Abstract
We investigated the therapeutic efficacy of a replication-competent oncolytic vaccinia virus, GLV-1h153, carrying human sodium iodide symporter (hNIS), in combination with radioiodine in an orthotopic triple-negative breast cancer (TNBC) murine model. In vitro viral infection was confirmed by immunoblotting and radioiodine uptake assays. Orthotopic xenografts (MDA-MB-231 cells) received intratumoral injection of GLV-1h153 or PBS. One week after viral injection, xenografts were randomized into 4 treatment groups: GLV-1h153 alone, GLV-1h153 and 131I (∼5 mCi), 131I alone, or PBS, and followed for tumor growth. Kruskal-Wallis and Wilcoxon tests were performed for statistical analysis. Radiouptake assay showed a 178-fold increase of radioiodine uptake in hNIS-expressing infected cells compared with PBS control. Systemic 131I-iodide in combination with GLV-1h153 resulted in a 6-fold increase in tumor regression (24 compared to 146 mm3 for the virus-only treatment group; P<0.05; d 40). We demonstrated that a novel vaccinia virus, GLV-1h153, expresses hNIS, increases the expression of the symporter in TNBC cells, and serves both as a gene marker for noninvasive imaging of virus and as a vehicle for targeted radionuclide therapy with 131I.—Gholami, S., Chen, C-H., Lou, E., Belin, L. J., Fujisawa, S., Longo, V. A. Chen, N. G., Gönen, M., Zanzonico, P. B., Szalay, A. A., Fong, Y. Vaccinia virus GLV-1h153 in combination with 131I shows increased efficiency in treating triple-negative breast cancer.
Keywords: symporter, radiation therapy
Triple-negative breast cancer (TNBC) is an aggressive cancer and is resistant to hormonal and target therapy because of a lack of hormonal targets and is in need of novel treatments. Oncolytic viral therapies are attractive therapeutic agents that selectively destroy tumor tissues through multiple mechanisms, including direct lysis, tumor vaccination, and insertion of genes that cause the infected cells to express antiangiogenic or immunostimulatory proteins. Vaccinia virus (VACV) is an ideal candidate virus due to its well-characterized safety profile as a result of its extensive use in the World Health Organization's smallpox eradication campaign during the 1960s, a large genome that accommodates multiple insertions, and efficient cytoplasmic replication minimizing recombination with host cells. We have previously shown that an engineered VACV, GLV-1h153, can kill multiple TNBC lines in vitro and in vivo (1).
The human sodium iodide symporter (hNIS) is a membrane-bound glycoprotein present on the basolateral surface of thyroid follicular cells. It facilitates the transport of iodine into the cytoplasm, where it is organificated in the process of thyroid hormone synthesis. This molecule has been successfully used to accumulate radioiodine in both imaging and treatment of differentiated thyroid cancer for >60 yr. Specifically, studies have shown that thyroid remnant ablation is effective in reducing recurrence rates in patients with differentiated thyroid cancer and reduces risk of death from the cancer in patients (2). Expression of hNIS has also allowed detection of metastatic deposits of thyroid cancer (3).
In addition to the thyroid gland, hNIS has been shown to be endogenously expressed in several extrathyroidal tissues, including the gastric mucosa, salivary glands, and lactating mammary glands (4). hNIS is known to be present in low levels in ∼70% of breast carcinomas (5). However, the extent to which hNIS levels found in breast carcinoma can be clinically useful for imaging and treatment remains unclear. Here, we report on the potential of a novel VACV carrying the hNIS, named GLV-1h153, to increase the expression of the symporter in TNBC cells. Its parent virus has been used against human anaplastic thyroid cancer (6, 7), as well as several other cancers, including mesothelioma (8), pancreatic cancers (9), and melanoma (10). We hypothesized that GLV-1h153 could serve as both a gene marker for noninvasive imaging of virus and a vehicle for targeted radionuclide therapy with 131I-iodide. We found that expression of hNIS in infected cells up-regulated this protein in a murine TNBC model at a level which is endogenously expressed and compatible with clinical use.
MATERIALS AND METHODS
Cell lines
The human TNBC cell line MDA-MB-231 was kindly provided by Dr. Koblinski (Northwestern University, Evanston, IL, USA) and was maintained in Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 5% fetal bovine serum, 100 IU/ml penicillin/streptomycin, 1 mM sodium pyruvate, 2× nonessential amino acids and 1 μg/ml blasticidin (Sigma-Aldrich, St. Louis, MO, USA). Cells were grown in a 5% CO2 humidified incubator at 37°C. Authentication of this cell line was performed by genetic resource fragment analysis at John Hopkins University (Baltimore, MD, USA; http://faf.grcf.jhmi.edu/str.html) using short tandem repeat (STR) profiling. Cell line was authenticated 2 wk prior to submission and matched the American Type Culture Collection cell profile reference profile (American Type Culture Collection, Manassas, VA, USA).
Virus
GLV-1h153 is a replication-competent, recombinant VACV derived from the parent VACV LIVP strain (Lister strain from the Institute for Research on Virus Preparations, Moscow, Russia; ref. 11). It was reconstituted from its parent virus, GLV-1h68, with the insertion of the hNIS gene, as described previously (12). PCR sequencing was used to confirm the genotype of hNIS-expressing GLV-1h153. GLV-1h153 also contains an enhanced green fluorescent protein (eGFP) transgene under the control of the VACV synthetic strong early/late promoter.
Immunofluorescence (IF) for hNIS in cell cultures
To visualize the expression and membrane localization of the hNIS protein following infection with GLV-1h153, MDA-MB-231 cells were cultured on coverglass slides and subsequently either infected with ∼1 plaque forming unit (PFU)/cell or mock-infected with PBS. At 24 h after infection, cells were fixed with 4% paraformaldehyde, permeabilized with methanol, blocked with PBS containing BSA, and incubated with a mouse anti-hNIS monoclonal antibody (AbCam Inc., Cambridge, MA, USA) at a dilution of 1:50 and anti-GFP chicken polyclonal antibody (AbCam) at a dilution of 1:200. This was then followed by incubation with a secondary Alexa Fluor 568-conjugated goat anti-mouse antibody (Invitrogen, Grand Island, NY, USA) at a dilution of 1:1000 and a secondary Alexa Fluor 488-conjugated goat anti-chicken antibody (Invitrogen) at a dilution of 1:000, respectively. IF images were taken with a Zeiss AxioPlan 2 imaging widefield microscope with ×20/0.5 NA objective and AxioCam MRM CCD camera (Carl Zeiss, Oberkochen, Germany). Slides plated with mock-infected cells were used as negative controls.
Immunoblotting for hNIS in vitro
To confirm whether the hNIS protein was being expressed in infected cells, cells were plated at 5 × 105 cells/well and infected with GLV-1h153 at various multiplicities of infection (MOIs). Proteins were subsequently harvested at regular time points (24, 48 and 72 h). Protein samples (30 μg) were loaded on 10% Bis-Tris-HCl buffered polyacrylamide gels using the Bio-Rad system (Bio-Rad Laboratories, San Francisco, CA, USA). Proteins were transferred to nitrocellulose membranes using electroblotting. Membranes were blocked for 1 h in 5% low-fat dried milk in TBS-T (20 mM Tris, 137 mM NaCl, and 0.1% Tween-20) and then incubated with a purified mouse antibody against hNIS at 1:100 dilution (AbCam) overnight at 4°C. Secondary antibody (horseradish peroxidase-conjugated goat anti-mouse IgG; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was applied for 1 h at room temperature at 1:5000 dilution. Peroxidase-bound protein bands were visualized using enhanced chemiluminescence Western blotting detection reagents (Amersham, Arlington Heights, IL, USA) at room temperature for ∼1 min and using Kodak Biomax MR films for exposure (Eastman Kodak, Rochester, NY, USA). Normal human thyroid lysate was used as a positive control, and cells treated with GLV-1h68 and PBS were used as negative controls.
In vitro radiouptake assay
To quantify hNIS-mediated 131I uptake, MDA-MB-231 cells were plated at 4 × 105 cells/well in 6-well plates. Cells were then infected with GLV-1h153 at an MOI of 1.0. After 24 h of infection, cells were incubated for 1 h with 1.5 μCi of either carrier-free 131I-iodide or 131I-iodide with 1 mM of sodium perchlorate (NaClO4), a competitive inhibitor of hNIS, as described previously (12). The 131I activity in the cell (cpm/gm protein) was calculated from the 131I measurements in a γ counter (Perkin Elmer, Waltham, MA, USA). Results are expressed as the change in uptake relative to negative (uninfected) control. All assays were performed in triplicate.
In vivo imaging with 124I
All mice were cared for and maintained in accordance with animal welfare regulations under a protocol approved by the Institutional Animal Care and Use Committee at Memorial Sloan-Kettering Cancer Center. MDA-MB-231 cells (1×107) were injected into the mammary fad pad of 6- to 8-wk-old female athymic nude mice (NCI:Hsd:Athymic Nude-Foxn1nu; Harlan, Indianapolis, IN, USA). GLV-1h153 (1×107 PFU in 100 μl of PBS) and 100 μl of PBS (controls) were injected intratumorally in 3 treatment mice and 3 control mice, respectively. At 4 d after viral injection, ∼ 300 μCi of carrier-free 124I was injected intravenously into all mice. 124I list-mode data were acquired 3, 6, 24, and 48 h after radiotracer injection on a Focus 120 microPET dedicated small-animal PET scanner (Concorde Microsystems Inc., Knoxville, TN, USA) by using an energy window of 350 to 700 keV and a coincidence timing window of 6 ns. These data were sorted into 2-dimensional histograms by Fourier rebinning and reconstructed by filtered back-projection. The count rates in the reconstructed images were converted to activity concentration [percentage of injected dose(%ID)/g] by using a system calibration factor (MBq/ml/cps/voxel) derived from imaging of a mouse-sized phantom filled with a uniform aqueous solution of 18F, with adjustment for the positron branching ratio of 124I. Image analysis was performed by using ASIPro (Siemens Preclinical Solutions, Knoxville, TN, USA), and tumor activity concentrations were corrected for radioactive decay back to the time of injection.
131I dosing analysis
Each animal's tumor 124I-iodide time-activity concentration curve (%ID/g vs. time postadministration) as measured by serial microPET imaging was corrected for radioactive 124I decay to the time of injection and was fitted to either a monoexponential or a biexponential curve. Each animal's fitted time-activity function was then integrated, taking into account the rate of 131I decay, to yield the cumulated activity concentration in tumor per unit administered activity of 131I (μCi-h/g/μCi 131I administered). The mean radiation absorbed dose to the tumor per microcurie of 131I administered (rad/μCi) was then calculated by multiplying the tumor 131I cumulated activity concentration (μCi-h/g/μCi) by the 131I equilibrium dose constant for nonpenetrating radiation (i.e., β-rays), 0.405 g-rad/μCi-h, assuming complete local absorption of the 131I β-rays and ignoring the much smaller dose contribution of the 131I γ-rays. The 131I tumor-absorbed dose ranged from 38 to 132 rad/mCi, meaning the 5-mCi administered activity per mouse would yield total tumor-absorbed doses of 190 to 660 rad.
In vivo combination treatment study using GLV-1h153 and 131I
Mammary fat-pad tumors were generated as described above. For the combination therapy study, 14 d after cell implantation, xenografts were treated with intratumoral injection of GLV-1h153 (1×106 PFU) or PBS. At 1 wk after viral injection to allow for hNIS expression in infected tumors (d 21), xenografts were further randomized into 4 treatment groups: GLV-1h153 alone, GLV-1h153 and iodine (∼5 mCi of 131I), iodine alone, or PBS (n=4/treatment group). Mice were followed for tumor growth by calculating tumor volumes with the equation V = (4/3) * π * [(a/2)2 * (b/2)], where a is the smallest diameter (mm) and b is the largest diameter (mm) of the tumor. Animals were observed for any sign of toxicity, and body weight was checked weekly.
Statistical analysis
Significance differences among the tumor volume growth rates of the 4 treatment groups (PBS, iodine, GLV-1h153, and GLV-1h153 with iodine) were determined via nonparametric 1-way analysis of variance (Kruskal-Wallis test). Differences for the combination study were calculated using a Wilcoxon test. A t test was used for the uptake assay comparisons. Values of P < 0.05 were considered significant. All analyses were performed using R 2.14 statistical software (http://www.r-project.org).
RESULTS
Infected MDA-MB-231 cells express hNIS on IF imaging and Western blotting
GLV-1h153 carries the GFP reporter gene and the hNIS gene. At 24 h after viral infection (at an MOI of 1) with GLV-1h153, infected MDA-MB-231 cells expressed GFP and stained positive for hNIS (Fig. 1A, bottom panel) in vitro compared to uninfected controls (Fig. 1A, top panel). Moreover, infection with GLV-1h153 caused expression of hNIS on Western blotting. hNIS expression was positively correlated with viral concentration (Fig. 1B, left panel) and infection time (Fig. 1B, right panel).
Figure 1.
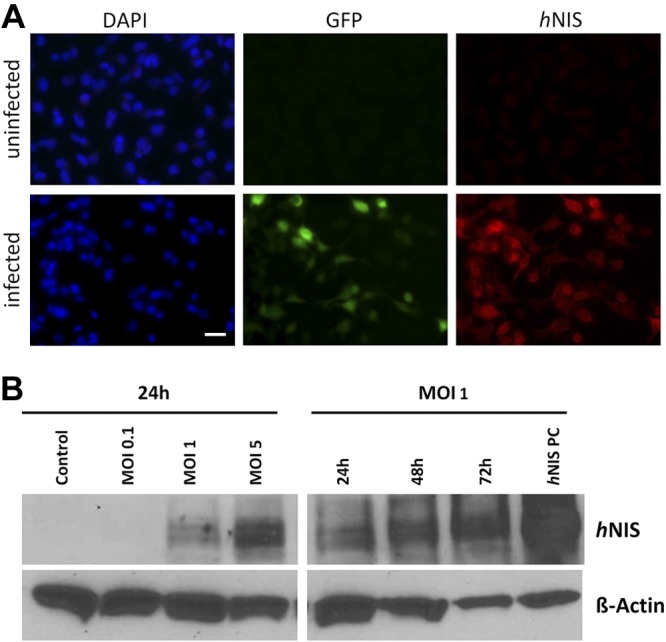
MDA-MB-231 cells infected with GLV-1h153 express hNIS, as detected with IF imaging and Western blots. A) IF imaging of uninfected (top panel) and infected (bottom panel) MDA-MB-231 cells in vitro. Cells were infected with GLV-1h153 at an MOI of 1 for 24 h. Uninfected controls were treated with medium only. (blue, DAPI; green, GFP; red, hNIS). Scale = 50 μm. B) Western blotting of hNIS in MDA-MB-231 cells infected with GLV-1h153. Left panel: MDA-MB-231 infected with GLV-1h153 at MOIs of 0.1, 1 and 5. Right panel: MDA-MB-231 infected with GLV-1h153 at an MOI of 1 for 24, 48, and 72 h. hNIS positive control (PC) represents normal thyroid tissue, which expresses high levels of hNIS.
Infected MDA-MB-231 cells demonstrate hNIS-specific radioiodine uptake
GLV-1h153-infected MDA-MB-231 cells demonstrated significantly enhanced hNIS-specific radioiodine uptake in cell cultures, with an average level of 228.9 cpm/g of protein compared to control cells (incubated without competitive hNIS inhibitor, NaClO4) with a background uptake of only 1.2 (P<0.05; Fig. 2). At 24 h after viral infection (at an MOI of 1), hNIS-specific radioiodine uptake was >178-fold greater than that in uninfected control cells (P<0.05).
Figure 2.
MDA-MB-231 cells infected with GLV-1h153 demonstrate hNIS-specific 131I radioactivity uptake in cell culture. Left panel: MDA-MB-231 cells in culture were treated with GLV-1h153 (MOI 1) or medium with or without the hNIS-blocking agent, sodium perchlorate (NaClO4). Radioactivity was measured 24 h after treatment. Right panel: Comparison between MDA-MB-231 treated with GLV-1h153 at an MOI of 1 for 24 h with or without NaClO4. Infected MDA-MB-231 without NaClO4 showed a >178-fold increase in 131I uptake (average level of 228.9 cpm/g protein; left panel, fourth bar). In comparison, infected cells with NaClO4 (left panel, third bar) show significantly less background uptake (1.2 cpm/g), suggesting that virally mediated radioiodine uptake was hNIS specific. No radioiodine uptake was observed when cells were incubated with iodine without the virus. Thus, uninfected control cells (with or without NaClO4) show only a background radioiodine uptake of <1 cpm/g protein.
hNIS-mediated imaging in GLV-1h153-infected xenografts via PET scanning and dosimetry calculations
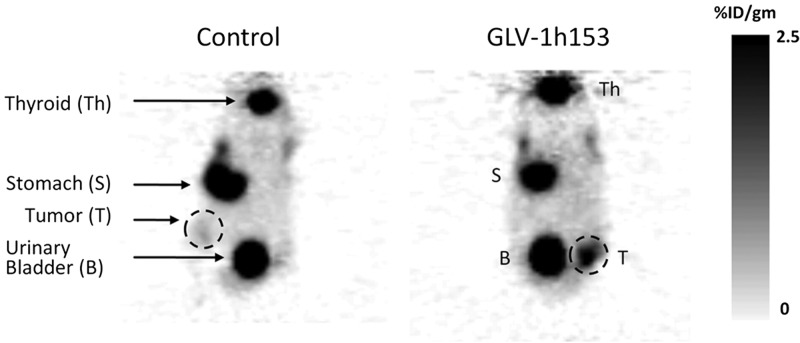
At 4 d after administration of intratumoral injection with GLV-1h153, hNIS protein expression in MDA-MB-231 xenografts allowed for tumor visualization by PET scanning (Fig. 3, right panel). Within 6 h of radiotracer (124I) injection, only infected mammary fat-pad tumors were positive on PET imaging, with an activity concentration of 2.5%ID/g; the uninfected control tumors were negative (not visualized) by PET scanning (Fig. 3, left panel). This finding suggests that GLV-1h153 can identify tumor cells in vivo via hNIS-mediated radioactive iodine (124I) uptake on PET imaging.
Figure 3.
PET scan showing hNIS-mediated deep tissue imaging in GLV-1h153-infected xenografts. GLV-1h153 or PBS (control) was injected intratumorally into the mammary fat pad of tumor-bearing mice 2 wk after tumor implantation. 124I PET-mediated imaging was obtained 4 d after viral infection and 6 h after radiotracer administration. Left panel: there was only background uptake at the tumor implantation site (T, dashed circle). Right panel: there was radioactivity concentration of 2.5%ID/g at the tumor implantation site (T, dashed circle). Shown are representative PET images from a total of 6 animals (3 treated, 3 control). B, bladder; S, stomach; Th, thyroid.
Combination treatment with GLV-1h153 and 131I in vivo achieves greater tumor growth suppression than single therapy with GLV-1h153 or 131I alone
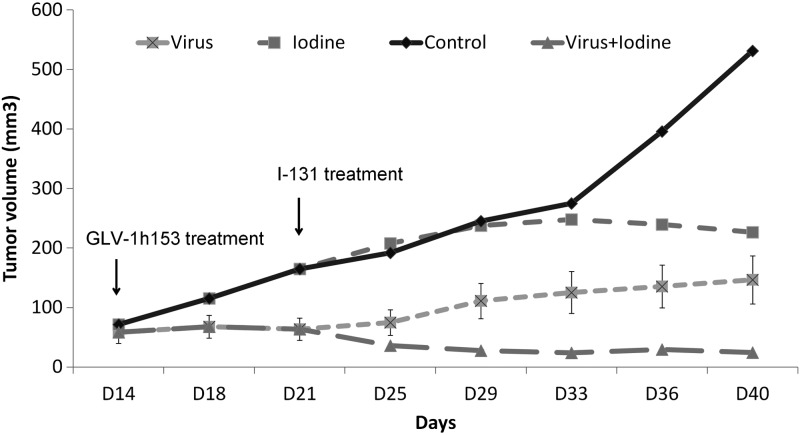
Mammary fat-pad xenografts were treated with GLV-1h153 at 2 wk after tumor injection (d 14). Within 1 wk of single treatment with GLV-1h153 (d 21), infected xenografts showed greater growth suppression compared to uninfected controls (P<0.05). At 1 wk after viral infection to allow for hNIS expression in infected tumors (d 21), xenografts treated with GLV-1h153 were randomized into 2 treatment groups: GLV-1h153 and combination of GLV-1h153 and 131I; untreated xenografts were randomized into another 2 groups: PBS and 131I alone. At the end of the treatment, GLV-1h153 alone significantly inhibited tumor growth (mean tumor size 146 mm3), with a >3.5-fold decrease compared to uninfected controls (P<0.05). Single therapy with 131I did not significantly decrease tumor size compared to PBS-treated controls. In contrast, administration of systemic radioiodine in combination with GLV-1h153 resulted in a significant regression of tumor size, exhibiting a >22-fold decrease in volume (tumor size 24 mm3) compared to untreated controls (tumor size 531 mm3, P<0.05) or iodine treatment alone (tumor size 226 mm3) (Fig. 4). Combination therapy thus resulted in a 6-fold greater tumor suppression than GLV-1h153 therapy alone (P<0.05). No significant difference in body weight was observed between the treatment groups in the experiment. Radioiodine significantly enhanced the antitumor effect of GLV-1h153 in this TNBC model.
Figure 4.
Combination treatment with GLV1h-153 and 131I in vivo achieves greater tumor growth suppression than single therapy with GLV-1h153 or 131I. At 14 d after xenograft implantation, mice were treated with intratumoral injections of GLV-1h153 or PBS. After 1 wk (d 21), virus-treated xenografts were randomized into virus alone and virus + iodine groups; PBS-treated xenografts were randomized into PBS alone (control) and iodine alone groups (n=4 animals/treatment group). Tumor volume growth rates and differences in the combination treatment were analyzed using a Kruskal–Wallis 1-way ANOVA analysis and a Wilcoxon test.
DISCUSSION
Although the notion of using radioactive iodine to treat nonthyroidal tumors has been proposed previously, to our knowledge, no one has yet identified an effective way of targeting TNBC with therapeutic radioiodine. In this study, we describe the cytotoxic effects of GLV-1h153 on TNBC cells in combination with radioactive iodine. We demonstrate that GLV-1h153 increases the expression of hNIS in TNBC cells both in vitro and in vivo, and thereby sensitizes TNBC to radioiodine therapy. Moreover, we demonstrate that combined therapy with GLV-1h153 and radioiodine is more effective than viral therapy alone at low dose, with a 6-fold increase in tumor regression compared to administration of GLV-1h153 alone.
VACV has been shown to treat a wide range of cancer cell types (6, 8, 9, 13), and its safety profile has been documented during the smallpox eradication. Recently, the parent strain, GLV-1h68, was tested in phase I human trials on multiple solid tumors (14). Our group has previously demonstrated that GLV-1h153 was able to infect and kill pancreatic, anaplastic thyroid and TNB cancers in in vitro and in vivo models (12, 15, 16). The key property of this next-generation strain of VACV is that it carries the gene for hNIS, a member of the sodium symporter family that can facilitate imaging via uptake of a variety of radionuclides, including 131I, 99mTc and 188Re (17). More notably, its expression allows for increased accumulation of 131I and thus therapeutic effectiveness of 131I-iodide.
131I is a highly effective therapy that is inexpensive, convenient, widely available, easy to administer, and well tolerated with minimal side effects. Once accumulated within cells, 131I undergoes β decay, releasing high-energy but relatively short (∼0.5-mm)-range electrons, delivering a localized radiation dose to immediately surrounding tissue. The ability of the virus to infect cells and allow focused hNIS expression increases the selectivity of this treatment and consequently should limit systemic toxicity. Moreover, the short but finite range of 131I β-rays means that a therapeutic dose of radiation may be delivered to tumor cells noninfected with the virus and/or not expressing hNIS.
131I has been widely and successfully used for the treatment of well-differentiated thyroid cancer for many years. For thyroid cancer, many studies have shown a decreased rate of recurrence, incidence of distant metastasis (2, 18, 19), and increased disease-specific and overall survival (2, 19, 20) in tumors treated with radioiodine (18, 21). Mazzaferri et al. (2) showed that radioiodine therapy decreased the risk of cancer death by half among post-thyroidectomy patients with thyroid cancer, as compared to hormone replacement or external radiation alone. In addition, radioactive iodine appears to be effective for treatment of microscopic disease (22), with pulmonary nodules responding best when the volume of disease was small or microscopic (23, 24). Probably one of the best examples relevant to the aggressive, metastatic TNBC model is that radioiodine therapy has been shown to slow the progression of bony metastases and decrease pain in patients with metastatic differentiated thyroid cancer (25).
As only the thyroid tissue organificates iodine naturally, the general consensus has been that only cancers derived from that organ would benefit therapeutically from this approach. In a study by Wapnir et al., (26) NIS expression was seen in a majority of breast cancers, but the degree to which NIS may be functional was not stated. Their study demonstrated that 48% of TNBC tumors express NIS, but that a standard therapeutic activity of 131I (∼75 to 200mCi) would not be sufficient to deliver a therapeutically effective dose to TNBC (26).
In our in vitro experience, very weak NIS expression was seen in our IF specimens, but these TNBC cells did not significantly concentrate radioiodine as compared to infected cells. Our uptake assays revealed that infection with GLV-1h153 resulted in expression of the hNIS protein in MDA-MB-231 cells, demonstrating a >178-fold increase in hNIS-specific uptake of 124I radiotracer as compared to uninfected controls as early as 24 h after infection at an MOI of 1. In vivo, infected xenografts showed enhanced sensitivity to the combination of GLV and 131I, with a 6-fold increase in tumor regression as compared to that of viral therapy alone. We therefore conclude that in our combination model, the increase in hNIS expression resulted in a functional effect in vivo. We note that for the purposes of this study, we specifically used a lower dose of the virus (1×106 PFU) than the dose (1×107 PFU) used in our previous work (1). We had previously demonstrated that GLV-1h153 is very effective as a single oncolytic agent. To prove that GLV-1h153 leads to functional hNIS expression that can take up radioiodine to achieve synergistic effects, we used a lower viral dose to avoid inducing complete viral oncolysis before this could be observed, treatment using a viral dose of 1 × 107 PFU would be extremely effective in reducing tumor size to the point of making accurate assessment difficult and nearly impossible.
In patients with thyroid cancer, radioiodine therapy has some side effects, including thyroiditis, nausea, taste dysfunction, and sialadenitis (27, 28), but most of these are minor and transient in nature. A possible adverse event associated with radioiodine therapy is the development of leukemia, but this risk is not markedly higher than in patients with thyroid cancer not treated with radioiodine (29, 30). More notably, however, is that side effects appear to be dose dependent. Since our results suggest increased cytotoxicity with combination therapy, we believe that our combination therapy may be effective at relatively low administered activities and side effects thereby minimized or eliminated.
Besides β decay, 131I also undergoes γ decay, which allows sites of radioactive iodine uptake to be visualized by γ camera imaging (21, 31) and unsuspected metastatic disease to be detected after treatment (32). In addition, hNIS allows for the uptake of the positron-emitting radioiodine 124I, which can be used for high-sensitivity detection and planning of radioiodine therapy of metastatic disease by using PET. One last added benefit of GLV-1h153 carrying the hNIS gene therefore is that one can image tumors via PET imaging. We demonstrated how GLV-1h153-infected MDA-MB-231 tumor-bearing mice can be visualized by PET imaging. Four days after infection with GLV-1h153, tumors were clearly detected by PET imaging; tumors in uninfected control mice were not visualized, however. This feature can be used to track metastatic lesions and monitor viral therapy. Other research groups have demonstrated different viral vectors, such as adenovirus and measles virus, to increase hNIS expression and allow for radionuclide uptake in prostate, medullary thyroid cancer, and multiple myeloma (33–35). Our data complements the scientific literature and we hope to add additional information to help answer the question of whether viral-mediated radiation therapy would be beneficial to patients with breast cancer.
In summary, this study demonstrates that a novel oncolytic VACV expressing exogenous hNIS can successfully induce expression of endogenous hNIS in TNBC. This greatly enhances intracellular accumulation of radioiodide in cells in vitro. In vivo, GLV-1h153 achieved a combined oncolytic cytotoxicity as well as an enhanced antitumor effect with radioiodine in a murine TNBC model. Our results support the continued investigation of this novel engineered VACV as a potentially important, readily translatable therapy and imaging tool for TNBC.
Acknowledgments
The authors thank Jason Aguilar and Mesruh Turkekul for their excellent technical assistance. Technical services provided by the Research Animal Resource Center (RARC), the Molecular Cytology facilities, and the Small-Animal Imaging Core facilities at Memorial Sloan-Kettering Cancer Center are gratefully acknowledged.
This work is supported by the William H. and Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center, the Flight Attendant's Medical Research Institute (FAMRI), and Cycle for Survival.
Footnotes
- eGFP
- enhanced green fluorescent protein
- hNIS
- human sodium iodide symporter
- IF
- immunofluorescence
- MOI
- multiplicity of infection
- PFU
- plaque forming unit
- TNBC
- triple-negative breast cancer
- VACV
- vaccinia virus
REFERENCES
- 1. Gholami S., Chen C. H., Lou E., De Brot M., Fujisawa S., Chen N. G., Szalay A. A., Fong Y. (2012) Vaccinia virus GLV-1h153 is effective in treating and preventing metastatic triple-negative breast cancer. Ann. Surg. 256, 437–445 [DOI] [PubMed] [Google Scholar]
- 2. Mazzaferri E. L. (1997) Thyroid remnant 131I ablation for papillary and follicular thyroid carcinoma. Thyroid 7, 265–271 [DOI] [PubMed] [Google Scholar]
- 3. Farina G. P., Pisano M., Baccoli A., Deserra A., Pani C., Cagetti M. (2000) [Therapeutic strategies in differentiated cancer of the thyroid: total thyroidectomy]. G. Chir. 21, 469–474 [PubMed] [Google Scholar]
- 4. Spitzweg C., Joba W., Eisenmenger W., Heufelder A. E. (1998) Analysis of human sodium iodide symporter gene expression in extrathyroidal tissues and cloning of its complementary deoxyribonucleic acids from salivary gland, mammary gland, and gastric mucosa. J. Clin. Endocrinol. Metab. 83, 1746–1751 [DOI] [PubMed] [Google Scholar]
- 5. Wapnir I. L., van de Rijn M., Nowels K., Amenta P. S., Walton K., Montgomery K., Greco R. S., Dohan O., Carrasco N. (2003) Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J. Clin. Endocrinol. Metab. 88, 1880–1888 [DOI] [PubMed] [Google Scholar]
- 6. Lin S. F., Price D. L., Chen C. H., Brader P., Li S., Gonzalez L., Zhang Q., Yu Y. A., Chen N., Szalay A. A., Fong Y., Wong R. J. (2008) Oncolytic vaccinia virotherapy of anaplastic thyroid cancer in vivo. J. Clin. Endocrinol. Metab. 93, 4403–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin S. F., Yu Z., Riedl C., Woo Y., Zhang Q., Yu Y. A., Timiryasova T., Chen N., Shah J. P., Szalay A. A., Fong Y., Wong R. J. (2007) Treatment of anaplastic thyroid carcinoma in vitro with a mutant vaccinia virus. Surgery 142, 976–983 [DOI] [PubMed] [Google Scholar]
- 8. Kelly K. J., Woo Y., Brader P., Yu Z., Riedl C., Lin S. F., Chen N., Yu Y. A., Rusch V. W., Szalay A. A., Fong Y. (2008) Novel oncolytic agent GLV-1h68 is effective against malignant pleural mesothelioma. Hum. Gene Ther. 19, 774–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu Y. A., Galanis C., Woo Y., Chen N., Zhang Q., Fong Y., Szalay A. A. (2009) Regression of human pancreatic tumor xenografts in mice after a single systemic injection of recombinant vaccinia virus GLV-1h68. Mol. Cancer Ther. 8, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly K. J., Brader P., Woo Y., Li S., Chen N., Yu Y. A., Szalay A. A., Fong Y. (2009) Real-time intraoperative detection of melanoma lymph node metastases using recombinant vaccinia virus GLV-1h68 in an immunocompetent animal model. Int. J. Cancer 124, 911–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Q., Yu Y. A., Wang E., Chen N., Danner R. L., Munson P. J., Marincola F. M., Szalay A. A. (2007) Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 67, 10038–10046 [DOI] [PubMed] [Google Scholar]
- 12. Gholami S., Haddad D., Chen C. H., Chen N. G., Zhang Q., Zanzonico P. B., Szalay A. A., Fong Y. (2011) Novel therapy for anaplastic thyroid carcinoma cells using an oncolytic vaccinia virus carrying the human sodium iodide symporter. Surgery 150, 1040–1047 [DOI] [PubMed] [Google Scholar]
- 13. Gentschev I., Stritzker J., Hofmann E., Weibel S., Yu Y. A., Chen N., Zhang Q., Bullerdiek J., Nolte I., Szalay A. A. (2009) Use of an oncolytic vaccinia virus for the treatment of canine breast cancer in nude mice: preclinical development of a therapeutic agent. Cancer Gene Ther. 16, 320–328 [DOI] [PubMed] [Google Scholar]
- 14. U.S. National Institutes of Health (2008–2013) Safety study of GL-ONC1, an oncolytic virus, in patients with advanced solid tumors. ClinicalTrials.gov ID: NCT00794131 Accessed August 2013 at http://clinicaltrials.gov/ct2/show/NCT00794131?term=GL-ONC1&rank=2
- 15. Haddad D., Chen N. G., Zhang Q., Chen C. H., Yu Y. A., Gonzalez L., Carpenter S. G., Carson J., Au J., Mittra A., Gonen M., Zanzonico P. B., Fong Y., Szalay A. A. (2011) Insertion of the human sodium iodide symporter to facilitate deep tissue imaging does not alter oncolytic or replication capability of a novel vaccinia virus. J. Transl. Med. 9, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gholami S., Chen C. H., Belin L. J., Lou E., Fujisawa S., Antonacci C., Carew A., Chen N. G., De Brot M., Zanzonico P. B., Szalay A. A., Fong Y. (2013) Vaccinia virus GLV-1h153 is a novel agent for detection and effective local control of positive surgical margins for breast cancer. BCR 15, R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee Y. J., Chung J. K., Shin J. H., Kang J. H., Jeong J. M., Lee D. S., Lee M. C. (2004) In vitro and in vivo properties of a human anaplastic thyroid carcinoma cell line transfected with the sodium iodide symporter gene. Thyroid 14, 889–895 [DOI] [PubMed] [Google Scholar]
- 18. Durante C., Haddy N., Baudin E., Leboulleux S., Hartl D., Travagli J. P., Caillou B., Ricard M., Lumbroso J. D., De Vathaire F., Schlumberger M. (2006) Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 91, 2892–2899 [DOI] [PubMed] [Google Scholar]
- 19. Mazzaferri E. L., Jhiang S. M. (1994) Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 97, 418–428 [DOI] [PubMed] [Google Scholar]
- 20. Mazzaferri E. L., Kloos R. T. (2001) Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 86, 1447–1463 [DOI] [PubMed] [Google Scholar]
- 21. Maxon H. R., 3rd, Smith H. S. (1990) Radioiodine-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinol. Metab. Clin. N. Am. 19, 685–718 [PubMed] [Google Scholar]
- 22. Robbins R. J., Schlumberger M. J. (2005) The evolving role of (131)I for the treatment of differentiated thyroid carcinoma. J. Nucl. Med. 46(Suppl. 1), 28S–37S [PubMed] [Google Scholar]
- 23. Schlumberger M., Tubiana M., De Vathaire F., Hill C., Gardet P., Travagli J. P., Fragu P., Lumbroso J., Caillou B., Parmentier C. (1986) Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 63, 960–967 [DOI] [PubMed] [Google Scholar]
- 24. Samaan N. A., Schultz P. N., Haynie T. P., Ordonez N. G. (1985) Pulmonary metastasis of differentiated thyroid carcinoma: treatment results in 101 patients. J. Clin. Endocrinol. Metab. 60, 376–380 [DOI] [PubMed] [Google Scholar]
- 25. Schlumberger M., Challeton C., De Vathaire F., Travagli J. P., Gardet P., Lumbroso J. D., Francese C., Fontaine F., Ricard M., Parmentier C. (1996) Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J. Nucl. Med. 37, 598–605 [PubMed] [Google Scholar]
- 26. Renier C., Yao C., Goris M., Ghosh M., Katznelson L., Nowles K., Gambhir S. S., Wapnir I. (2009) Endogenous NIS expression in triple-negative breast cancers. Ann. Surg. Oncol. 16, 962–968 [DOI] [PubMed] [Google Scholar]
- 27. Allweiss P., Braunstein G. D., Katz A., Waxman A. (1984) Sialadenitis following I-131 therapy for thyroid carcinoma: concise communication. J. Nucl. Med. 25, 755–758 [PubMed] [Google Scholar]
- 28. Van Nostrand D., Neutze J., Atkins F. (1986) Side effects of “rational dose” iodine-131 therapy for metastatic well-differentiated thyroid carcinoma. J. Nucl. Med. 27, 1519–1527 [PubMed] [Google Scholar]
- 29. Hall P., Holm L. E., Lundell G., Bjelkengren G., Larsson L. G., Lindberg S., Tennvall J., Wicklund H., Boice J. D., Jr. (1991) Cancer risks in thyroid cancer patients. Br. J. Cancer 64, 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bitton R., Sachmechi I., Benegalrao Y., Schneider B. S. (1993) Leukemia after a small dose of radioiodine for metastatic thyroid cancer. J. Clin. Endocrinol. Metab. 77, 1423–1426 [DOI] [PubMed] [Google Scholar]
- 31. Sweeney D. C., Johnston G. S. (1995) Radioiodine therapy for thyroid cancer. Endocrinol. Metab. Clin. N. Am. 24, 803–839 [PubMed] [Google Scholar]
- 32. Sherman S. I., Tielens E. T., Sostre S., Wharam M. D., Jr., Ladenson P. W. (1994) Clinical utility of posttreatment radioiodine scans in the management of patients with thyroid carcinoma. J. Clin. Endocrinol. Metab. 78, 629–634 [DOI] [PubMed] [Google Scholar]
- 33. Rajecki M., Sarparanta M., Hakkarainen T., Tenhunen M., Diaconu I., Kuhmonen V., Kairemo K., Kanerva A., Airaksinen A. J., Hemminki A. (2012) SPECT/CT imaging of hNIS-expression after intravenous delivery of an oncolytic adenovirus and 131I. PloS ONE 7, e32871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barton K. N., Stricker H., Elshaikh M. A., Pegg J., Cheng J., Zhang Y., Karvelis K. C., Lu M., Movsas B., Freytag S. O. (2011) Feasibility of adenovirus-mediated hNIS gene transfer and 131I radioiodine therapy as a definitive treatment for localized prostate cancer. Mol. Ther. 19, 1353–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spitzweg C., Baker C. H., Bergert E. R., O'Connor M. K., Morris J. C. (2007) Image-guided radioiodide therapy of medullary thyroid cancer after carcinoembryonic antigen promoter-targeted sodium iodide symporter gene expression. Hum. Gene Ther. 18, 916–924 [DOI] [PubMed] [Google Scholar]