Abstract
Evidence suggests putative interactions of leptin and C-reactive protein (CRP) in the pathogenesis of adiposity-related atherosclerotic cardiovascular disease (CVD). Therefore, we investigated whether CRP levels modify the relationship of leptin levels with coronary artery calcium (CAC). We examined 1,460 asymptomatic individuals from two community-based cross-sectional studies coordinated at a single, university-based research center. We focused on subjects who were overweight or obese (BMI ≥25) given greater biologic plausibility in this setting. In multivariable CAC models, we analyzed the interaction of log-transformed plasma leptin levels with higher CRP levels as defined by three cut-points: two clinically based (2 mg/l, 3 mg/l) and one dataset specific (sex-specific upper quartile). The association of plasma leptin with CAC was modified by higher CRP regardless of cut-point (interaction term P values all <0.01 in fully adjusted models). Leptin levels were associated with CAC in those with high, but not low CRP levels (e.g., tobit ratio for a 1 unit increase in ln(leptin) (95% CI): 2.18 (1.29–3.66) if CRP level ≥3 mg/l; N = 461 vs. 0.94 (0.67–1.31) if CRP levels <3 mg/l; N = 999) in fully adjusted models. No interaction with CRP was present in control analyses with adiponectin, BMI and waist circumference. In conclusion, in asymptomatic overweight and obese adults, increased leptin levels were independently associated with increased CAC in the presence of high, but not low CRP levels, supporting a leptin-CRP interface in atherosclerosis risk.
INTRODUCTION
Obesity is pandemic in the developed world. In the United States, for every three individuals, two are overweight (1). Individuals with excess weight characteristically exhibit chronically high levels of the pleiotropic cytokine-hormone leptin due to increased production by excess adipose tissue and loss of negative feedback (2). Several lines of evidence suggest a link between hyperleptinemia and atherosclerotic cardiovascular disease (CVD) via direct atherogenicity as well as unfavorable influences on CVD risk factors such as insulin resistance (2).
Recent studies indicate that elevated plasma C-reactive protein (CRP), or underlying inflammatory processes, could modify leptin effects in CVD (2–6). First, leptin stimulates CRP expression in vascular endothelial cells (3,4). Second, in HEK293 cells (5) and human aortic endothelial cells (6), CRP interferes with cellular actions of human leptin. Third, Chen et al. (5) found a physical interaction, with putative functional consequence, between circulating human CRP and leptin. Finally, in a human epidemiologic analysis, leptin levels are most strongly associated with myocardial infarction or stroke in the presence of elevated CRP levels (7).
Previously, in asymptomatic adults, we reported independent association of leptin with coronary artery calcium (CAC) (8,9) and sex-dependent CAC association of CRP (10,11) in the Study of Inherited Risk of Coronary Atherosclerosis (SIRCA) and the Penn Diabetes Heart Study (PDHS). In this SIRCA/PDHS report, we aimed to test the hypothesis that CRP levels modify the relationship of leptin levels with CAC in individuals with excess adiposity.
METHODS AND PROCEDURES
Study participants
The SIRCA and PDHS protocols have been described previously (8–10). These community-based, cross-sectional studies were coordinated at the University of Pennsylvania using the same research center, staff, and CT scanner. Inclusion criteria were age 30–79 years and a family history of premature CVD in SIRCA, or the presence of type 2 diabetes mellitus (T2DM) in PDHS. Exclusion criteria included a clinical history of CVD (as evidenced by myocardial infarction, coronary revascularization, angiographic disease, or positive stress test) and elevated creatinine in both studies. In addition, SIRCA excluded those with clinical evidence of T2DM. This report focuses on unrelated subjects with a BMI ≥25 and data for both circulating leptin and CRP (N = 1,460). We focus on overweight and obese subjects because our hypothesis is targeted at adiposity-related CVD based on stronger biologic plausibility in this setting (2). The studies were approved by the institutional review board of University of Pennsylvania and all participants provided informed consent.
Evaluated parameters
Participants were evaluated at the University of Pennsylvania Medical Center General Clinical Research Center after a 12-h overnight fast. Plasma levels of leptin, adiponectin, and insulin (Linco Research, St Charles, MO), as well as interleukin-6 (IL-6) (R&D Systems, Minneapolis, MN), were measured by ELISA. CRP was assayed with an ultrahigh-sensitivity immunoturbidimetric assay (Wako Pure Chemical Industries, Osaka, Japan) on a Cobas Fara II analyzer (Roche Diagnostics, Indianapolis, IN). Lipid parameters were measured in Penn's Centers for Disease Control-certified lipid laboratory. Laboratory test results were generated by personnel blinded to the clinical characteristics and CAC scores of subjects.
Clinical parameters, including blood pressure, BMI, and waist circumference, were assessed in standardized fashion as previously reported (8,10). Smoking (active vs. nonsmoker or ex-smoker), alcohol use (none vs. any), and exercise (none vs. any) were evaluated by a standardized written questionnaire. Framingham risk scores, using low-density lipoprotein cholesterol, were determined as described by Wilson et al. (12). The homeostasis model assessment [HOMA-IR = fasting glucose (mmol/l) × fasting insulin (mU/ml)/22.5] was used as a measure of insulin resistance in those not on insulin therapy. Subjects were classified as having the metabolic syndrome using the revised National Cholesterol Education Program definition (glucose cut-point 100 mg/dl; all subjects with T2DM were classified as meeting glucose criteria) (13). Global CAC scores were quantified according to the method of Agatston et al. (14) by electron beam tomography.
Statistical analysis
Data are reported as median (interquartile range = IQR) or mean ± standard deviation for continuous variables and as proportions for categorical variables. In all regressions, leptin, CRP, IL-6 and HOMA-IR were logarithmically transformed when included as continuous variables given non-Gaussian distributions. Leptin's correlation to CRP and IL-6 levels was assessed by unadjusted then multivariable linear regression. Likelihood ratio testing (LRT) quantified the incremental value of adding an independent variable in adjusted models. Where sufficient power exists, sex-adjusted and sex-stratified results are presented given known variation by sex in leptin and CRP levels (8–11).
Multivariable CAC modeling employed tobit conditional regression to accommodate the unusual distribution of CAC data (many zero scores but also a marked right skew) (15). Tobit combines logistic regression of the presence of CAC (any CAC present vs. CAC zero score) with a linear regression (of log-transformed CAC) when CAC is present to produce a single estimate for the relationship of risk factors with CAC data.
To address our primary hypothesis, we created interaction terms for leptin with three different cut-points of CRP. Two of these cut-points were clinically based, including the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial cut-point of 2 mg/l (16) and the Centers for Disease Control and American Heart Association high risk cut-point of 3 mg/l (17). The third cut-point was the sex-specific upper quartile of CRP for our dataset.
We performed CAC interaction analyses in a basic model adjusted for age, sex, race, T2DM and waist circumference, then in a full model additionally adjusted for multiple CVD risk factors and medications as indicated in Figure 1. Missing covariate data constituted 13.7% of IL-6 values, 1.1% of blood pressure values, 0.6% of adiponectin values, 0.5% of family history values, 0.1% of cholesterol and smoking values, and <0.1% alcohol use, exercise, and medication values. For missing values, we performed sequential regression multiple imputation by standard methodology (18) using all analysis variables to generate 10 imputed datasets (m = 10). Results were similar in models with non-imputed values and in models with Framingham risk score and metabolic syndrome in place of their individual components (data not shown). We conducted CAC control interaction analyses in which we interacted CRP with adiposity parameters other than leptin and in which we replaced CRP with Framingham risk score. To assess the specificity of CAC as the outcome measure, we also conducted leptin-CRP interaction analyses in relation to other CVD risk factors as outcomes.
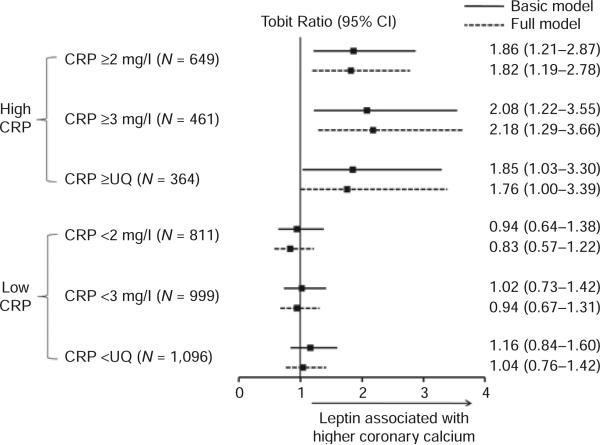
Figure 1.
Association of leptin levels with coronary calcium at high and low C-reactive protein levels. Tobit ratios for the increase in coronary artery calcium (CAC) score for a one unit increase in ln(leptin) demonstrating an independent positive association of leptin with CAC at high, but not low C-reactive protein (CRP) levels. Basic model included age, sex, race, type 2 diabetes mellitus, and waist circumference. Full model further adjusted for apolipoprotein B, ln(triglycerides), high-density lipoprotein cholesterol, systolic blood pressure, diastolic blood pressure, family history of premature cardiovascular disease, menopause, active smoking, alcohol use, exercise, adiponectin, ln(interleukin-6), and medications (same as Table 2). UQ = sex-specific upper quartile of CRP levels (≥6.5 mg/l in women; ≥2.6 mg/l in men).
Statistical analyses were performed using Stata 10.0 software (Stata Corp, College Station, TX). CAC models used the tobit command with the 11(0) option to indicate left censoring at a CAC score of 0. Stata's ice software add-on was used for multiple imputation; results from imputed datasets were averaged into a single inference. Analyses employed the xi interaction expansion with interaction terms for i.CRP × leptin where CRP = a binary CRP variable for levels < or ≥ one of the three cut-points defined above and leptin = natural log of leptin levels.
RESULTS
Characteristics of study sample
Table 1 displays demographics in all subjects and stratified by sex. This 30–77 year-old sample included 35% women and 24% nonwhite subjects. Compared with men, women were less likely white, had higher HDL-C, lower blood pressure, higher BMI, but smaller waists. They were less likely to drink alcohol, more sedentary and fewer were on cardiometabolic medications. Plasma leptin and CRP levels were higher in women vs. men, as were adiponectin and IL-6. Coronary calcium and Framingham risk scores were lower in women. Overall, coronary calcification was prevalent in the study population.
Table 1.
Characteristics of study sample
| All subjects (N = 1,460) | Women (N = 513) | Men (N = 947) | Women vs. men (P value) | |
|---|---|---|---|---|
| Age, years; full range | 55 (30–77) | 55 (31–76) | 55 (30–77) | 0.80 |
| White | 75.9% | 70.2% | 79.0% | <0.001 |
| Diabetes | 61.2% | 58.3% | 62.8% | 0.09 |
| Apolipoprotein B, mg/dl | 89 (76–105) | 90 (76–107) | 89 (76–104) | 0.70 |
| HDL-cholesterol, mg/dl | 45 (37–54) | 53 (44–63) | 42 (36–49) | <0.001 |
| Systolic blood pressure, mm Hg | 130 (122–140) | 129 (120–138) | 131 (123–141) | 0.001 |
| Diastolic blood pressure, mm Hg | 77 (72–84) | 75 (70–81) | 79 (73–84) | <0.001 |
| BMI, kg/m2 | 31 (28–35) | 32 (29–37) | 30 (28–34) | <0.001 |
| Waist circumference, inches | 41 (37–44) | 39 (35–44) | 41 (38–44) | <0.001 |
| Leptin, ng/ml | 12.1 (6.5–23.2) | 26.8 (18.4–36.3) | 7.9 (5.1–12.7) | <0.001 |
| CRP, mg/l | 1.7 (0.8–3.8) | 3.0 (1.4–6.5) | 1.3 (0.7–2.6) | <0.001 |
| Adiponectin, μg/ml | 10.7 (6.7–16.9) | 14.2 (8.7–22.1) | 9.4 (6.0–14.5) | <0.001 |
| Interleukin-6, pg/ml | 1.4 (0.9–2.2) | 1.7 (1.1–2.6) | 1.3 (0.9–2.0) | <0.001 |
| HOMA-IR | 2.9 (1.6–4.8) | 2.6 (1.6–4.6) | 2.9 (1.7–4.9) | 0.06 |
| Metabolic syndrome | 64.7% | 65.7% | 64.1% | 0.57 |
| Framingham risk score, 10 year | 9% (6–15%) | 8% (4–13%) | 11% (7–18%) | <0.001 |
| Active smoker | 11.0% | 9.6% | 11.8% | 0.22 |
| Alcohol drinker | 57.6% | 47.2% | 63.3% | <0.001 |
| Sedentary | 30.1% | 33.7% | 28.2% | 0.05 |
| Postmenopausal | N/A | 69.6% | N/A | N/A |
| Aspirin | 32.5% | 28.3% | 34.9% | 0.01 |
| Statin | 38.1% | 31.8% | 41.5% | <0.001 |
| Other lipid lowering agent | 13.9% | 10.5% | 15.7% | 0.007 |
| Antihypertensive | 48.8% | 46.4% | 50.1% | 0.19 |
| Insulin | 11.6% | 12.3% | 11.3% | 0.61 |
| Other antidiabetic agent | 49.7% | 44.4% | 52.6% | 0.003 |
| HRT | 11.8% | 33.5% | N/A | N/A |
| CAC Median (IQR) | 15 (0–185) | 1 (0–40) | 48 (1–318) | <0.001 |
| CAC >0 | 72.1% | 57.5% | 79.9% | <0.001 |
| CAC >100 | 32.1% | 16.2% | 40.8% | <0.001 |
| CAC >400 | 15.6% | 4.7% | 21.5% | <0.001 |
Continuous variables were compared by the Student's t-test or Mann–Whitney test and categorical variables were compared by Fisher's exact test. HOMA-IR = homeostasis model assessment of insulin resistance; 63 women and 107 men on insulin excluded. Other lipid lowering agent = niacin, fibrate, ezetimibe, bile acid resin, fish oil. Antihypertensive = β-blocker, ACE-inhibitor, calcium channel blocker, diuretic, other antihypertensive. Other antidiabetic agent = metformin, sulfonylurea, thiazolidin-edione, meglitidine, α-glucosidase inhibitor, exenatide.
CAC, coronary artery calcium score; CRP, C-reactive protein; HRT, hormone replacement therapy.
Leptin-CRP correlation
Unadjusted correlations between leptin and CRP levels in women (r = 0.41, P < 0.001) and men (r = 0.29, P < 0.001) were higher than those between leptin and IL-6 (r = 0.13, P < 0.001 in women; r = 0.12, P < 0.001 in men) or CRP and adiponectin (r = 0.33, P < 0.001 in women; r = 0.12, P < 0.001 in men). Multivariable leptin-CRP association was independent of age, race, metabolic/lifestyle factors and medications (χ2 >15 and P < 0.001 in both sexes; Table 2) whereas the leptin-IL-6 association was weaker in the total population after multivariable adjustment and lost in sex-stratified analyses.
Table 2.
Association of leptin levels with C-reactive protein vs. interleukin-6 levels in multivariable models
| All subjects (N = 1,460) |
Women (N = 513) |
Men (N = 947) |
||||
|---|---|---|---|---|---|---|
| r | χ 2 | r | χ 2 | r | χ 2 | |
| CRP | ||||||
| Unadjusted | 0.43 | 301.8* | 0.41 | 94.3* | 0.29 | 84.1* |
| Age, (sex), race | 0.71 | 147.5* | 0.45 | 79.8* | 0.36 | 75.2* |
| Age, (sex), race, medications, metabolic and lifestyle factors | 0.81 | 44.9* | 0.64 | 34.1* | 0.69 | 15.9* |
| Interleukin-6 | ||||||
| Unadjusted | 0.18 | 50.4* | 0.13 | 9.4* | 0.12 | 13.5* |
| Age, (sex), race | 0.68 | 15.1* | 0.28 | 6.1† | 0.25 | 8.6† |
| Age, (sex), race, medications, metabolic and lifestyle factors | 0.80 | 5.8‡ | 0.61 | 3.2 | 0.68 | 1.6 |
Correlation coefficients (r) represent the amount of the dependent variable (leptin) explained by the linear regression model. Likelihood ratio testing quantifies with χ2 the incremental explanatory value of C-reactive protein or interleukin-6 levels to the model.
P < 0.001
P < 0.01
P < 0.05.
A parenthesis around sex indicates adjustment in models of all subjects only. Medications = aspirin, β-blocker, ACE-inhibitor, calcium channel blocker, diuretic, other antihypertensive, statin, niacin, fibrate, ezetimibe, bile acid resin, fish oil, metformin, sulfonylurea, thiazolidinedione, meglitidine, α-glucosidase inhibitor, exenatide, insulin and hormone replacement therapy; each medication controlled for individually. Metabolic factors = type 2 diabetes mellitus, waist circumference, menopause (in women), systolic blood pressure, diastolic blood pressure, apolipoprotein B, ln(triglycerides), high-density lipoprotein cholesterol and adiponectin. Lifestyle factors = active smoking, alcohol use and exercise.
Interaction of leptin and CRP levels in relation to CAC
In a CAC model adjusted for age, sex, race, T2DM and waist circumference, leptin levels interacted with higher CRP levels as defined by specific cut-points (P = 0.025, 0.005, and 0.012 for 2 mg/l, 3 mg/l, and sex-specific upper quartile cut-points, respectively). After further adjustment for traditional CVD risk factors, medications, adiponectin and IL-6 in our full model, leptin levels interacted with higher CRP (P = 0.006, 0.002, 0.004 for respective cut-points). Interaction was such that increased leptin levels were associated with increased CAC in those with high, but not low CRP levels (e.g., Figure 1). Exploratory subgroup analyses by sex, menopause, T2DM, and race were underpowered and showed a trend toward modest heterogeneity by sex and menopause (not shown).
Specificity of CAC outcome and leptin-CRP interaction
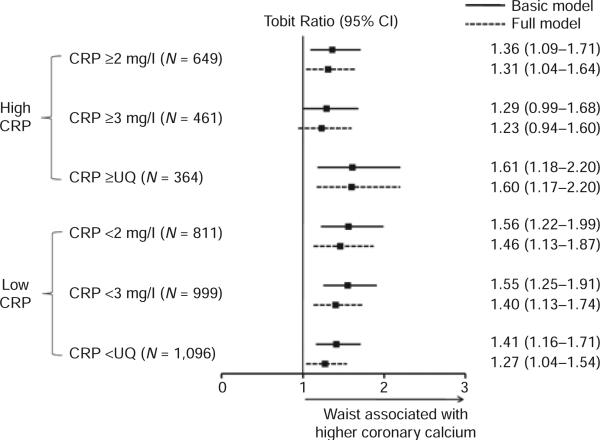
Leptin levels did not interact with higher CRP in relation to outcomes other than CAC, including metabolic syndrome, HOMA-IR, systolic and diastolic blood pressure, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and apolipoprotein B. In CAC models adjusted for age, sex, race, T2DM, and waist circumference, leptin levels did not interact with higher Framingham risk scores (cut-point < or ≥ the upper quartile; P = 0.90). In similar modeling, adiponectin did not interact with higher CRP (P = 0.72, 0.62, 0.31 for 2 mg/l, 3 mg/l, and sex-specific upper quartile cut-points respectively). In age, sex, race and T2DM adjusted models, BMI (P = 0.62, 0.59, 0.54) and waist circumference (P = 0.46, 0.25, 0.51) also did not interact with higher CRP. As an example, Figure 2 illustrates the stable independent association of waist circumference with CAC across CRP cut-points.
Figure 2.
Association of waist circumference with coronary calcium at high and low C-reactive protein levels. Tobit ratios for the increase in coronary artery calcium (CAC) score for a one standard deviation increase in waist circumference (5.30) in basic and full models (same adjustment as Figure 1 with waist circumference transitioned to independent variable of interest) demonstrating the absence of interaction across C-reactive protein (CRP) cut-points.
DISCUSSION
In a sample of asymptomatic overweight and obese individuals, we found that circulating leptin was associated with increased CAC when CRP levels were high, but not when they were low. Control analyses, considering other measures of adiposity and inflammation, suggested selectivity in the interaction between CRP and leptin.
Our results extend an emerging literature of putative atherogenic leptin effects that seem to interface with chronic inflammation (2,19). Leptin stimulates T lymphocytes towards a Th1 (proinflammatory) adaptive immune response while promoting monocyte recruitment, macrophage foam cell formation and secretion of proinflammatory cytokines (2). This functional profile, along with structural relation to proinflammatory cytokines, and signaling through the IL-6 glycoprotein-130 cytokine receptor, frame leptin as an “adipocytokine.” The discovery of the leptin receptor in human atherosclerotic lesions (20,21) and epidemiologic data associating leptin levels with measures of CVD and CVD risk (2,8,9,19) support a role for leptin in human atherogenesis.
Recent experimental investigations have revealed specific cross-talk between leptin and CRP. Leptin stimulated CRP expression in vascular endothelial cells (3,4) and CRP impaired leptin-induced endothelial nitric oxide synthase activation in human aortic endothelial cells (6). While such findings are provocative for direct functional leptin-CRP interplay in the endothelium, CRP itself may not be a causal mediator of CVD (22,23). A role for CRP, or related inflammatory pathways, however, in modulating causal atherogenic signals including leptin is certainly possible and may contribute to confounding in the observed association of plasma CRP with atherosclerotic CVD.
In this report, we used an epidemiological strategy to examine CRP's influence on plasma leptin's relationship to human atherosclerosis. We previously showed in SIRCA and PDHS that leptin is independently associated with CAC (8,9) and that CRP has a modest association with CAC that is most evident in women (10,11). Here we find evidence that CRP itself, or the inflammatory pathways that it captures, modulates the relationship of plasma leptin to the burden of underlying atherosclerosis in overweight and obese individuals, a clinical setting where chronic inflammation and leptin resistance converge. In this sample, the positive association of leptin levels with CAC was isolated to the setting of high CRP levels, defined by either clinical or dataset driven cut-points. This pattern was stable across a spectrum of models that varied in the degree of multivariable adjustment. Little prior epidemiologic literature is available for comparison. However, when Romero-Corral et al. examined 6,251 adult participants from the Third National Health and Nutrition Examination Survey, the area under the curve for the association of risk factors with the presence of myocardial infarction or stroke was highest with the inclusion of high levels of both leptin and CRP together (7). Additionally, Figure 1 of Romero-Corral et al.'s manuscript suggests that leptin levels were most strongly associated with cardiovascular events in the setting of high CRP levels, which is concordant with our data.
Our findings might be due to direct effects of CRP in modulating leptin atherogenicity or alternatively could relate to indirect mechanisms whereby CRP is a marker for pathways that alter leptin signaling. In control analyses to assess for such confounding in CRP's modification, CRP's modulation of leptin's relationship with CAC did not seem to simply represent it tracking with a higher risk milieu based on Framingham risk scores. Further, CRP did not interact with adiponectin or direct measures of adiposity (BMI or waist circumference) in CAC models. There was also specificity by outcome as CRP did not modify leptin's relationship with metabolic syndrome, insulin resistance, blood pressure or lipid parameters. Thus, our data point to a more direct CRP-related inflammatory modulation of leptin's atherogenic signaling.
The positive independent correlation between circulating leptin and CRP concentrations in our study agrees with previous reports (24–27). We found that their correlation is relatively stronger than that of leptin with IL-6 or adiponectin with CRP. Consistent with published data for exercise (28,29) and alcohol consumption (30,31), in SIRCA and PDHS, we found that leptin and CRP levels demonstrated an inverse association with exercise and alcohol intake (data not shown). While a differential univariate association has been observed in relation to active smoking (lower leptin (32); higher CRP (33)), leptin was not independently associated with smoking in multiple regression analysis (32), which is consistent with our data (not shown).
Circulating leptin and CRP levels appear to be linked by an adipo-hepato regulatory loop in which leptin stimulates hepatic CRP expression, as evidenced by both in vitro (5) and clinical investigations (34,35). In turn, CRP may regulate leptin bioavailabilty as one of five serum leptin-interacting proteins (SLIPs) in human blood (5). The concomitant role of the soluble leptin receptor in modulating plasma leptin bioavailability (36,37) provides one potential explanation for common variation at the leptin receptor locus being associated with CRP blood levels (38,39). Indeed, aside from the CRP locus itself, the leptin-receptor locus contains the most number of single-nucleotide polymorphisms independently related to CRP levels (39). Overall, the chronic inflammatory element of obesity, and specifically CRP, may promote leptin's pathological transition by worsening leptin resistance and thus contribute to the pathogenesis of CVD.
Study limitations
Our study carries several potential limitations. First, our modeling assumes that a cross-sectional representation of risk factors reflects prior exposures that contributed to atherosclerosis over time. Second, our observational analysis did not directly address molecular causality and is open to the influence of unmeasured confounders as leptin has such broad physiologic function. However, we made a concerted effort to minimize potential confounding by performing multivariable modeling in a well-phenotyped sample and we also employed comparative control interaction testing to assess specificity. Third, subgroup analyses were underpowered in our study limiting more refined interpretations. Fourth, plasma levels of biomarkers vary with time and physiological influences. For this reason, blood sampling in our study was performed between 8–10 am in a fasting state and we employed validated assays for all laboratory variables. Nevertheless, the presence of T2DM and premature subclinical atherosclerosis represent potential sources of selection bias that could result in higher levels of leptin and CRP. Fifth, CAC is an estimate (40,41), and not a direct measure of coronary atherosclerosis, thus it may fail to detect some coronary atherosclerotic plaques. Despite this limitation, CAC scores are clinically relevant because they are strong, independent predictor predictors of CVD (41–43).
Conclusion
We found in a sample of asymptomatic overweight and obese individuals that circulating leptin was associated CAC when CRP levels were high but not when they were low. For cardiovascular risk stratification, there may be utility in considering leptin and CRP levels in tandem, and in discerning which patterns of biomarkers, rather than individual levels, are most informative. More broadly, therapeutic interventions that attenuate metabolic and inflammatory disturbances concurrently may have the greatest clinical potential.
ACKNOWLEDGMENTS
This work was supported by a Clinical and Translational Science Award (UL1RR024134) from the National Center for Research Resources and by the Diabetes and Endocrine Research Center at the University of Pennsylvania (P20-DK 019525). M.P.R. is also supported by RO1 HL-073278 and P50 HL-083799-SCCOR from the National Institutes of Health.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh P, Hoffmann M, Wolk R, Shamsuzzaman AS, Somers VK. Leptin induces C-reactive protein expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:e302–e307. doi: 10.1161/ATVBAHA.107.148353. [DOI] [PubMed] [Google Scholar]
- 4.De Rosa S, Cirillo P, Pacileo M, et al. Leptin stimulated C-reactive protein production by human coronary artery endothelial cells. J Vasc Res. 2009;46:609–617. doi: 10.1159/000226229. [DOI] [PubMed] [Google Scholar]
- 5.Chen K, Li F, Li J, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–432. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 6.Procopio C, Andreozzi F, Laratta E, et al. Leptin-stimulated endothelial nitric-oxide synthase via an adenosine 5’-monophosphate-activated protein kinase/Akt signaling pathway is attenuated by interaction with C-reactive protein. Endocrinology. 2009;150:3584–3593. doi: 10.1210/en.2008-0921. [DOI] [PubMed] [Google Scholar]
- 7.Romero-Corral A, Sierra-Johnson J, Lopez-Jimenez F, et al. Relationships between leptin and C-reactive protein with cardiovascular disease in the adult general population. Nat Clin Pract Cardiovasc Med. 2008;5:418–425. doi: 10.1038/ncpcardio1218. [DOI] [PubMed] [Google Scholar]
- 8.Reilly MP, Iqbal N, Schutta M, et al. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3872–3878. doi: 10.1210/jc.2003-031676. [DOI] [PubMed] [Google Scholar]
- 9.Qasim A, Mehta NN, Tadesse MG, et al. Adipokines, insulin resistance, and coronary artery calcification. J Am Coll Cardiol. 2008;52:231–236. doi: 10.1016/j.jacc.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reilly MP, Wolfe ML, Localio AR, Rader DJ, Study of Inherited Risk of Coronary Atherosclerosis C-reactive protein and coronary artery calcification: The Study of Inherited Risk of Coronary Atherosclerosis (SIRCA). Arterioscler Thromb Vasc Biol. 2003;23:1851–1856. doi: 10.1161/01.ATV.0000092327.60858.4A. [DOI] [PubMed] [Google Scholar]
- 11.Qasim AN, Budharaju V, Mehta NN, et al. Gender differences in the association of C-reactive protein with coronary artery calcium in type-2 diabetes. Clin Endocrinol (Oxf) 2011;74:44–50. doi: 10.1111/j.1365-2265.2010.03879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Reilly MP, Wolfe ML, Localio AR, Rader DJ. Coronary artery calcification and cardiovascular risk factors: impact of the analytic approach. Atherosclerosis. 2004;173:69–78. doi: 10.1016/j.atherosclerosis.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 17.Pearson TA, Mensah GA, Alexander RW, et al. Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 18.He Y. Missing data analysis using multiple imputation: getting to the heart of the matter. Circ Cardiovasc Qual Outcomes. 2010;3:98–105. doi: 10.1161/CIRCOUTCOMES.109.875658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 21.Kang SM, Kwon HM, Hong BK, et al. Expression of leptin receptor (Ob-R) in human atherosclerotic lesions: potential role in intimal neovascularization. Yonsei Med J. 2000;41:68–75. doi: 10.3349/ymj.2000.41.1.68. [DOI] [PubMed] [Google Scholar]
- 22.Zacho J, Tybjaerg-Hansen A, Jensen JS, et al. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 23.Nordestgaard BG. Does elevated C-reactive protein cause human atherothrombosis? Novel insights from genetics, intervention trials, and elsewhere. Curr Opin Lipidol. 2009;20:393–401. doi: 10.1097/MOL.0b013e3283307bfe. [DOI] [PubMed] [Google Scholar]
- 24.Piemonti L, Calori G, Mercalli A, et al. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: impact on cardiovascular mortality. Diabetes Care. 2003;26:2883–2889. doi: 10.2337/diacare.26.10.2883. [DOI] [PubMed] [Google Scholar]
- 25.Kazumi T, Kawaguchi A, Hirano T, Yoshino G. C-reactive protein in young, apparently healthy men: associations with serum leptin, QTc interval, and high-density lipoprotein-cholesterol. Metab Clin Exp. 2003;52:1113–1116. doi: 10.1016/s0026-0495(03)00184-7. [DOI] [PubMed] [Google Scholar]
- 26.Shamsuzzaman AS, Winnicki M, Wolk R, et al. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109:2181–2185. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- 27.Abdullah SM, Khera A, Leonard D, et al. Sex differences in the association between leptin and CRP: results from the Dallas Heart Study. Atherosclerosis. 2007;195:404–410. doi: 10.1016/j.atherosclerosis.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Reseland JE, Anderssen SA, Solvoll K, et al. Effect of long-term changes in diet and exercise on plasma leptin concentrations. Am J Clin Nutr. 2001;73:240–245. doi: 10.1093/ajcn/73.2.240. [DOI] [PubMed] [Google Scholar]
- 29.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–1061. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 30.Otaka M, Konishi N, Odashima M, et al. Effect of alcohol consumption on leptin level in serum, adipose tissue, and gastric mucosa. Dig Dis Sci. 2007;52:3066–3069. doi: 10.1007/s10620-006-9635-x. [DOI] [PubMed] [Google Scholar]
- 31.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 32.Kajikawa Y, Ikeda M, Takemoto S, et al. Association of circulating levels of leptin and adiponectin with metabolic syndrome and coronary heart disease in patients with various coronary risk factors. Int Heart J. 2011;52:17–22. doi: 10.1536/ihj.52.17. [DOI] [PubMed] [Google Scholar]
- 33.O'Loughlin J, Lambert M, Karp I, et al. Association between cigarette smoking and C-reactive protein in a representative, population-based sample of adolescents. Nicotine Tob Res. 2008;10:525–532. doi: 10.1080/14622200801901997. [DOI] [PubMed] [Google Scholar]
- 34.Mackintosh RM, Hirsch J. The effects of leptin administration in non-obese human subjects. Obes Res. 2001;9:462–469. doi: 10.1038/oby.2001.60. [DOI] [PubMed] [Google Scholar]
- 35.Hukshorn CJ, Lindeman JH, Toet KH, et al. Leptin and the proinflammatory state associated with human obesity. J Clin Endocrinol Metab. 2004;89:1773–1778. doi: 10.1210/jc.2003-030803. [DOI] [PubMed] [Google Scholar]
- 36.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–988. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 37.Zastrow O, Seidel B, Kiess W, et al. The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord. 2003;27:1472–1478. doi: 10.1038/sj.ijo.0802432. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YY, Gottardo L, Mlynarski W, et al. Genetic variability at the leptin receptor (LEPR) locus is a determinant of plasma fibrinogen and C-reactive protein levels. Atherosclerosis. 2007;191:121–127. doi: 10.1016/j.atherosclerosis.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Pare G, Parker A, et al. Loci related to metabolic-syndrome pathways including LEPR,HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women's Genome Health Study. Am J Hum Genet. 2008;82:1185–1192. doi: 10.1016/j.ajhg.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mautner GC, Mautner SL, Froehlich J, et al. Coronary artery calcification: assessment with electron beam CT and histomorphometric correlation. Radiology. 1994;192:619–623. doi: 10.1148/radiology.192.3.8058924. [DOI] [PubMed] [Google Scholar]
- 41.Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243–252. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 42.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164:1285–1292. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 43.Elkeles RS, Godsland IF, Feher MD, et al. PREDICT Study Group Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29:2244–2251. doi: 10.1093/eurheartj/ehn279. [DOI] [PubMed] [Google Scholar]