Abstract
The capability of currently available echocardiography-based strain estimation techniques to fully map myocardial abnormality at early stages of myocardial ischemia is yet to be investigated. In this study, myocardial elastography (ME), a radio-frequency (RF)-based strain imaging technique that maps the full 2D transmural angle-independent strain tensor in standard echocardiographic views at both high spatial and temporal resolution is presented. The objectives were to (1) evaluate the performance of ME on mapping the onset, extent and progression of myocardial ischemia at graded coronary constriction levels (from partial to complete coronary flow reduction), and (2) validate the accuracy of the strain estimates against sonomicrometry (SM) measurements. A non-survival canine ischemic model (n = 5) was performed by gradually constricting the left anterior descending (LAD) coronary blood flow from 0% (baseline blood flow) to 100% (zero blood flow) at 20% increments. An open-architecture ultrasound system was used to acquire RF echocardiograms in a standard full short-axis view at the frame rate of 211 fps, at least twice higher than what is typically used in conventional echocardiographic systems, using a previously developed, fully automated composite technique. Myocardial deformation was estimated by ME and validated against sonomicrometry. ME estimates and maps transmural (1) 2D displacements using RF cross-correlation and recorrelation; and (2) 2D polar (radial and circumferential) strains, derived from 2D (i.e. both lateral and axial) displacement components, at high accuracy. Full-view strain images were shown and found to reliably depict decreased myocardial function in the region at risk at increased levels of coronary flow reduction. The ME radial strain was deemed to be a more sensitive, quantitative, regional measure of myocardial ischemia as a result of coronary flow reduction when compared to the conventional wall motion score index and ejection fraction. Good agreement (0.22% strain bias, 95% limits of agreement) using Bland–Altman analysis and good correlation (r = 0.84) were found between the ME and SM measurements. These findings demonstrate for the first time that ME could map angle-independent strains to non-invasively detect, localize and characterize the early onset of myocardial ischemia, i.e. at 40%, and possibly as low as 20%, LAD flow reduction, which could be further associated with the severity of coronary stenosis.
1. Introduction
Early diagnosis of coronary artery disease, such as myocardial ischemia caused by reduced coronary blood flow associated with coronary stenosis (Nichols and O’Rourke 2005), is critical for timely and appropriate treatment. The relationship between reduced coronary blood flow and impaired left-ventricular deformation has previously been investigated in vivo in anesthetized (Lekven et al 1973, Kerber et al 1975, Waters et al 1977, Gallagher et al 1980, 1987, Stowe et al 1978) and awake animals (Vatner 1980, Gallagher et al 1983, 1987). As a result, techniques that can be employed to quantify regional myocardial deformation can shed light on the detection of myocardial ischemia resulting from stenosed coronary arteries. Non-invasive imaging techniques, such as echocardiography and magnetic resonance imaging (MRI), have emerged to quantify myocardial deformation (or, strain). Compared to MRI, echocardiography typically enjoys lower cost, portability, and compatibility with pacemakers as well as higher temporal and spatial resolution in its functional estimates such as blood flow, valve function and strain imaging.
Several groups have validated their echocardiography-based strain analysis techniques against sonomicrometry in animal models in vivo (Amundsen et al 2006, Langeland et al 2005, 2006, Papademetris et al 2001, Urheim et al 2000), where myocardial ischemia was induced by performing complete occlusion (i.e. ligation) of the coronary arteries. Amundsen et al (2006) validated their speckle tracking echocardiography technique against sonomicrometry in dogs in both normal and ischemic (5 to 15 min ligation of the left anterior descending (LAD) coronary artery) states. Langeland et al (2005, 2006) validated their echocardiographic strain estimates against sonomicrometry measurements in the ovine inferolateral wall, under both normal and ischemic (ligation of the distal left circumflex (LCx) coronary artery) conditions in vivo. Papademetris et al (2001) correlated their shape-tracking algorithm in three-dimensional (3D) echocardiograms against sonomicrometry before and after LAD ligation. Urheim et al (2000) validated the longitudinal strains estimated by Doppler echocardiography against sonomicrometry in apical views of canine left ventricles during both control and ischemic (LAD ligation) conditions.
Even though the aforementioned studies have validated their echocardiography-based techniques in the normal and coronary artery ligation cases against sonomicrometry in vivo, temporal strain profiles, i.e. without always including 2D strain images, have mainly been shown due to the additional challenge presented in high quality strain imaging, which may facilitate the instantaneous identification of abnormal myocardial regions across the entire echocardiographic view.
Despite the aforementioned studies on extreme coronary flow compromise, strain-imaging-based assessment of myocardial dysfunction at earlier stages (or earlier onset) of myocardial ischemia caused by partial coronary stenosis is much less known by comparison. Jamal et al (2001) and Reant et al (2008) reported on the change of regional myocardial function quantified by echocardiography-based strains under graded hypoperfusion of the coronary arteries in pigs. Jamal et al 2001 measured the radial strain based on color Doppler myocardial imaging (CDMI), while Reant et al 2008 measured radial, circumferential and longitudinal strains from 2D short-axis and apical views using a commercial software, and validated against sonomicrometry. Although their derived strains detected the regional myocardial abnormality under partially reduced coronary flow, full-view strain images were not provided at each level of the hypoperfusion.
In this paper, the performance of strain imaging using an echocardiographic, angle-independent technique, namely myocardial elastography (ME) (Konofagou et al 2002, Lee et al 2007, 2008, Lee and Konofagou 2008, Luo and Konofagou 2008, Wang et al 2008, Zervantonakis et al 2007, Luo et al 2007, Luo et al 2009), under graded coronary flow reduction is evaluated. Different from previously reported speckle-tracking-based strain imaging methods, ME, which is radio-frequency (RF)-based and angle-independent (Lee et al 2007, Zervantonakis et al 2007), aims at imaging transmural 2D displacements and angle-independent strains in full echocardiographic views at simultaneously high spatial and temporal resolution for the detection, localization, and identification of dysfunctional myocardial segments. Its reliable performance in imaging transmural myocardial deformation has previously been demonstrated in both a theoretical framework (Lee et al 2007, Lee and Konofagou 2008) and clinical setting (Lee and Konofagou 2008, Lee et al 2008), in normal and pathological cases. In this study, ME is evaluated for the first time in mapping and quantifying myocardial function under graded myocardial ischemia conditions. Ischemia was induced by steadily reduced LAD flow in anesthetized open-chest dogs in vivo, and the findings were validated against sonomicrometry and compared to independently obtained wall motion score index and ejection fraction measurements.
2. Methods
2.1. Experimental protocol
2.1.1. Animal preparation
Five mongrel male dogs, weighing 27.6 ± 2.7 kg, were anesthetized with an intravenous injection of thiopental (10–17 mg kg−1) and morphine (0.15 mg kg−1), intubated and maintained with isoflurane (0.5–5%). Lidocaine was infused at a constant rate of 50 μg kg−1 h−1. The respiration of the animal was controlled by a ventilator. The electrocardiogram (ECG), arterial blood pressure and oxygen saturation were monitored throughout every experiment. A heating pad was used to maintain body temperature. Upon lateral thoracotomy, the pericardium was removed for further constrictor placement and crystal implantation. The removal of the pericardium did not influence the results of this study since this work dealt with changes in the myocardium as a result of progressive coronary flow reduction with respect to the baseline.
2.1.2. Instrumentation
A pressure catheter (SPR-360, Millar Instruments Inc., Houston, TX, USA) was inserted into the left ventricle via the right carotid artery to monitor the left-ventricular pressure. A flow probe (figure 1) (2.5SB, Transonic Systems Inc., Ithaca, NY, USA) connected to a Transonic® flowmeter (T206) was placed around the LAD to measure the coronary flow.
Figure 1.
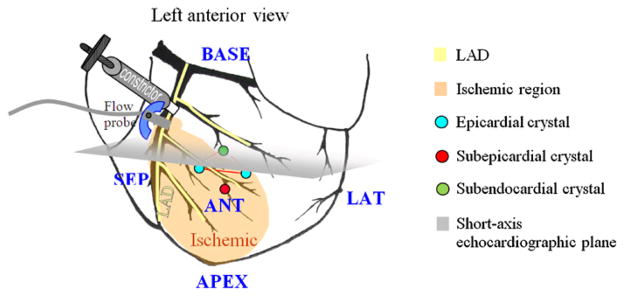
Left anterior view of a schematic heart. Sub-endocardial, sub-epicardial, and epicardial crystals form a tetrahedron and are indicated. A constrictor and a flow probe were placed after the first diagonal of the left anterior descending (LAD) coronary artery. The expected ischemic region is shown in shaded yellow. The short-axis echocardiographic plane is shown in gray. Anterior (ANT), lateral (LAT), posterior (POST), septal (SEP), basal (BASE) and apical (APEX) regions are indicated.
A customized constrictor (figure 1) was placed around the LAD, distal to the first diagonal branch, and was manually adjusted to gradually restrict the coronary flow in the LAD, from 0% to 100% at 20% increments to induce regional myocardial ischemia. Note that the constriction increments throughout this paper refer to the percentage of LAD flow reduction. The flow was monitored and allowed enough time to stabilize. The stabilization duration, depending on the animal’s coronary autoregulation response, ranged between 15 and 40 min. The 100% (i.e. complete) LAD flow reduction level was sustained for 30 min. In two animals, reperfusion at the full release of the constrictor was thereafter performed and lasted for 15 min prior to acquiring echocardiographic RF and sonomicrometry data for the determination of myocardial viability. All animals were euthanized with pentobarbital (100 mg kg−1) at the termination of each experiment.
A total of ten piezoelectric crystals (Sonometrics Corp., London, Ontario, Canada) were placed on the left ventricle of each dog, and the distance between all crystal pairs was continuously monitored. Four piezoelectric crystals were used in the apical, basal and mid-ventricular levels for global function measurements (e.g., P–V loops) and to validate ischemia conditions. A set of four piezoelectric crystals (figure 1) was thus positioned in a tetrahedral configuration in the anticipated ischemic (i.e. anterior) region. The average separation of the tetrahedral crystals was equal to 13.5 mm. The sub-epicardial crystal was implanted immediately beneath the epicardium, while the sub-endocardial crystal was inserted using an introducer, ensuring implantation depth. Even though the extent of the ischemic region can reliably be predicted when constricting the LAD, at lower flow reduction levels, such predictions become more challenging. In addition, the difference in coronary architecture in different animals poses additional challenges in the implantation of the crystals. One-minute long sonomicrometry data acquisition at a sampling rate of 379 Hz was separately acquired in each one of the seven (i.e. six constriction and the reperfusion) configurations immediately prior to each echocardiographic data acquisition to ensure similar functional conditions while avoiding ultrasonic signal interference.
At the end of each experiment, the animals were sacrificed, and their hearts excised, stored at 4 °C for 6–10 h, sliced at 10 mm thickness, immersed in 1% triphenyltetrazolium chloride (TTC) solution, incubated at 37 °C for 1.5 h and fixed in a 10% formalin solution for 30 min. The region unstained by TTC appeared pale in color, indicating the infarct formed at reperfusion, while the TTC-stained region appeared red, indicating the viable myocardium (Khalil et al 2006). This pathology procedure was considered as a preliminary step for examining the myocardial viability in the case of the graded coronary flow reduction model. All animal procedures were approved prior to use by the Institutional Animal Care and Use Committee of Columbia University.
2.1.3. RF echocardiographic data acquisition
A Sonix RP system (Ultrasonix Medical Corp., Richmond, BC, Canada) with a 3.3 MHz phased array probe (beam density of 128) was used to acquire RF echocardiograms in a full, standard 2D short-axis view at the frame rate of 211 fps, higher than other commercially available echocardiography systems, using a customized, automated composite technique developed by our group (Wang et al 2008). A stabilizer (Medtronic Inc., Minneapolis, MN, USA) was used to keep the ultrasound transducer fixed during imaging and data acquisition. Degassed ultrasound gel added in the sternal cavity was deemed essential in improving the image quality. The ventilator was switched off to avoid respiratory artifacts during acquisition for optimal, off-line RF-image reconstruction using ECG gating.
2.2. Strain calculation
2.2.1. Sonomicrometry strain measurement
The regional strain was calculated in cardiac coordinates based on the shape change of the crystal tetrahedron. Further details are provided in the appendix.
2.2.2. ME strain estimation
The two in-plane orthogonal displacement components (lateral and axial) were first iteratively estimated on the RF signals using cross-correlation and recorrelation in a 2D search (matching kernel of 3.5 mm; 80% overlap) (Lee et al 2007) to ensure spatial resolution of 0.7 mm and then integrated to obtain the cumulative displacements occurred during one cardiac cycle with end-diastole (ED) as the reference configuration. Finite Lagrangian lateral and axial strains were computed from the cumulative displacements (Lee et al 2007) using a least-squares strain estimator (LSQSE) (Kallel and Ophir 1997) with a kernel of 8.5 mm (lateral) by 3.4 mm (axial). Radial and circumferential strains, which are inherently angle-independent, were further obtained from Lagrangian finite lateral and axial strains through coordinate transformation in order to depict the myocardial deformation in polar coordinates and facilitate the detection of the ischemic region (Lee et al 2007, Zervantonakis et al 2007). Note that each finite strain component involves the gradient operation from both the lateral and axial displacement components (Lee et al 2007). That is to say, good estimation accuracy for both lateral and axial displacements is critical for strain imaging of good quality throughout the entire myocardial region.
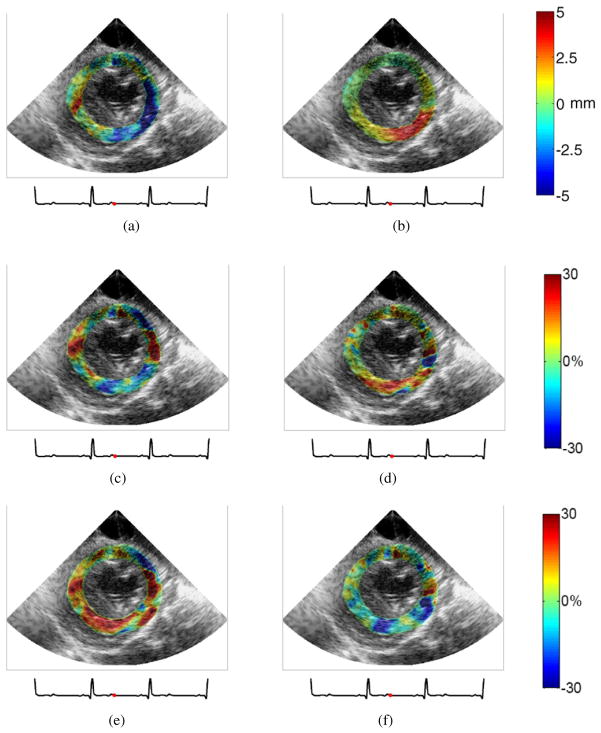
Figure 2 shows the initial (cumulative lateral (a) and axial (b) displacements), intermediate (cumulative lateral (c) and axial (d) strains) and the final (cumulative radial (e) and circumferential (f) strains) outputs of ME in the short-axis view at end systole in the case of reperfusion. Positive and negative lateral displacements indicate rightward and leftward motion, respectively (figure 2(a)), while positive and negative axial displacements indicate upward and downward motion, respectively (figure 2(b)). Positive and negative lateral/axial strains represent tension and compression in the lateral/axial direction, respectively (figures 2(c) and (d)). Radial thickening and thinning are denoted by positive and negative strain values, respectively (figure 2(e)), whereas circumferential shortening and stretching are denoted by negative and positive strains, respectively (figure 2(f)). This example again demonstrated that good quality strain images necessitated accurate displacement estimates.
Figure 2.
Cumulative systolic myocardial displacement and deformation images of a canine left ventricle after reperfusion in a short-axis view mapped at end systole: (a) lateral displacement, (b) axial displacement, (c) lateral strain, (d) axial strain, (e) radial strain, and (f) circumferential strain.
Some previous studies reported that radial thickening was more sensitive than circumferential deformation for the detection of the myocardial dysfunction, especially in the subendocardial region, which is more vulnerable to hypoperfusion (Armstrong et al 2000, Gallagher et al 1986). Furthermore, wall systolic thickening (i.e. radial deformation) was better documented for the verification of our findings. Thus, the remainder of this paper focused on the radial strain component and its change with variable stages of myocardial ischemia.
The advantage of ME was to provide transmural strain estimates of high spatial resolution. In contrast to ME, sonomicrometry measures strains in an average fashion, outputting only one single strain value within each crystal tetrahedron (figure 1; figure A1) despite it being served as the gold standard in this study. In order to preserve the high strain resolution provided by ME while ensuring a fair comparison with sonomicrometry measurements, the transmural radial ME strains were averaged within a region-of-interest (ROI) of 3 × 3 mm2 centered around the mid-wall enclosed by the implanted crystals, in order to match the lowest wall thickness at systolic radial thinning (i.e. in the case of 100% coronary flow reduction).
2.3. Wall motion score index and ejection fraction calculation
The wall motion score index (WMSI) (using reverse scoring) and ejection fraction (EF) were evaluated by two experienced cardiologists and echocardiography experts in the short-axis views and both apical four- and two-chamber, manually traced echocardiographic views based on the modified Simpson’s rule (Otto 2004), respectively.
3. Results
The short-axis, systolic radial strain images in a canine left ventricle, over all six coronary flow and reperfusion cases are shown in figure 3. Positive and negative radial strains indicate radial thickening and radial thinning, respectively. There are two important observations. First, the finite radial strain is derived from both the lateral and axial displacement components (Lee et al 2007). Second, in the short-axis view, radial strain in the lateral/septal wall region is mainly contributed from the lateral displacement, whereas in the anterior/posterior wall region it is dominated by the axial displacement. Hence, both accurate lateral and axial displacement estimates are the key to obtaining reliable radial strain maps across the entire section of the left ventricle.
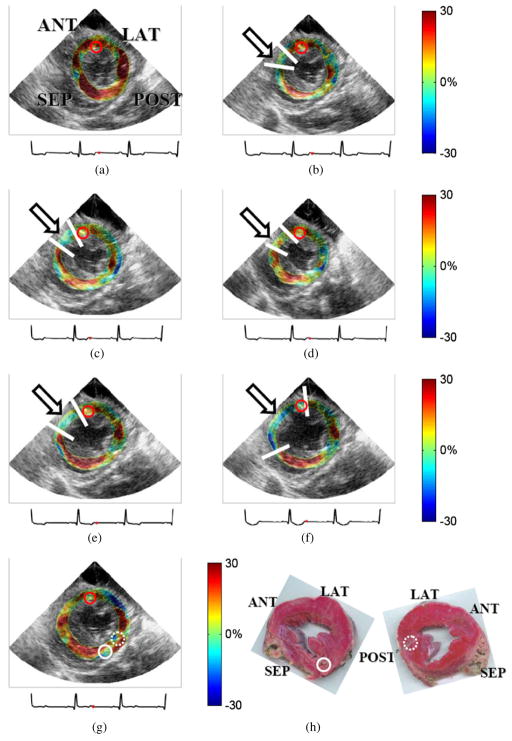
Figure 3.
Cumulative systolic radial strain images of the same canine left ventricle as in figure 2 at (a) 0%, (b) 20%, (c) 40%, (d) 60%, (e) 80% and (f) 100% LAD flow reduction levels and (g) after reperfusion. The red dot on the ECG (below each image) indicates the corresponding cardiac phase. The arrows and the white lines on the anterior wall region indicate the ischemic region. Regions are indicated as in figure 1. (h) Both sides of one canine mid-ventricular slice stained using 1% TTC solution with pale (unstained by TTC) and red (stained by TTC) colors indicating infarcted and normal tissues, respectively. The left and right pathology images are the apical and basal sides of the same heart slice, respectively. The pathology image viewed from the apical side was oriented in accordance with the echocardiogram. Solid, dotted and red solid circles indicate the implanted SM crystal locations and transmural recordings.
At baseline (i.e., 0% LAD flow reduction; flow: 21 mL min−1), radial thickening throughout the entire myocardium was observed (figure 3(a)). At 20% flow reduction (figure 3(b)), reduced radial thickening and thinning were observed in the anterior sub-epicardium and sub-endocardium, respectively. At 40% (figure 3(c)), 80% (figure 3(e)) and 100% (figure 3(f)) flow reduction, the anterior wall regions underwent radial thinning, while the remaining myocardium continued to exhibit radial thickening. The reduced radial strains indicated myocardial dysfunction due to ischemia in the anterior region in this canine heart.
Figure 3(g) shows systolic radial strain images of the reperfused left ventricle. The anterior segment in figure 3(g) presented systolic radial thickening after reperfusion. Figure 3(h) shows both apical and basal pathology findings, corresponding to the short-axis image of figure 3(g). The radial strain (figure 3(g)) in the anterior wall region, indicating viability of that muscle after reperfusion, was confirmed by the red color on the TTC-stained slice (figure 3(h)).
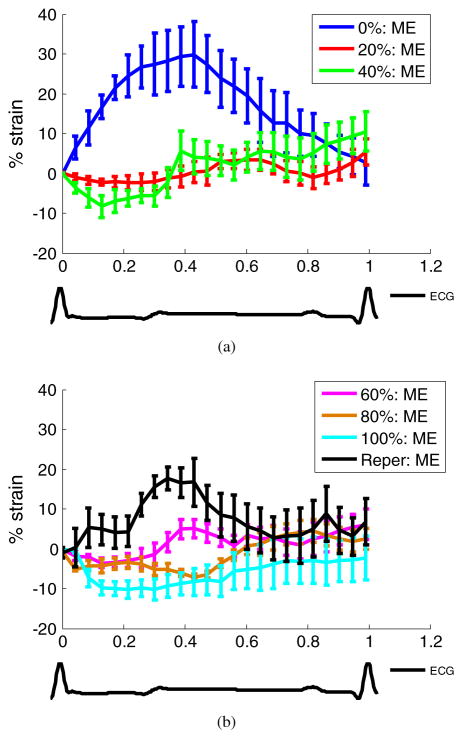
Temporal cumulative radial strain profiles of the same canine left ventricle (figure 3) in the anterior wall segment over the entire cardiac cycle are shown in figure 4. The error bars (equal to one standard deviation) indicate the transmural variation of the radial strain within the segment. The overall decreasing radial strain magnitude showed that regional myocardial deformation steadily decreased with increasing coronary flow reduction. Anterior radial thinning during systole was first identified at 20% flow reduction (figure 4(a)) and was increasingly pronounced in both strain magnitude and spatial extent at higher constriction levels up to 100% (figure 4(b)). However, radial thickening in the same region was detected upon reperfusion, indicating that the function had been reinstated.
Figure 4.
Temporal radial strain curves of the same canine heart as shown in figure 3 in an anterior wall region of 3 × 3 mm2 at (a) 0%, 20%, 40%, and (b) 60%, 80%, and 100% LAD flow reduction levels and after reperfusion (Reper). Below the figures is shown the ECG at baseline. ME denotes myocardial elastography. Error bars equal one standard deviation.
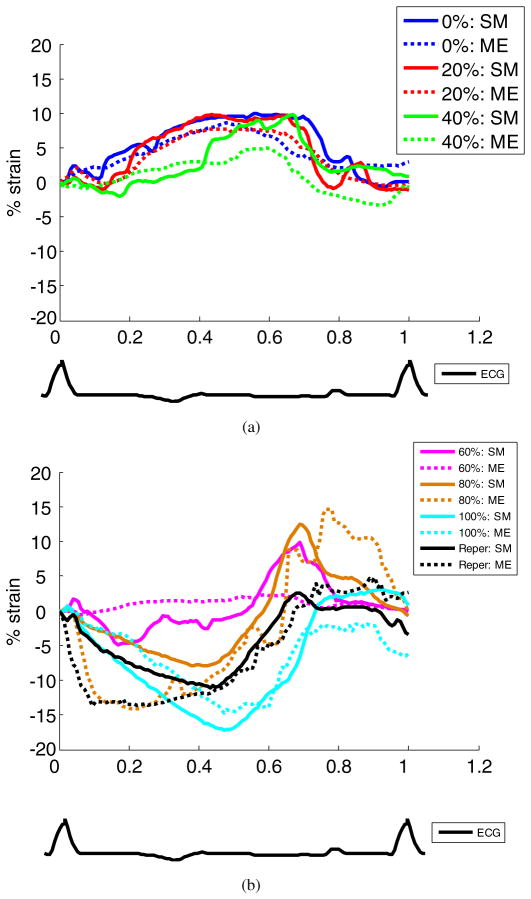
Temporal cumulative radial strain profiles of a second canine left ventricle, from both sonomicrometry and ME, over the entire cardiac cycle are shown in figure 5. Not only was the ME radial strain in good agreement with the sonomicrometry strain, but it also reliably depicted the progression of ischemia across the myocardial wall (figure 5).
Figure 5.
Temporal radial strain curves of another canine heart in an anterior–lateral wall region of 3 × 3 mm2 at (a) 0%, 20%, 40%, and (b) 60%, 80%, and 100% LAD flow reduction levels and after reperfusion (Reper). Below the figure is shown the ECG at baseline. ME and SM denote myocardial elastography and sonomicrometry, respectively.
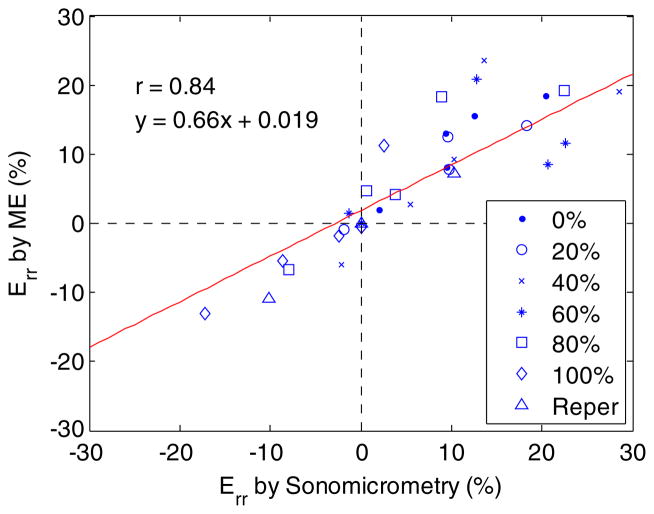
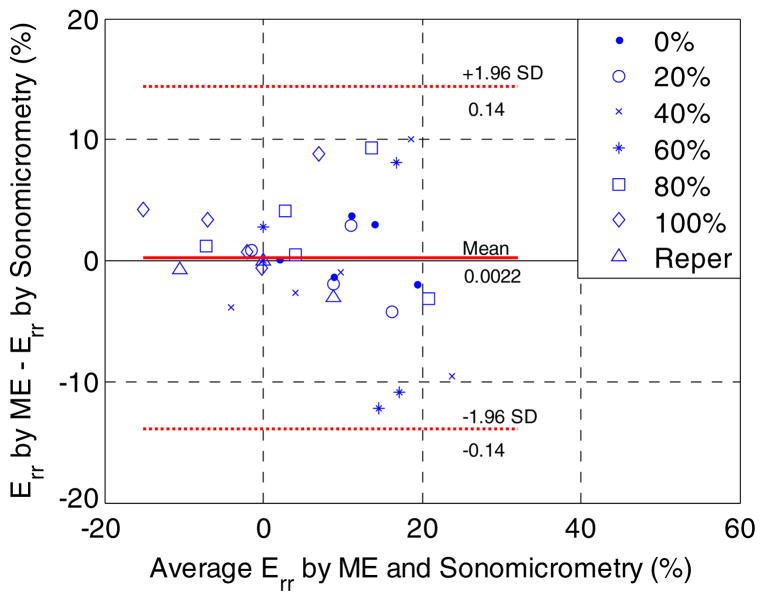
Good correlation (r = 0.84) was found in the end-systolic radial strain of the ischemic region between sonomicrometry and ME at all graded flow reduction levels in all animals studied (figure 6). The Bland–Altman analysis (Altman and Bland 1983) in all flow reduction and reperfusion cases (figure 7) confirmed good agreement in the end-systolic radial strain between the two methods with a bias of 0.22% strain and 95% limits of agreement (−13.9% to 14.3% strain). Compared to the range of the average systolic radial strain measured (between −20% and 25% strain), the bias measured was deemed insignificant.
Figure 6.
A scatter plot of the end-systolic radial strain (Err) in an anterior wall region of 3 × 3 mm2 between myocardial elastography (ME) and sonomicrometry at six different LAD flow reduction levels and reperfusion (Reper) in five dogs. A correlation coefficient of 0.84 was found.
Figure 7.
A Bland–Altman plot of the end-systolic radial strains (Err) of five canine left ventricles in an anterior wall region of 3 × 3 mm2 at six different LAD flow reduction levels and reperfusion (Reper). SD denotes standard deviation. The bias and 95% limits of agreement were found to be 0.22% strain and −13.9% to 14.3% strain, respectively.
Table 1 summarizes the end-systolic ME radial strain (Err), WMSI, EF, and end-diastolic left-ventricular pressure (EDLVP) in all five dogs at six graded LAD flow reduction levels and after reperfusion. Overall, Err and WMSI consistently decreased with increasing levels of LAD flow reduction. This showed the progression of ischemia based on the decline of myocardial deformation, Err. The onset of myocardial dysfunction could be determined by a pronounced decrease in Err. If a decrease of 5% strain is assumed to be significant regardless of the initial strain value, the decline in myocardial function was first observed at 80%, 40%, 80%, 40%, and 20% for canine hearts 1 to 5, respectively.
Table 1.
Wall motion score index (WMSI), Err, ejection fraction (EF) and end-diastolic left-ventricular pressure (EDLVP) in five dogs under graded myocardial ischemia. WMSI was assessed with 5: Normal; 4: Mild hypokinetic; 3: Hypokinetic; 2: Severe hypokinetic; 1: Akinetic; 0: Dyskinetic. Err denotes the ME end-systolic radial strain.
| LAD flow reduction | 0% | 20% | 40% | 60% | 80% | 100% | Reperfusion | ||
|---|---|---|---|---|---|---|---|---|---|
| Animal 1 | WMSI | Expert 1 | 5 | 5 | 5 | 5 | 5 | 1 | N/A |
| Expert 2 | 5 | 5 | 5 | 5 | 5 | 0 | N/A | ||
| Err (%) | 23.65 | 28.57 | 27.68 | 27.76 | 19.24 | −7.52 | N/A | ||
| EFecho (%) | 73.86 | 74.11 | 63.83 | 46.43 | 39.51 | 44.26 | N/A | ||
| EDLVP (mmHg) | 13.56 | 16.00 | 17.82 | 18.13 | 14.78 | 18.13 | N/A | ||
| Animal 2 | WMSI | Expert 1 | 5 | 5 | 5 | 3 | 3 | 1 | N/A |
| Expert 2 | 4 | 4 | 4 | 3 | 3 | 1 | N/A | ||
| Err (%) | 37.48 | 30.77 | 24.95 | 11.59 | 2.01 | −4.95 | N/A | ||
| EFecho (%) | 60.17 | 66.92 | 66.90 | 75.45 | 60.33 | 40.68 | N/A | ||
| EDLVP (mmHg) | 17.54 | 24.14 | 22.88 | 24.77 | 24.77 | 31.05 | N/A | ||
| Animal 3 | WMSI | Expert 1 | 5 | 4 | 2 | N/A | 2 | 1 | N/A |
| Expert 2 | 4 | 4 | 3 | N/A | 2 | 1 | N/A | ||
| Err (%) | 17.72 | 16.02 | 12.93 | N/A | 7.67 | −0.45 | N/A | ||
| EFecho (%) | 45.48 | 53.80 | 52.43 | N/A | 44.50 | 35.92 | N/A | ||
| EDLVP (mmHg) | 11.35 | 16.69 | 13.55 | N/A | 28.62 | 30.20 | N/A | ||
| Animal 4 | WMSI | Expert 1 | 2 | 3 | 2 | 2 | 1 | 1 | 2 |
| Expert 2 | 3 | 3 | 3 | 2 | 1 | 1 | 1 | ||
| Err (%) | 8.15 | 7.79 | 2.73 | 1.44 | −2.29 | −13.30 | −11.45 | ||
| EFecho (%) | 38.91 | 41.19 | 44.05 | 33.57 | 20.12 | 19.56 | 28.20 | ||
| EDLVP (mmHg) | 16.93 | 16.93 | 16.93 | 16.61 | 33.27 | 21.33 | 20.70 | ||
| Animal 5 | WMSI | Expert 1 | 5 | 5 | 4 | 5 | 2 | 1 | 4 |
| Expert 2 | 4 | 3 | 3 | 3 | 0 | 0 | 3 | ||
| Err (%) | 28.01 | −1.27 | −1.24 | 1.98 | −5.03 | −9.31 | 18.07 | ||
| EFecho (%) | 64.34 | 58.68 | 38.93 | 33.46 | 27.65 | 17.48 | 40.87 | ||
| EDLVP (mmHg) | 16.55 | 14.98 | 15.61 | 15.91 | 14.98 | 20.01 | 13.41 | ||
4. Discussion
In this paper, the sensitivity of an echocardiographic RF-based, angle-independent strain imaging technique to early onset of disease was investigated. More specifically, the lowest degree of myocardial ischemia (i.e. coronary blood flow reduction) detectable on the systolic strain image of myocardial elastography (ME) was investigated as manifested as an abnormal regional deformation relative to the baseline. The reliability of the technique in early detection of ischemia was assessed against sonomicrometry. To our knowledge, 2D transmural angle-independent strain imaging of the canine left ventricle was shown for the first time in vivo at steadily increasing levels of coronary flow reduction (0% to 100% at 20% increments).
At 20% LAD flow reduction, radial thinning in the sub-endocardium and reduced radial thickening in the sub-epicardium were detected in the anterior wall region (figure 3(b)), in agreement with the fact that the subendocardial region is more susceptible to reduced coronary blood flow (Nichols and O’Rourke 2005). This suggested that non-transmural ischemia may have been induced at 20% flow reduction level and detected on the strain images through depiction of the associated transmural variation (figure 3(b)). Significant decrease in percent shortening in the subendocardial wall at 20% flow reduction has previously been reported with sonomicrometry (Lekven et al 1973, Vatner 1980).
At 60% coronary flow reduction, reduced radial thickening in the anterior wall (figure 3(d)) was observed compared with that at the baseline (figure 3(a)). Nonetheless, no further decreased radial thickening compared with that at 40% flow reduction (figure 3(c)) was noted. Confirmed by experienced cardiologists, the images at the 60% coronary flow reduction level were acquired at a different short-axis plane (figure 3(d)). At the complete constriction (100% reduction), dyskinesia in the anterior wall was clearly detected and mapped (figure 3(f)). This was in agreement with the existing literature that the ischemic region behaved as a passively tethered tissue, exhibiting dyskinesia during systole (Holmes et al 2005).
Figure 3(g) shows partial restoration of the myocardial function in the anterior wall segment after reperfusion but abnormal myocardial deformation in part of the remote myocardium (solid and dotted circles in figure 3(g)). Evident damage caused by implanted crystals in the posterior region was indicated and in pathology (figure 3(h)), justifying the atypical radial deformation present in the remote region on the ME radial strain image. Note that the sutures used for the implantation of crystals remained in position on the excised heart to serve as the co-registration markers between pathology and echocardiographic images.
According to the temporal cumulative ME radial strain profiles shown in figures 4(a) and 5(a), at 40% LAD flow reduction, decreased radial thickening during systole and delayed radial thickening were observed. Up to 80% flow reduction, reversed systolic strain (i.e. radial thinning) was first noted followed by delayed radial thickening (figures 4(b) and 5(b)). This delayed deformation phenomenon has previously been reported by several groups (Korinek et al 2008, Skulstad et al 2002, Takayama et al 1988) under acute ischemia and termed ‘post-systolic shortening/thickening’, a potential indicator of myocardial viability.
The systolic radial strains of another canine left ventricle at 0% coronary flow reduction (figure 5) ranged from −10% to 10% strain, which was low compared to the strain values of figure 4 and those previously reported in the literature (Papademetris et al 2001, Waldman et al 1985). Although this canine left ventricle was found to be hypokinetic at baseline prior to surgery, the entire graded ischemia procedure was successfully performed, and the dyskinetic anterior wall was observed at greater LAD flow reduction levels based on the ME radial strain estimates, in good agreement with the sonomicrometry measurements (figure 5).
The drift of cumulative systolic strain curves in figures 4 and 5 toward the end of the cardiac cycle was noted. This drift was known to possibly stem from insufficient imaging frame rate and estimation errors but could be compensated by forcing the cumulative strain at the end of the cardiac cycle to be zero, assuming that the estimation error was present throughout the entire cardiac cycle (D’hooge et al 2000a, 2000b). The precise sources of the drift are still yet to be studied, and whether this drift should be corrected highly depends on the strain calculation process (D’hooge et al 2000a) and requires a separate study to investigate the drift. In our study, the major cause of the drift in figures 4 and 5 might have been the accumulation of estimation errors starting from the fast filling phase where frame rate was not optimized due to the tradeoff among image resolution, frame rate, and acquisition time in the in vivo configuration. Moreover, our study focused on utilizing strain images during the systolic (i.e. active contraction) phase to characterize the ischemic myocardium. The presence of large out-of-plane motion may also have caused 2D estimation and accumulation errors, which may have further impacted the drift of the strain curve. Should both the sufficient frame rate for the fast filling phase and full 3D imaging be achieved, the compensation of the drift can be fairly performed and justified.
Similar to the EF and WMSI, the end-systolic ME radial strain overall decreased at greater flow reductions (table 1). Unlike the ME radial strains and WMSI, the EF is a global volumetric measure and was shown to be less sensitive to the detection of regional myocardial abnormality at early ischemic stages (40% flow reduction and below). In contrast to WMSI, the ME radial strain images provided detailed (i.e. transmural) quantitative myocardial deformation maps and were immune to the intra- or inter-observer variability.
4.1. Study limitations
The radial strain images shown in figure 3, the standard deviations in figure 4 and radial strain movies provided in the supplementary materials available at stacks.iop.org/PMB/56/1155/mmedia altogether demonstrated that the transmural variation of myocardial deformation could be depicted by ME at high spatial and temporal resolution and that good full 2D displacement and strain tensor estimation advanced reliable and fully detailed strain maps. However, the validation of the radial strains across the wall were only performed in a specific region of the myocardidum given that only one tetrahedron of sonomicrometers was implanted in the myocardial region at risk so as to avoid damaging the myocardial region of interest and to prevent undesirable loading on the myocardium.
Our findings showed that the change in the estimated radial strain was sensitive to graded levels of coronary flow reduction and could be reliably used to depict regional myocardial dysfunction at earlier stages. However, recent studies reported that circumferential and longitudinal strains measured by their echocardiography-based methods were more sensitive than the radial strain to detect myocardial abnormality at early hypoperfusion (e.g., 25% flow-limiting stenosis) (Mizuguchi et al 2008, Reant et al 2008). Their findings, in contradiction with some other studies (Armstrong et al 2000, Gallagher et al 1986), may infer the different accuracy in measuring different strain components inherent in each technique. Our findings that the radial strain images reliably depicted myocardial function throughout the myocardium under graded levels of myocardial ischemia (figure 3) and that ME provided all 2D displacement and strain estimates at good quality (figure 2) merit further assessment of all 3D strain components on their sensitivity in detecting myocardial abnormalities.
In the canine hearts used in this study, physiologic preconditions, well developed collateral circulation and different autoregulation responses hindered the evaluation of the association of graded coronary flow reduction levels with the regional myocardial function. Additionally, the blood flow inside the main LAD, instead of the regional blood flow distribution of the myocardium itself, was measured. These combined reasons may explain why two coronary flow reduction cases (i.e. animals 1 and 3) in table 1 did not exhibit significant reduction in the myocardial function until 80% flow reduction.
Regardless of these limitations, ME was shown capable of imaging myocardial function and localizing the myocardial region mostly affected by the early onset of ischemia as evidenced by reduced transmural thickening or thinning. Ongoing studies entail expansion to 3D strain imaging, in an enhanced protocol (e.g., with the use of colored-microspheres for the evaluation of the region-at-risk) and further validation of those results in larger sample sizes.
5. Conclusion
Strain images in standard, full echocardiographic views were shown at variable levels of myocardial ischemia as caused by coronary flow reduction, at both high spatial and temporal resolution, for the first time to our knowledge. ME was shown capable of non-invasively identifying and characterizing the region of altered myocardial deformation starting at 40%, and possibly as low as 20%, LAD blood flow reduction in vivo as well as identifying viable myocardium upon reperfusion. Not only were the strain estimates at all coronary flow levels confirmed by independent sonomicrometry measurements, but the increasing size of region at risk was clearly mapped on the strain images as the coronary blood flow was progressively reduced, at equal or higher sensitivity with wall motion scoring measurements. These findings thus unveil the potential of strain imaging using ME as a diagnostic tool for the early detection, localization and quantification of myocardial ischemia as well as reliable diagnosis of myocardial viability. Clinical studies on the sensitivity of identifying abnormal myocardial function at early stages of coronary stenosis in a clinical setting are currently ongoing.
Supplementary Material
Acknowledgments
This study was supported in part by the National Institutes of Health (R01EB006042) and the Wallace H Coulter Foundation. The authors wish to thank Heather S Duffy, PhD, previously affiliated with Peter Danilo, PhD, and Iryna N Shlapakova, currently affiliated with the Department of Pharmacology at Columbia University for their help on the experimental procedure. The authors also wish to thank Jeffrey W Holmes, MD, PhD, and Katy Parker, MS, Department of Biomedical Engineering at University of Virginia for advice on the sonomicrometry acquisition and analysis, and Shunichi Homma, MD, and Eiichi Hyodo, MD, with the Department of Medicine, Jianwen Luo, PhD, Shougang Wang, PhD, Jaimie Lee, and Siddhi Dessai, MS, with the Department of Biomedical Engineering at Columbia University for helpful discussions.
Appendix
A.1. Method—sonomicrometry strain measurement
Knowledge of the crystal location relative to the imaging transducer and the visible crystals shown on the acquired images were used as landmarks for the selection of the ROIs. The size of the ROI was determined according to the average thickness (10.03 mm) of the myocardial wall region of the canine left ventricles used and the diameter of the crystals implanted (see section 2.1.2).
The acquired sonomicrometry data were denoised semi-automatically using SonoSOFT® (Sonometrics Corp., London, Ontario, Canada). The 3D coordinates of each crystal and the pressure–volume relationship were also obtained using SonoSoft® with user-defined axes and a reference crystal. The anterior–posterior, lateral–septal and apex–base axes were used to compute the left-ventricular volume. The crystal at the apex was selected as the reference crystal.
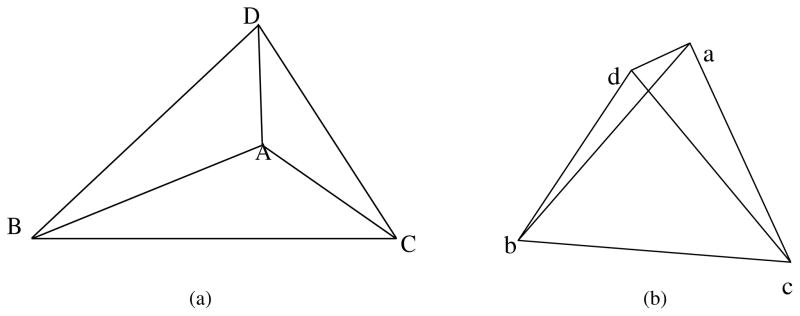
In order to calculate the in-plane transmural strains in a short-axis view, the four crystals, which form a tetrahedron in the anterior or posterior region, were used. An illustration of the tetrahedron in the undeformed and deformed configurations is shown in figure A1, where capital and lowercase letters indicate the vertices of the tetrahedron before and after deformation, respectively.
The normal and shear components of a 3D strain tensor were computed from the changes in squared vector lengths defined by these four crystals using the following equation (Lai et al 1993, Meier et al 1980, Waldman et al 1985):
| (A.1) |
where ds2 and dS2 denote the squared vector lengths in the deformed and undeformed configurations, respectively; the alphabetical subscripts in upper and lower cases represent the six edges in the undeformed and deformed configurations, respectively; X1, X2, and X3 denote the three orthogonal components of the vector defined by each edge of the tetrahedron; the numerical subscripts 1–3 indicate the three orthogonal axes in the Cartesian coordinates; and E is the strain tensor.
The strain tensor was computed in Cartesian coordinates, which were computed in the SonoSoft® software. The computed sonomicrometry strains needed to be further transformed from Cartesian to polar coordinates, in which the myocardial deformation could be depicted more adequately and the two different modalities could be appropriately compared. A coordinate transformation matrix was thus obtained by defining the centroid of the left ventricle and then determining the left-ventricular polar coordinates.
Figure A1.
An illustration of the tetrahedron composed of four crystals in (a) an undeformed and (b) a deformed configuration. A–D indicate the vertices of the tetrahedron in the undeformed case, while a–d in the deformed case.
A.2. Results
The evolution of myocardial radial deformation during the systolic phase at graded levels of coronary flow reduction was provided in separate video clips available at stacks.iop.org/PMB/56/1155/mmedia. Videos ‘0p-err-sys.flv’, ‘20p-err-sys.flv’, ‘40p-err-sys.flv’, ‘60p-err-sys.flv’, ‘80p-err-sys.flv’, ‘100p-err-sys.flv’, ‘reper-err-sys.flv’ correspond to the 0%, 20%, 40%, 60%, 80% and 100% flow reduction levels and reperfusion. Note that the systolic deformation was displayed in real time and that the white bars in the videos signify the region at risk.
Footnotes
Online supplementary data available from stacks.iop.org/PMB/56/1155/mmedia
References
- Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician. 1983;32:307–17. [Google Scholar]
- Amundsen BH, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography—validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–93. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Armstrong G, Pasquet A, Fukamachi K, Cardon L, Olstad B, Marwick T. Use of peak systolic strain as an index of regional left ventricular function: comparison with tissue Doppler velocity during dobutamine stress and myocardial ischemia. J Am Soc Echocardiogr. 2000;13:731–7. doi: 10.1067/mje.2000.105912. [DOI] [PubMed] [Google Scholar]
- D’hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, Hatle L, Suetens P, Sutherland GR. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr. 2000a;1:154–70. doi: 10.1053/euje.2000.0031. [DOI] [PubMed] [Google Scholar]
- D’hooge J, Jamal F, Bijnens B, Heimdal A, Thoen J, Van de Werf F, Sutherland GR, Suetens P. Calculation of strain values from strain rate curves: how should this be done? In: Schneider SC, editor. IEEE Ultrasonics Symp Proc, 2000. 1 and 2. New York: IEEE; 2000b. pp. 1269–72. [Google Scholar]
- Gallagher KP, Gerren RA, Choy M, Stirling MC, Dysko RC, et al. Subendocardial segment length shortening at lateral margins of ischemic myocardium in dogs. Am J Physiol. 1987;253:H826–37. doi: 10.1152/ajpheart.1987.253.4.H826. [DOI] [PubMed] [Google Scholar]
- Gallagher KP, Gerren RA, Stirling MC, Choy M, Dysko RC, McManimon SP, Dunham WR. The distribution of functional impairment across the lateral border of acutely ischemic myocardium. Circ Res. 1986;58:570–83. doi: 10.1161/01.res.58.4.570. [DOI] [PubMed] [Google Scholar]
- Gallagher KP, Kumada T, Koziol JA, McKown MD, Kemper WS, Ross J. Significance of regional wall thickening abnormalities relative to transmural myocardial perfusion in anesthetized dogs. Circulation. 1980;62:1266–74. doi: 10.1161/01.cir.62.6.1266. [DOI] [PubMed] [Google Scholar]
- Gallagher KP, Matsuzaki M, Osakada G, Kemper WS, Ross J. Effect of exercise on the relationship between myocardial blood flow and systolic wall thickening in dogs with acute coronary stenosis. Circ Res. 1983;52:716–29. doi: 10.1161/01.res.52.6.716. [DOI] [PubMed] [Google Scholar]
- Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–53. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- Jamal F, Kukulsi T, Strotmann J, Szilard M, D’Hooge J, Bijnens B, Rademakers F, Hatle L, De Scheerder I, Sutherland GR. Quantification of the spectrum of changes in regional myocardial function during acute ischemia in closed chest pigs: an ultrasonic strain rate and strain study. J Am Soc Echocardiogr. 2001;14:874–84. doi: 10.1067/mje.2001.112037. [DOI] [PubMed] [Google Scholar]
- Kallel F, Ophir J. A least-squares strain estimator for elastography. Ultrason Imaging. 1997;19:195–208. doi: 10.1177/016173469701900303. [DOI] [PubMed] [Google Scholar]
- Kerber RE, Marcus ML, Ehrhardt J, Wilson R, Abboud FM. Correlation between echocardiographically demonstrated segmental dyskinesis and regional myocardial perfusion. Circulation. 1975;52:1097–104. doi: 10.1161/01.cir.52.6.1097. [DOI] [PubMed] [Google Scholar]
- Khalil PN, Siebeck M, Huss R, Pollhammer M, Khalil MN, Neuhof C, Fritz H. Histochemical assessment of early myocardial infarction using 2,3,5-triphenyltetrazolium chloride in blood-perfused porcine hearts. J Pharmacol Toxicol Methods. 2006;54:307–12. doi: 10.1016/j.vascn.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Konofagou EE, D’hooge J, Ophir J. Myocardial elastography—a feasibility study in vivo. Ultrasound Med Biol. 2002;28:475–82. doi: 10.1016/s0301-5629(02)00488-x. [DOI] [PubMed] [Google Scholar]
- Korinek J, et al. Doppler strain imaging closely reflects myocardial energetic status in acute progressive ischemia and indicates energetic recovery after reperfusion. J Am Soc Echocardiogr. 2008;21:961–8. doi: 10.1016/j.echo.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WM, Rubin D, Krempl E. Introduction to Continuum Mechanics. 3. Oxford: Butterworth-Heinemann; 1993. [Google Scholar]
- Langeland S, D’hooge J, Wouters PF, Leather HA, Claus P, Bijnens B, Sutherland GR. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation. 2005;112:2157–62. doi: 10.1161/CIRCULATIONAHA.105.554006. [DOI] [PubMed] [Google Scholar]
- Langeland S, Wouters PF, Claus P, Leather HA, Bijnens B, Sutherland GR, Rademakers FE, D’Hooge J. Experimental assessment of a new research tool for the estimation of two-dimensional myocardial strain. Ultrasound Med Biol. 2006;32:1509–13. doi: 10.1016/j.ultrasmedbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Lee WN, Ingrassia CM, Fung-Kee-Fung SD, Costa KD, Holmes JW, Konofagou EE. Theoretical quality assessment of myocardial elastography with in vivo validation. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:2233–45. doi: 10.1109/tuffc.2007.528. [DOI] [PubMed] [Google Scholar]
- Lee WN, Konofagou EE. Angle-independent and multi-dimensional myocardial elastography—from theory to clinical validation. Ultrasonics. 2008;48:563–7. doi: 10.1016/j.ultras.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WN, Qian Z, Tosti CL, Brown TR, Metaxas DN, Konofagou EE. Preliminary validation of angle-independent myocardial elastography using MR tagging in a clinical setting. Ultrasound Med Biol. 2008;34:1980–97. doi: 10.1016/j.ultrasmedbio.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekven J, Mjos OD, Kjekshus JK. Compensatory mechanisms during graded myocardial ischemia. Am J Cardiol. 1973;31:467–73. doi: 10.1016/0002-9149(73)90296-8. [DOI] [PubMed] [Google Scholar]
- Luo J, Fujikura K, Homma S, Konofagou EE. Myocardial elastography at both high temporal and spatial resolution for the detection of infarcts. Ultrasound Med Biol. 2007;33:1206–23. doi: 10.1016/j.ultrasmedbio.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Luo J, Konofagou EE. High-frame rate, full-view myocardial elastography with automated contour tracking in murine left ventricles in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:240–8. doi: 10.1109/TUFFC.2008.633. [DOI] [PubMed] [Google Scholar]
- Luo J, Lee W-N, Konofagou EE. Fundamental performance assessment of 2-D myocardial elastography in a phased array configuration. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56:2320–7. doi: 10.1109/TUFFC.2009.1313. [DOI] [PubMed] [Google Scholar]
- Meier GD, Ziskin MC, Santamore WP, Bove AA. Kinematics of the beating heart. IEEE Trans Biomed Eng. 1980;27:319–29. doi: 10.1109/TBME.1980.326740. [DOI] [PubMed] [Google Scholar]
- Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain Imaging. J Am Soc Echocardiogr. 2008;21:1138–44. doi: 10.1016/j.echo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O’Rourke MF. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 5. London: Hodder Arnold; 2005. [Google Scholar]
- Otto CM. Textbook of Clinical Echocardiography. 3. Philadelphia, PA: Elsevier Saunders; 2004. [Google Scholar]
- Papademetris X, Sinusas AJ, Dione DP, Duncan JS. Estimation of 3D left ventricular deformation from echocardiography. Med Image Anal. 2001;5:17–28. doi: 10.1016/s1361-8415(00)00022-0. [DOI] [PubMed] [Google Scholar]
- Reant P, et al. Experimental validation of circumferential, longitudinal, and radial 2-dimensional strain during dobutamine stress echocardlography in ischemic conditions. J Am Coll Cardiol. 2008;51:149–57. doi: 10.1016/j.jacc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- Skulstad H, Edvardsen T, Urheim S, Rabben SI, Stugaard M, Lyseggen E, Ihlen H, Smiseth OA. Postsystolic shortening in ischemic myocardium—active contraction or passive recoil? Circulation. 2002;106:718–24. doi: 10.1161/01.cir.0000024102.55150.b6. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Mathey DG, Moores WY, Glantz SA, Townsend RM, Kabra P, Chatterjee K, Parmley WW, Tyberg JV. Segment stroke work and metabolism depend on coronary blood flow in the pig. Am J Physiol. 1978;234:H597–607. doi: 10.1152/ajpheart.1978.234.5.H597. [DOI] [PubMed] [Google Scholar]
- Takayama M, Norris RM, Brown MA, Armiger LC, Rivers JT, White HD. Postsystolic shortening of acutely ischemic canine myocardium predicts early and late recovery of function after coronary artery reperfusion. Circulation. 1988;78:994–1007. doi: 10.1161/01.cir.78.4.994. [DOI] [PubMed] [Google Scholar]
- Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography: validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–64. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- Vatner SF. Correlation between acute reductions in myocardial blood flow and function in conscious dogs. Circ Res. 1980;47:201–7. doi: 10.1161/01.res.47.2.201. [DOI] [PubMed] [Google Scholar]
- Waldman LK, Fung YC, Covell JW. Transmural myocardial deformation in the canine left-ventricle: normal in vivo three-dimensional finite strains. Circ Res. 1985;57:152–63. doi: 10.1161/01.res.57.1.152. [DOI] [PubMed] [Google Scholar]
- Wang SG, Lee WN, Provost J, Luo JW, Konofagou EE. A composite high-frame-rate system for clinical cardiovascular imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:2221–33. doi: 10.1109/TUFFC.921. [DOI] [PubMed] [Google Scholar]
- Waters DD, Daluz P, Wyatt HL, Swan HJC, Forrester JS. Early changes in regional and global left ventricular function induced by graded reductions in regional coronary perfusion. Am J Cardiol. 1977;39:537–43. doi: 10.1016/s0002-9149(77)80163-x. [DOI] [PubMed] [Google Scholar]
- Zervantonakis IK, Fung-Kee-Fung SD, Lee W-N, Konofagou EE. A novel, view-independent method for strain mapping in myocardial elastography: eliminating angle- and centroid-dependence. Phys Med Biol. 2007;52:4063–80. doi: 10.1088/0031-9155/52/14/004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.