Abstract
Telehomecare is considered one of the most successful applications of telehealth. However, despite increasing evidence of telehomecare benefits, the diffusion of these services is still limited. Decision-makers need strong evidence in order to expand the development of telehomecare to various populations, regions, and health conditions. The objective of this review is to provide a basis for decision-making by identifying common indicators from the literature on telehomecare. A comprehensive review of the literature on the cost-effectiveness of telehomecare was conducted in specialized bibliographic databases. A total of 23 studies met the inclusion criteria. First, selected studies were analyzed to identify and classify the indicators that better addressed the cost-effectiveness impacts of telehomecare projects. Then, a synthesis of the evidence was done by exploring the relative cost-effectiveness of telehomecare applications. The analyses show that there is fair evidence of cost-effectiveness for many telehomecare applications. However, the heterogeneity among cost-effectiveness indicators in the applications reviewed and the methodological limitations of the studies impede the possibility of generalizing the findings. This suggests the need for a set of common indicators that could be applied for assessing the cost-effectiveness of telehomecare projects. This review provides knowledge on the indicators available for assessing cost-effectiveness in telehomecare projects. It appears that the specific context in which the projects take place, meaning different patients, environments, technologies, and healthcare systems, should be taken into account when selecting indicators for assessing telehomecare cost-effectiveness.
Keywords: telehomecare, cost-effectiveness, literature review
Introduction
Telehomecare (THC), the application of telemedicine technologies to the home environment,1 is one of its most successful applications. However, despite increasing evidence of THC benefits, its diffusion remains limited in part due to a lack of evidence about the most relevant tools for its assessment.2
In that sense, economic analysis has been included in health evaluation with the aim of supporting healthcare policy and organizational decision-making.3 As a consequence, cost-effectiveness analyses have gained an important role in the assessment of health interventions and THC projects are no exception to this tendency. Nevertheless, there is scant evidence found in the literature about the main indicators used in THC projects evaluation.
The objective of this paper is to systematically review the cost-effectiveness indicators that have been most commonly used to measure the impact of THC in order to identify and provide reliable indicators that could guide further projects assessment.
Economic Tools to Assess Health Interventions
Different approaches have been used for assessing the economic impact of telehealth projects with diverse levels of acceptability. Jester and Hicks4,5 and Dixon6 identify three methods to calculate the cost-effectiveness between two health interventions: cost-minimization analysis (CMA), cost–benefit analysis (CBA), and cost-effectiveness analysis (CEA). Reardon3 also identifies the return of investment, and the functional economic analysis to be used in telemedicine projects.
CMA assumes that both alternatives (THC and standard care) have the same effectiveness in terms of health outcomes, but that they are different in terms of costs. In CMA, changes in costs are considered but health outcomes are kept unchanged. This assumes that, for instance, THC and standard care have the same reduction in hospitalization days, but that their cost differs, so decision-makers have to consider only the differences in costs to decide which alternative is less expensive.
CBA acknowledges that very few projects are equally effective but different in costs. Thus, changes in costs and health outcomes are considered simultaneously by giving a monetary or numeric value to the changes in health outcomes. Although this method takes into account changes in both variables, the idea of giving numeric values to outcomes such as patient satisfaction or quality of life is not always acceptable for decision-makers in the healthcare field.
CEA appears as a solution to this issue since it measures the benefits in nonmonetary terms. In CEA, each outcome is defined according to its specific unit so the final ratios will show a relation between economic and health outcomes. Therefore, the final decision will depend on the ratio that the decision-maker considers the best.3,6 CEA compares the cost and health outcomes of an intervention to assess whether it is worth doing from the economic perspective.7 This arises from the concept of opportunity cost, that is, the value of resources given up in a decision.3,7 This definition accepts the fact that there is always a trade-off in a decision between two alternatives. Hence, whatever may be the final choice would always represent a “sacrifice.” In economic terms, a sacrifice is understood as something valued that is given up.3 In THC projects, given resources are usually monetary and received benefits are in terms of health outcomes. As such, the idea is to select the option that represents the smallest sacrifice.
CEA implies the selection of a unique outcome to be measured in terms of benefits and costs.3,6 Represented as a ratio—the incremental cost-effectiveness ratio (ICER)—this corresponds to the sum of all benefits in medical terms as the denominator, and the costs incurred to obtain those benefits as the numerator. The United States Public Health Services Consensus Panel8 provides a theoretically consistent cost-assessment framework for CEA, which implies:
Defining a cost or resource allocation decision, including decision-making objectives, and defining the program or intervention;
Determining the decision model of costs and benefits;
Specifying data and measures;
Performing appropriate statistical, modeling, and sensitivity analyses;
Reporting results understandable by decision-makers and researchers.
This methodology highlights the sensitivity of the CEA to the specific context.7 As an example, a special type of CEA, the cost–utility analysis, has used the quality-adjusted life years as an outcome.9–11 Suppose a CEA with an ICER of $10,000/life year gained and one of $25,000/life year gained for the standard care and THC project, respectively. Even though the first impulse is to select the first option (the cheapest one), there are other restrictions (such as shortage of beds or professionals in the hospital) that could shift the decision-maker in the other direction.
Measuring Cost-Effectiveness in THC Projects
Before reviewing cost-effectiveness indicators of THC projects, some aspects have to be considered. Thus, we provide a framework to synthesize THC cost-effectiveness indicators based on the following questions.
-
Is this really a THC project?
System design. Is the system designed to let the patient stay at home? If the intervention includes any movement from the patient’s home, it is not considered THC. We also considered nursing homes as “patient’s home” in THC projects.
Technology. In some studies, telehomecare included telephone monitoring or triage systems.12–14 According to Britton et al.,1 telephones, arrhythmia monitors, and pacemaker monitoring devices are not currently considered as telehomecare systems.
-
How should the indicators be selected?
The indicators should be selected and interpreted according to the following:
Decision-makers. Who is going to use these indicators to make a decision? The type of decision-maker (government, hospitals, home care agency, researcher, etc.) determines the variables involved and the values assigned to the costs and benefits. This is why cost-effectiveness indicators in THC are hard to generalize. Decision-makers will also determine the levels at which cost-effectiveness will be measured: Would it be an organizational or a societal perspective?6
Objectives. The indicators must be considered according to the context of the decision, which implies that each indicator must align with the project’s objectives.3
System design. What are the technology and the transmission media used? If the program implies the use of phone lines, indicators should be interpreted differently than for a video-based system. For instance, if two projects measure visit time for the professionals, it could be expected that more time will be spent with a phone-based system if the patient has to describe a wound. In this case, it would be inappropriate to compare a phone-based alternative and a video-based alternative.
Methods
A systematic literature search was conducted using the following electronic databases: MEDLINE, PUBMED, EMBASE, COCHRANE, CINAHL, PROQUEST; and two databases for economic papers: RePEC, and EconLit. The inclusion criteria were: Period from January 1997 to October 2007; Language: English; Population: all patients and professionals. Only documents for which full text could be obtained were reviewed.
Key words included telemedicine and telehomecare terms in the first set, and economic terms in the second set. The search strategy was very sensitive, including roots and variations of these words. Some of the abstracts and full-text documents were searched by traditional Web searchers such as Google.com, and documents that seemed to be relevant but did not have a digital version were hand searched.
Results
DESCRIPTION OF TELEHOMECARE STUDIES
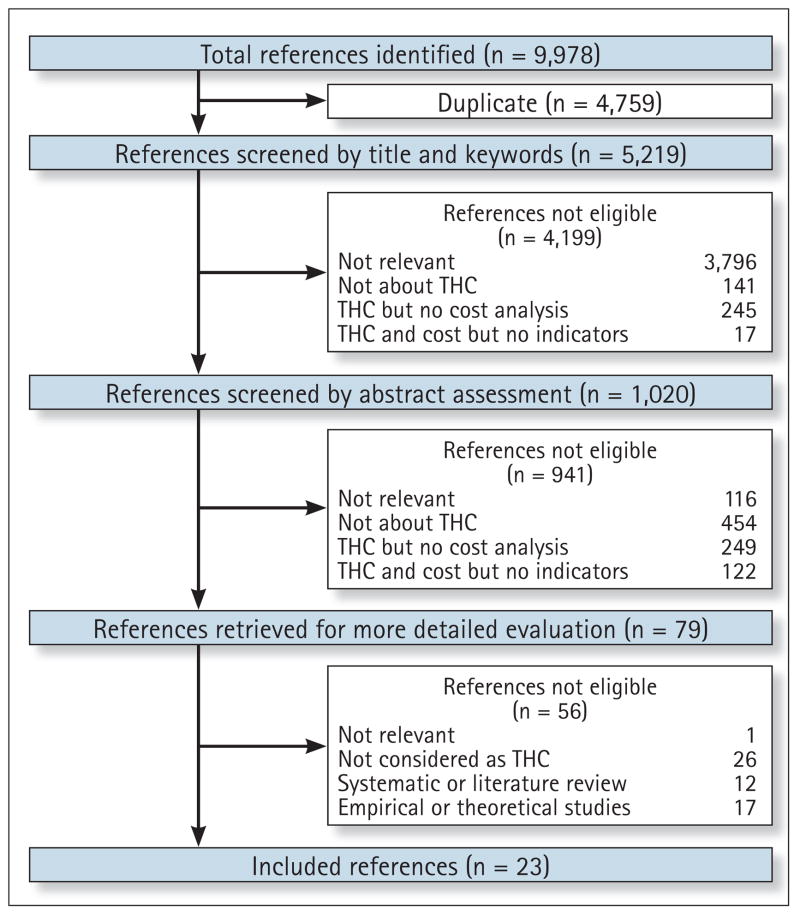
The search strategy yielded 9,978 papers. After eliminating duplicates, the final database contained a total of 5,219 papers. These papers were reviewed by title and categorized in the following categories: not relevant at all; not about THC; THC but no cost analysis included; THC and costs included but no indicators; and potentially relevant, with a total of 1,020 potentially relevant papers. The abstracts of these papers were read carefully and categorized again according to criteria described above, yielding 79 potentially relevant papers for which full-text documents were obtained. As presented in Figure 1, we applied specific exclusion criteria and papers reporting theoretical studies, systematic or literature reviews, studies based on simulation data only, and projects in which technology design did not fit the THC definition (for instance, projects that used only a telephone call as the monitoring system) were rejected. Finally, 23 papers15–37 met the inclusion criteria and were included in the review (Fig. 1).
Fig. 1.
Search strategy. THC, telehomecare.
Sixteen studies (70%) were from the United States. Other studies were from Canada (2), China (2), Germany (1), Italy (1), and UK (1). Only one paper (4%) was published before 2000; 11 (48%) between 2000 and 2002; and 11 (48%) between 2003 and 2007.
Fifteen studies (65%) were randomized controlled trials, three (13%) were longitudinal observational studies, two (9%) were case controlled trials, two (9%) were retrospective studies, and one (4%) used cost comparison from databases. Thirteen studies (57%) adopted a quantitative approach while the rest (43%) used a mix of quantitative and qualitative approaches. Twelve studies (52%) made a comparison between THC and traditional homecare, while remaining studies compared THC and other types of care, such as outpatient or inpatient care that does not imply a service in the patient’s home.
Health services were most frequently dedicated to elderly (48%) or adult patients (35%). The diseases that were targeted by THC are shown in Table 1. Congestive health failure and diabetes were the most targeted diseases. Fourteen (61%) studies covered only one disease while six (26%) covered more than three diseases. From the studies reviewed, it seems that THC has been mostly (83%) used as a complement or replacement for chronic healthcare.
Table 1.
Diseases Targeted by Telehomecare
| TYPES OF DISEASE | NO. | % |
|---|---|---|
| CHF, COPD, CWC, diabetes, cancer or/and others | 6 | 26.1% |
| CHF | 4 | 17.4% |
| Diabetes | 4 | 17.1% |
| Acute infections | 1 | 4.3% |
| COPD | 1 | 4.3% |
| CWC | 1 | 4.3% |
| Dermatology | 1 | 4.3% |
| Spinal cord injuries | 1 | 4.3% |
| Women with high-risk pregnancy | 1 | 4.3% |
| Other, NS | 3 | 13.0% |
| Total | 23 | 100% |
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CWC, chronic wound care; NS, not specified.
Teleconsulting was the most common type of THC service used (39%), followed by telemonitoring (35%). One study (4%) focused on telerehabilitation while the others included a combination of telemonitoring and teleconsulting. The most common providers of the THC service were nurses (Table 2). Eleven studies (48%) involved two caregivers in the THC project, usually a nurse and a physician, and five studies (22%) included more than three care-givers. This was the case when multiple specialists were involved due to the type or number of diseases covered by the project. At the technological level, the design of the THC projects included a combination of multiple devices (stethoscopes, blood pressure and glucose meters, etc.) with direct data transmission through telephone (52%) or videophones (48%). The most common transmission media were telephone lines (73%) and Internet (32%).
Table 2.
Caregivers Involved in Telehomecare
| CAREGIVERS | NO. | % |
|---|---|---|
| Nurse | 19 | 83% |
| General physician | 13 | 57% |
| Specialist physician | 11 | 48% |
| Other, not specified | 15 | 65% |
Table 3 shows the level at which cost-effectiveness was measured. The indicators related to patients and organizations were the most frequently reported in THC projects.
Table 3.
Levels at Which Cost-Effectiveness Is Assessed
| LEVELS | NO. | % |
|---|---|---|
| Organization | 3 | 13% |
| Patient and organization | 12 | 52% |
| Patient and professionals | 1 | 4% |
| Patient, organization and community | 1 | 4% |
| Patient, organization and professionals | 6 | 26% |
PATIENT SATISFACTION
Fourteen (61%) studies measured patient satisfaction. Among those, eight (57%) found that THC was preferred to other alternatives by patients; five (36%) studies showed that patients were indifferent between both alternatives, and one study (7%) did not provide any results.
COST-EFFECTIVENESS INDICATORS
Tables 4 and 5 report the effectiveness and cost indicators used for assessing THC projects. The expected impact of THC on each indicator is reported in the second column. References to studies in which the expected impact was found (Yes), not found (No) and those for which no measure of impact was provided or that showed inconclusive results (NS) are presented in the third, fourth, and fifth columns, respectively.
Table 4.
Effectiveness Indicators
| EFFECTIVENESS INDICATORS | EXPECTED IMPACT WITH THE THC PROJECT | IMPACT IN THE EXPECTED DIRECTION (REFERENCES) | TOTAL NO. OF STUDIES | ||
|---|---|---|---|---|---|
| YES | NO | NS | |||
| No. of THC video visits | Increase | 18, 19, 21, 23–25, 27–30, 37 | 32 | 36 | 13 |
| No. of outpatient visits | Reduction | 15, 19, 20, 22, 27, 33, 37 | 18, 29 | 9 | |
| No. of home visits | Reduction | 26, 28, 29, 31, 34, 37 | 22, 32, 33 | 21, 24, 36 | 12 |
| No. of telephone interventions | No consensus about impact | 18, 28, 29, 31, 34 | 5 | ||
| No. of ER visits | Reduction | 15, 20, 27–29, 32, 33, 37 | 31 | 9 | |
| No. of hospitalizations | Reduction | 15, 18, 20, 22, 32–34, 37 | 31 | 9 | |
| No. of readmissions | Reduction | 17, 28, 35 | 3 | ||
| Average length of all visits (minutes) | No consensus about impact | 19, 21–24, 26, 29, 32, 34, 36 | 10 | ||
| Average travel time for professionals (minutes) | Reduction | 19, 23, 26, 30, 34, 36 | 18, 22 | 24 | 9 |
| Average travel time for patients (minutes) | Reduction | 18 | 1 | ||
| Average distance covered by the professionals (km) | Reduction | 23, 26, 30, 34, 36 | 5 | ||
| Average length of call intervention (minutes) | No consensus about impact | 22, 28, 34 | 3 | ||
| Average length of hospitalization (days) | Reduction | 15, 17, 25, 27, 29, 32, 33 | 34 | 8 | |
| Quality of life | Increase or no change | 16, 17, 20, 31–33, 35, 37 | 8 | ||
THC, telehomecare; ER, emergency room; NS, not specified.
Table 5.
Cost Indicators
| EFFECTIVENESS INDICATORS | EXPECTED IMPACT WITH THE THC PROJECT | IMPACT IN THE EXPECTED DIRECTION | TOTAL NO. OF STUDIES | ||
|---|---|---|---|---|---|
| YES | NO | NS | |||
| DIRECT COSTS | |||||
| Remuneration of professionals: visit time | Reduction | 15, 18, 21, 26, 30–35, 37 | 19, 22, 28, 29 | 17, 24, 25, 36 | 19 |
| Remuneration of professionals: travel/driving time | Reduction | 21, 23, 30, 34 | 26 | 5 | |
| Remuneration of professionals: telephone intervention | Increase | 18, 28, 31, 34 | 21 | 5 | |
| Remuneration and cost of technical support | Increase | 25, 26 | 2 | ||
| Traveling expenses for the professionals | Reduction | 15, 18, 19, 21, 23, 30, 32–34, 36, 37 | 22 | 12 | |
| ER cost | Reduction | 15, 27–29, 32, 33, 37 | 7 | ||
| Hospitalization costs | Reduction | 15, 21, 22, 25, 27, 29, 32–34 | 9 | ||
| Readmission costs | Reduction | 17, 28, 35 | 3 | ||
| Software licenses | Increase | 34, 35 | 2 | ||
| Purchase of equipment | Increase | 15, 18, 19, 22–26, 28–31, 33–35, 37 | 17 | 17 | |
| Installation, support and maintenance of equipment | Increase | 18, 19, 22, 26, 28, 29, 32, 34–37 | 27 | 17, 24 | 14 |
| INDIRECT COSTS | |||||
| Lost productivity for patient and relatives | Reduction | 18, 22 | 2 | ||
| Traveling expenses for the patient | Reduction | 33 | 1 | ||
| Insurance, supplies, administrative costs | No impact expected | 21, 24, 26, 30–32, 37 | 7 | ||
| Miscellaneous costs | No impact expected | 15, 24, 35 | 3 | ||
| Laboratory tests, pharmacy | No impact expected | 24, 29, 37 | 3 | ||
| OTHER DIRECT COSTS | |||||
| Specific to a specialty | No consensus about impact | 19, 22 | 2 | ||
| Remuneration of project coordinator | No impact expected | 25 | 1 | ||
| TOTAL COSTS | Reduction | 15–18, 21, 23, 25, 27, 28, 30, 34–37 | 14 | ||
| COST PER PATIENT | Reduction | 15, 19–22, 29, 31, 33, 34, 37 | 17 | 11 | |
| COST PER VISIT | Reduction | 23, 24, 26, 32 | 4 | ||
ER, emergency room; NS, not specified.
We found that some studies expected a different impact for the same indicator. For instance, Pare et al.,34 Biermann et al.,18 and Johnston et al.29 expected a higher number of telephone interventions because of the system design and patient’s anxiety, whereas Jerant et al.28 expected a reduction in this indicator due to a higher level of care that made telephone interventions unnecessary. Furthermore, some indicators (for example, miscellaneous costs) are usually shared for both alternatives; thus, no specific impact was expected. Finally, quality of life was found in only eight studies as an effectiveness indicator. As shown in Table 4, THC increased or did not impact the quality of life of the patients in all those studies.
To provide an overview of THC cost-effectiveness, we considered the average change in cost-effectiveness indicators for each study. To do so, a global change score was calculated from the information provided in the paper. This score represents the average change found across all effectiveness indicators used in each study. Table 6 provides an example of the calculation of the global change score. This example compares two effectiveness indicators (number of outpatient visits and number of home visits made by healthcare professionals) between THC and standard care (SC). The percentage of change for each indicator is calculated by this formula:
Table 6.
Example of the Calculation of the Global Change Score
| INDICATORS | THC | SC | % CHANGE |
|---|---|---|---|
| No. of outpatient visits | 10 | 15 | −33% |
| No. of home visits | 5 | 25 | −80% |
| Average change | −57% | ||
| Global change score | 57% |
THC, telehomecare; SC, standard care.
The global change score represents the mean change for these two indicators.
It is important to consider that a negative change represents a benefit in that case, since a diminution in the number of visits favors THC over SC. So, the global change score is reported in absolute value.
We decided to apply this methodology since each indicator used different measures (km, days, minutes, etc.), which made impossible to add them up, as suggested in the literature.3,6,7 This led us to take into account the number and sign (±) of the variables for each study. For the cost indicators, we used only the change in the total costs for each alternative since the individual change in each cost indicator is not as relevant as the change in the total costs. Given that some studies did not find statistically significant differences between the alternatives, two tendency lines were drawn.
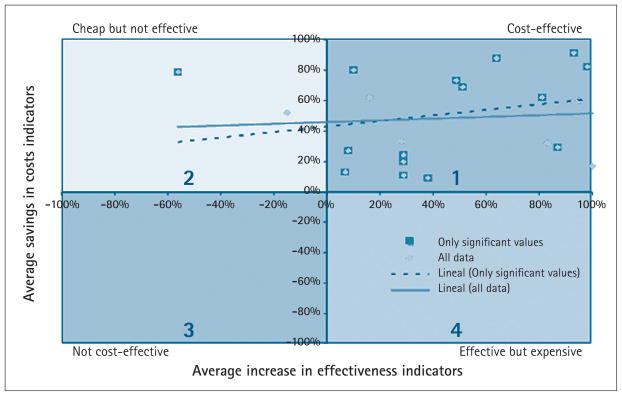
Barnett et al.16 was the only study that calculated an ICER, using the QALYs as the only effectiveness indicator. As this study only provided the ICER, it did not show effectiveness or cost changes; thus, it was excluded from the synthesis presented in Figure 2. Noel and Vogel32 made a cost-minimization analysis, so the changes in effectiveness indicators were mentioned but not as a number. Thus, it was not possible to compute a percentage of change, and this study was also excluded from Figure 2.
Fig. 2.
Cost-effectiveness in telehomecare.
Figure 2 represents the four possible scenarios for a THC project. In the first quadrant (upper right), the best scenario, there are substantial savings in costs and an increase in effectiveness indicators. The opposite of this scenario is the third quadrant, which corresponds to an expensive and not effective alternative. The second quadrant represents alternatives that are cheap but not effective, which is not desirable for health interventions. Finally, in the fourth quadrant, the effective but expensive alternative could be chosen if the decision-maker is willing to pay a higher cost, perhaps because of other potential benefits of the project or because external or intangible variables are taken into account. Seventeen (91%) studies belong to the first quadrant, and only two (9%) are found in the second quadrant. Tendency lines show a direct relation between higher benefits and higher costs savings, which are more pronounced when excluding results that are not significant.
Two studies concluded that THC reduces costs but is not effective.22,28 In Jerant et al.,28 a comparison was made between an intervention group with THC, one with telephone calls, and one with standard care. As a result, THC was more effective than standard care but telephone care represented a higher increase in effectiveness, so the investments were recovered in a shorter time. In Dawson et al.,22 the system was designed to include home visits and video visits. At the end of the project, the number of home visits was higher than before the THC project. Even though this was a benefit for the patients, it was not in the direction expected by the researchers.
COST-EFFECTIVENESS INDICATORS OF THC
A general conclusion from the results above is the lack of a set of common indicators that have been applied in all THC studies. However, some effectiveness and cost indicators are found present in several studies. For these indicators, most of the studies found an impact in the expected direction. This could be interpreted as the reliability of the indicator since it shows a tendency in the indicator’s behavior even though it is applied to different projects. The cost and effectiveness indicators most frequently used are shown in Table 7.
Table 7.
Common Cost and Effectiveness Indicators in THC Projects
| COST INDICATORS | NUMBER OF STUDIES |
|---|---|
| Purchase of equipment | 18 |
| Remuneration of professionals: visit time | 17 |
| Installation, support and maintenance of equipment | 14 |
| Total costs | 14 |
| Traveling expenses for professionals | 12 |
| Total cost per patient | 11 |
| Hospitalization costs | 10 |
| EFFECTIVENESS INDICATORS | |
| No. of THC video visits | 23 |
| No. of home visits | 12 |
| Average length of all visits (minutes) | 10 |
| No. of outpatient visits | 9 |
| No. of ER visits | 9 |
| No. of hospitalizations | 9 |
| Average travel time for professionals (minutes) | 9 |
| Average length of hospitalization (days) | 9 |
THC, telehomecare; ER, emergency room.
Discussion
Our results show that in recent years, economic analysis has risen in the field of THC since all papers but one were published after 2000. It is also worth noting that the majority of these studies are from the United States. This is important since most studies took place in home care agencies, which are usually government supported. In that sense, the conclusions of these studies have an impact not only for healthcare organizations, but also for the public healthcare system as a whole.
THC resulted to be a cost-effective alternative in 91% of the studies. Main THC benefits included: decreased hospital utilization; improved patient compliance with treatment plans; improved patient satisfaction with health services; and improved quality of life. THC also improved cognitive status, cognitive level, and self-rated health status. The studies also found disadvantages related to THC, such as technical problems and reluctance from patients, caregivers, nurses, and physicians.25
However, we found that although the papers promoted benefits of CEA, the number of variables studied, the methodologies followed, and the interpretation of the results are far from providing the basis to make a good decision. Indeed, results show a tendency for THC cost-effectiveness, but they certainly do not allow for a generalization of the conclusions. CEA implies the selection of a unique effectiveness indicator to then measure the cost of such benefit to calculate a ratio (ICER). We found only one study that calculated the ICER.16 CEA methodology also suggests amortizing equipment costs and conducting a sensitivity analysis; both aspects were barely covered in the studies.
Moreover, it would be desirable to have a set of indicators common to all THC studies in order to make easier comparisons between projects. To get a general picture of cost-effectiveness across the studies, we have considered the overall effectiveness of the THC project by calculating a global change score. To do so, a simple methodology was developed in order to compare the cost-effectiveness across different studies. However, due to the great heterogeneity between THC applications tested and cost-effectiveness indicators, it was not possible to calculate any effect size, as required for a meta-analysis. The fact that the studies included in this review were not appraised for their quality constitutes another limitation to this synthesis. As THC evaluations become increasingly available, it would be important to identify good-quality studies that could serve as models for assessing cost-effectiveness.
Another important issue to consider is how to decide which indicators should be used to assess THC and how to interpret their results. Accordingly, we admit that it is difficult to determine which indicators could be generalized, since each one must be interpreted within its unique context. This includes the type of decision-maker, the project’s objectives, the THC system design, the healthcare system, and others.
This review has highlighted the lack of uniformity in studies that have assessed THC cost-effectiveness. The main contribution of this systematic review is to present the indicators that are mostly used to assess THC projects, their expected impact, and some evidence regarding the cost-effectiveness of THC. As a result, this study provides a starting point for decision-makers interested in THC projects.
It is also important to consider the limitations of CEA. As mentioned previously, cost-effectiveness indicators are highly influenced by the type of decision-maker, and as a consequence, by the objectives, context, and study design. This certainly represents an advantage since it provides contextualized evaluation,38 but also a limit since it is difficult to synthesize and interpret these results to provide a generalized basis for decision-making.3 Finally, the synthesis presented here would be mostly helpful as guideline.
Footnotes
Disclosure Statement
No competing financial interests exist.
References
- 1.Britton BP, Martha Keehner E, Dan BR, Kahlid M. Measuring costs and quality of TeleHomecare. Home Health Care Manage Pract. 2000;12:27. [Google Scholar]
- 2.Hebert MA, Korabek B, Scott RE. Moving research into practice: A decision framework for integrating home telehealth into chronic illness care. Int J Med Informatics. 2006;75:786–794. doi: 10.1016/j.ijmedinf.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Reardon T. Research findings and strategies for assessing telemedicine costs. Telemed e-Health. 2005;11:348–369. doi: 10.1089/tmj.2005.11.348. [DOI] [PubMed] [Google Scholar]
- 4.Jester R, Hicks C. Using cost-effectiveness analysis to compare Hospital at Home and in-patient interventions. Part 1. J Clin Nurs. 2003;12:13–19. doi: 10.1046/j.1365-2702.2003.00685.x. [DOI] [PubMed] [Google Scholar]
- 5.Jester R, Hicks C. Using cost-effectiveness analysis to compare Hospital at Home and in-patient interventions. Part 2. J Clin Nurs. 2003;12:20–27. doi: 10.1046/j.1365-2702.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- 6.Dixon I, Lundeen A. Cost-effectiveness analysis: An employer decision support tool. Center for Prevention and Health Services: Issue Brief. 2004 Aug;:1–20. [Google Scholar]
- 7.Phillips C, Thompson G. What is cost-effectiveness? Hayward Medical Communications. 2001;1:1–6. [Google Scholar]
- 8.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. p. 446. [Google Scholar]
- 9.Chapman RH, Berger M, Weinstein MC, Weeks JC, Goldie S, Neumann PJ. When does quality-adjusting life-years matter in cost-effectiveness analysis? Health Economics. 2004;13:429–436. doi: 10.1002/hec.853. [DOI] [PubMed] [Google Scholar]
- 10.McGregor M. Cost-utility analysis: Use QALYs only with great caution. Can Med Assoc J. 2003;168:433–434. [PMC free article] [PubMed] [Google Scholar]
- 11.Tengs TO. Cost-effectiveness versus cost-utility analysis of interventions for cancer: Does adjusting for health-related quality of life really matter? Value Health. 2004;7:70–78. doi: 10.1111/j.1524-4733.2004.71246.x. [DOI] [PubMed] [Google Scholar]
- 12.Adkins JW, Storch EA, Lewin AB, Williams L, Silverstein JH, Malasanos T, Geffken GR. Home-based behavioral health intervention: Use of a telehealth model to address poor adherence to type-1 diabetes medical regimens. Telemed J e-Health. 2006;12:370–372. doi: 10.1089/tmj.2006.12.370. [DOI] [PubMed] [Google Scholar]
- 13.Maiolo C, Mohamed EI, Fiorani CM, De Lorenzo A. Home telemonitoring for patients with severe respiratory illness: The Italian experience. J Telemed Telecare. 2003;9:67–71. doi: 10.1258/135763303321327902. [DOI] [PubMed] [Google Scholar]
- 14.Subirana Serrate R, Ferrer-Roca O, Gonzalez-Davila E. A cost-minimization analysis of oncology home care versus hospital care. J Telemed Telecare. 2001;7:226–232. doi: 10.1258/1357633011936444. [DOI] [PubMed] [Google Scholar]
- 15.Alwan M, Sifferlin EB, Turner B, Kell S, Brower P, Mack DC, Dalal S, Felder RA. Impact of passive health status monitoring to care providers and payers in assisted living. Telemed e-Health. 2007;13:279–286. doi: 10.1089/tmj.2006.0056. [DOI] [PubMed] [Google Scholar]
- 16.Barnett TE, Chumbler NR, Vogel WB, Beyth RJ, Ryan P, Figueroa S. The cost-utility of a care coordination/home telehealth programme for veterans with diabetes. J Telemed Telecare. 2007;13:318–321. doi: 10.1258/135763307781644843. [DOI] [PubMed] [Google Scholar]
- 17.Benatar D, Bondmass M, Ghitelman J, Avitall B. Outcomes of chronic heart failure. Arch Intern Med. 2003;163:347–352. doi: 10.1001/archinte.163.3.347. [DOI] [PubMed] [Google Scholar]
- 18.Biermann E, Dietrich W, Rihl J, Standl E. Are there time and cost savings by using telemanagement for patients on intensified insulin therapy? A randomised, controlled trial. Computer Methods Programs Biomed. 2002;69:137–146. doi: 10.1016/s0169-2607(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 19.Chan HH, Woo J, Chan WM, Hjelm M. Teledermatology in Hong Kong: A cost-effective method to provide service to the elderly patients living in institutions. Int J Dermatol. 2000;39:774–748. doi: 10.1046/j.1365-4362.2000.00034.x. [DOI] [PubMed] [Google Scholar]
- 20.Cherry JC, Moffatt TP, Rodriguez C, Dryden K. Diabetes disease management program for an indigent population empowered by telemedicine technology. Diabetes Technol Therapeut. 2002;4:783–791. doi: 10.1089/152091502321118801. [DOI] [PubMed] [Google Scholar]
- 21.Dansky KH, Palmer L, Shea D, Bowles KH. Cost analysis of telehomecare. Telemed e-Health. 2001;7:225–232. doi: 10.1089/153056201316970920. [DOI] [PubMed] [Google Scholar]
- 22.Dawson A, Cohen D, Candelier C, Jones G, Sanders J, Thompson A, Arnall C, Coles E. Domiciliary midwifery support in high-risk pregnancy incorporating telephonic fetal heart rate monitoring: A health technology randomized assessment. J Telemed Telecare. 1999;5:220–230. doi: 10.1258/1357633991933756. [DOI] [PubMed] [Google Scholar]
- 23.Dimmick SL, Mustaleski C, Burgiss SG, Welsh T. A case study of benefits & potential savings in rural home telemedicine. Home Healthcare Nurse. 2000;18:124–135. doi: 10.1097/00004045-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Doolittle GC. A cost measurement study for a home-based telehospice service. J Telemed Telecare. 2000;6(suppl 1):S193–S195. doi: 10.1258/1357633001934645. [DOI] [PubMed] [Google Scholar]
- 25.Eron L, Marineau M. Telemedicine: Treating infections in the home. Infect Med. 2006;23:517–524. [Google Scholar]
- 26.Finkelstein SM, Speedie SM, Potthoff S. Home telehealth improves clinical outcomes at lower cost for home healthcare. Telemed e-Health. 2006;12:128–136. doi: 10.1089/tmj.2006.12.128. [DOI] [PubMed] [Google Scholar]
- 27.Hui E, Woo J. Telehealth for older patients: The Hong Kong experience. J Telemed Telecare. 2002;8(suppl 3):39–41. [PubMed] [Google Scholar]
- 28.Jerant AF, Azari R, Nesbitt TS. Reducing the cost of frequent hospital admissions for congestive heart failure: A randomized trial of a home telecare intervention. Med Care. 2001;39:1234–1245. doi: 10.1097/00005650-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Johnston B, Wheeler L, Deuser J, Sousa KH. Outcomes of the Kaiser Permanente tele-home health research project. Arch Fam Med. 2000;9:40–45. doi: 10.1001/archfami.9.1.40. [DOI] [PubMed] [Google Scholar]
- 30.Litzinger G, Rossman T, Demuth B, Roberts J. In-home wound care management utilizing information technology. Home Healthcare Nurse. 2007;25:119–130. doi: 10.1097/00004045-200702000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Myers S, Grant RW, Lugn NE, Holbert B, Kvedar JC. Impact of home-based monitoring on the care of patients with congestive heart failure. Home Health Care Manage Pract. 2006;18:444–451. [Google Scholar]
- 32.Noel HC, Vogel DC. Resource costs and quality of life outcomes for homebound elderly using telemedicine integrated with nurse case management. Care Manage. 2000;6:22. [Google Scholar]
- 33.Noel HC, Vogel DC, Erdos JJ, Cornwall D, Levin F. Home telehealth reduces healthcare costs. Telemed e-Health. 2004;10:170–183. doi: 10.1089/tmj.2004.10.170. [DOI] [PubMed] [Google Scholar]
- 34.Pare G, Sicotte C, St-Jules D, Gauthier R. Cost-minimization analysis of a telehomecare program for patients with chronic obstructive pulmonary disease. Telemed e-Health. 2006;12:114–121. doi: 10.1089/tmj.2006.12.114. [DOI] [PubMed] [Google Scholar]
- 35.Scalvini S, Capomolla S, Zanelli E, Benigno M, Domenighini D, Paletta L, Glisenti F, Giordano A. Effect of home-based telecardiology on chronic heart failure: Costs and outcomes. J Telemed Telecare. 2005;11(suppl 1):16–18. doi: 10.1258/1357633054461688. [DOI] [PubMed] [Google Scholar]
- 36.Tousignant M, Boissy P, Corriveau H, Moffet H. In home telerehabilitation for older adults after discharge from an acute hospital or rehabilitation unit: A proof-of-concept study and costs estimation. Disabil Rehabil Assist Technol. 2006;1:209–216. doi: 10.1080/17483100600776965. [DOI] [PubMed] [Google Scholar]
- 37.Townsend WA. The impact of implementing telemedicine (telehomecare) technology in a spinal cord injury (SCI) home environment. Tampa, FL: University of South Florida; 2002. [Google Scholar]
- 38.May C, Mort M, Williams T, Mair F, Gask L. Health technology assessment in its local contexts: Studies of telehealthcare. Soc Sci Med. 2003;57:697–710. doi: 10.1016/s0277-9536(02)00419-7. [DOI] [PubMed] [Google Scholar]