi: 10.1523/JNEUROSCI.4588-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–8. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–96. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder JS, Ferrante SC, Cameron HA. Late maturation of adult-born neurons in the temporal dentate gyrus. PLoS One. 2012;7:e48757. doi: 10.1371/journal.pone.0048757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez-Amaya V, Angulo-Perkins A, Chawla MK, Barnes CA, Rosi S. Sustained Transcription of the Immediate Early Gene Arc in the Dentate Gyrus after Spatial Exploration. J Neurosci. 2013;33:1631–9. doi: 10.1523/JNEUROSCI.2916-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, et al. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22:451–5. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–42. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 44.Bilbo SD, Yirmiya R, Amat J, Paul ED, Watkins LR, Maier SF. Bacterial infection early in life protects against stressor-induced depressive-like symptoms in adult rats. Psychoneuroendocrinology. 2008;33:261–9. doi: 10.1016/j.psyneuen.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89:567–73. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci. 2001;14:1009–20. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- 47.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 48.de Bruin JP, Sanchez-Santed F, Heinsbroek RP, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Res. 1994;652:323–33. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 49.Furtado JC, Mazurek MF. Behavioral characterization of quinolinate-induced lesions of the medial striatum: relevance for Huntington's disease. Exp Neurol. 1996;138:158–68. doi: 10.1006/exnr.1996.0054. [DOI] [PubMed] [Google Scholar]
- 50.Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–9. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- 51.Weissenbock H, Hornig M, Hickey WF, Lipkin WI. Microglial activation and neuronal apoptosis in Bornavirus infected neonatal Lewis rats. Brain Pathol. 2000;10:260–72. doi: 10.1111/j.1750-3639.2000.tb00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iida R, Yamada K, Mamiya T, Saito K, Seishima M, Nabeshima T. Characterization of learning and memory deficits in C57BL/6 mice infected with LP-BM5, a murine model of AIDS. J Neuroimmunol. 1999;95:65–72. doi: 10.1016/s0165-5728(98)00259-8. [DOI] [PubMed] [Google Scholar]
- 53.Williamson LL, Bilbo SD. Chemokines and the hippocampus: A new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]


