Abstract
Transcriptomic and biochemical analyses of the experimental pathosystem constituted by Ustilago maydis and Arabidopsis thaliana were performed. Haploid or diploid strains of U. maydis inoculated in A. thaliana plantlets grew on the surface and within the plant tissues in the form of mycelium, inducing chlorosis, anthocyanin formation, malformations, necrosis and adventitious roots development, but not teliospores. Symptoms were more severe in plants inoculated with the haploid strain which grew more vigorously than the diploid strain. RNA extracted at different times post-infection was used for hybridization of one-channel microarrays that were analyzed focusing on the fungal genes involved in the general pathogenic process, biogenesis of the fungal cell wall and the secretome. In total, 3,537 and 3,299 genes were differentially expressed in the haploid and diploid strains, respectively. Differentially expressed genes were related to different functional categories and many of them showed a similar regulation occurring in U. maydis infecting maize. Our data suggest that the haploid strain behaves as a necrotrophic pathogen, whereas the diploid behaves as a biotrophic pathogen. The results obtained are evidence of the usefulness of the U. maydis-A. thaliana pathosystem for the analysis of the pathogenic mechanisms of U. maydis.
Keywords: microarrays, experimental pathosystem, Ustilago maydis, Arabidopsis thaliana, plant virulence, necrotrophic fungus
Introduction
The use of alternative hosts to understand pathogenic processes is a strategy widely used since the earliest microbiological studies on human diseases. More recently, these studies extended to fungal infections, e.g., rabbits and mice have been used as hosts to study fungal human pathogens as Cryptococcus neoformans and Candida albicans1,2 and even Drosophila melanogaster has served as alternative host for studying human mycoses.3 Additionally, model plants are used to study fungal pathogens of commercial interest. Thus Nicotiana benthamiana was employed as alternative host for mutants of Colletotrichum orbiculare, a pathogen of melon and cucumber4 and Brachypodium distachyon was used as alternative host of Magnaporthe grisea, a rice and barley pathogen.5 Additionally, in the pathosystems Cryptococcus-Arabidopsis and Cryptococcus-Eucalyptus, the human pathogen completed its sexual cycle.6
Similarly, our research group demonstrated that Ustilago maydis infected different plant species, unrelated to its natural host, Zea mays under axenic conditions,7 establishing the Ustilago maydis-Arabidopsis thaliana pathosystem as model to study some virulence aspects of the fungus.8 A. thaliana has the attractive characteristics of its small size, short life cycle and its complete genome sequence. Furthermore, Arabidopsis is a model for plant-pathogen interactions.9,10
U. maydis is a pathogen of maize (Zea mays) and teozintle (Zea mays ssp parviglumis), where it completes its known sexual life cycle. This starts when two haploid yeast-like cells of compatible mating types fuse giving rise to a dikaryotic hypha that infects the plant. Inside the host, the mycelium undergoes morphological changes eventually giving rise to teliospores that accumulate within tumors induced by the pathogen. Teliospores germinate to form haploid basidiospores that reinitiate the life cycle.11,12 Interestingly, we demonstrated that U. maydis performs a completely different sexual life cycle with the surprising formation of basidiocarps, when incubated under defined environmental conditions.13
Considering the complexity of the sexual-pathogenic process of U. maydis in maize, the Ustilago maydis-Arabidopsis pathosystem appears as an attractive alternative, since no sexual cycle occurs, and haploid strains are pathogenic. In this study we analyzed the infection of Arabidopsis by haploid or diploid strains of U. maydis and compared the transcriptomic differences of both strains to identify factors that explained the specificity of maize infection by dikaryons or diploids and to define similarities and differences in the infection of Arabidopsis and maize.
Results
Symptoms in plantlets infected with U. maydis strains
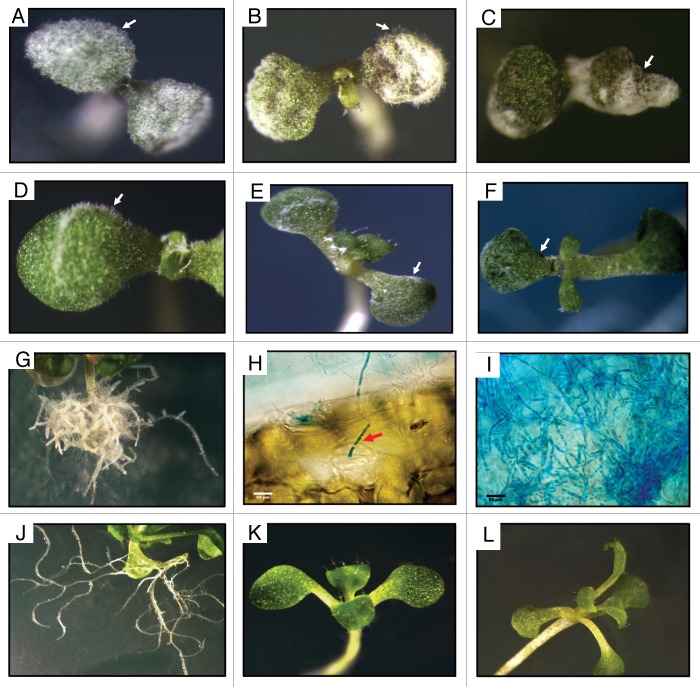
Haploid or diploid strains of U. maydis infected Arabidopsis plantlets as described,8 but the haploid induced more severe symptoms. Aerial mycelium of the haploid strain developed at the inoculation sites after 24 h, spreading to other regions (Fig. 1A and B, arrows), necrotic areas developed 4 d post-infection (dpi) and tissue detachment at 8 dpi (Fig. 1B and C, respectively, arrows). Mycelium developed extensively within plant tissues (Fig. 1I), possibly penetrating through stomata (Fig. 1H, red arrow). The haploid strain also induced severe alterations in the root system at 20 dpi (Fig. 1G). On the other hand, plantlets infected with Uid1 developed scant mycelium at early periods (Fig. 1D and E) and necrosis points only after 8 dpi (Fig. 1F, arrow).
Figure 1. Appearance of A. thaliana plantlets infected with U. maydis strains. General aspect of plants inoculated with the FB2 haploid strain (A–C), or the Uid1 diploid strain (D–F) at 2, 4 or 8 d post-infection respectively. Notice in (B) the necrotic area (arrow), in (C) necrotic tissue detachment (arrow) and in (F) necrotic tissue points in the infected plantlets. (G) Severe alteration in the root system in haploid-infected plantlets 20 dpi. (H) Mycelium of the haploid strain entering through stomata (red arrow).(I) mycelium of the haploid strain growing within the infected tissue. (J–L) Aspect of roots and leaves of control not-inoculated plantlets. Scale bar in (H) and (I), 20 μm.
Effect of U. maydis inoculation on growth of Arabidopsis plantlets
Growth of Arabidopsis plantlets infected with the haploid was reduced approximately from 50 to 90 per cent after 4 dpi, whereas diploid-infected plantlets were similar to controls until 12 dpi, before slowing down. Dry weight (DW) of all plantlets was similar until 4 dpi, but thereafter DW of haploid-inoculated plantlets remained constant, while that of diploid-inoculated plantlets remained similar to controls until 8 dpi, after which they showed similar growth as the control plantlets (Fig. 2).
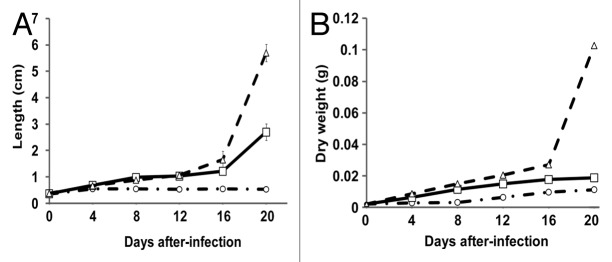
Figure 2. Growth of A. thaliana plantlets infected with U. maydis strains. (A) Plantlets length and (B) plantlets dry weight. Dotted lines with circles, plantlets infected with the haploid strain. Solid lines with squares, plantlets infected with the diploid strain. Dotted lines with triangles, control plantlets that received sterile distilled water. Results from three independent experiments with six plants in each one. Results expressed in cm or g per plantlet respectively in each graph. Bars represent standard error values.
Growth of U. maydis within Arabidopsis plantlets
Growth of U. maydis was measured by ergosterol and chitin, compounds that are present in fungi, but absent in plants. Amounts of chitin and ergosterol in plants infected with either strain were similar at 4 dpi, but afterwards, the levels of both compounds were higher in haploid-infected plants, whereas they decreased in diploid-infected plants, suggesting curtailment of fungal growth by the plant, something not occurring with the haploid strain. As expected, in the un-inoculated plants, no ergosterol or chitin were detected (Fig. 3).
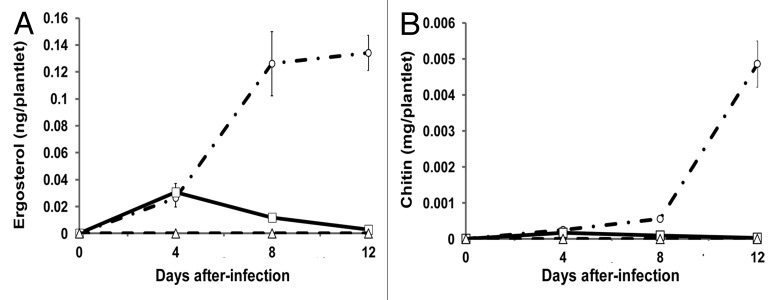
Figure 3. Development of U. maydis within infected A. thaliana plantlets. (A) Ergosterol determination. (B) Chitin determination. Empty circles with dotted lines, plantlets infected with the haploid. Empty squares with solid lines, plantlets infected with the diploid. Empty triangles with dotted lines, control plantlets that received sterile distilled water. Results from three independent experiments with 100 plant batches in each one. Results expressed as ng ergosterol or mg GlcNAC per plantlet. Bars represent standard error values.
Differential gene expression in U. maydis strains infecting Arabidopsis
Analysis of the genes differentially expressed in Ustilago strains during Arabidopsis infection was compared with their expression in yeast-like cells grown in liquid culture. As considered significant in most microarray analyses, a two-fold change up or down was used to consider differential expression of genes. The results obtained revealed that in the haploid 2,636 genes were differentially expressed at 1 dpi, 1,294 up-regulated and 1,342 down-regulated. In the diploid the corresponding numbers were 2,389; 1,170 and 1,219, respectively. In both cases the numbers remained almost constant during the following dpi (Table 1). These numbers reveal the extreme changes occurring when U. maydis changes its mode of life from saprophytic to pathogenic. The total number of genes differentially expressed along the infection process were 3,537; 1,703 up-regulated and 1,834 down-regulated in the haploid and 3,299; 1,621 and 1,678 respectively in the diploid; genes commonly regulated in both strains were respectively, 696, 407 and 289. Table S1 describes the genes with the highest difference in expression (at least a twenty-fold change up or down).
Table 1. Differential gene expression in U. maydis strains during infection of Arabidopsis thaliana plantlets.
| Strain | Genes | Days post-infection | |||
|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | ||
| Haploid (FB2) |
Differential total | 2,636 | 2,315 | 2,503 | 2,320 |
| Upregulated | 1,294 | 1,132 | 1,216 | 1,152 | |
| Downregulated | 1,342 | 1,183 | 1,287 | 1,168 | |
|
Diploid (Uid1) |
Differential total | 2,389 | 2,420 | 2,162 | 2,162 |
| Upregulated | 1,170 | 1,203 | 1,084 | 1062 | |
| Downregulated | 1,219 | 1,217 | 1,078 | 1,100 | |
Functional classification of differential genes
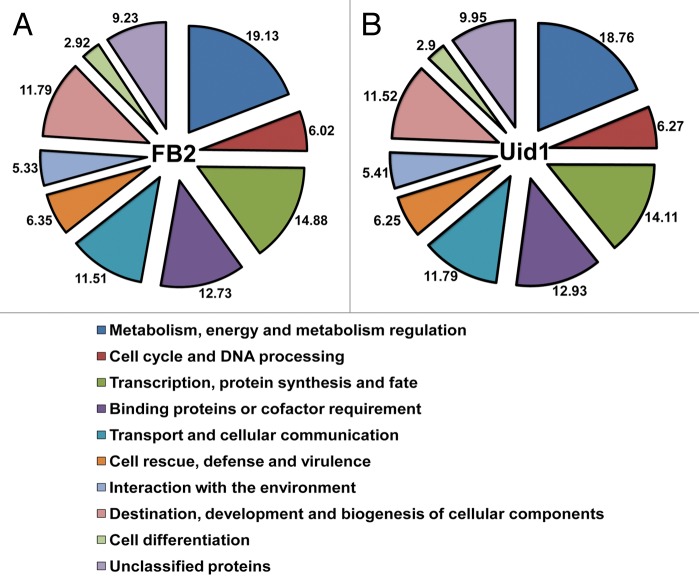
Functional grouping of the differentially expressed genes in either strain gave similar data (Fig. 4). The categories with higher gene numbers were related to metabolism, energy and metabolism regulation (19.1% in the haploid and 18.7% in the diploid of the total differential genes), transcription, protein synthesis and fate (respective values of 14.9% and 14.1%). The categories with lower gene numbers were interaction with the environment (respectively, 5.3% and 5.4%) and cell differentiation (respectively, 2.9% and 2.9 %) (Fig. 4).
Figure 4. Functional categorization of U. maydis genes regulated during the infective process of Arabidopsis. (A) Differentially regulated genes in the haploid. (B) Differentially regulated genes in the diploid. Numbers represent the percentage of genes in a given functional category and text corresponds to the names of each category.
Classes of differentially regulated genes
Considering the high number of differentially regulated genes during Arabidopsis infection, we investigated their nature according to classes related to pathogenicity, some of which are regulated during maize infection.
Genes regulated by the bE/bW heterodimer
It has been described that U. maydis infection in maize by heterokarions or diploids, is regulated by a heterodimer made of the bE and bW gene products coming from the two sexually-interacting strains; this heterodimer acts as a master transcription factor for a number of genes 11,14,15 and it has been hypothesized that it is also involved in the U. maydis biotrophic phase.16 Accordingly, we analyzed their regulation during Arabidopsis infection considering that only the diploid contains the heterodimer. PRF1, the gene directly regulating transcription of the heterodimer was repressed in the haploid, whereas no differential expression was observed in the diploid. Of the genes directly regulated by the heterodimer: CLP1 (required for intracellular hyphal proliferation), FRB52 (of unknown function) and RBF1,16 only RBF1 was up-regulated in the diploid (Table 2). In turn, Rbf1 regulates BIZ1, HDP1, HDP2 and FOX1 encoding transcription factors and two additional proteins: KPP6 and PCL12. HDP1 was up-regulated only in the haploid, FOX1 was up-regulated in the diploid and down-regulated in the haploid, whereas no differential expression of BIZ1 and HDP2 was observed in any strain. PCL12 and KPP6 were up-regulated in both strains (Table 2).
Table 2. Differential expression of genes regulated by the bE/bW heterodimer during U. maydis infection of A. thaliana.
| Identity | Gene | Description | Regulation | ||
|---|---|---|---|---|---|
| Haploid strain FB2 | Diploid strain Uid1 | ||||
| um03172 | Rbf1 | Related to Zinc finger protein | - | up | |
| um02438 | Clp1 | Related to clp1, essential for A-regulated sexual development | - | - | |
| um01262 | Frb52 | DNA polymerase X-putative | up | up | |
| um02331 | Kpp6 | MAP kinase | up | up | |
| um02549 | Biz1 | b-induced zinc finger protein | - | - | |
| um12024 | um05762 | Hdp1 | Potential homeodomain transcription factor | up | - |
| um04928 | Hdp2 | Uncharacterized protein | - | - | |
| um10529 | um10529.2 | Pcl12 | b-dependently cyclin | up | up |
| um01523 | Fox1 | Transcriptional regulator | down | up | |
Genes belonging to 12 pathogenicity clusters
Expression of genes belonging to the twelve pathogenic clusters in the diploid strain was similar to the one observed in maize.11,15 Other genes, especially those belonging to cluster 2A whose deletion increases virulence, were down-regulated in the haploid and up-regulated in the diploid as occurs in maize (Table S2).
Effector genes
Effectors are proteins secreted by plant pathogens that manipulate the physiology of their hosts to their benefit.17 In biotrophic fungi, these effectors accumulate in the zone of interaction with the host inducing the suppression of plant immune responses.18 Fox1, controlling the expression of effector-encoding genes in U. maydis,19 specifically PEP1, the EFF1 family and PIT2,20-22 was up-regulated in the diploid and down-regulated in the haploid. PIT2 was down-regulated in the haploid and was not differentially expressed in the diploid; EFF1-5 to EFF1-11 were up-regulated in the diploid, whereas in the haploid EFF1-6 and EFF1-8 were not differentially expressed and EFF1-7 was down-regulated. EFF1-9, EFF1-10 and EFF1-11 were up-regulated but at most at two different dpi. In contrast, EFF1-1 to EFF1-4 and PEP1 were not differentially expressed in either strain (Table 3).
Table 3. Differential expression of genes encoding U.maydis effector proteins during Arabidopsis infection.
| Identity | Gene | Regulation in Haploid (FB2) | Regulation in Diploid (Uid1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 dpi | 2 dpi | 4 dpi | 8 dpi | 1 dpi | 2 dpi | 4 dpi | 8 dpi | ||
| um01375 | Pit2 | 2.0 down | 2.2 down | - | - | - | - | - | - |
| um02135 | Eff1-5 | - | - | 3.1 up | 2.7 up | 5.2 up | 5.4 up | 6.5 up | 5.8 up |
| um02136 | Eff1-6 | - | - | - | - | 2.6 up | 2.7 up | - | 2.1 up |
| um02137 | Eff1-7 | 2.2 down | 2.2 down | - | - | - | - | 2.6 up | 2.5 up |
| um02138 | Eff1-8 | - | - | - | - | 4.0 up | 4.4 up | 3.3 up | 4.2 up |
| um02139 | Eff1-9 | - | - | 2.2 up | 2.2 up | - | - | 3.3 up | 2.1 up |
| um02140 | Eff1-10 | - | - | 2.1 up | 3.3 up | 2.6 up | - | - | - |
| um02141 | Eff1-11 | - | 2.1 up | - | 2.0 up | 4.8 up | 4.4 up | 2.4 up | 2.5 up |
Genes encoding hydrophobins and repellent proteins
Hydrophobins and repellent proteins are involved in U. maydis virulence in maize. HUM2 has essential functions in maize infection and REP1 is involved in aerial hyphae formation.23,24 We found that during Arabidopsis infection REP1, HUM2 and HUM3 were up-regulated in the haploid strain, particularly REP1 (39.3 of fold change at 1 dpi). In contrast, only REP1 and HUM2 were up-regulated in the diploid (not shown).
Genes encoding transcription factors
We identified 111 genes encoding potential transcription factors differentially expressed during Arabidopsis infection. In the haploid, 45 were up-regulated and 66 down-regulated. In the diploid 47 were up-regulated and 63 down-regulated. Seventy-two genes were up- or down-regulated in common in both strains (27 up- and 45 down-regulated). In the haploid, gene um02301 was highly over-expressed (25.5-fold change) and um06283 was highly repressed (15.1-fold change). In the diploid, genes um00841 and um05966 were highly over-expressed (9.0 and 7.7 of fold change, respectively), whereas um06283 and um04208 were severely repressed (8.7 and 11.1 of fold change, respectively) (Table S3). The importance of regulation of transcription factors during maize infection is known,16,25 in agreement with our data on Arabidopsis infection.
Other genes reported as involved in maize infection
Around fifty genes have been described to be involved in maize infection by U. maydis, as deduced from the phenotype of their corresponding mutants. In Arabidopsis infection we identified that 21 of them were differentially regulated. In the haploid, 12 were up-regulated and 9 were down-regulated and in the diploid 8 were up-regulated and 10 down-regulated (Table 4).
Table 4. Regulation of Ustilago maydis genes described as important for virulence in maize, during Arabidopsis infection.
| Identity | Description | Reference | Fold change during Arabidopsis thaliana infection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Days post-infection with the haploid strain FB2 | Days post-infection with the diploid strain Uid1 | ||||||||||
| 1 | 2 | 4 | 8 | 1 | 2 | 4 | 8 | ||||
| um00641 | um11450 | Hgl1; Hgl1p, required for dimorphism and teliospore formation | Deletion67 | 4.4 down | 5.2 down | - | - | 3.0 down | 3.9 down | 3.1 down | - |
| um01374 | Pit1 | Deletion21 | - | 2.2 up | 3.9 up | 3.1 up | - | - | 2.2 up | 2.2 up | |
| um01597 | Hap2 | Deletion68 | 5.2 down | 2.8 down | 2.6 down | - | 2.9 down | 2.2 down | - | - | |
| um01947 | Related to cytochrome-c peroxidase precursor | Deletion69 | 2.6 down | 16.5 down | 14.8 down | 48.2 down | - | - | 3.0 down | 3.6 down | |
| um02374 | Srt1; related to monosaccharide transporter | Deletion70 | 3.1 down | 2.7 down | 3.2 down | - | 3.9 down | 3.5 down | - | - | |
| um02377 | Probable cytochrome c peroxidase precursor | Deletion69 | - | - | - | - | 3.7 up | 3.3 up | 2.2 up | 2.9 up | |
| um03280 | um10828 | Tup1; probable TUP1-general transcription repressor | Deletion28 | - | - | - | - | 2.2 down | - | - | - |
| um03305 | Ubc3; MAP kinase | Deletion71 | 3.5 up | 3.1 up | 3.6 up | 3.4 up | 2.3 up | 2.3 up | - | - | |
| um03315 | Ukb1; serine/threonine protein kinase b-related Ukb1 | Deletion72 | 3.1 up | 2.4 up | 2.8 up | 2.3 up | - | - | - | - | |
| um04258.2 | um04258 | Ubc4 - MAPKK kinase | Deletion73 | - | 2.0 down | - | - | - | - | - | - |
| um04474 | Gpa3; guanine nucleotide-binding protein α-3 subunit | Deletion74 | 2.3 up | 2.8 up | 2.8 up | 4.3 up | 3.2 up | 3.3 up | 3.9 up | 3.8 up | |
| um04580 | O-mannosylation | Deletion75 | 3.3 up | - | 2.3 up | 2.0 up | 2.5 up | 2.0 up | - | 2.0 up | |
| um05261 | Ubc2; MAP kinase pathway-interacting protein | Deletion76 | 2.9 down | 2.7 down | 2.8 down | 2.0 down | 4.2 down | 4.2 down | 3.6 down | 2.5 down | |
| um05433 | Pmt4; probable PMT4; dolichyl-phosphate-mannose–protein O-mannosyltransferase | Deletion44 | 3.3 up | 2.8 up | 3.1 up | 2.6 up | - | - | - | - | |
| um05818 | Probable chimeric spermidine synthase/saccharopine reductase | Deletion77 | - | - | 2.4 up | 2.7 up | 2.0 down | - | - | 2.0 down | |
| um05850 | um05850.2 | Polyamine oxidase (PAO) | Deletion78 | 3.5 up | 2.4 up | 2.4 up | 2.2 up | 2.3 up | - | 2.2 up | 2.0 up |
| um10417 | um04252 | Nit2; related to transcription factor ScGATA-6 | Deletion29 | - | - | - | - | 2.7 down | 2.7 down | 2.2 down | - |
| um10672 | um10672.2 | Peroxidases | Deletion69 | 2.7 down | - | 2.3 down | 2.3 down | 4.5 down | 5.2 down | 4.1 down | 5.7 down |
| um10792 | um00999 | Related to S-adenosylmethionine decarboxylase (spe-2) | Deletion79 | 3.0 down | 2.0 down | 2.1 down | - | - | - | - | - |
| um10803 | um01516 | Sql2; guanyl nucleotide exchange factor Sql2 | Deletion80 | 2.7 down | 2.6 down | 2.5 down | 2.2 down | 2.1 down | 2.5 down | - | - |
| um11410 | um02513 | Crk1; Cdk-related kinase 1 | Deletion81 | - | 2.1 up | 2.1 up | 2.5 up | - | - | - | 2.3 up |
| um11453 | um00648 | Ubc5; probable UBC5 - E2 ubiquitin-conjugating enzyme | Deletion73 | - | - | - | - | 2.5 up | 3.1 up | - | - |
| um12033 | um06025 | Rop1 HMG-box transcription factor (C-terminal fragment) | Deletion82 | - | - | 3.1 up | - | - | - | - | - |
| um04456 | um10537 | Adr1 - protein kinase A, catalytic subunit | Described83 | 3.3 up | 3.2 up | 2.9 up | 2.5 up | - | - | - | - |
| um12024 | um05762 | hdp1 | Described16 | - | - | 5.6 up | 2.0 up | - | - | - | - |
Genes involved in the synthesis and structure of the cell wall
Genes involved in the synthesis of structural polysaccharides
In the haploid, CHS1 one of the genes encoding chitin synthases, was up-regulated during the whole infection period, whereas CHS3 was down-regulated, but only at 8 dpi. In the diploid, no CHS gene was differentially expressed. Genes involved in the synthesis of β-1,3- or β-1,6-glucans behaved differently; GLS encoding β-1,3-glucan synthase was constitutively expressed in both strains, but of the genes involved in β-1,6-glucan synthesis, CWH41 and ROT2 homologs and three KRE6 homologs were up-regulated (at least at one dpi), whereas three other homologs of KRE6 were down-regulated in the haploid. In the diploid, two KRE6 homologs were up-regulated and two down-regulated (Table 5). Genes encoding chitin deacetylases (CDAs), enzymes involved in chitosan synthesis, were up-regulated in both strains, but their fold changes were greater in the haploid. In contrast, three other CDA genes were down-regulated in the haploid and only two of them in the diploid (Table 5).
Table 5. Differential expression of genes involved in the synthesis of structural polysaccharides of U.maydis during Arabidopsis infection.
| Identity | Gene | Subcelular localization of protein | Haploid (FB2) | Diploid (Uid1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 dpi | 2 dpi | 4 dpi | 8 dpi | 1 dpi | 2 dpi | 4 dpi | 8 dpi | |||
| um10718 | CHS1 | Cell wall | 3.2 up | 3.4 up | 2.1 up | 2.3 up | - | - | - | - |
| um10120 | CHS3 | - | - | - | 2.3 down | - | - | - | - | |
| um01143 | CDA | - | 2.5 down | - | 3.8 down | 3.1 down | 3.0 down | 2.1 | 2.0 down | |
| um01788 | 2.6 down | 2.2 down | - | - | 2.5 down | 2.8 down | - | - | ||
| um02019 | - | - | 2.7 down | - | - | - | - | - | ||
| um05792 | - | 2.7 up | 5.4 up | 8.1 up | 2.6 up | - | 2.4 up | - | ||
| um11922 | 24.2 up | 31.0 up | 29.0 up | 27.1 up | 10.9 up | 9.9 up | 15.1 up | 12.0 up | ||
| um01639 | GLS | - | - | - | - | - | - | - | - | |
| um11723 | CWH41a | Endoplasmic Reticulum | 3.4 up | 3.1 up | 2.7 up | 2.7 up | - | - | - | - |
| um04405 | ROT2a | 2.1 up | 2.1 up | 2.0 up | - | - | - | - | - | |
| um00857 | KRE6/SKN1a | Golgi Apparatus | 2.0 up | - | 2.5 up | - | - | - | - | - |
| um03569 | 6.0 down | 4.3 down | 7.9 down | 5.6 down | - | - | - | - | ||
| um05718 | 3.4 down | 3.7 down | 3.0 down | 3.2 down | 2.2 down | 2.1 down | - | - | ||
| um05807 | 3.3 up | 5.2 up | - | - | - | - | - | - | ||
| um05809 | 18.9 up | 27.0 up | 8.1 up | 10.1 up | 3.7 up | 3.9 up | 6.8 up | 4.9 up | ||
| um05811 | 4.0 down | 10.3 down | 7.9 down | 2..5 down | 5.4 down | 4.8 down | 3.4 down | 3.2 down | ||
a Homologs to Saccharomyces cerevisie genes.
Genes involved in polysaccharide biosynthesis precursors
One gene encoding a hexokinase and one encoding a N-glucose acetyl transferase involved in the synthesis of uridine diphosphate GlcNAc (UDP-GlcNAc) were up-regulated in both strains, whereas a gene encoding a phosphoacetylglucosamine mutase was down-regulated in both strains. Genes involved in the synthesis of uridine diphosphoglucose (UDPGlc) and guanosine diphospho manose (GDPMan) were also differentially expressed, thus, the gene encoding UDP-glucose-hexose-1-phosphate uridylyltransferase was up-regulated in the diploid strain and the gene encoding mannose-1-phosphate guanylyltransferase showed a decrease in the level of transcription in the same strain (not shown).
Genes involved in the synthesis of glycoproteins
In the haploid, almost all the genes involved in the synthesis of the N-linked glycan moiety of glycoproteins were up-regulated, with the exception of SEC59, encoding a dolichol kinase, that was down-regulated, same as happened in the diploid, where only a few genes were up-regulated (Table 6). In contrast, fewer genes involved in the synthesis of the O-glycan moiety of glycoproteins were differentially regulated. Of PMT genes encoding enzymes that transfer the first mannose to Ser/Thre residues of proteins, only PMT4 was up-regulated in the haploid and genes involved in further addition of mannosyl units (KRE2 homologs) were up-regulated in both strains (Table 6).
Table 6. Differential expression of U.maydis genes involved in N- and O glycosylation during Arabidopsis infection.
| Sequence identity |
Homolog genea | Function | Haploid (FB2) | Diploid (Uid1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 dpi | 2 dpi | 4 dpi | 8 dpi | 1 dpi | 2 dpi | 4 dpi | 8 dpi | |||
| um10484 | ALG7 | Oligosaccharide synthesis | 5.4 up | 4.1 up | 2.7 up | 2.5 up | 8.1 up | 4.6 up | 4.0 up | 4.3 up |
| um11771 | ALG14 | 3.1 up | - | - | - | 2.5 up | 2.1 up | - | - | |
| um11547 | ALG1 | 2.3 up | - | 2.2 up | - | - | - | - | - | |
| um05209 | ALG11 | 2.1 up | 2.2 up | 2.3 up | - | - | - | - | - | |
| um02433 | RFT1 | 2.5 up | 2.6 up | 2.1 up | - | - | - | - | - | |
| um11341 | ALG12 | 4.2 up | 3.8 up | 3.7 up | 2.8 up | 6.5 up | 5.3 up | 4.7 up | 3.6 up | |
| um04779 | SEC59 | 2.7 down | 2.4 down | 2.2 down | 2.1 down | 2.4 down | 2.5 down | 2.1 down | - | |
| um01231 | ALG5 | 2.3 up | 2.2 up | 2.3 up | 2.0 up | 2.0 up | 2.0 up | 2.1 up | 2.3 up | |
| um05547 | ALG10/DIE2 | - | - | - | - | 2.1 up | - | - | - | |
| um01062 | VRG4 | 2.1 up | - | - | - | - | - | - | - | |
| um04198 | OST3 | Oligosaccharide transference | 3.8 up | 2.8 up | 3.6 up | 2.8 up | 2.8 up | 2.9 up | 2.2 up | 2.0 up |
| um05293 | STT3 | 3.5 up | 3.8 up | 3.6 up | 3.6 up | 2.4 up | 2.2 up | 2.5 up | 2.3 up | |
| um11723 | CWH41/GLS1 | Modification of oligosaccharide | 3.4 up | 3.1 up | 2.7 up | 2.7 up | - | - | - | - |
| um04405 | ROT2 | 2.1 up | 2.1 up | 2.0 up | - | - | - | - | - | |
| um01957 | MNS1, MNL1/HTM1 | 4.0 up | 1.6 up | 3.0 up | 2.1 up | 7.3 up | 4.6 up | 8.1 up | 5.7 up | |
| um02227 | 2.8 up | 2.5 up | 2.6 up | 2.7 up | 4.9 up | 5.6 up | 6.8 up | 5.5 up | ||
| um10494 | 3.7 up | 2.3 up | 2.5 up | 2.1 up | - | - | - | - | ||
| um05433 | PTM4 | Transfer of first mannose to Ser/Thre | 3.1 up | 2.8 up | 3.0 up | 2.6 up | - | - | - | - |
| um01154 | KRE2 | Modification with additional mannose | 3.8up | 3.6 up | 3.2 up | 3.2 up | 2.9 up | 2.8 up | 3.4 up | 3.2 up |
| um01821 | 2.0 up | - | - | - | 2.1 up | 2.4 up | 2.2 up | 2.5 up | ||
a Homologs to S. cerevisiae genes
Genes GPI12, GPI14 and GUP1 involved in the synthesis of the GPI anchor of GPI proteins were up-regulated in both strains. Additionally, GPI17 and GPI18 were up-regulated and PER1 down-regulated in the haploid, whereas in the diploid GPI1 and GPI8 were up-regulated and GPI3 was down-regulated at different dpi (Table 7).
Table 7. Expression of U.maydis genes involved in GPI-anchor synthesis during Arabidopsis infection.
| Identity | Homolog gene | Function | Process | FB2 | Uid1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 dpi | 2 dpi | 4 dpi | 8 dpi | 1 dpi | 2 dpi | 4 dpi | 8 dpi | ||||
| um00213 | GPI1 | Modified of PI with GlcNAc (GPI-GnT complex) | Synthesis of GPI anchor from PI | - | - | - | - | 3.4 up | 3.2 up | 2.6 up | 2.5 up |
| um00302 | GPI12 | Deacetylation | - | 2.0 up | - | - | 2.5 up | 2.0 up | 2.0 up | - | |
| um11347 | GPI14 | Transfer of mannose | 2.9 up | 4.5 up | 3.3 up | 4.1 up | - | - | 2.0 up | 2.4 up | |
| um05554 | GPI18 | Transfer of mannose | 2.4 up | 2.9 up | 2.6 up | 2.3 up | - | - | - | - | |
| um05709 | GPI8 | Catalytic site | - | - | - | - | 2.3 up | 2.0 up | - | - | |
| um01565 | GPI17 | 2.3 up | 2.5 up | 2.9 up | 2.2 up | - | - | - | - | ||
| um04859 | PER1 | Removal of acyl group of GPI-anchor | Lipid Remodeling | 4.5 down | 3.9 down | 4.3 down | 3.9 down | - | - | - | - |
| um03003 | GUP1 | Lipid remodeling with longer fatty acids | 5.3 up | 4.1 up | 4.9 up | 4.5 up | 2.8 up | 2.9 up | 3.0 up | 3.1 up | |
Genes encoding GPI proteins and proteins of the secretome
CDA genes and other genes encoding GPI-hydrolytic enzymes were up-regulated in both strains (not shown), and numerous genes encoding secreted proteins classified as unknown or with degradative, synthetic, redox or non-enzymatic functions were differentially expressed in either strain. A greater number of genes encoding enzymes that degrade polysaccharides, lipids and proteins were up-regulated, in the haploid, as compared to the diploid strain (Table 8).
Table 8. Numbers of U.maydis genes encoding secreted proteins regulated during Arabidopsis infection.
| Function | Regulate | Haploid (FB2) | Diploid (Uid1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 dpi | 2 dpi | 4 dpi | 8 dpi | 1 dpi | 2 dpi | 4 dpi | 8 dpi | |||
| Unknown | Up | 54 | 50 | 62 | 57 | 61 | 72 | 62 | 76 | |
| Down | 29 | 37 | 27 | 32 | 17 | 20 | 30 | 22 | ||
| Degradation | Polysaccharidases | Up | 17 | 16 | 19 | 15 | 10 | 9 | 11 | 9 |
| Down | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | ||
| Peptidases | Up | 9 | 7 | 8 | 6 | 4 | 4 | 4 | 5 | |
| Down | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 2 | ||
| Nucleases | Up | 6 | 5 | 5 | 5 | 5 | 6 | 5 | 5 | |
| Down | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Lipases and esterases | Up | 4 | 5 | 6 | 8 | 4 | 5 | 3 | 5 | |
| Down | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | ||
| Phytases | Up | 3 | 2 | 3 | 2 | 2 | 3 | 3 | 1 | |
| Down | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Synthesis | Up | 3 | 4 | 5 | 4 | 4 | 2 | 4 | 2 | |
| Down | 1 | 2 | 3 | 3 | 1 | 4 | 2 | 3 | ||
| Redox | Up | 12 | 11 | 12 | 9 | 7 | 5 | 7 | 8 | |
| Down | 3 | 3 | 3 | 3 | 8 | 8 | 8 | 7 | ||
| Non-enzymatic | Up | 4 | 4 | 2 | 2 | 4 | 4 | 4 | 4 | |
| Down | 3 | 3 | 5 | 5 | 5 | 5 | 5 | 6 | ||
Discussion
Ustilago maydis is a useful model for understanding phenomena of fungal pathogenesis,11,12 and Arabidopsis thaliana is an excellent model for understanding plant responses to pathogens.10 For these reasons, our working group established the experimental pathosystem Ustilago maydis-Arabidopsis thaliana, as model system for understanding some aspects of Ustilago pathogenesis.8 In this work we analyzed the transcriptome of U. maydis infecting Arabidopsis plantlets comparing a diploid and a haploid strain of the fungus, considering that diploids (as Uid1, used in this work) and heterokaryons, but not haploids, are infective in maize, but both infect Arabidopsis.
We observed a drastic alteration in gene expression in U. maydis during Arabidopsis infection, in numbers similar to maize infection,15 corresponding to more than one third of the whole genome. These numbers indicate that U. maydis drastically alters the levels of transcription of a great number of genes to cope with the changes taking place during its adaptation from a more or less comfortable environment where its nutritional necessities are satisfied, to the harsh conditions existing in the host, where it has to deal with the plant defenses and struggle for nutrients.
The damage caused to Arabidopsis plantlets by the haploid was noticeably more severe than that caused by the diploid and drastically inhibited plant growth, besides causing severe alterations in their roots. Growth of the haploid in the plant, measured by two specific parameters: ergosterol and chitin accumulation was also more abundant than growth of the diploid. These results reveal the higher virulence of the haploid, suggesting that it behaves as a necrotrophic parasite in Arabidopsis. These phytopathogens overturn the host defense mechanisms, destroy the plant,26 and are resistant to host hypersensitive reactions.27 In contrast, the diploid behaves as a biotrophic pathogen, as occurs in maize. This suggestion is supported by several pieces of evidence, e.g., the up-regulation in the diploid, contrasting with the haploid of RBDF1 (a master regulator required for all b-dependent processes) controlled by the bE/bW heterodimer and the genes that it regulates.16 Moreover, of the genes regulated by Rbf1, HDP1, involved in filamentous growth, was up-regulated only in the haploid, and FOX1 that regulates genes encoding effector proteins, was down-regulated in the haploid and up-regulated in the diploid. These data reveal, firstly, that genes encoding both transcription factors, as well as genes encoding Pcl12 and Kpp6, can be regulated by mechanisms alternative to Rbf1, and more important, that the opposite effect on HDP1 and FOX1 may be related to the different pathogenic behavior of both strains, biotrophic or necrotrophic. Additionally, a larger number of effectors, important for the biotrophic stage, were up-regulated in the diploid and were even down-regulated in the haploid. Further evidence to explain the different behavior of both strains and data of gene expression revealing homologies and differences in the pathogenic behavior of U. maydis in maize and Arabidopsis are described below.
In this sense, we must indicate that genes encoding transcription factors TUP1 and NIT2 involved in maize infection,28,29 were also regulated during Arabidopsis infection, TUP1 being down-regulated in both strains and NIT2 repressed only in the diploid strain. Tup1 is repressed during maize infection and NIT2 disruption reduced virulence in maize. Behavior of homologs of genes encoding transcription factors related to virulence in different fungi was also significant: two homolog genes of HSF1 of C. albicans (that is up-regulated during formation of invasive mycelium)30,31 and are up-regulated in U. maydis infecting maize, were differentially expressed only in the haploid, one up- and the other one down-regulated. Additionally, a homolog of SKN7 encoding a transcription factor required for virulence in C. albicans and C. neoformans32,33 was up-regulated only in the haploid strain. These data provide evidence of the similarity in the pathogenic mechanisms of U maydis in maize and Arabidopsis.
Functional grouping identified only a limited number of genes involved in pathogenesis, as occurs in maize.34 This result agrees with the knowledge that U. maydis possesses fewer pathogenesis genes than other phytopathogenic fungi,11 possibly in relation to its biotrophic lifestyle. Among the factors involved in pathogenesis, genes encoding G proteins and transcription factors can be cited. The number of genes involved in cell rescue and defense that may be involved in response to stress and in detoxification was small, and interestingly, were mainly up-regulated in the haploid. Worth mentioning is that genes involved in response to stress, such as oxidases were repressed in the diploid, contrasting with the haploid where significant numbers of genes responding to oxidative stress were up-regulated, possibly as a defense mechanism. Other up-regulated genes mainly in the haploid strain are involved in homeostasis, cell migration, chemotaxis, mechanical stimulus perception and perception and response to nutrients. These functions are important for the formation of intracellular hyphae involved in acquisition of nutrients, signaling, communication and avoidance.35
The process of Arabidopsis invasion, as occurs in maize, involves cell differentiation and dimorphic transition of yeast cells to invading hyphae. This process involves changes in expression of genes involved in cell wall biogenesis.36 Accordingly, we analyzed the regulation of genes involved in this process. Some genes encoding glucanases or chitinases, probably involved in structural changes of the wall were differentially expressed during Arabidopsis infection. The higher number of CDA genes up-regulated in comparison to CHS genes, agrees with the observation that the hyphal surface of invasive biotrophic rust fungi, contains chitosan instead of chitin,37 probably because chitosan, in contrast to chitin, lacks elicitor activity.
Expression of GLS encoding the single β-1,3-glucan synthase of Ustilago was constitutive during infection by both strains, agreeing with data from in vitro dimorphism or maize infection.38 In contrast homologs of ROT2 and CWH41 from the pathway of β-1,6-glucans synthesis and N-glycosylation were up-regulated during Arabidopsis infection only in the haploid. Considering that mutants of the six homologs of KRE6/SKN1 in C. neoformans were avirulent to mouse,39 the data reveal the importance of β-1,6-glucans synthesis in fungal pathogenesis, agreeing with the more aggressive behavior of the haploid strain.
Increased protein glycosylation is required for fungal-host interaction and virulence.40 Accordingly, genes involved in the synthesis of N-glycans such as CWH41 and ROT2 and MSN1 and VRG4, have been related to virulence.41 In this regard, the observation that three MSN1 homologs from U. maydis were up-regulated in the haploid and only two in the diploid and that the homolog of VRG4 was up-regulated in the haploid is relevant. Regarding O-glycans, it was reported that PMT4, a gene involved in their synthesis, is involved in C. albicans and C. neoformans virulence,42,43 and that its U. maydis mutants were avirulent to maize.44 Agreeing with these data, we observed that this gene was up-regulated only in the haploid, correlating again with its higher virulence.
GPI proteins are important components of fungal membranes and walls and among them Yps aspartyl proteases are important in Candida pathogenesis,45 agreeing with the observation that a gene encoding a Yps was up-regulated in both Ustilago strains. Taking into consideration that genes involved in the synthesis of the glycosylphosphatidyl inositol moiety of GPI-proteins such as GPI12, GPI17, GPI8 are important in virulence of fungi and protozoa,46-48 it was significant to observe that in U. maydis the homolog of GPI12 was up-regulated in both strains and that GPI17 was up-regulated only in the haploid and GPI8 only in the diploid.
Hydrophobins and repellent proteins are important in Ustilago infection of maize.23,24,49 We observed that REP1 and HUM2 genes were up-regulated in both strains, REP1 to higher levels in the haploid, whereas HUM3 was up-regulated only in this strain, suggesting their role in Arabidopsis infection.
Finally, it must be indicated that a higher number of genes encoding hydrolytic enzymes, some degrading the plant cell wall, were up-regulated in the haploid. This result may help to explain the greater damage produced by the haploid in Arabidopsis plants, agreeing with the observation that biotrophic fungi secrete a reduced number of degradative enzymes50 and supporting our hypothesis of the different behavior of haploid and diploid strains of U. maydis during Arabidopsis infection.
In conclusion, our data that provide evidence of the importance of a number of genes in Ustilago virulence and that the genetic machinery used in Arabidopsis infection is similar to that used in maize infection. According to the evidence presented, we propose the hypothesis that the haploid strain of U. maydis behaves in Arabidopsis as a necrotrophic pathogen, in contrast to the diploid that, as occurs in maize, behaves as a biotrophic agent. We attribute this different behavior to alterations in the expression of genes encoding virulence factors, degradation enzymes, effector genes and transcription factors under the control of the bE/bW heterodimer, that obviously is absent in the haploid strain.
Materials and Methods
Fungal and plant strains, culture media and growth conditions
Fungal strains. U. maydis wild type strain FB2 (a2b2)51 and the diploid strain Uid1 (a1b1Δpan/a2b2Δodc::HygR)52 were maintained and grown as described by Ruiz-Herrera et al.36Arabidopsis thaliana L. Landsberg erecta (Ler) plantlets were grown on MS synthetic medium according to Mendez-Morán et al.8 (see below).
Arabidopsis growth conditions
A.thaliana Landsberg erecta seeds were sterilized with chlorine gas. Open Eppendorf tubes containing the seeds were placed in a desiccator that contained a beaker with 100 mL of concentrated hydrochloric acid and 5 mL sodium hypochlorite. The desiccator was kept closed during 4–6 h. After of sterilization, the seeds were maintained at 4°C during 2 d. For plant growth, 80–100 seeds were placed over plates of sterile solid MS medium and incubated in a chamber at 25°C with photoperiods of 12 h.
Inoculation and measurement of plantlets growth
U. maydis strains were grown in shaken liquid MC at 28°C for 18 h. The cells were recovered by centrifugation at 1,000 g for 10 m and washed twice with sterile distilled water (SDW) by centrifugation. Finally the cells were suspended in 5 mL of SDW and cell concentration was determined with a Neubauer chamber. A. thaliana plantlets were inoculated with 1–2 μl of a cell suspension containing 106 cells/mL on the vegetative apex, 6 d post-planting. Control plantlets received SDW only. Plantlets were incubated under controlled conditions in a growth chamber as described above. At intervals, some plants were observed by light microscopy, directly or stained with cotton blue-lactophenol and photographed. At different periods post-inoculation plantlets were recovered and their dry weight and stem length were measured.
Ustilago maydis growth in A. thaliana seedlings
The biomass of U. maydis in inoculated plantlets was determined by ergosterol53 and chitin54 measurements. N-acetylglucosamine (GlcNAC) was determined as described by Reissig et al.55
Isolation of RNA and microarrays hybridization of microarrays
Total RNA from Ustilago cells (three experiments in triplicates) or Arabidopsis plantlets (four experiments with 150 plants each) was isolated using Trizol (Invitrogen) and purified with QIAGEN columns (Cat. 28104). RNA concentration was measured by its absorbance at 260 nm and its integrity observed by electrophoresis in denaturing agarose gels. cDNA synthesis and labeling and hybridization of the microarrays were performed by Roche NimbleGen Inc.
Design, image analysis, normalization and analysis of microarrays
The microarrays used were high density (one channel) arrays from NimbleGene, according to a design from Scott Gold (University of Georgia). Five different oligonucleotide probes per gene (60 nt in length) per duplicate represented each one of the 6,883 genes of the U. maydis genome. GenePix 4000B scan and associated software were used to scan the arrays. Roche NimbleScan software was used to import the scanned images and data extraction. Normalization was made with NimbleScan software using quantile56 and the algorithm Robust Multi-array Analysis (RMA).57 Microarray analyses were made with DNAStar ArrayStar software, comparing data from Arabidopsis infected plants against U. maydis grown in culture medium. Data obtained from plants that received SDW were used as controls. P-values obtained were adjusted by the false discovery rate (FDR) method.58 p-values inferior to 0.05 were considered significant. A two-fold change up or down was used to consider differential expression of genes. The Functional Catalogue (FunCat)59 was used for functional annotation of differentially expressed genes.
Bioinformatic searches
Besides a classification of all regulated genes, in silico searches of specific genes previously reported in the following groups, were made: belonging to pathogenesis clusters,11 encoding proteins from the secretome,60,61 encoding hydrophobins or repellent proteins24, regulated by the bE/bW heterodimer,16,25 involved in the synthesis and organization of the cell wall,62 and those previously reported as important during maize infection (specific references cited in “Results”). Additionally, we performed in silico searches of genes encoding transcription factors using online programs UniProt,63 KEGG,64 Pfam,65 SUPERFAMILY66 and MIPS Ustilago maydis DataBase (http://mips.helmholtz-muenchen.de/genre/proj/ustilago/).
Supplementary Material
Acknowledgments
This work was partially supported by Consejo Nacional de Ciencia y Tecnología (CONACYT), México. Thanks are given to Dr Lucila Ortiz and Dr Doralinda Guzmán for assistance in some analyses and Dr Scott Gold for permission to use his microarray design. D.M.S. and A.M.R.B. are doctoral students with fellowships from CONACYT.
Glossary
Abbreviations:
- dpi
days post-infection
- DW
dry weight
- FDR
false discovery rate
- FunCat
functional catalogue
- RMA
robust multi-array analysis
- SDW
sterile distilled water
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25059
References
- 1.Perfect JR, Savani DV, Durack DT. Comparison of itraconazole and fluconazole in treatment of cryptococcal meningitis and candida pyelonephritis in rabbits. Antimicrob Agents Chemother. 1986;29:579–83. doi: 10.1128/AAC.29.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–60. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontoyiannis DP, Lewis RE. Studying fungal pathogenesis in Drosophila. Microbe. 2010;5:291–5. [Google Scholar]
- 4.Chen N, Hsiang T, Goodwin PH. Use of green fluorescent protein to quantify the growth of Colletotrichum during infection of tobacco. J Microbiol Methods. 2003;53:113–22. doi: 10.1016/S0167-7012(02)00234-8. [DOI] [PubMed] [Google Scholar]
- 5.Parker D, Beckmann M, Enot DP, Overy DP, Rios ZC, Gilbert M, et al. Rice blast infection of Brachypodium distachyon as a model system to study dynamic host/pathogen interactions. Nat Protoc. 2008;3:435–45. doi: 10.1038/nprot.2007.499. [DOI] [PubMed] [Google Scholar]
- 6.Xue C, Tada Y, Dong X, Heitman J. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe. 2007;1:263–73. doi: 10.1016/j.chom.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 7.León-Ramírez C, Cabrera-Ponce JL, Martínez-Espinoza A, Herrera-Estrella L, Méndez-Morán L, Reynaga-Peña G, et al. Infection of alternative host plant species by Ustilago maydis. New Phytol. 2004;164:337–46. doi: 10.1111/j.1469-8137.2004.01171.x. [DOI] [PubMed] [Google Scholar]
- 8.Méndez-Morán L, Reynaga-Peña CG, Springer PS, Ruiz-Herrera J. Ustilago maydis infection of the nonnatural host Arabidopsis thaliana. Phytopathology. 2005;95:480–8. doi: 10.1094/PHYTO-95-0480. [DOI] [PubMed] [Google Scholar]
- 9.Davis KR, Hammershmidt R. Arabidopsis thaliana as a model for plant-pathogen interactions. In: The American Phytopathological Society, ed. St. Paul, USA, 1993:134. [Google Scholar]
- 10.Innes R. Arabidopsis as a model host in molecular plant pathology. In: Singh RP, Singh US, ed(s). Molecular methods in plant pathology. FL, USA: Boca Raton CRC Press, 1995:203-19. [Google Scholar]
- 11.Kämper J, Kahmann R, Bölker M, Ma LJ, Brefort T, Saville BJ, et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature. 2006;444:97–101. doi: 10.1038/nature05248. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Herrera J, León-Ramírez CG. Development and dimorphism of the phytopathogenic basidiomycota Ustilago maydis In: Ruiz-Herrera J, ed. Dimorphic fungi. Their importance as models for differentiation and fungal pathogenesis. Ed. Bentham eBooks elSBN: 978-1-60805-364-3, 2012:105-16. [Google Scholar]
- 13.Cabrera-Ponce JL, León-Ramírez CG, Verver-Vargas A, Palma-Tirado L, Ruiz-Herrera J. Metamorphosis of the Basidiomycota Ustilago maydis: transformation of yeast-like cells into basidiocarps. Fungal Genet Biol. 2012;49:765–71. doi: 10.1016/j.fgb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Brefort T, Doehlemann G, Mendoza-Mendoza A, Reissmann S, Djamei A, Kahmann R. Ustilago maydis as a Pathogen. Annu Rev Phytopathol. 2009;47:423–45. doi: 10.1146/annurev-phyto-080508-081923. [DOI] [PubMed] [Google Scholar]
- 15.Skibbe DS, Doehlemann G, Fernandes J, Walbot V. Maize tumors caused by Ustilago maydis require organ-specific genes in host and pathogen. Science. 2010;328:89–92. doi: 10.1126/science.1185775. [DOI] [PubMed] [Google Scholar]
- 16.Heimel K, Scherer M, Vranes M, Wahl R, Pothiratana C, Schuler D, et al. The transcription factor Rbf1 is the master regulator for b-mating type controlled pathogenic development in Ustilago maydis. PLoS Pathog. 2010;6:e1001035. doi: 10.1371/journal.ppat.1001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torto TA, Li S, Styer A, Huitema E, Testa A, Gow NA, et al. EST mining and functional expression assays identify extracellular effector proteins from the plant pathogen Phytophthora. Genome Res. 2003;13:1675–85. doi: 10.1101/gr.910003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemetsberger C, Herrberger C, Zechmann B, Hillmer M, Doehlemann G. The Ustilago maydis effector Pep1 suppresses plant immunity by inhibition of host peroxidase activity. PLoS Pathog. 2012;8:e1002684. doi: 10.1371/journal.ppat.1002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahiri A, Heimel K, Wahl R, Rath M, Kämper J. The Ustilago maydis forkhead transcription factor Fox1 is involved in the regulation of genes required for the attenuation of plant defenses during pathogenic development. Mol Plant Microbe Interact. 2010;23:1118–29. doi: 10.1094/MPMI-23-9-1118. [DOI] [PubMed] [Google Scholar]
- 20.Doehlemann G, van der Linde K, Assmann D, Schwammbach D, Hof A, Mohanty A, et al. Pep1, a secreted effector protein of Ustilago maydis, is required for successful invasion of plant cells. PLoS Pathog. 2009;5:e1000290. doi: 10.1371/journal.ppat.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doehlemann G, Reissmann S, Assmann D, Fleckenstein M, Kahmann R. Two linked genes encoding a secreted effector and a membrane protein are essential for Ustilago maydis-induced tumour formation. Mol Microbiol. 2011;81:751–66. doi: 10.1111/j.1365-2958.2011.07728.x. [DOI] [PubMed] [Google Scholar]
- 22.Khrunyk Y, Münch K, Schipper K, Lupas AN, Kahmann R. The use of FLP-mediated recombination for the functional analysis of an effector gene family in the biotrophic smut fungus Ustilago maydis. New Phytol. 2010;187:957–68. doi: 10.1111/j.1469-8137.2010.03413.x. [DOI] [PubMed] [Google Scholar]
- 23.Teertstra WR, Deelstra HJ, Vranes M, Bohlmann R, Kahmann R, Kämper J, et al. Repellents have functionally replaced hydrophobins in mediating attachment to a hydrophobic surface and in formation of hydrophobic aerial hyphae in Ustilago maydis. Microbiology. 2006;152:3607–12. doi: 10.1099/mic.0.29034-0. [DOI] [PubMed] [Google Scholar]
- 24.Müller O, Schreier PH, Uhrig JF. Identification and characterization of secreted and pathogenesis-related proteins in Ustilago maydis. Mol Genet Genomics. 2008;279:27–39. doi: 10.1007/s00438-007-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vollmeister E, Schipper K, Baumann S, Haag C, Pohlmann T, Stock J, et al. Fungal development of the plant pathogen Ustilago maydis. FEMS Microbiol Rev. 2012;36:59–77. doi: 10.1111/j.1574-6976.2011.00296.x. [DOI] [PubMed] [Google Scholar]
- 26.Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:751–7. doi: 10.1016/S0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 27.Mayer AM, Staples RC, Gil-ad NL. Mechanisms of survival of necrotrophic fungal plant pathogens in hosts expressing the hypersensitive response. Phytochemistry. 2001;58:33–41. doi: 10.1016/S0031-9422(01)00187-X. [DOI] [PubMed] [Google Scholar]
- 28.Elías-Villalobos A, Fernández-Álvarez A, Ibeas JI. The general transcriptional repressor Tup1 is required for dimorphism and virulence in a fungal plant pathogen. PLoS Pathog. 2011;7:e1002235. doi: 10.1371/journal.ppat.1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horst RJ, Zeh C, Saur A, Sonnewald S, Sonnewald U, Voll LM. The Ustilago maydis Nit2 homolog regulates nitrogen utilization and is required for efficient induction of filamentous growth. Eukaryot Cell. 2012;11:368–80. doi: 10.1128/EC.05191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholls S, MacCallum DM, Kaffarnik FAR, Selway L, Peck SC, Brown AJP. Activation of the heat shock transcription factor Hsf1 is essential for the full virulence of the fungal pathogen Candida albicans. Fungal Genet Biol. 2011;48:297–305. doi: 10.1016/j.fgb.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du H, Guan G, Xie J, Sun Y, Tong Y, Zhang L, et al. Roles of Candida albicans Gat2, a GATA-type zinc finger transcription factor, in biofilm formation, filamentous growth and virulence. PLoS ONE. 2012;7:e29707. doi: 10.1371/journal.pone.0029707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh P, Chauhan N, Ghosh A, Dixon F, Calderone RS. SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect Immun. 2004;72:2390–4. doi: 10.1128/IAI.72.4.2390-2394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wormley FL, Jr., Heinrich G, Miller JL, Perfect JR, Cox GM. Identification and characterization of an SKN7 homolog in Cryptococcus neoformans. Infect Immun. 2005;73:5022–30. doi: 10.1128/IAI.73.8.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brachmann A, Weinzierl G, Kämper J, Kahmann R. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol Microbiol. 2001;42:1047–63. doi: 10.1046/j.1365-2958.2001.02699.x. [DOI] [PubMed] [Google Scholar]
- 35.Smith SE, Smith FA. Structure and function of the interfaces in biotrophic symbioses as they relate to nutrient transport. New Phytol. 1990;114:1–38. doi: 10.1111/j.1469-8137.1990.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Herrera J, León-Ramirez C, Guevara-Olvera L, Cárabez-Trejo A. Yeast-mycelial dimorphism of haploid and diploid strains of Ustilago maydis. Microbiology. 1995;141:695–703. doi: 10.1099/13500872-141-3-695. [DOI] [Google Scholar]
- 37.El Gueddari NE, Rauchhaus U, Moerschbacher BM, Deising HB. Developmentally regulated conversion of surface-exposed chitin to chitosan in cell walls of plant pathogenic fungi. New Phytol. 2002;156:103–12. doi: 10.1046/j.1469-8137.2002.00487.x. [DOI] [Google Scholar]
- 38.Robledo-Briones M, Ruiz-Herrera J. Transcriptional regulation of the genes encoding chitin and β-1,3-glucan synthases from Ustilago maydis. Curr Microbiol. 2012;65:85–90. doi: 10.1007/s00284-012-0129-0. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert NM, Donlin MJ, Gerik KJ, Specht CA, Djordjevic JT, Wilson CF, et al. KRE genes are required for β-1,6-glucan synthesis, maintenance of capsule architecture and cell wall protein anchoring in Cryptococcus neoformans. Mol Microbiol. 2010;76:517–34. doi: 10.1111/j.1365-2958.2010.07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates S, Hughes HB, Munro CA, Thomas WP, MacCallum DM, Bertram G, et al. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J Biol Chem. 2005;281:90–8. doi: 10.1074/jbc.M510360200. [DOI] [PubMed] [Google Scholar]
- 41.Nishikawa A, Poster JB, Jigami Y, Dean N. Molecular and phenotypic analysis of CaVRG4, encoding an essential Golgi apparatus GDP-mannose transporter. J Bacteriol. 2002;184:29–42. doi: 10.1128/JB.184.1.29-42.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rouabhia M, Schaller M, Corbucci C, Vecchiarelli A, Prill SK, Giasson L, et al. Virulence of the fungal pathogen Candida albicans requires the five isoforms of protein mannosyltransferases. Infect Immun. 2005;73:4571–80. doi: 10.1128/IAI.73.8.4571-4580.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson GM, Fox DS, Wang P, Alspaugh JA, Buchanan KL. Role of protein O-mannosyltransferase Pmt4 in the morphogenesis and virulence of Cryptococcus neoformans. Eukaryot Cell. 2007;6:222–34. doi: 10.1128/EC.00182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández-Alvarez A, Elías-Villalobos A, Ibeas JI. The O-mannosyltransferase PMT4 is essential for normal appressorium formation and penetration in Ustilago maydis. Plant Cell. 2009;21:3397–412. doi: 10.1105/tpc.109.065839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur R, Ma B, Cormack BP. A family of glycosylphosphatidylinositol-linked aspartyl proteases is required for virulence of Candida glabrata. Proc Natl Acad Sci USA. 2007;104:7628–33. doi: 10.1073/pnas.0611195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richard M, Ibata-Ombetta S, Dromer F, Bordon-Pallier F, Jouault T, Gaillardin C. Complete glycosylphosphatidylinositol anchors are required in Candida albicans for full morphogenesis, virulence and resistance to macrophages. Mol Microbiol. 2002;44:841–53. doi: 10.1046/j.1365-2958.2002.02926.x. [DOI] [PubMed] [Google Scholar]
- 47.Lillico S, Field MC, Blundell P, Coombs GH, Mottram JC. Essential roles for GPI-anchored proteins in African trypanosomes revealed using mutants deficient in GPI8. Mol Biol Cell. 2003;14:1182–94. doi: 10.1091/mbc.E02-03-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang T, Milne KG, Güther ML, Smith TK, Ferguson MA. Cloning of Trypanosoma brucei and Leishmania major genes encoding the GlcNAc-phosphatidylinositol de-N-acetylase of glycosylphosphatidylinositol biosynthesis that is essential to the African sleeping sickness parasite. J Biol Chem. 2002;277:50176–82. doi: 10.1074/jbc.M208374200. [DOI] [PubMed] [Google Scholar]
- 49.Wösten HA, Bohlmann R, Eckerskorn C, Lottspeich F, Bölker M, Kahmann R. A novel class of small amphipathic peptides affect aerial hyphal growth and surface hydrophobicity in Ustilago maydis. EMBO J. 1996;15:4274–81. [PMC free article] [PubMed] [Google Scholar]
- 50.Mendgen K, Hahn M. Plant infection and the establishment of fungal biotrophy. Trends Plant Sci. 2002;7:352–6. doi: 10.1016/S1360-1385(02)02297-5. [DOI] [PubMed] [Google Scholar]
- 51.Banuett F, Herskowitz I. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc Natl Acad Sci USA. 1989;86:5878–82. doi: 10.1073/pnas.86.15.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruiz-Herrera J, León-Ramírez C, Cabrera-Ponce JL, Martínez-Espinoza AD, Herrera-Estrella L. Completion of the sexual cycle and demonstration of genetic recombination in Ustilago maydis in vitro. Mol Gen Genet. 1999;262:468–72. doi: 10.1007/s004380051107. [DOI] [PubMed] [Google Scholar]
- 53.Martin F, Delareulle C, Hilbert JL. An improved ergosterol assay to estimate fungal biomass in ectomycorrhizas. Mycol Res. 1990;94:1059–64. doi: 10.1016/S0953-7562(09)81333-6. [DOI] [Google Scholar]
- 54.Specht CA, Liu Y, Robbins PW, Bulawa CE, Iartchouk N, Winter KR, et al. The chsD and chsE genes of Aspergillus nidulans and their roles in chitin synthesis. Fungal Genet Biol. 1996;20:153–67. doi: 10.1006/fgbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 55.Reissig JL, Storminger JL, Leloir LF. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955;217:959–66. [PubMed] [Google Scholar]
- 56.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 57.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 58.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, B. 1995;57:289–300. [Google Scholar]
- 59.Ruepp A, Zollner A, Maier D, Albermann K, Hani J, Mokrejs M, et al. The FunCat, a functional annotation scheme for systematic classification of proteins from whole genomes. Nucleic Acids Res. 2004;32:5539–45. doi: 10.1093/nar/gkh894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller O, Kahmann R, Aguilar G, Trejo-Aguilar B, Wu A, de Vries RP. The secretome of the maize pathogen Ustilago maydis. Fungal Genet Biol. 2008;45:S63–70. doi: 10.1016/j.fgb.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz-Herrera J, Ortiz-Castellanos L, Martínez AI, León-Ramírez C, Sentandreu R. Analysis of the proteins involved in the structure and synthesis of the cell wall of Ustilago maydis. Fungal Genet Biol. 2008;45:S71–6. doi: 10.1016/j.fgb.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 62.Robledo-Briones M, Ruiz-Herrera J. Regulation of genes involved in cell wall synthesis and structure during Ustilago maydis dimorphism. FEMS Yeast Res. 2013;13:74–84. doi: 10.1111/1567-1364.12011. [DOI] [PubMed] [Google Scholar]
- 63.UniProt Consortium Update on activities at the Universal Protein Resource (UniProt) in 2013. Nucleic Acids Res. 2013;41:D43–7. doi: 10.1093/nar/gks1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEEG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:290–301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gough J, Karplus K, Hughey R, Chothia C. Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J Mol Biol. 2001;313:903–19. doi: 10.1006/jmbi.2001.5080. [DOI] [PubMed] [Google Scholar]
- 67.Dürrenberger F, Laidlaw RD, Kronstad JW. The hgl1 gene is required for dimorphism and teliospore formation in the fungal pathogen Ustilago maydis. Mol Microbiol. 2001;41:337–48. doi: 10.1046/j.1365-2958.2001.02528.x. [DOI] [PubMed] [Google Scholar]
- 68.Mendoza-Mendoza A, Eskova A, Weise C, Czajkowski R, Kahmann R. Hap2 regulates the pheromone response transcription factor prf1 in Ustilago maydis. Mol Microbiol. 2009;72:683–98. doi: 10.1111/j.1365-2958.2009.06676.x. [DOI] [PubMed] [Google Scholar]
- 69.Molina L, Kahmann R. An Ustilago maydis gene involved in H2O2 detoxification is required for virulence. Plant Cell. 2007;19:2293–309. doi: 10.1105/tpc.107.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wahl R, Wippel K, Goos S, Kämper J, Sauer N. A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis. PLoS Biol. 2010;8:e1000303. doi: 10.1371/journal.pbio.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mayorga ME, Gold SE. Characterization and molecular genetic complementation of mutants affecting dimorphism in the fungus ustilago maydis. Fungal Genet Biol. 1998;24:364–76. doi: 10.1006/fgbi.1998.1078. [DOI] [PubMed] [Google Scholar]
- 72.Abramovitch RB, Yang G, Kronstad JW. The ukb1 gene encodes a putative protein kinase required for bud site selection and pathogenicity in Ustilago maydis. Fungal Genet Biol. 2002;37:98–108. doi: 10.1016/S1087-1845(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 73.Andrews DL, Egan JD, Mayorga ME, Gold SE. The Ustilago maydis ubc4 and ubc5 genes encode members of a MAP kinase cascade required for filamentous growth. Mol Plant Microbe Interact. 2000;13:781–6. doi: 10.1094/MPMI.2000.13.7.781. [DOI] [PubMed] [Google Scholar]
- 74.Regenfelder E, Spellig T, Hartmann A, Lauenstein S, Bölker M, Kahmann R. G proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 1997;16:1934–42. doi: 10.1093/emboj/16.8.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernández-Álvarez A, Marín-Menguiano M, Lanver D, Jiménez-Martín A, Elías-Villalobos A, Pérez-Pulido AJ, et al. Identification of O-mannosylated virulence factors in Ustilago maydis. PLoS Pathog. 2012;8:e1002563. doi: 10.1371/journal.ppat.1002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mayorga ME, Gold SE. The ubc2 gene of Ustilago maydis encodes a putative novel adaptor protein required for filamentous growth, pheromone response and virulence. Mol Microbiol. 2001;41:1365–79. doi: 10.1046/j.1365-2958.2001.02606.x. [DOI] [PubMed] [Google Scholar]
- 77.Valdés-Santiago L, Cervantes-Chávez JA, Ruiz-Herrera J. Ustilago maydis spermidine synthase is encoded by a chimeric gene, required for morphogenesis, and indispensable for survival in the host. FEMS Yeast Res. 2009;9:923–35. doi: 10.1111/j.1567-1364.2009.00539.x. [DOI] [PubMed] [Google Scholar]
- 78.Valdés-Santiago L, Guzmán-de-Peña D, Ruiz-Herrera J. Life without putrescine: disruption of the gene-encoding polyamine oxidase in Ustilago maydis odc mutants. FEMS Yeast Res. 2010;10:928–40. doi: 10.1111/j.1567-1364.2010.00675.x. [DOI] [PubMed] [Google Scholar]
- 79.Valdés-Santiago L, Cervantes-Chávez JA, Winkler R, León-Ramírez CG, Ruiz-Herrera J. Phenotypic comparison of samdc and spe mutants reveals complex relationships of polyamine metabolism in Ustilago maydis. Microbiology. 2012;158:674–84. doi: 10.1099/mic.0.055954-0. [DOI] [PubMed] [Google Scholar]
- 80.Müller P, Katzenberger JD, Loubradou G, Kahmann R. Guanyl nucleotide exchange factor Sql2 and Ras2 regulate filamentous growth in Ustilago maydis. Eukaryot Cell. 2003;2:609–17. doi: 10.1128/EC.2.3.609-617.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garrido E, Voss U, Müller P, Castillo-Lluva S, Kahmann R, Pérez-Martín J. The induction of sexual development and virulence in the smut fungus Ustilago maydis depends on Crk1, a novel MAPK protein. Genes Dev. 2004;18:3117–30. doi: 10.1101/gad.314904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orth AB, Rzhetskaya M, Pell EJ, Tien M. A serine (threonine) protein kinase confers fungicide resistance in the phytopathogenic fungus Ustilago maydis. Appl Environ Microbiol. 1995;61:2341–5. doi: 10.1128/aem.61.6.2341-2345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zavrel M, Majer O, Kuchler K, Rupp S. Transcription factor Efg1 shows a haploinsufficiency phenotype in modulating the cell wall architecture and immunogenicity of Candida albicans. Eukaryot Cell. 2012;11:129–40. doi: 10.1128/EC.05206-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shah JC, Clancy MJ. IME4, a gene that mediates MAT and nutritional control of meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:1078–86. doi: 10.1128/mcb.12.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aréchiga-Carvajal ET, Ruiz-Herrera J. The RIM101/pacC homolog from the basidiomycete Ustilago maydis is functional in multiple pH-sensitive phenomena. Eukaryot Cell. 2005;4:999–1008. doi: 10.1128/EC.4.6.999-1008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brefort T, Müller P, Kahmann R. The high-mobility-group domain transcription factor Rop1 is a direct regulator of prf1 in Ustilago maydis. Eukaryot Cell. 2005;4:379–91. doi: 10.1128/EC.4.2.379-391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.