Abstract
The antiviral drug 2′,3′-didehydro-3′-deoxythymidine (D4T; also know as stavudine and Zerit), which is used against human immunodeficiency virus (HIV), causes delayed toxicity (peripheral neuropathy) in long-term use. After examining a series of 2′,3′-didehydro-3′-deoxy-4′-substituted thymidine (4′-substituted D4T) analogs, 4′-ethynyl D4T was found to have a fivefold-better antiviral effect and to cause less cellular and mitochondrial toxicity than D4T. The antiviral activity of this compound can be reversed by dThd but not by dCyd. The compound acted synergistically with β-l-2′,3′-deoxy-3′-thiacytidine (also known as lamivudine) and β-l-2′,3′-dideoxy-2′,3′-didehydro-5-fluorocytidine (also known as elvucitabine) and additively with 2′,3′-dideoxyinosine (also known as didanosine and Videx) and 3′-azido-3′-deoxythymidine (also known as Retovir and zidovudine) against HIV. 4′-Ethynyl D4T is phosphorylated by purified human thymidine kinase 1 (TK-1) from CEM cells with a faster relative Vmax and a lower Km value than D4T. The efficiency of TK-1 in the phosphorylation of 4′-ethynyl D4T is fourfold better than that of D4T. While D4T is broken down by the catabolic enzyme thymidine phosphorylase, the level of breakdown of 4′-ethynyl D4T was below detection. Since 4′-ethynyl D4T has increased anti-HIV activity and decreased toxicity and interacts favorably with other currently used anti-HIV drugs, it should be considered for further development as an anti-HIV drug.
Human immunodeficiency virus (HIV [AIDS]) has become the leading infectious cause of death worldwide, surpassing malaria and tuberculosis. Data from the World Health Organization AIDS Epidemic Update for December 2002 list 3.1 million deaths and 42 million people currently living with AIDS. The need for new therapeutic agents with better efficacy is evident. Dideoxynucleosides are an important group of antiviral compounds (18, 29, 30). A member of this group, 3′-azido-3′-deoxythymidine (AZT; also known as Retovir and zidovudine) was the first drug approved for the treatment of HIV. Its dose-limiting adverse effect is myelosuppression (16, 39, 42), which may be worsened by the concurrent administration of other drugs that cause bone marrow suppression or that are hepatically metabolized. 2′,3′-Didehydro-3′-deoxythymidine (D4T; also known as stavudine and Zerit) was then approved because of better bioavailability and lower acute toxicity levels (1). The use of D4T is limited by a long-term delayed toxicity, peripheral sensory neuropathy (5), which is related to mitochondrial damage (4, 6, 7, 15, 20, 25, 33, 36, 37). 2′,3′-Dideoxyinosine (ddI; also known as didanosine and Videx) and 2′,3′-dideoxycytidine (ddC; also known as zalcitabine) are dideoxynucleoside anti-HIV compounds that also have peripheral neuropathy as their leading adverse effect. In the search to find anti-HIV nucleoside analogs that had less neuropathy, many classes of compounds were synthesized and assessed for their antiviral activity and cytotoxicity (including their impact on mitochondrial DNA [mtDNA]). Dideoxynucleosides in the unnatural l conformation represented by β-l-2′,3′-dideoxy-3′-thiacytidine (3TC; also known as lamivudine), its 5-fluoro analog (FTC; also known as emtricitabine), and β-l-2′,3′-dideoxy-2′,3′-didehydro-5-fluorocytidine (LFd4C; also known as elvucitabine) have been shown by Bridges et al., Doong et al., Dutschman et al., and Lin et al. (3, 13, 14, 26-28) and others (10, 11, 17, 41) to have good antiviral activity and low mitochondrial toxicity levels. However, even with compounds relatively nontoxic to mitochondria there is a lack of a durable response. This condition can be caused by either the rapid emergence of resistant virus or host changes that cause differences in drug metabolism (12, 22, 38).
One approach taken to combat this problem is that of developing compounds with less toxicity and a lack of cross-resistance to other antiviral drugs. When used in combinations, these compounds may decrease the dosage of existing drugs needed to achieve the same antiviral effect with less toxicity. Furthermore, these compounds can even delay the onset of resistance; such a delay might result from the decreased viral load during treatment. In the search for a new antiviral compounds, others have looked at 4′-substituted dThd analogs (32, 35), while we synthesized a series of 2′,3′-didehydro-3′-deoxy-4′-substituted thymidine (4′-substituted D4T) analogs. Screening revealed the 4′-ethynyl D4T to be the most active compound among those tested (19). In the studies described within, we determined the structure activity relationship of this class of compounds and characterized 4′-ethynyl D4T in more detail with respect to its mode of action against HIV and its interaction with key cellular enzymes that mediate its activity.
MATERIALS AND METHODS
Chemicals.
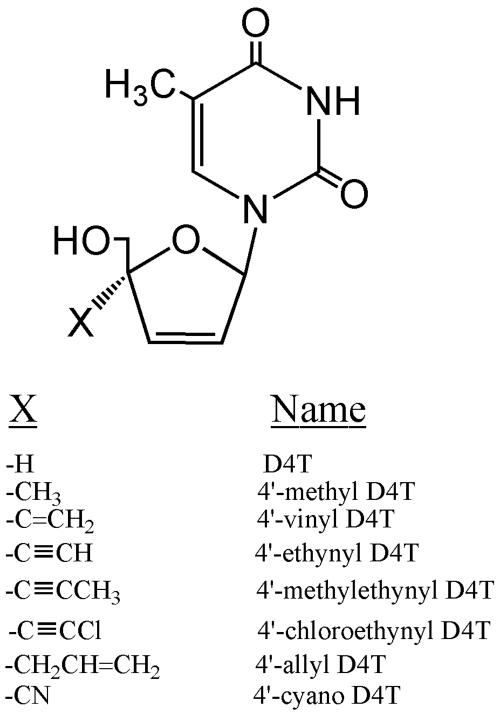
The 4′-D4T analogs (Fig. 1) were synthesized in the laboratory of Hiromichi Tanaka, School of Pharmaceutical Sciences, Showa University, Tokyo, Japan. dThd, D4T, and 3′-azido-3′-deoxythymidine (AZT) were purchased from Sigma-Aldrich Corp., St. Louis, Mo. ddI was purchased from ICN Biochemicals Inc., Aurora, Ohio. 3TC was received from Triangle Pharmaceuticals, Durham, N.C. LFd4C was received from Vion Inc., New Haven, Conn. All other chemicals used were of reagent grade or higher.
FIG. 1.
The chemical structures of the 4′-substituted D4T analogs.
Cell lines and virus.
Both the H9 cell line (used for toxicity studies and virus propagation) and the MT-2 cell line (used for the antiviral activity studies) were received from the AIDS Research and Reference Reagent Program of the National Institutes of Health and were contributed by Robert Gallo and Douglas Richman, respectively. The HIV-1 strain IIIB was received from John Mellors.
Determination of antiviral activity.
Compounds were tested in MT-2 cells infected with HIV-1 strain IIIB essentially as described previously (34). Briefly, serial dilutions of drugs were placed in triplicate wells of a 96-well tissue culture plate and then MT-2 cells grown in RPMI 1640 medium supplemented with 10% dialyzed fetal bovine serum and 100 μg of kanamycin/ml were added at 104 cells/100 μl (± 0.1 multiplicity of infection) of HIV-1 IIIB. Five days later, MTT dye was added to the wells and the color of the tetrazolium dye (measured at 595 nm) was used to quantitate the cellular viability (23). Calculations of the percentage of protection and isobologram combination studies have been described previously (14). Briefly, using the same antiviral assay method the 50% effective concentrations (EC50) of compounds alone and in combination with a second compound were plotted using Cartesian coordinates. This graphic display of drug effect is configured in the form of an isobologram. If the paired points fall on the line connecting the axial points the compounds are additive, but if the point is shifted to the left the combination is considered to be synergistic (2, 9, 21, 40).
Cellular toxicity of nucleoside analogs.
These dThd analogs have been evaluated in several cell lines (H9, CEM, MT-2, and HepG2). The basic procedures are similar. The cells are seeded a low concentration, and then serial dilutions of test compound are added. The CEM, MT-2, and H9 cell lines we used are grown in RPMI 1640 supplemented with 10% dialyzed fetal bovine serum and 10 μg of kanamycin/ml. After 48 to 96 h of incubation at 37°C in a 5% CO2 humidified incubator, the assay is ended. The drug-treated samples are compared to the untreated controls. This is accomplished with suspension cell lines by counting cell numbers with a hemocytometer or by using a Coulter counter. The HepG2 cells (representing a human hepatoma cell line) were grown in Dulbecco's modified Eagle's medium supplemented with 10% dialyzed fetal bovine serum and 10 μg of kanamycin/ml. The effect on HepG2 (a monolayer cell line) was quantified by staining with 1.0% methylene blue dye dissolved in 50% ethanol after the growth medium was decanted. The cell layer was then solubilized in a 5% sarkosyl solution and the resulting color was measured at 595 nm on a model Vmax plate-reading spectrophotometer (Molecular Devices, Menlo Park, Calif.). The color of the untreated controls was compared to that of the drug-treated samples.
mtDNA.
The effect of nucleoside analogs on mtDNA content was assessed as described previously (3). Briefly, CEM cells maintained in RPMI 1640 supplemented with 10% dialyzed fetal bovine serum were plated at 2 × 105/ml into a 24-well tissue culture plate. Cells (treated with drugs used at various concentrations either as single agents or in combination) were grown for 4 days. Cells were then harvested and treated with proteinase K and DNase-free RNase. Extracts were applied to nylon membranes and hybridized with an mtDNA probe. After the membrane was stripped, the load was normalized by rehybridizing the membrane with an Alu probe. Blots were quantitated with a Molecular Dynamics personal densitometer synergy index (SI) and ImageQuant analysis software.
Monophosphorylation of analogs by TK.
All the analogs were tested for their ability to be phosphorylated by thymidine kinase 1 (TK-1) from CEM cells. This enzyme was purified by an affinity column technique developed in this laboratory (8). Thymidine analogs (250 μM) were incubated in a mixture that contained 150 mM Tris HCl (pH 7.5), 2.4 mM ATP, 2.4 mM MgCl2, 0.6 mg of creatine phosphate, 5.8 U of creatine phosphokinase, 0.19 mg of albumin, and 0.07 U of TK-1 in a total volume of 200 μl. At the end of the incubation time the reaction was stopped by the addition of 3 parts of cold high-pressure liquid chromatography (HPLC) grade methanol. After being incubated on ice for at least 10 min, the methanol-insoluble material was precipitated by centrifugation and the methanol-soluble supernatants were placed into clean microcentrifuge tubes. These samples were brought to dryness in a Speedvac centrifuge. The samples were dissolved in water and separated on a Shimatzu HPLC model SCL 10Avp apparatus using a gradient of water to 300 mM potassium phosphate and a Whatman 10/25 particle SAX column. Km and relative Vmax studies were done in a similar fashion, using the same mixture and different amounts of substrate and enzyme.
Acid stability studies.
Nucleoside samples were mixed with 1 N HCl and incubated at 37°C for 2.5 h. The samples were then examined by HPLC using a Beckman ODS column employing a gradient of water to 80% methanol.
TP assays.
Nucleoside analogs (100 μM) were incubated in 75 mM potassium phosphate buffer (pH 7.3) at 37°C through the use of a partially purified preparation of human liver extract (31) as a source of the thymidine phosphorylase (TP). After incubation, the reaction was stopped by the addition of trichloroacetic acid to a final concentration of 15%. The samples were then incubated on ice. After the acid-insoluble components were removed by centrifugation, the supernatant was neutralized by two extractions with one-half volume of trioctylamine-freon (45:55). The aqueous supernatants were examined by HPLC using the Beckman ODS column method as described in the previous paragraph.
TK assays.
The TK assays were performed as described previously (24). Briefly, the assay used [14C]dThd (100 μM, 6.7 mCi/mmol) in a mixture that contained 2.4 mM ATP-Mg, 156 mM Tris-HCl (pH 7.5), 0.23 mg of creatine phosphate, 7 μg of creatine phosphokinase, 67 μg of bovine serum albumin, and 1.9 mM dithiothreitol in a 75-μl volume. The reaction mixtures were incubated for various amounts of time and were then terminated by spotting 50-μl aliquots onto DE-81 anion exchange disks (Whatman Inc., Clifton, N.J.) that were immersed immediately in 95% ethanol. After two additional washes in ethanol, the disks were dried and placed in scintillation vials that contained 5 ml of SafeScint scintillation cocktail (American Bioanalytical, Natick, Mass.). The amount of radioactivity (which represents the amount of dTMP formed) was quantitated in an LS5000TD scintillation counter (Beckman Instruments Inc., Palo Alto, Calif.).
RESULTS
Antiviral effect of 4′-substituted D4T analogs.
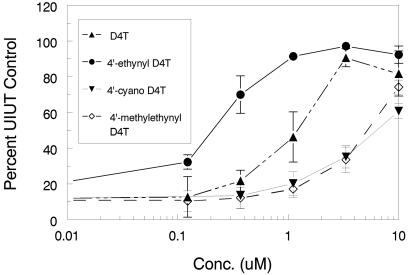
Experiments were performed in the MT-2/IIIB anti-HIV-1 system by adding compounds with a substitution of a methyl, vinyl, ethynyl, methylethynyl, chloroethynyl, allyl, or cyano group at the 4′ position of D4T (Fig. 1). The results indicated that the 4′-ethynyl analog was more effective against HIV and less toxic than the parental compound D4T. Whereas 4′-cyano D4T and 4′-methylethynyl D4T were less active than D4T against HIV (Fig. 2), the 4′-methyl-, 4′-vinyl-, 4′-chloroethynyl-, and 4′-allyl-substituted D4T analogs did not achieve an EC50 at a concentration of 100 μM. A summary of the EC50 against HIV of these compounds together with D4T is shown in Table 1.
FIG. 2.
Anti-HIV activity of 4′-substituted D4T analogs. The antiviral activities of 4′-ethynyl D4T, D4T, 4′-methylethynyl D4T, and 4′-cyano D4T were determined in the MT-2/HIV IIIB system as described in Materials and Methods. Inhibition was determined by comparison of the results determined at an optical density of 595 nm to those determined with uninfected untreated (UIUT) control MT-2 cells. The symbols represent the means of triplicate determinations within a single experiment, and the error bars represent standard deviations. This graph is representative of several similar experiments.
TABLE 1.
Effect of 4′-substituted D4T analogs on HIV
| Compound | EC50 (μM)a | ID50 (μM)b |
|---|---|---|
| D4T | 1.3 ± 0.4 | 98.0 ± 10.8 |
| 4′-Methyl D4T | >100d | >100 |
| 4′-Vinyl D4T | >100d | >100 |
| 4′-Ethynyl D4T | 0.25 ± 0.14d | >256c |
| 4′-Methylethynyl D4T | 4.0 ± 1.6 | >100 |
| 4′-Chloroethynyl D4T | >100 | 63.3 ± 20.8 |
| 4′-Allyl D4T | >100 | >100 |
| 4′-Cyano D4T | 7.0 ± 2.6 | >100 |
EC required to achieve 50% protection from HIV in MT-2 cells. Numbers represent means and standard deviations obtained from three separate experiments and derived from antiviral curves generated using triplicate samples at each concentration.
Concentration required to inhibit MT-2 cell growth by 50%. Numbers represent means and standard deviations obtained from three separate experiments and derived from toxicity curves generated using triplicate samples at each concentration.
The highest concentration tested.
EC50 reported previously (19).
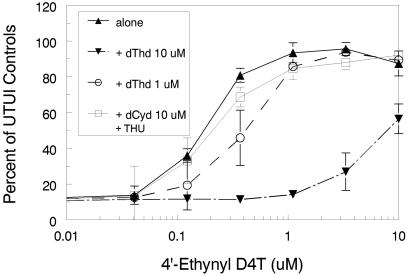
To determine whether 4′-ethynyl D4T acts as a dThd analog against HIV, the effect of the addition of dThd or dCyd on the antiviral activity of 4′-ethynyl D4T was examined. To prevent the possibility of deamination of dCyd to dUrd in cells, a cytidine deaminase inhibitor, tetrahydro uridine, was also added (at a nontoxic level). It was observed that dThd decreased the antiviral effect of 4′-ethynyl D4T in a concentration-dependent manner. However, dCyd had no significant effect on the activity of 4′-ethynyl D4T against HIV (Fig. 3).
FIG. 3.
Reversal of the anti-HIV effect of 4′-ethynyl D4T. dThd (10 μM), dThd (1 μM), and dCyd (10 μM) were added to the standard antiviral assay in the presence of THU (5 μM). Inhibition was determined by comparison of the results determined at an optical density of 595 nm to those determined with uninfected untreated (UIUT) control MT-2 cells. The symbols represent the means of triplicate determinations within a single experiment, and the error bars represent standard deviations. This figure is representative of several similar experiments.
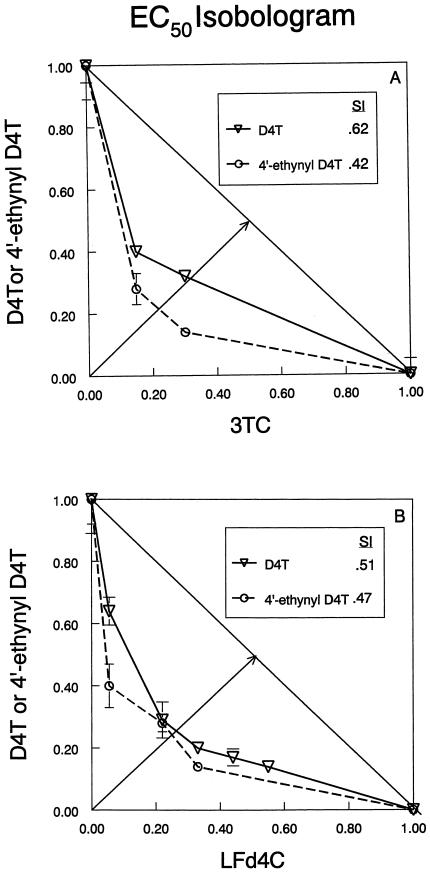
To assess the interaction of 4′-ethynyl D4T with other antiviral nucleoside analogs, the antiviral isobolograms of 4′-ethynyl D4T in combination with 3TC, LFd4C, ddI, and AZT were generated. 4′-Ethynyl D4T was shown to have synergistic interactions with 3TC and LFd4C against HIV (Fig. 4), and the SI was determined by measuring the relative distance from the line indicating an additive drug effect. However, the antiviral effect of 4′-ethynyl D4T in combination with ddI and AZT was only additive (data not shown).
FIG. 4.
Antiviral isobolograms of D4T and 4′-ethynyl D4T in combination with (A) 3TC and (B) LFd4C data obtained with the MT-2/HIV IIIB system. The numbers along each axis represent values proportional to that of the EC50 (assigned a value of 1) for the drug indicated as a single agent. EC50 values for single agents are 1.4 μM (D4T), 0.5 μM (4′-ethynyl D4T), 1.0 μM (3TC), and 0.18 μM (LFd4C). Each datum point represents a combination that produces an effect equivalent to that of the EC50 for either drug alone. SI values were calculated according to the fractional distance from the 45° line to the line (drawn between the 1.00 values on the x and y axes) that indicates that the drug interaction is additive; the total distance was assigned a value of 1.0. The symbols represent the means of three determinations, and the error bars represent the standard deviations of the slope at the concentrations indicated. This isobologram was generated from one of several similar experiments.
Cellular toxicity.
The effect of the 4′-substituted D4T analogs on cell growth and mtDNA content was determined with CEM cells (Table 2). None of those analogs (with the exception of 4′-chloroethynyl D4T) could retard 4-day cell growth with a 50% infective dose (ID50) of less than 100 μM. Results of 72-h toxicity studies of HepG2 cells also showed that the ID50 values for D4T, 4′-vinyl D4T, and 4′-ethynyl D4T were more than 100 μM. 4′-Ethynyl D4T decreased intracellular mtDNA with an ID50 of 100 μM, which is a concentration 10 times higher than that required with D4T. In view of the synergistic interaction of 4′-ethynyl D4T with 3TC and LFd4C against HIV, the effect of the interaction of those compounds on cell growth was also assessed. With H9 cells observed during a 48-h assay, no significant increase in toxic interactions was seen (Table 3).
TABLE 2.
Toxicity of nucleoside analogs in CEM cells
| Compound | ID50 (μM)
|
|
|---|---|---|
| Cellular toxicityb | mtDNA contentc | |
| D4T | 60.0 ± 18.0 | 9.3 ± 1.4 |
| 4′-Methyl D4T | >100 (114 ± 2) | NDd |
| 4′-Vinyl D4T | >100 (78 ± 21) | ND |
| 4′-Ethynyl D4T | >100 (77 ± 16) | >100 (94 ± 4) |
| 4′-Methylethynyl D4T | >100 (94 ± 20) | >100 (116 ± 26) |
| 4′-Chloroethynyl D4T | 62.6 ± 10.0 | ND |
| 4′-Allyl D4T | >100 | ND |
| 4′-Cyano D4T | >100 (60 ± 1) | >100 (264 ± 23) |
| ddC | 5.5 ± 1.8 | 0.15 ± 0.12 |
| 3TC | >200 (77 ± 28) | >200 (114 ± 2) |
Procedures were as described in Materials and Methods.
Toxicity determined by cell counts compared to results obtained with untreated controls. Two wells were counted in duplicate for each condition on a Coulter counter, and the experiment was repeated three times.
mtDNA content results were determined by Southern blot analysis; densitometer readings were compared to those obtained with untreated CEM control cells. The numbers are means and standard deviations of micromolar concentrations that cause 50% inhibition of control cells. The numbers in parentheses represent the means and standard deviations of percentages of untreated CEM control cells at the concentrations indicated.
ND, not determined.
TABLE 3.
Toxicity in H-9 cells of 4′-ethynyl D4T alone and in combination with other anti-HIV compounds as percentages of the results obtained with untreated controlsa
| Additive and concn (μM) | % of results obtained with untreated controls at 4′-ethynyl D4T concn (μM) b of
|
||
|---|---|---|---|
| 0 | 25 | 50 | |
| None | 100 ± 5 | 97 ± 7 | 102 ± 6 |
| LFd4C | |||
| 5 | 73 ± 9 | 62 ± 3 | 72 ± 5 |
| 10 | 56 ± 4 | 58 ± 4 | 64 ± 5 |
| 3TC | |||
| 5 | 99 ± 3 | 98 ± 2 | 99 ± 10 |
| 20 | 94 ± 3 | 88 ± 3 | 87 ± 6 |
| 100 | 108 ± 12 | 85 ± 3 | 84 ± 6 |
H-9 cells were grown for 48 h in the presence of single compounds or in combination as described in Materials and Methods.
Three or more wells for each condition were counted in duplicate using a Coulter counter. Numbers represent means and standard deviations obtained from three experiments.
Interaction of 4′-substituted D4T analogs with TK-1.
The potential of these compounds to be phosphorylated by purified human TK-1 was assessed (Table 4). AZT was converted to the monophosphate form half as fast as dThd, while the rates for 4′-methyl D4T and 4′-vinyl D4T were similar to the rate for D4T (approximately 2% of the rate seen with dThd). The conversion rate of 4′-ethynyl D4T was superior to that of D4T (with a confidence level of 0.06). There was no significant difference in the phosphorylation rates of 4′-ethynyl D4T and 4′-chloroethynyl D4T (with a confidence level of 0.91 [two-tailed test]). The Km of 4′-ethynyl D4T was assessed to be 52 μM, which is lower than the 133 μM assessed for D4T but higher than the Km seen with dThd. To be sure that none of these dThd analogs act as a potent inhibitor of TK-1 (even if they are not substrates), dThd, AZT, D4T, and the 4′-substituted analogs of D4T were added to a TK assay at a concentration 10-fold higher than that of the [14C]dThd and then the amount of conversion to [14C]dTMP was compared to the results seen with reaction mixtures with no additions (Table 5). Compounds (such as AZT) that are phosphorylated well by TK-1 can affect the amount of phosphorylated dThd. The addition (even in a 10-fold excess) of D4T or its analogs that are poorly phosphorylated has less effect than that of AZT on [14C]dThd phosphorylation by TK-1.
TABLE 4.
Phosphorylation by human cytoplasmic TKa
| Compound | Km (μM) | Relative Vmaxd |
|---|---|---|
| dThd | 2.6b | 100 |
| AZT | NDc | 55.5 ± 9.7 |
| D4T | 133 | 2.1 ± 0.7 |
| 4′-Methyl D4T | ND | 1.6 ± 0.5 |
| 4′-Vinyl D4T | ND | 1.8 ± 0.5 |
| 4′-Ethynyl D4T | 52 | 3.8 ± 0.8 |
| 4′-Methylethynyl D4T | ND | 2.5 ± 0.9 |
| 4′-Chloroethynyl D4T | ND | 3.9 ± 1.0 |
| 4′-Allyl D4T | ND | 0.4 ± 0.2 |
| 4′-Cyano D4T | ND | 1.1 ± 0.2 |
Data represent results obtained with 250 μM dThd or an analog and 2.4 mM ATP incubated with 0.07 U of TK-1 at 37°C for 285 min.
Value published previously (24).
ND, not determined.
Numbers represent means and standard deviations of at least three separate determinations.
TABLE 5.
Effect of the addition of thymidine analogs to a TK assaya
| Nucleoside added | % Activityb |
|---|---|
| None | 100 |
| dThd | 9.1 ± 3.2 |
| AZT | 5.4 ± 2.3 |
| D4T | 106.3 ± 7.7 |
| 4′-Methyl D4T | 103.8 ± 9.9 |
| 4′-Vinyl D4T | 99.5 ± 6.9 |
| 4′-Ethynyl D4T | 83.9 ± 6.1 |
| 4′-Methylethynyl D4T | 74.0 ± 7.9 |
| 4′-Chloroethynyl D4T | 53.2 ± 9.2 |
| 4′-Allyl D4T | 110.8 ± 9.0 |
| 4′-Cyano D4T | 71.8 ± 8.2 |
The assays were performed essentially as described in Materials and Methods except that the [14C]dThd concentration in the assay was reduced to 25 μM and the concentration of the added nucleoside was 250 μM.
Numbers represent means and standard deviations of six to eight determinations.
Interaction with TP and acid stability of 4′-ethynyl D4T.
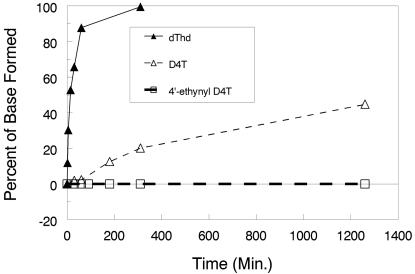
A partially purified preparation of TP from human liver was utilized for these studies. dThd broke down very quickly, while D4T broke down at least 10 times more slowly. The breakdown of 4′-ethynyl D4T was below the detection level during the whole incubation period studied (Fig. 5). The stability of D4T and 4′-ethynyl D4T at pH 1 and 37°C was examined for 2.5 h. No breakdown of either compound was detected.
FIG. 5.
TP treatment of D4T analogs. dThd, D4T, and 4′-ethynyl D4T were incubated with a partially purified preparation of TP from human liver extract. The ratio of base to nucleoside was then determined by reverse-phase HPLC on a Beckman ODS column as described in Materials and Methods. Each data point is from a single HPLC determination, and the figure is representative of several experiments with similar results.
DISCUSSION
D4T is an effective anti-HIV d-dideoxy-thymidine analog. Its limiting clinical toxicity in long-term treatment is peripheral neuropathy, which is associated with its action of decreasing the mtDNA content of peripheral neurons (5, 6, 36, 37). The biochemical determinants of the action of D4T are different from those of 3TC, ddI, or ddC. An analog of D4T which has more potent anti-HIV activity and less impact on nuclear or mtDNA synthesis could have a better therapeutic effect than D4T and could substitute for D4T in anti-HIV combination therapy. Thus, the synthesis of D4T analogs with better pharmacological properties is a direction which has been taken in anti-HIV drug discovery. Among all the 4′-substituted D4T analogs synthesized by us and others, 4′-ethynyl D4T is the compound most active against HIV in culture. Maag et al. described a 4′-azido D4T that was inactive against HIV at nontoxic levels (32), and O-Yang et al. described three 4′-substituted D4T analogs that were nontoxic and had no anti-HIV activity (35).
D4T is catabolized (rather quickly) into beta-aminoisobutyric acid and thymine by the hepatocytes of the liver (J. P. Sommadossi, Z. Zhou, M. J. Hitchcock, H. M. McClure, M. el Kouni, and E. Cretton, abstract from the Proceedings of the Annual Meeting of the American Association for Cancer Research, Cancer Res. 33:A3253, 1992). The enzyme responsible for this breakdown is TP, which in the presence of phosphate breaks dThd into thymine and 2-deoxy-d-ribose-1-phosphate. By the incubation of 4′-ethynyl D4T and D4T with a partially purified preparation of human liver TP, it was shown that 4′-ethynyl D4T was much more resistant to TP than D4T. This indicates that 4′-ethynyl has an additional advantage over D4T from a pharmacokinetic point of view. Furthermore, 4′-ethynyl D4T is also as stable as D4T in an acidic condition that mimics the stomach (data not shown). This suggests that 4′-ethynyl D4T (like D4T) could be an orally active agent. Detailed pharmacokinetic studies will be performed in the future. Since 4′-ethynyl D4T is more potent than D4T, it is conceivable that 4′-ethynyl D4T could present fewer viral drug resistance issues. When 4′-ethynyl D4T is employed at the same dosage as D4T for the patient, the viral load is much less, thereby decreasing the probable occurrence of resistant strains. It may also be possible to give 4′-ethynyl D4T at higher dosage than D4T, since 4′-ethynyl D4T is less inhibitory to cell growth and causes less of a decrease in the levels of mtDNA than D4T. However, the determination of the amount of 4′-ethynyl D4T that can be safely used will require further investigation.
Monotherapy allows the development of resistant strains of virus to occur more readily than combination therapy. It is therefore necessary for an antiviral compound to work in conjunction with other approved antiviral drugs which have different biochemical determinants of drug resistance. If the compounds are synergistic (or at least additive) with respect to their antiviral activity, but not with respect to their cytotoxic effect on the host cells, improved therapy can be achieved. Indeed, combination therapy for HIV has made tremendous progress in the management of AIDS and D4T is often used as one of the drugs in combination protocols. To assess the potential use of 4′-ethynyl D4T in combination therapy, we examined the interaction of this compound with four antiviral nucleoside analogs. 4′-Ethynyl D4T is synergistic with 3TC and LFd4C (Fig. 3) and additive with AZT and ddI (data not shown) with respect to anti-HIV activity but not with respect to cytotoxicity (Table 3). This suggests that 4′-ethynyl D4T could be a useful compound for combination therapy and could be useful against viruses resistant to currently used nucleosides by increasing the effectiveness of those nucleosides through a synergistic response. The activity of 4′-ethynyl D4T against viruses resistant to other nucleoside analogs is currently being investigated.
The underlying mechanism that makes 4′-ethynyl D4T more active against HIV than the other 4′-substituted D4T analogs studied is not clear. Deoxynucleoside analogs typically are converted into 5′-triphosphate metabolites that are substrates for viral DNA polymerases. Triphosphate metabolites of known anti-HIV dideoxynucleosides interact preferentially with viral reverse transcriptase and act as chain terminators when they are incorporated into a DNA strand. The formation of the monophosphate metabolite is the first step in the process of the formation of a triphosphate metabolite. The 4′-substituted D4T compounds are (like D4T) dThd analogs, so we used purified TK-1 to test whether it could phosphorylate these analogs to their respective monophosphate forms. The results showed that 4′-ethynyl D4T was phosphorylated twice as fast as D4T (although at a much slower rate than that seen with dThd or AZT). It is interesting that the 4′-methyl D4T and 4′-vinyl D4T analogs were phosphorylated at the same rate as D4T, but neither had significant anti-HIV activity. Thus, it can be concluded that the lack of activity against HIV of some of these 4′-substituted D4T analogs is not due to their inability to be phosphorylated by TK-1. The phosphorylation of 4′-ethynyl D4T by TK-1 is an essential step but is not sufficient to generate antiviral activity. Since its antiviral effect could be neutralized by dThd but not by dCyd, 4′-ethynyl D4T (like D4T) acts as a dThd analog; however, the antiviral mechanism of action of 4′-ethynyl D4T could still be quite different from that of D4T. Our unpublished results indicate that D4T could be more efficiently phosphorylated (using a CEM cellular extract supplemented with partially purified TK-1 and recombinant human dTMP kinase) to the triphosphate metabolite than 4′-ethynyl D4T. This raises the issue of whether the 4′-ethynyl D4TMP is the active metabolite instead of 4′-ethynyl D4TTP and requires further investigation.
In conclusion, 4′-ethynyl D4T is more potent against HIV and less toxic than D4T in cell culture. It could also have pharmacokinetic advantages over D4T, since it is not a substrate of TP. The potential of 4′-ethynyl D4T as a new anti-HIV drug is suggested by these studies and warrants further investigation.
Acknowledgments
This work was supported by Public Heath Service grant AI-38204 from the National Institutes of Health to Y.-C.C. It was also supported in part by grants from Japan Health Sciences Foundation (SA14718 to M.B.), Japan Society for the Promotion of Science (KAKENHI 15590100 to K.H. and 15590020 to H.T.), and the Research Foundation for Pharmaceutical Sciences (to K.H.). Y.-C. C. is a Fellow of the National Foundation for Cancer Research.
REFERENCES
- 1.August, E. M., M. E. Marongiu, T. S. Lin, and W. H. Prusoff. 1988. Initial studies on the cellular pharmacology of 3′-deoxythymidin-2′-ene (d4T): a potent and selective inhibitor of human immunodeficiency virus. Biochem. Pharmacol. 37:4419-4422. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini, J., E. De Clercq, A. Carbonez, V. Burt, and J.-P. Kleim. 2000. Long-term exposure of HIV type 1-infected cell cultures to combinations of novel quinoxaline GW420867X with lamivudine, abacavir, and a variety of nonnucleoside reverse transcriptase inhibitors. AIDS Res. Hum. Retrovir. 16:517-528. [DOI] [PubMed] [Google Scholar]
- 3.Bridges, E. G., G. E. Dutschman, E. A. Gullen, and Y. C. Cheng. 1996. Favorable interaction of beta-l(−) nucleoside analogues with clinically approved anti-HIV nucleoside analogues for the treatment of human immunodeficiency virus. Biochem. Pharmacol. 51:731-736. [DOI] [PubMed] [Google Scholar]
- 4.Brinkman, K., H. J. ter Hofstede, D. M. Burger, J. A. Smeitink, and P. P. Koopmans. 1998. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS 12:1735-1744. [DOI] [PubMed] [Google Scholar]
- 5.Browne, M. J., K. H. Mayer, S. B. Chafee, M. N. Dudley, M. R. Posner, S. M. Steinberg, K. K. Graham, S. M. Geletko, S. H. Zinner, S. L. Denman, L. M. Dunkle, S. Kaul, C. McLaren, G. Skowron, N. M. Kouttab, T. A. Kennedy, A. B. Weitberg, and G. A. Curt. 1993. 2′,3′-Didehydro-3′-deoxythymidine (D4T) in patients with AIDS or AIDS-related complex: a phase I trial. J. Infect. Dis. 167:21-29. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. H., and Y. C. Cheng. 1989. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2′,3′-dideoxycytidine. J. Biol. Chem. 264:11934-11937. [PubMed] [Google Scholar]
- 7.Chen, C. H., M. Vazquez-Padua, and Y. C. Cheng. 1991. Effect of anti-human immunodeficiency virus nucleoside analogs on mitochondrial DNA and its implication for delayed toxicity. Mol. Pharmacol. 39:625-628. [PubMed] [Google Scholar]
- 8.Cheng, Y. 1978. Thymidine kinase from blast cells of myelocytic leukemia. Methods Enzymol. 51:365-371. [DOI] [PubMed] [Google Scholar]
- 9.Chou, T. C. 1991. The median-effect principle and the combination index for quantitation of synergism and antagonism, p. 61-102. In T. C. Chou and D. C. Rideout (ed.), Synergism and antagonism in chemotherapy. Academic Press, Inc., San Diego, Calif.
- 10.Coates, J. A., N. Cammack, H. J. Jenkinson, A. J. Jowett, M. I. Jowett, B. A. Pearson, C. R. Penn, P. L. Rouse, K. C. Viner, and J. M. Cameron. 1992. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob. Agents Chemother. 36:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coates, J. A., N. Cammack, H. J. Jenkinson, I. M. Mutton, B. A. Pearson, R. Storer, J. M. Cameron, and C. R. Penn. 1992. The separated enantiomers of 2′-deoxy-3′-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob. Agents Chemother. 36:202-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Clercq, E. 1994. HIV resistance to reverse transcriptase inhibitors. Biochem. Pharmacol. 47:155-169. [DOI] [PubMed] [Google Scholar]
- 13.Doong, S. L., C. H. Tsai, R. F. Schinazi, D. C. Liotta, and Y. C. Cheng. 1991. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc. Natl. Acad. Sci. USA 88:8495-8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutschman, G. E., E. G. Bridges, S.-H. Liu, E. Gullen, X. Guo, M. Kukhanova, and Y.-C. Cheng. 1998. Metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine and its activity in combination with clinically approved anti-human immunodeficiency virus β-d(+) nucleoside analogs in vitro. Antimicrob. Agents Chemother. 42:1799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, J. Y., A. A. Johnson, K. A. Johnson, and K. S. Anderson. 2001. Insights into the molecular mechanism of mitochondrial toxicity by AIDS drugs. J. Biol. Chem. 276:23832-23837. [DOI] [PubMed] [Google Scholar]
- 16.Gelmon, K., J. S. Montaner, M. Fanning, J. R. Smith, J. Falutz, C. Tsoukas, J. Gill, G. Wells, M. O'Shaughnessy, M. Wainberg, and J. Ruedy. 1989. Nature, time course and dose dependence of zidovudine-related side effects: results from the Multicenter Canadian Azidothymidine Trial. AIDS 3:555-561. [DOI] [PubMed] [Google Scholar]
- 17.Gosselin, G., R. F. Schinazi, J.-P. Sommadossi, C. Mathé, M.-C. Bergogne, A.-M. Aubertin, A. Kirn, and J.-L. Imbach. 1994. Anti-human immunodeficiency virus activities of the β-l enantiomer of 2′,3′-dideoxycytidine and its 5-fluoro derivative in vitro. Antimicrob. Agents Chemother. 38:1292-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamamoto, Y., H. Nakashima, T. Matsui, A. Matsuda, T. Ueda, and N. Yamamoto. 1987. Inhibitory effect of 2′,3′-didehydro-2′,3′-dideoxynucleosides on infectivity, cytopathic effects, and replication of human immunodeficiency virus. Antimicrob. Agents Chemother. 31:907-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haraguchi, K., S. Takeda, H. Tanaka, T. Nitanda, M. Baba, G. E. Dutschman, and Y. C. Cheng. 2003. Synthesis of a highly active new anti-HIV agent 2′,3′-didehydro-3′deoxy-4′-ethynylthymidine. Bioorg. Med. Chem. Lett. 13:3775-3777. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, A. A., A. S. Ray, J. Hanes, Z. Suo, J. M. Colacino, K. S. Anderson, and K. A. Johnson. 2001. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J. Biol. Chem. 276:40847-40857. [DOI] [PubMed] [Google Scholar]
- 21.King, R. W., S. K. Ladner, T. J. Miller, K. Zaifert, R. B. Perni, S. C. Conway, and M. J. Otto. 1998. Inhibition of human hepatitis B virus replication by AT-61, a phenylpropenamide derivative, alone and in combination with (−)β-l-2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 42:3179-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larder, B. A. 1995. Viral resistance and the selection of antiretroviral combinations. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10(Suppl. 1):S28-S33. [PubMed] [Google Scholar]
- 23.Larder, B. A., B. Chesebro, and D. D. Richman. 1990. Susceptibilities of zidovudine-susceptible and -resistant human immunodeficiency virus isolates to antiviral agents determined by using a quantitative plaque reduction assay. Antimicrob. Agents Chemother. 34:436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, L. S., and Y. C. Cheng. 1976. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J. Biol. Chem. 251:2600-2604. [PubMed] [Google Scholar]
- 25.Lewis, W., and M. C. Dalakas. 1995. Mitochondrial toxicity of antiviral drugs. Nat. Med. 1:417-422. [DOI] [PubMed] [Google Scholar]
- 26.Lin, T. S., M. Z. Luo, M. C. Liu, S. B. Pai, G. E. Dutschman, and Y. C. Cheng. 1994. Antiviral activity of 2′,3′-dideoxy-beta-l-5-fluorocytidine (beta-l-FddC) and 2′,3′-dideoxy-beta-L-cytidine (beta-l-ddC) against hepatitis B virus and human immunodeficiency virus type 1 in vitro. Biochem. Pharmacol. 47:171-174. [DOI] [PubMed] [Google Scholar]
- 27.Lin, T. S., M. Z. Luo, M. C. Liu, S. B. Pai, G. E. Dutschman, and Y. C. Cheng. 1994. Synthesis and biological evaluation of 2′,3′-dideoxy-l-pyrimidine nucleosides as potential antiviral agents against human immunodeficiency virus (HIV) and hepatitis B virus (HBV). J. Med. Chem. 37:798-803. [DOI] [PubMed] [Google Scholar]
- 28.Lin, T. S., M. Z. Luo, M. C. Liu, Y. L. Zhu, E. Gullen, G. E. Dutschman, and Y. C. Cheng. 1996. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-beta-l-cytidine (beta-l-d4C) and 2′,3′-dideoxy 2′,3′-didehydro-beta-l-5-fluorocytidine (beta-l-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent inhibitors of human immunodeficiency virus (HIV) in vitro. J. Med. Chem. 39:1757-1759. [DOI] [PubMed] [Google Scholar]
- 29.Lin, T. S., R. F. Schinazi, M. S. Chen, E. Kinney-Thomas, and W. H. Prusoff. 1987. Antiviral activity of 2′,3′-dideoxycytidin-2′-ene (2′,3′-dideoxy-2′,3′-didehydrocytidine) against human immunodeficiency virus in vitro. Biochem. Pharmacol. 36:311-316. [DOI] [PubMed] [Google Scholar]
- 30.Lin, T. S., R. F. Schinazi, and W. H. Prusoff. 1987. Potent and selective in vitro activity of 3′-deoxythymidin-2′-ene (3′-deoxy-2′,3′-didehydrothymidine) against human immunodeficiency virus. Biochem. Pharmacol. 36:2713-2718. [DOI] [PubMed] [Google Scholar]
- 31.Lu, Z. H., R. Zhang, and R. B. Diasio. 1993. Comparison of dihydropyrimidine dehydrogenase from human, rat, pig and cow liver. Biochemical and immunological properties. Biochem. Pharmacol. 46:945-952. [DOI] [PubMed] [Google Scholar]
- 32.Maag, H., R. M. Rydzewski, M. J. McRoberts, D. Crawford-Ruth, J. P. Verheyden, and E. J. Prisbe. 1992. Synthesis and anti-HIV activity of 4′-azido- and 4′-methoxynucleosides. J. Med. Chem. 35:1440-1451. [DOI] [PubMed] [Google Scholar]
- 33.Medina, D. J., C.-H. Tsai, G. D. Hsiung, and Y.-C. Cheng. 1994. Comparison of mitochondrial morphology, mitochondrial DNA content, and cell viability in cultured cells treated with three anti-human immunodeficiency virus dideoxynucleosides. Antimicrob. Agents Chemother. 38:1824-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellors, J. W., G. E. Dutschman, G. J. Im, E. Tramontano, S. R. Winkler, and Y. C. Cheng. 1992. In vitro selection and molecular characterization of human immunodeficiency virus-1 resistant to non-nucleoside inhibitors of reverse transcriptase. Mol. Pharmacol. 41:446-451. [PubMed] [Google Scholar]
- 35.O-Yang, C., H. Y. Wu, B. Fraser-Smith, and K. A. M. Walker. 1992. Synthesis of 4′-cyanothymidine and analogs as potent inhibitors of HIV. Tetrahedron Lett. 33:37-40. [Google Scholar]
- 36.Parker, W. B., and Y. C. Cheng. 1995. Disruption of energy metabolism and mitochondrial function, p. 483-490. In L. W. Chang and W. Slikker, Jr. (ed.), Neurotoxicology: approaches and methods. Academic Press Inc., New York, N.Y.
- 37.Parker, W. B., and Y. C. Cheng. 1994. Mitochondrial toxicity of antiviral nucleoside analogs. J. NIH Res. 6:57-61. [Google Scholar]
- 38.Richman, D. D. 1993. Resistance of clinical isolates of human immunodeficiency virus to antiretroviral agents. Antimicrob. Agents Chemother. 37:1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richman, D. D., M. A. Fischl, M. H. Grieco, M. S. Gottlieb, P. A. Volberding, O. L. Laskin, J. M. Leedom, J. E. Groopman, D. Mildvan, M. S. Hirsch, G. G. Jackson, D. T. Durack, S. Nusinoff-Lerhman, and the AZT Collaborative Working Group. 1987. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N. Engl. J. Med. 317:192-197. [DOI] [PubMed] [Google Scholar]
- 40.Rideout, D. C. 1991. Synergism, antagonism, and potentiation in chemotherapy: an overview, p. 3-60. In T. C. Chou and D. C. Rideout (ed.), Synergism and antagonism in chemotherapy. Academic Press, Inc., San Diego, Calif.
- 41.Schinazi, R. F., C. K. Chu, A. Peck, A. McMillan, R. Mathis, D. Cannon, L. S. Jeong, J. W. Beach, W. B. Choi, S. Yeola, and D. C. Liotta. 1992. Activities of the four optical isomers of 2′,3′-dideoxy-3′-thiacytidine (BCH-189) against human immunodeficiency virus type 1 in human lymphocytes. Antimicrob. Agents Chemother. 36:672-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarchoan, R., J. M. Pluda, R. V. Thomas, H. Mitsuya, P. Brouwers, K. M. Wyvill, N. Hartman, D. G. Johns, and S. Broder. 1990. Long-term toxicity/activity profile of 2′,3′-dideoxyinosine in AIDS or AIDS-related complex. Lancet 336:526-529. [DOI] [PubMed] [Google Scholar]