Abstract
A dense mucous layer in the large intestine prevents inflammation by shielding the underlying epithelium from luminal bacteria and food antigens. This mucous barrier is organized around the hyperglycosylated mucin MUC2. Here we show that the small intestine has a porous mucous layer, which permitted the uptake of MUC2 by antigen-sampling dendritic cells (DCs). Glycans associated with MUC2 imprinted DCs with anti-inflammatory properties by assembling a galectin-3-Dectin-1-FcγRIIB receptor complex that activated β-catenin. This transcription factor interfered with DC expression of inflammatory but not tolerogenic cytokines by inhibiting gene transcription through nuclear factor-κB. MUC2 induced additional DC-conditioning signals via intestinal epithelial cells. Thus, mucus does not merely form a nonspecific physical barrier, but also constraints the immunogenicity of gut antigens by delivering tolerogenic signals.
Mechanisms whereby the gut mucosa tolerates commensal bacteria and food antigens without developing inflammation remain elusive. Though traditionally viewed as a nonspecific barrier between the host and the environment, mucus also regulates gut homeostasis. The building block of gut mucus is MUC2, a gel-forming mucin secreted by goblet cells (GCs) (1). In the large intestine (LI), MUC2 prevents inflammation by generating an outer nonattached mucous layer inhabited by the microbiota and an inner mucous layer adherent to intestinal epithelial cells (IECs) and impervious to bacteria (1). The structure and function of mucus in the small intestine (SI) are less well understood.
The SI harbors bacteria that promote homeostasis by inducing Foxp3+ T regulatory (Treg) cells and B cell production of immunoglobulin A (IgA) antibodies (Abs) in Peyer’s patches (PPs) and mesenteric lymph nodes (MLNs) (2, 3). These responses involve sampling of bacteria by CD11c+ dendritic cells (DCs) (4), including macrophage-like CD103−CD11b+CX3CR1+ DCs and myeloid CD103+CD11b+CX3CR1− DCs (2, 5, 6). In the lamina propria (LP), myeloid DCs sample soluble antigens from GC-associated passages and cooperate with lymphoid CD103+CD11b−CX3CR1− DCs to generate Treg cells (7–9). Such antigen-sampling activities require a porous mucous barrier, raising questions as to how MUC2 prevents inflammation in the SI.
MUC2 Mitigates Inflammatory Responses in DCs
We first analyzed the structure of gut mucus in wild type (WT) C57BL/6 mice. While LI mucus formed a dense bilayered barrier that segregated bacteria from IECs, SI mucus was less organized and thus permitted the adhesion of bacteria to IECs (fig. S1A–C). Consistent with the presence of some MUC2-coated bacteria on IECs and inside DCs (fig. S2A–C), CX3CR1+ DCs from both PPs and SI-LP captured carboxyfluorescein succinimidyl ester (CFSE)-labeled MUC2 bound to fluorescent bacteria (fig. S3A–C). In agreement with recent studies (6), also some CD103+ DCs captured MUC2-coated bacteria (fig. S3A–D). Similarly, human monocyte-derived DCs internalized MUC2-bound bacteria across IECs sealed by occludin-containing tight junctions (fig. S4A–D). Moreover, MUC2 was detected in human SI-LP CD103+ DCs proximal to GCs (Fig. 1A and movies S1 and S2).
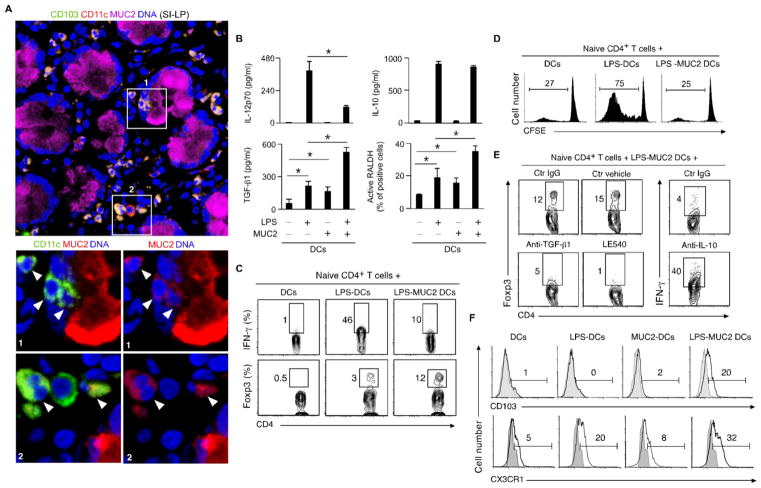
Fig. 1. MUC2 imprints DCs with tolerogenic properties.
(A) Confocal microscopy of human SI-LP stained for CD11c, MUC2, CD103 and DNA-capturing 4′-6- diamidino-2-phenylindole (DAPI). Boxes and arrowheads: MUC2+CD103+ DCs. Original magnification, ×63. (B) Enzyme-linked immunosorbent assay (ELISA) of IL-12p70, IL-10 and TGF-β1 and flow cytometry (FC) of active RALDH in human DCs cultured for 2 days with or without LPS and/or MUC2. (C–E) FC of IFN-γ, Foxp3, CD4 and CFSE in human naïve CD4+ T cells cultured for 4 days with allogeneic DCs stimulated with or without LPS and/or MUC2 for 2 or 5 days in the absence or presence of control (ctr) IgG Ab, ctr vehicle, neutralizing Abs to TGF-β1 or IL-10 or LE540. (F) FC of CD103 and CX3CR1 on DCs cultured for 2 days with or without LPS and/or MUC2. Data summarize 3 experiments (error bars, s.d.; unpaired t test, *P <0.05) or show one of 4 experiments with similar results.
In the presence of MUC2, human DCs exposed to bacteria or LPS across IECs or directly incubated with LPS secreted less interleukin 12 (IL-12) (Fig. 1B and fig. S4E), a cytokine that induces proinflammatory interferon-γ (IFN-γ)-producing CD4+ T helper 1 (Th1) cells (2). This effect was comparably induced by human, murine or porcine MUC2 and was not due to elevated endotoxin, impaired DC uptake of bacteria or increased DC apoptosis (figs. S4F and S5A–D). Unlike native MUC2, deglycosylated MUC2, a MUC2 peptide or the mucin-interacting protein trefoil factor 3 did not inhibit LPS-induced IL-12 secretion (fig. S5E). MUC2 also impaired IL-12 as well as IL-6, IL-8 and tumor necrosis factor (TNF) transcription in response to bacterial Toll-like receptor (TLR) ligands such as LPS and flagellin or cytokines such as TNF (Fig. 1B and fig. S6A–E). MUC2 elicited similar anti-inflammatory effects in monocyte-derived and myeloid CD1c+ DCs (Fig. 1B and fig. S6A–E), which include precursors of mucosal DCs (2, 8, 10). Thus, uptake of MUC2 causes carbohydrate-dependent attenuation of proinflammatory cytokine production by DCs.
MUC2 Delivers Tolerogenic Signals to DCs
Next, we established whether MUC2 induces IL-10, an anti-inflammatory cytokine that inhibits IL-12 and IFN-γ (2). Besides sustaining or augmenting IL-10 transcription and secretion in monocyte-derived DCs exposed to LPS or flagellin, MUC2 enhanced IL-10 secretion in LPS-activated myeloid CD1c+ DCs (Fig. 1B and fig. S6C-E). MUC2 alone or combined with LPS also increased DC transcription and secretion of transforming growth factor-β1 (TGF-β1), a SMAD-signaling cytokine (Fig. 1B and figs. S6E and S7A) that helps the induction of Treg cells by tolerogenic CD103+ DCs (2, 7, 11). Moreover, MUC2 augmented DC transcription and activation of retinaldehyde de-hydrogenase (RALDH or ALDH1), a 4-diethylaminobenzaldehyde (DEAB)-sensitive enzyme (Fig. 1B and figs. S6E and S7B) with A1-3 isoforms that help CD103+ DCs to induce Treg cells by converting dietary vitamin A into retinoic acid (RA) (2, 7, 11).
Accordingly, human DCs exposed to LPS in the presence of MUC2 decreased CD4+ T cell proliferation and IFN-γ production, but increased Foxp3 expression (Fig. 1C and D and fig. S8A). These effects were reversed by neutralizing Abs to IL-10 or TGF-β1 and by the RA antagonist LE540 (Fig. 1E). In spite of up-regulating the antigen-presenting molecule human leukocyte antigen-DR (HLA-DR), MUC2 down-regulated the T-cell co-stimulatory molecules CD80 and CD86 and the maturation molecule CD83 on LPS-activated DCs (fig. S8B). Consistent with its ability to autonomously induce TGF-β1 and RA, MUC2 induced regulatory DCs even in the absence of LPS priming (fig. S9A and B). In addition to up-regulating CD103 and CX3CR1 on LPS-activated DCs (Fig. 1F), MUC2 stimulated CD103 expression and Treg cell-inducing signals in DCs undergoing transepithelial sampling of bacteria (fig. S10A–C).
In the presence of MUC2, LPS-primed mouse bone marrow-derived DCs produced less IL-12 but more IL-10 (fig. S11A). When pulsed with the soluble protein ovalbumin (OVA) in the presence of MUC2, these DCs triggered less IFN-γ production by OVA-specific transgenic OT-II CD4+ T cells, which concurrently generated more Treg cells (fig. S11B and C). MUC2 also attenuated flagellin-induced TNF but sustained or augmented IL-10 production in SI-LP CD103+ and CX3CR1+ DCs, respectively (Fig. 2A). Thus, MUC2 elicits tolerogenic IL-10, TGF-β1 and RA signals that somewhat vary in distinct subsets of DCs.
Fig. 2. MUC2 delivers anti-inflammatory signals to gut DCs.
(A) ELISA of TNF and IL-10 from mouse SI-LP DCs cultured for 2 days with or without flagellin and/or MUC2. (B) Light microscopy of Alcian blue-stained mucin and fluorescence in situ hybridization (FISH) of bacterial 16S ribosomal RNA in DAPI-stained SI-LP from WT and Muc2−/− mice. Original magnification, ×10. (C–E) Quantitative real-time polymerase chain reaction (qRT-PCR) of mRNAs for TNF, IL-12p35 (Il12a), IL-12p40 (Il12b), IL-10, RALDH1 (Aldh1a1) and TGF-β1 in SI-LP DCs and FC of Foxp3 and CD4 on SI-LP T cells from WT and Muc2−/− mice before and after oral antibiotics. RE, relative expression compared to Gapdh encoding glyceraldehyde 3-phosphate dehydrogenase. (F) ELISA of proliferation-induced bromodeoxyuridine (BrdU) and IFN-γ from OT-II cells activated for 5 days by OVA-pulsed SI-LP DCs from WT and Muc2−/− mice with or without MUC2. Data summarize 2 experiments with ≥3 mice/group (error bars, s.d.; unpaired Student’s t test, *P <0.05) or show one of 4 experiments with similar results.
MUC2 Enhances Gut Homeostasis
The immunoregulatory function of MUC2 was further explored in MUC2-deficient (Muc2−/−) mice (12). Compared to WT controls, Muc2−/− mice showed more IEC-adherent bacteria and their SI-LP CD103+ and CX3CR1+ DCs expressed more TNF and IL-12, but less IL-10, TGF-β1 and ALDH1A1 and thus induced fewer Treg cells (Fig. 2B and C and fig. S12A). Accordingly, Muc2−/− mice had fewer SI-LP Treg cells but more proinflammatory Th1 and IL-17-producing Th17 cells (fig. S12B). These changes were associated with increased bacteria-bound IgA Abs and SI-LP DC expression of B cell-activating factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL) (fig. S12C and D), two IgA-inducing cytokines induced by inflammatory signals (3). Compared to WT mice, Muc2−/− mice sterilized of gut bacteria normalized SI-LP DC expression of BAFF and APRIL, but neither augmented SI-LP DC expression of IL-10, TGF-β1 and ALDH1A2 nor increased SI-LP Treg cells (Fig. 2E and fig. S12D and E). Thus, perturbations of gut homeostasis in Muc2−/− mice cannot be solely ascribed to increased IEC-adherent bacteria.
We then verified whether MUC2 regulates IECs. Compared to controls, IECs from Muc2−/− mice expressed less IL-10, TGF-β1, ALDH1A1 and thymic stromal lymphopoietin (TSLP) (fig. S13A), which generates tolerogenic DCs (13, 14). Gut sterilization failed to restore WT-like levels of IL-10, ALDH1A1 and TSLP, but did normalize TGF-β1 and RegIIIγ (fig. S13A), an antimicrobial protein induced by bacteria (15). In humans, IECs expressed more TSLP in response to MUC2 (fig. S13A), which confirmed the tolerogenic function of MUC2 on IECs.
The immune and barrier functions of MUC2 were further uncoupled by gavaging Muc2−/− mice with MUC2 from WT controls. When exposed to MUC2, SI-LP CD103+ and CX3CR1+ DCs from Muc2−/− mice reduced OVA-specific CD4+ T cell proliferation and IFN-γ production as SI-LP DCs from WT mice did (Fig. 2F). Gavaged MUC2 did not restore a visible barrier, but its capture not only augmented IL-10, TGF-β and ALDH1A1 and decreased IL-12 in SI-LP DCs, but also increased SI-LP Treg cells and reduced SI-LP Th1 and Th17 cells (Fig. 3A and B and fig. S14A). Similarly, gavaged MUC2 helped SI-LP CD103+ DCs from Muc2−/− mice to induce more OVA-specific Treg cells after intra-gastric OVA immunization (Fig. 3C).
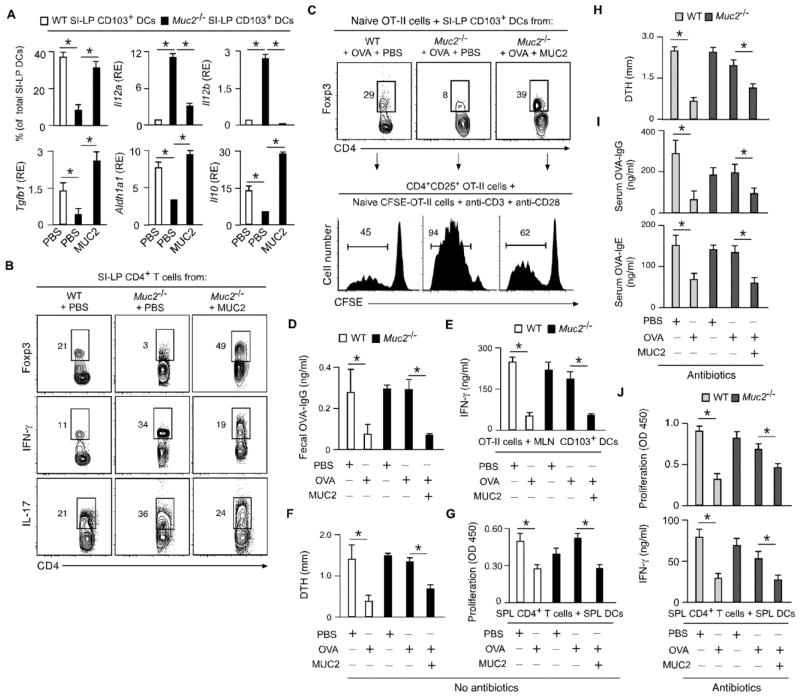
Fig. 3. MUC2 enhances gut homeostasis and oral tolerance.
(A) FC of CD103 and qRT-PCR of Il12a, Il12b, Il10, Aldh1a1 and Tgfb1 in SI-LP CD103+ DCs from WT or Muc2−/− mice gavaged for 5 days with phosphate buffer solution (PBS) or MUC2. RE, relative expression compared to Gapdh. (B) FC of Foxp3, IFN-γ, IL-17 and CD4 in SI-LP T cells from WT or Muc2−/− mice treated as in (A). (C) FC of Foxp3 and CD4 in naïve OT-II cells cultured for 5 days with SI-LP CD103+ DCs from WT or Muc2−/− mice treated as in (A) and intragastrically immunized with OVA. CD4+CD25+ OT-II cells from these cultures were incubated for 5 days with CFSE-labeled naïve OT-II cells and Abs to CD3 and CD28; divided CFSElow cells were quantified by FC. (D) ELISA of fecal OVA-specific IgG from WT and Muc2−/− mice tolerized with PBS, OVA or OVA plus MUC2 for 5 days and immunized as in (C). (E) ELISA of IFN-γ from OT-II cells incubated for 5 days with MLN CD103+ DCs from WT or Muc2−/− mice tolerized and immunized as in (D). (F) OVA-induced DTH in WT or Muc2−/− mice tolerized as in (C) and subcutaneously immunized with OVA. (G) ELISA of proliferation-induced BrdU from SPL CD4+ T cells activated for 5 days with OVA-pulsed SPL DCs from WT or Muc2−/− mice tolerized and immunized as in (F). (H–J) DTH, OVA-specific serum IgG and IgE, and SPL CD4+ T cell proliferation and IFN-γ secretion in WT or Muc2−/− mice immunized and tolerized as in (F) after oral antibiotics. Data summarize 2 experiments with ≥4 mice/group (error bars, s.d.; unpaired Student’s t test, *P <0.05) or show one of 4 experiments with similar results.
Importantly, gavaged MUC2 enhanced the resistance of Muc2−/− mice to dextran sodium sulfate (DSS), a colitogenic agent that disrupts the epithelial barrier. Although unable to attenuate inflammation-induced shortening of the LI, gavaged MUC2 ameliorated both clinical symptoms and histological lesions in DSS-treated Muc2−/− mice, which showed less weight loss than WT or Muc2−/− mice challenged with DSS in the absence of exogenous MUC2 (fig. S14B–D). Thus, MUC2 attenuates bacteria-induced gut inflammation by delivering DC and IEC conditioning signals.
MUC2 Promotes Oral Tolerance
Oral tolerance consists in the attenuation of T and B cell responses to an antigen by prior oral administration of that antigen and involves MLN induction of Treg cells by migratory CD103+ DCs (2). WT mice gavaged and later intragastrically immunized with OVA showed decreased gut B cell production of OVA-specific IgG Abs, which correlated with reduced IFN-γ production by OVA-reactive CD4+ T cells in response to OVA-pulsed MLN, PP or SI-LP CD103+ DCs (Fig. 3D and E and fig. S15A and B).
Tolerization also reduced OVA-induced delayed-type hypersensitivity (DTH) – an inflammatory skin reaction involving Th1 cells – as well as DC-dependent splenic CD4+ T cell proliferation and IFN-γ secretion following systemic immunization of Muc2−/− mice with OVA (Fig. 3F and G and fig. S15C and D). Unlike WT mice, Muc2−/− mice gavaged with OVA developed neither intestinal nor systemic tolerance (Fig. 3D–G and fig. S15A–D). However, Muc2−/− mice restored oral tolerance when gavaged with OVA in the presence of MUC2 (Fig. 3D–G and fig. S15A–D). The impairment of tolerance in Muc2−/− mice was not merely due to bacteria-induced inflammation, as Muc2−/− mice lacking gut bacteria did not attenuate OVA-specific DTH, IgG and IgE responses after gavage with OVA (Fig. 3H–J). Yet, tolerance was restored when OVA was gavaged in combination with MUC2 (Fig. 3H–J). Thus, mucus actively constrains the immunostimulating properties of oral soluble antigens.
MUC2 Binds a Galectin-3-Dectin-1-FcgRIIB Receptor Complex on DCs
Carbohydrates account for 80% of the weight of MUC2 (1) and may thus mediate its binding to DCs. Indeed, unlabeled glycosylated MUC2 inhibited the binding of CFSE-labeled native MUC2 to human DCs, whereas unlabeled deglycosylated MUC2 or a MUC2 peptide did not (Fig. 4A and fig. S16A). C-type lectin receptors (CLRs) and soluble galectins form carbohydrate-binding DC platforms with tolerogenic function (16–20). Saturation of CLRs and galectins with mannan and lactose, respectively, attenuated MUC2 binding to DCs (Fig. 4A). DCs express galectin-1, -3 and -9 (21), but only galectin-3 interacted with glycosylated MUC2 and increased its binding to DCs through a process that was inhibited by MUC2 deglycosylation or Ab-mediated blockade of galectin-3 (Fig. 4B–D). In agreement with their ability to bind galectin-3 following its secretion (16, 21), DCs increased both soluble and membrane-bound galectin-3 in response to LPS (fig. S16B). Thus, DCs may form a galectin-3-based MUC2-binding platform after sensing gut microbial signals.
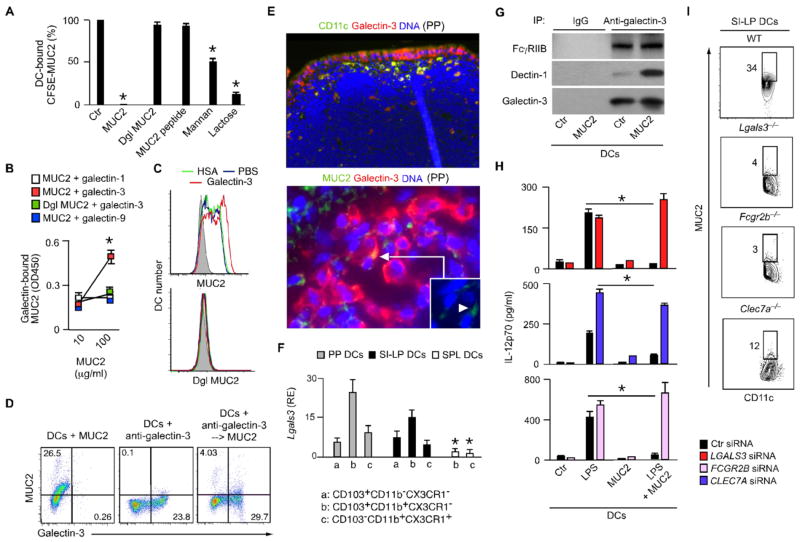
Fig. 4. MUC2 binds galectin-3, dectin-1 and FcγRIIB on DCs.
(A) FC of CFSE-MUC2 on human DCs pre-incubated with unlabeled native MUC2, deglycosylated (dgl) MUC2, MUC2 peptide, mannan or lactose. % of MUC2 binding compared to medium alone. (B) ELISA of native or dgl MUC2 interaction with galectins. (C) CFSE-MUC2 or CFSE-dgl MUC2 binding to DCs pre-incubated with PBS, human serum albumin (HSA) or galectin-3. (D) CFSE-MUC2 binding to DCs before and after pre-incubation with a fluorescent Ab to galectin-3. (E) IFA of CD11c, galectin-3, Muc2 and DAPI in mouse PP sections. Original magnification ×5 (upper panel) and °—63 (bottom panel). (F) qRT-PCR of mRNA for galectin-3 in DC subsets from mouse PPs, SI-LP and SPL. RE, relative expression compared to Gapdh. (G) Immunoprecipitation (IP) with control or anti-galectin-3 Abs of proteins from human DCs treated without (ctr) or with MUC2 for 30 min, followed by immunoblotting of FcγRIIB, Dectin-1 and galectin-3. (H) ELISA of IL-12p70 from human DCs exposed to scrambled (ctr) or LGALS3 (galectin-3), FCGR2B (FcγRIIB) or CLEC7A (Dectin-1) small interfering RNAs (siRNAs) and cultured with or without LPS and/or MUC2 for 2 days. (I) Binding of CFSE-MUC2 to SI-LP DCs from WT, Lgals3−/−, Clec7A−/− or Fcgr2b−/− mice. Data summarize experiments with 3 donors or 3 mice from each strain (error bars, s.d.; unpaired t test, *P <0.05) or show one of 3 experiments with similar results.
Accordingly, PP and SI-LP CD103+ and CX3CR1+ DCs produced more galectin-3 than splenic DCs did and showed intracellular galectin-3 co-localized with MUC2 (Fig. 4E and F and fig. S16C-E). SI-LP DCs also displayed more surface galectin-3 (Fig. 4F and fig. S16F), part of which may have an epithelial origin (21). Indeed, galectin-3 was detected in both IECs and GCs and its secretion augmented upon IEC exposure to bacteria (fig. S16G and H). Consequently, DCs acquired more surface galectin-3 after exposure to IEC supernatant or migration across IECs (fig. S16I and J).
We then determined how soluble galectin-3 anchors itself and MUC2 to DCs. Galectin-3 binds Dectin-1, a CLR that induces tolerogenic DCs and enhances gut homeostasis by recognizing fungal carbohydrates (16, 17, 20). Dectin-1 further interacts with the anti-inflammatory receptor FcγRIIB in response to glycans from therapeutic IgG Abs (22). In human embryonic kidney 293 cells, MUC2 bound transfected Dectin-1 and FcγRIIB in the presence of galectin-3 (fig. S17A and B). In DCs, MUC2 binding correlated with elevated surface levels of galectin-3, Dectin-1 and FcγRIIB and caused Dectin-1 recruitment to a pre-formed galectin-3-FcγRIIB complex (Fig. 4G and fig. S17C). Accordingly, DCs lacking galectin-3, Dectin-1 or FcγRIIB neither effectively bound MUC2 nor decreased IL-12 production in response to MUC2 (Fig. 4H and I and fig. S17D–G).
In WT mice, SI-LP CD103+ DCs expressed less Dectin-1 but more galectin-3 and FcγRIIB than CX3CR1+ DCs did (Fig. 5A). Compared to Lgals3 (galectin-3)−/−, Clec7a (Dectin-1)−/− or Fcgr2b (FcγRIIB)−/− mice, WT mice had a comparable SI mucous layer, but showed more SI-LP DCs containing MUC2 (Fig. 5B and fig. S18A and B). Unlike IECs, these SI-LP DCs did not express MUC2 (Fig. 5C), which confirmed that gut DCs acquire MUC2 from the external environment. Compared to controls, Lgals3−/−, Clec7a−/− or Fcgr2b−/− mice had SI-LP DCs that produced more IL-12, but less ALDH1A1 and TGF-β1 and did not respond to MUC2 (Fig. 5D and fig. S19). These mice also had fewer SI-LP Treg cells, more SI-LP Th1 cells, and impaired tolerogenic T and B cell responses to OVA (Fig. 5E–G). Thus, MUC2 may generate gut homeostasis and tolerance by assembling a signal-transducing Dectin-1-FcγRIIB complex on DCs with the help of soluble galectin-3.
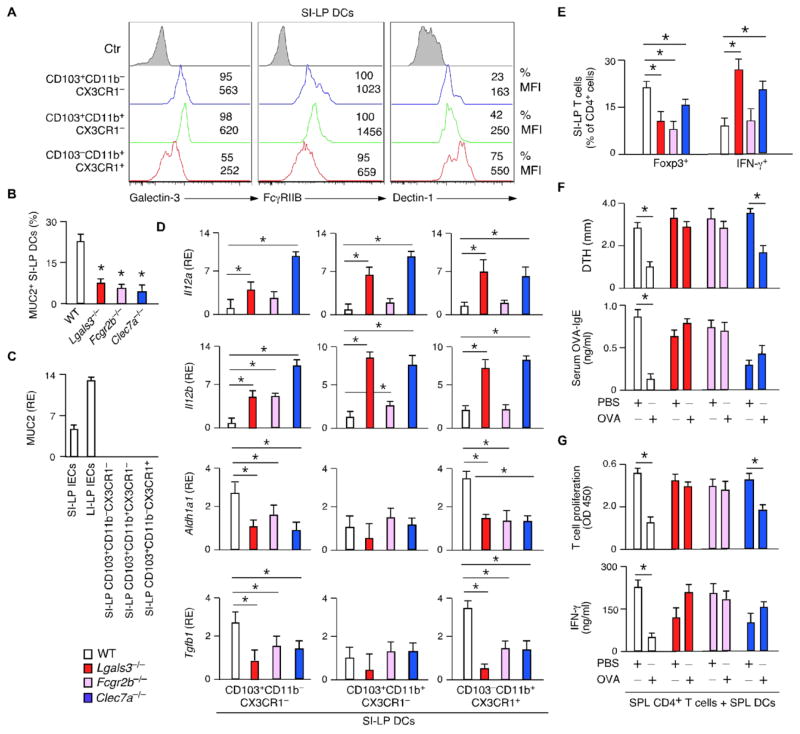
Fig. 5. Galectin-3, Dectin-1 and FcγRIIB enhance gut homeostasis and oral tolerance.
(A) FC of galectin-3, FcγRIIB and Dectin-1 on SI-LP DCs from WT mice. MFI, mean fluorescence intensity. (B) Quantification of MUC2+ SI-LP DCs from WT, Lgals3−/−, Clec7A−/− or Fcgr2b−/− mice by IFA of 10–12 SI-LP sections/group. (C) qRT-PCR of Muc2 in IECs and SI-LP DCs from WT mice. RE, relative expression compared to Gapdh. (D and E) qRT-PCR of Il12a, Il12b, Aldh1a1 and Tgfb1 in SI-LP DCs and FC of Foxp3 and IFN-γ in SI-LP CD4+ T cells from WT, Lgals3−/−, Clec7A−/− or Fcgr2b−/− mice. RE, relative expression compared to Gapdh. (F) DTH and ELISA of OVA-specific IgE in WT, Lgals3−/−, Clec7A−/− or Fcgr2b−/− mice intragastrically tolerized with PBS or OVA for 5 days and subcutaneously immunized with OVA. (G) ELISA of proliferation-induced BrdU from SPL CD4+ T cells activated for 5 days by OVA-pulsed SPL DCs from WT, Lgals3−/−, Clec7A−/− or Fcgr2b−/− mice tolerized and immunized as in (F). Data summarize 2 experiments with ≥4 mice/group (error bars, s.d.; unpaired Student’s t test, *P <0.05) or show one of 4 experiments with similar results.
MUC2 Tolerizes DCs by Inducing β-Catenin via Dectin-1
How do Dectin-1 and FcγRIIB convey tolerogenic signals to DCs exposed to MUC2? Dectin-1 phosphorylates AKT (23) and may thus induce β-catenin, a transcription factor required by gut tolerogenic DCs (24). Indeed, MUC2 alone or combined with LPS phosphorylated AKT and glycogen synthase kinase-3β (GSK-3β) (Fig. 6A), two events that cause GSK-3β inactivation and inhibition of β-catenin degradation (24, 25). Consequently, MUC2 elicited cytoplasmic accumulation and nuclear translocation of β-catenin in WT but not Clec7a−/− DCs (Fig. 6A and fig. S20A and B).
Fig. 6. MUC2 impairs NF-κB-driven inflammatory signals via β-catenin.
(A) WB of cytoplasmic or nuclear phospho (p)-AKT, AKT, pGSK-3β, GSK-3β, β-catenin (β-cat), dephospho (dp)-β-cat, actin and octamer-1 (Oct-1) from human DCs cultured with or without LPS and/or MUC2 for 10 min. (B) Electrophoretic mobility gel shift assay of IL12A-bound NF-κB p65-p50 and consensus DNA-bound Oct-1 in DCs cultured as in (A) for 3 hours. (C) Chromatin IP (ChIP) of IL12A-bound NF-κB p65 in DCs cultured as in (A) for 3 hours. RDQ, relative DNA quantity. (D) IP with control (ctr) IgG Ab or anti-dp-β-cat Ab of nuclear proteins from DCs cultured with or without LPS and/or MUC2 for 10 min, followed by WB of NF-κB p65 and dp-β-cat. (E) IL12A transcription in DCs cultured as in (A) for 2 days. (F and G) NF-κB-mediated transcription and ELISA of IL-12p70 and IL-10 in DCs cultured as in (E) in the presence of scrambled (ctr) or CTNNB1 (β-cat) siRNAs. (H) IFA of pAKT, pGSK-3β, β-cat, galectin-3, CD11c and DAPI in mouse PPs. Original magnification, ×5. Data show one of 3 experiments yielding similar results or summarize 3 experiments (error bars, s.d.; unpaired t test, *P <0.05).
In some tumors, β-catenin interacts with nuclear factor κB (NF-κB) p65 to impede the binding of p50-p65 complexes to death-inducing genes (25). In DCs, MUC2 alone or coupled with LPS enhanced nuclear β-catenin interaction with NF-κB p65 and decreased NF-κB p50-p65 binding to both minimal and IL12 gene promoters, thus impairing their transcription (Fig. 6B–E and fig. S20C). Accordingly, β-catenin deficiency impeded MUC2-mediated inhibition of LPS-induced IL-12 but not IL-10 transcription and/or secretion, whereas β-catenin overexpression dampened TNF-induced NF-κB-driven transcription (Fig. 6F and G and fig. S20D–F). Consistent with these data, galectin-3-expressing DCs from PPs contained abundant β-catenin in addition to activated AKT and inactive GSK-3β (Fig. 6H).
Besides AKT, Dectin-1 phosphorylates SYK, which activates NF-κB through a pathway mitigated by FcγRIIB via SH2 domain-containing inositol 5-phosphatase-1 (SHIP-1) (22, 26). SYK also activates cAMP responsive element binding protein (CREB), a calcium-dependent IL-10-inducing protein that removes the co-activator CREB-binding protein (CBP) from DNA-bound NF-κB (27, 28). Besides triggering SYK and SHIP-1 phosphorylation, MUC2 alone or combined with LPS decreased NF-κB p65 but not p50 nuclear translocation and increased galectin-dependent calcium fluxes, phosphorylation of CREB-targeting AKT, ERK1/2 and p38 kinases, phosphorylation and nuclear translocation of CREB, binding of CREB to IL10 and IL12 promoters, and loss of CBP from the IL12 promoter (figs. S20G and S21A-G). Thus, similar to hyperglycosylated IgG Abs used to treat autoimmune disorders (22), MUC2 may recruit SHIP-1 via FcγRIIB to constrain proinflammatory NF-κB but not tolerogenic CREB signals emanating from Dectin-1 and SYK. In addition to inducing IL-10, CREB may cooperate with dectin-1-induced β-catenin to inhibit IL-12.
Conclusions
We have shown here that MUC2 enhances gut homeostasis and oral tolerance by conditioning DCs and IECs. Antigen-sampling DCs assemble galectin-3, Dectin-1 and FcγRIIB to acquire MUC2 across IECs and possibly from GCs (fig. S22A). The MUC2 receptor complex suppresses inflammatory but not tolerogenic DC responses by inhibiting NF-κB via β-catenin and possibly CREB (fig. S22B). How DCs tune down these signals during infection remains unclear, but pathogen-induced perturbations of MUC2 glycosylation and polymerization patterns may be involved (1). A full understanding of the immunoregulatory function of MUC2 could help to devise better vaccines and treatments against infections and food allergies and to unravel how alterations of MUC2 and its receptors aggravate inflammatory bowel disease (1, 20), thus leading to safer therapies against this disorder.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Allergy and Infectious Diseases, NIH (AI61093, AI57653, AI95613, AI96187 and AI74378 to A.C. and AI073899, DK072201 and AI095245 to J.B.) and by Redes Temáticas de Investigación Cooperativa en Salud/Fondo Europeo de Desarrollo Regional (RD12/0036/0054 to AB). pRSETb-mRFP used for red bacteria is under MTA with Roger Y. Tsien at UCSD/HHMI. The data presented in this manuscript are tabulated in the main paper and the supplementary materials.
Footnotes
References and Notes
- 1.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 5.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 6.Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, Furtado GC, Lira SA, Shakhar G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581–595. doi: 10.1016/j.immuni.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, Anguiano E, Pourpe S, Goller K, Pascual V, Banchereau J, Merad M, Palucka K. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-β. Immunity. 2013;38:818–830. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 12.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 13.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 14.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 15.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteban A, Popp MW, Vyas VK, Strijbis K, Ploegh HL, Fink GR. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc Natl Acad Sci USA. 2011;108:14270–14275. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Kawasaki H, Hsu SC, Lee RT, Yao X, Plunkett B, Fu J, Yang K, Lee YC, Huang SK. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. 2010;16:1128–1133. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, Rotter JI, Wang HL, McGovern DP, Brown GD, Underhill DM. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 22.Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, Blanchard V, Winkler A, Hess C, Reid DM, Majoul IV, Strait RT, Harris NL, Köhl G, Wex E, Ludwig R, Zillikens D, Nimmerjahn F, Finkelman FD, Brown GD, Ehlers M, Köhl J. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med. 2012;18:1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur J Immunol. 2009;39:1379–1386. doi: 10.1002/eji.200838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, Lin SY, Hung MC. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–334. doi: 10.1016/S1535-6108(02)00154-X. [DOI] [PubMed] [Google Scholar]
- 26.Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, Brown GD, Leitges M, Ruland J. Syk kinase-coupled C-type lectin receptors engage protein kinase C-σ to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly EK, Wang L, Ivashkiv LB. Calcium-activated pathways and oxidative burst mediate zymosan-induced signaling and IL-10 production in human macrophages. J Immunol. 2010;184:5545–5552. doi: 10.4049/jimmunol.0901293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Herguido C, Guiu J, D’Altri T, Inglés-Esteve J, Dzierzak E, Espinosa L, Bigas A. Hematopoietic stem cell development requires transient Wnt/β-catenin activity. J Exp Med. 2012;209:1457–1468. doi: 10.1084/jem.20120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stadtfeld M, Graf T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development. 2005;132:203–213. doi: 10.1242/dev.01558. [DOI] [PubMed] [Google Scholar]
- 31.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Axelsson MA, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem. 1998;273:18864–18870. doi: 10.1074/jbc.273.30.18864. [DOI] [PubMed] [Google Scholar]
- 33.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baró T, de Heredia CD, Torán N, Català A, Torrebadell M, Fortuny C, Cusí V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Aróstegui JI, Juan M, Yagüe J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alpan O, Rudomen G, Matzinger P. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J Immunol. 2001;166:4843–4852. doi: 10.4049/jimmunol.166.8.4843. [DOI] [PubMed] [Google Scholar]
- 35.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Förster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cols M, Barra CM, He B, Puga I, Xu W, Chiu A, Tam W, Knowles DM, Dillon SR, Leonard JP, Furman RR, Chen K, Cerutti A. Stromal endothelial cells establish a bidirectional crosstalk with chronic lymphocytic leukemia cells through the TNF-related factors BAFF, APRIL, and CD40L. J Immunol. 2012;188:6071–6083. doi: 10.4049/jimmunol.1102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, Shan M, Xiong H, Bussel JB, Chiu A, Puel A, Reichenbach J, Marodi L, Döffinger R, Vasconcelos J, Issekutz A, Krause J, Davies G, Li X, Grimbacher B, Plebani A, Meffre E, Picard C, Cunningham-Rundles C, Casanova JL, Cerutti A. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.