Abstract
Mitochondria are equipped with an efficient machinery for Ca2+ uptake and extrusion and are capable of storing large amounts of Ca2+ ions. Furthermore, key steps of mitochondrial metabolism (ATP production) are Ca2+-dependent. In the field of cardiac physiology and pathophysiology, two main questions have dominated the thinking about mitochondrial function in the heart: 1) how does mitochondrial Ca2+ buffering shape cytosolic Ca2+ levels and affect excitation-contraction coupling, particularly the Ca2+ transient, on a beat-to-beat basis, and 2) how does mitochondrial Ca2+ homeostasis influence cardiac energy metabolism. To answer these questions a thorough understanding of the kinetics of mitochondrial Ca2+ transport and buffer capacity is required. Here, we summarize the role of mitochondrial Ca2+ signaling in the heart, discuss the evidence either supporting or arguing against the idea that Ca2+ can be taken up rapidly by mitochondria during excitation-contraction coupling and highlight some interesting new areas for further investigation.
Keywords: calcium uniporter, sodium-calcium exchange, mitochondrial inner membrane, Ca2+ transients
Intro
Like historic vintages, certain great questions of physiology have managed to span more than a century and continue to generate interest and debate. An example is the mechanism behind the Frank-Starling “law of the heart”[1], which in an early form described the relationship between diastolic cardiac volume and oxygen consumption (an indirect measurement of mechanical energy expenditure)[2], evolving more recently into current discussions about how the Ca2+ sensitivity of myofilament force generation is determined by changes in sarcomere length. The second component of this question, which is also incompletely understood at the present time, is the mechanism by which “the metabolism of the heart increases or diminishes in proportion to the mechanical demands made upon this organ, so that the chemical sources of energy are drawn upon in proportion to its requirements”, as stated by Starling and coworkers in 1914[1].
Based on studies of suspensions of isolated mitochondria, several potential mechanisms have been elucidated by which mitochondrial ATP production can be regulated to match energetic demand. Respiratory (or acceptor) control by ADP and Pi availability was described in the 1950s[3, 4] and is indisputably a robust determinant of respiration in isolated mitochondria. On the other hand, the inability to detect changes in the bulk adenine nucleotide phosphorylation potential (i.e., [ATP]/([ADP] + [Pi]) in intact hearts at different workloads using phosphorous NMR[5] has led investigators to examine other potential factors regulating oxidative phosphorylation. For example, control of mitochondrial function through the allosteric effects of Pi on the rates of both NADH production by the tricarboxylic acid (TCA) cycle and electron transport has been put forward as a potentially important metabolic signal[6].
Since mitochondria have a high permeability to Ca2+[7] and key enzymes in the tricarboxylic acid (TCA) cycle are known to be Ca2+ sensitive, metabolic signaling by Ca2+ has been a leading hypothesis to explain the control of mitochondrial oxidative phosphorylation in vivo. Moreover, evidence suggests that Ca2+ might also regulate various sites of the electron transport chain and the mitochondrial ATP synthase[8, 9]. Changes in cardiac output often (but not always) involve changes in the time averaged cytoplasmic Ca2+ concentration, so it is important to determine the kinetics and magnitude of mitochondrial Ca2+ transport in intact cardiomyocytes in order to answer two main questions: 1) how does mitochondrial Ca2+ buffering shape the Ca2+ transient and 2) how does mitochondrial Ca2+ uptake influence metabolism. This subject been a subject of intense investigation and numerous reviews[10, 11]. Here, we will briefly summarize some of the evidence either supporting or arguing against the idea that Ca2+ can be taken up rapidly by mitochondria during excitation-contraction coupling and highlight some interesting new areas for further investigation.
POINT: Evidence in favor of fast mitochondrial Ca2+ uptake in the heart
The development of new tools and techniques of measuring mitochondrial Ca2+ over the years has led to a gradual accumulation of support for the idea that mitochondria can respond rapidly to changes in Ca2+ during the heartbeat. This evidence spans from the level of isolated mitochondria to the intact heart, as described below.
Isolated mitochondria
Ca2+ is taken up by the mitochondria via the Ca2+ uniporter (mCU), a still undefined protein in the inner membrane, driven by the large electrochemical gradient for Ca2+ across the inner mitochondrial membrane (ΔΨm ≈ −180 mV). The mCU studied in isolated mitochondria has second order kinetics, indicating an allosteric effect of Ca2+ on the transport rate. The turnover for Ca2+ uptake was previously estimated to be approximately 2×104 Ca2+ s−1 per single mCU molecule, in the range of a fast gated pore[7, 10]. In support of the notion that the mCU is an ion channel, a recent patch clamp study of intact mitoplasts has identified a mitochondrial Ca2+ channel (MiCa) possessing a high affinity binding site for Ca2+ (2 nM) that confers high Ca2+ selectivity at resting cytoplasmic Ca2+ but with an enormous capacity for Ca2+ uptake (half-saturation of IMiCa was ~20 mM)[12]. The channel activated almost instantaneously at negative membrane potentials with some inward rectification and showed partial fast inactivation and block by known mCU inhibitors like ruthenium red. Notably the Vmax of MiCa was significantly higher than earlier estimates of mCU (Vmax = 5×106 Ca2+ s−1 per single mCU molecule), as was the channel density (~10–40 channels per μm2). The authors suggested that the true mCU Vmax is usually underestimated in intact mitochondria due to the fact that ΔΨm will be rapidly dissipated at high Ca2+ concentrations, a problem which is circumvented by voltage clamping the membrane. Similarly, the true Km of Ca2+ uptake through the mCU, which is on the order of ~10–20 μM for intact isolated mitochondria, might be much higher.
Indirect support for the idea that Ca2+ uptake is very fast in isolated mitochondria comes from experiments examining the effects of Ca2+ on respiration using rapid solution switching or stopped-flow methods. As long ago as 1965, Chance[12] reported that the oxidation of cytochrome b occurs with a halftime of ~70 msec (an upper limit estimate) in isolated liver mitochondria exposed to a 200 μM pulse of Ca2+. In more recent experiments, Territo et al[13] showed that changes in mitochondrial oxygen consumption, NADH, and light scattering (a measure of mitochondrial matrix volume) occurred with an initial response time of <100 msec after the rapid application of an optimal level of 535 nM Ca2+ in porcine heart mitochondria.
A rapid mode of mitochondrial Ca2+ uptake, termed RaM, has been reported[14], with kinetics approximately 100-fold faster than those measured for mCU (which are in the range of seconds). Rapid mitochondrial Ca2+ uptake via RaM was observed on the first of a series of Ca2+ pulses delivered by rapid mixing in liver or heart mitochondria[14]. RaM is less sensitive to ruthenium red than the mCU, but it is rapidly inactivated if the Ca2+ concentration in the medium exceeds ~100 nM[15]. Recovery from inactivation of this mode of Ca2+ transport occurs over 10s of seconds, so it is unlikely to account for beat-to-beat mitochondrial Ca2+ transients in paced cells.
Other cytosolic factors can dramatically influence the apparent affinity for mitochondrial Ca2+ uptake in isolated mitochondria, including the concentrations of Na+ and Mg2+ in the medium. For example, the addition of 10 mM Na+ plus 1–2 mM Mg2+, levels not very different from physiological conditions, can shift the midpoint of Ca2+ uptake from ~0.1 μM to >1.5 μM[16]. Moreover, the midpoint of activation of TCA cycle enzymes by extramitochondrial Ca2+ can be shifted to the right by about one log unit when 0.5 Mg2+ plus 15 mM Na+ is present[17]. The effects of Mg2+ are thought to be mediated by a direct interaction of the divalent ion with the mCU, and Ca2+ current through MiCa2+ is inhibited by almost 60% in the presence of 0.5mM Mg2+. The Na+ effect follows logically from the consensus that the primary mitochondrial Ca2+ efflux mechanism (at least in cardiac mitochondria) is the mitochondrial Na+/Ca2+ exchanger (mNCE)[7, 17], which is presumably electrogenic[18, 19], exchanging 1 Ca2+ for 3 Na+, with a Km for [Na+]i of ~8 mM[20]. Steady-state matrix Ca2+ concentration would therefore depend on the balance between fast mitochondrial Ca2+ uptake and slow efflux via mNCE.
Electron-probe microanalysis
Electron-probe microanalysis studies were among the first to suggest that mitochondrial Ca2+ uptake in intact cardiac myocytes can be several orders of magnitude faster than that in traditional isolated mitochondrial suspensions. Wendt-Gallitelli and Isenberg[21, 22] reported transients in total mitochondrial Ca2+ that peaked ~15–20 ms after the cytosolic transient in shock frozen guinea-pig cardiac, with flux estimates on the order of ~100 nmol/s/mg protein for the mCU. Mitochondrial Ca2+ decayed with a rate of ~36 nmol/s/mg protein, accompanied by a mitochondrial Na+ transient, consistent with matrix Ca2+ rapidly exchanging with Na+ via the mNCE[22]. This extra increment of matrix [Na+] is then removed by exchange of Na+ with H+ via the mitochondrial Na+/H+-exchanger (mNHE) at the expense of the pH gradient[7, 16, 23]. Notably, the observation of fast mitochondrial transients was only possible after paired-pulse stimulation, which will enhance the SR Ca2+ load.
Loading the mitochondrial compartment with fluorescent Ca2+ indicators
The other main strategy for examining the kinetics of mitochondrial Ca2+ uptake has been to load the mitochondrial matrix with fluorescent Ca2+ indicators. Among the inorganic dyes, Rhod-2 is often used, because its negative charge helps to preferentially retain it in the matrix due to the large negative ΔΨm. The challenge with these methods is to avoid contamination from dye in the cytosolic component when intact myocytes are employed. Several papers from the Lemasters group tackled this issue by using confocal microscopy in rabbit cardiac myocytes loaded with either fluo-3/AM[24, 25], rhod-2/AM-[26, 27] or indo-1/AM[25]. They argued that the high spatial resolution of the confocal permitted them to distinguish cytosolic and mitochondrial signals and they found that Ca2+ signals arising from mitochondria (i.e., colocalizing with the ΔΨm probe TMRE) had rapid kinetics almost identical to the cytosolic Ca2+ transient (although the time resolution of the recordings was not sufficient to analyze the rising phase of the transients in detail). β-Adrenergic stimulation increased amplitudes of both the cytosolic and mitochondrial Ca2+ transients[24, 25]. Although it has been argued that this experimental design inadequately excludes contamination of the fluorescence signal from the cytosolic compartment[11], it was shown that the mCU-inhibitor ruthenium red preferentially inhibited the mitochondrial Ca2+ signal without altering the cytoplasmic transient[26].
To more rigorously exclude contamination of mitochondrial Ca2+ signals with cytoplasmic dye, others have either quenched the cytosolic fluorescence by incubation with Mn2+, at concentrations that don’t significantly impair contraction[28–33], allowed the cytoplasmic component to leak out over hours at warm temperatures[32]., or equilibrated the cytoplasm with dye-free pipette solution using the whole cell patch clamp method[33]. The majority of these studies came to the conclusion that mitochondrial Ca2+ uptake occurred over seconds rather than milliseconds, when myocytes were electrically-stimulated, even at rates up to 4Hz with β-adrenergic activation electrical stimulation of the myocyte[28, 29]. When the experimental protocols of these papers are specifically broken down and critically analyzed, there is still room to argue in favor of fast mitochondrial Ca2+ uptake. For instance, Mn2+ quench methods do not take into account the inhibitory effect of Mn2+ on the mCU and oxidative phosphorylation that could alter the kinetics of mitochondrial Ca2+ uptake. Additionally, some of the evidence for a high Km and slow uptake of Ca2+ by mitochondria in several studies was based on changing the cytosolic Ca2+ through slow transsarcolemmal process, that is, Ca2+ was raised by activating reverse-mode NCX current by removing external Na+[28] or by giving long voltage clamp pulses[33]. In the latter instances, the extracellular location of the source of Ca2+, and the diffusion pathway to reach the mitochondria may be very different from Ca2+ released as a spark form the SR right next to the mitochondrion.
The importance of the kinetics, amplitude and location of the Ca2+ pulse applied to the mitochondria cannot be understated, as emphasized by studies of partially-permeabilized cells. Sharma et al.[34], loaded rat cardiomyocytes with rhod-2/AM and then permeabilized the sarcolemma with saponin. Ca2+ in the bath solution was measured using fura-2 salt. SR Ca2+ release initiated by the application of caffeine caused bath Ca2+ to rise rapidly in a few seconds and then decay within 13 seconds. Mitochondrial Ca2+ rose with similar kinetics, had a longer peak and then also decayed rapidly within 15s. The mitochondrial location of the rhod-2 signal was confirmed by the differential sensitivity of the transient to Ruthenium Red and its abolishment by dissipation of the protonmotive force. Most notably, while the caffeine-induced increase in free Ca2+ in the medium could be prevented by adding the fast Ca2+ buffer BAPTA to the bath, the mitochondria still took up Ca2+, indicating that the sites of mitochondrial Ca2+ uptake were in a microdomain that was very close to the release sites. The rate of mitochondrial Ca2+ uptake upon caffeine exposure was strictly controlled by the rate of SR Ca2+ release, which could be slowed by the addition of thapsigargin, an inhibitor of SERCA. In a more recent study, Belmonte et al[35] also reported that caffeine-induced SR Ca2+ release rapidly loads the mitochondrial compartment in adult rat cardiomyocyte, even if the sarcolemma is permeabilized and bulk Ca2+ is buffered. The important novel finding of this study was that the mitochondrial Ca2+ store could be discharged back into the cytoplasm under the mechanical strain imposed by a change in the perfusion flow (a “pressure puff”).
Similar evidence for an SR-mitochondrial Ca2+ microdomain was also obtained by Szalai et al.[36] in cardiac-derived H9c2 cells. In permeabilized cells, SR Ca2+ release induced by caffeine or ryanodine produced rapid increases in mitochondrial Ca2+ synchronized with the rising phase of the cytosolic transient and these mitochondrial Ca2+ transients were not inhibted by adding Ca2+ buffer to the medium. Addition of 30μM Ca2+ to the bath was required to match the rates of rise of the caffeine-induced mitochondrial Ca2+ transients, again indicating that the local Ca2+ in the microdomain near the mitochondria during SR Ca2+ release is much higher than the average cytoplasmic concentration. In another study by the Hajnóczky group, more direct evidence of local fast communication between the SR Ca2+ release units and the mitochondria was obtained. In permeabilized H9C2 myotubes, in which the mitochondria were loaded with rhod-2, low concentrations of caffeine elicited repeated Ca2+ sparks that generated Ca2+ transients in adjacent single mitochondria, referred to as Ca2+ marks (i.e. mitochondrial Ca2+ sparks)[37]. Marks had fast rise times of <50ms and decayed very slowly, with a half width of 215 msec. The duration of the Ca2+ marks was prolonged to 374 msec by the mNCE inhibitor CGP-37157, while the decay of cytosolic Ca2+ sparks was unaffected. More recently, this group reported that the functional complex contining the SR Ca2+ release unit is retained even after isolation of the mitochondria[38]. Acute caffeine treament could initiate a rise in mitochondrial Ca2+ even after partial purification of mitochondria to remove SR membranes, indicative of a close physical coupling between the SR Ca2+ release apparatus and the mitochondria.
Although most of the early studies using Mn2+ quench to eliminate the cytoplasmic component of the Ca2+ indicators did not support fast mitochondrial Ca2+ transients, Griffiths[39] made the observation that indo-1 loaded guinea-pig, but not rat, cardiac myocytes showed beat-to-beat mitochondrial Ca2+ transients using the heat treatment method. In fact, this species selectivity may provide an important clue to explaining the disparate results reported in the literature. Considering potential explanations, either there is an intrinsic difference in the mechanism of mitochondrial Ca2+ uptake in rats versus guinea pigs or there are different regulatory factors present that influence SR-mitochondrial Ca2+ transport.
In light of our own recent studies, one such factor could be the resting cytosolic Na+ concentration ([Na+]i). As discussed above, it is well known that [Na+]i shifts the apparent Km for mitochondrial Ca2+ uptake, presumably by affecting the Ca2+ efflux rate via the mNCE. It is also known that resting [Na+]i is highly species dependent[40]. To study the effects of changing [Na+]i on mitochondrial Ca2+ uptake in intact guinea pig cardiac myocytes, we recently developed a method to monitor both cytosolic and mitochondrial Ca2+ in guinea pig myocytes using a combination of differential dye loading and whole cell dialysis into the cytoplasmic and mitochondrial matrix compartments[41]. We found that a rapid mitochondrial Ca2+ transient could be observed during every cytosolic Ca2+ transient elicited by voltage clamp pulses to initiate SR Ca2+ release. Remarkably, the upstroke of the [Ca2+]m transient preceded the upstroke of [Ca2+]c by several ms, whereas the decay of [Ca2+]m was ~2.5-fold slower than of [Ca2+]c. The fast uptake, slow release kinetics led to a stepwise accumulation [Ca2+]m during rapid pacing (up to 4Hz), particularly in the presence of concomitant β-adrenergic stimulation. Directionally opposite effects on the mitochondrial and cytosolic Ca2+ transients were affected by inhibitors of mitochondrial Ca2+ uptake or efflux, confirming the mitochondrial origin of the signal and indicating that a significant fraction of the Ca2+ released by the SR is buffered by the mitochondria on each beat. Most relevant to the present discussion, the slope of the relationship between the amplitude of the cytosolic Ca2+ transient and the mitochondrial Ca2+ transient was strongly dependent on [Na+]i. An increase of [Na+]i from 5 mM to 15 mM, within the range of measured values of Na+ in intact myocytes or muscles, blunted the amplitude of the mitochondrial Ca2+ transient and decreased the extent of mitochondrial Ca2+ loading upon electrical stimulation. Importantly, the diminished mitochondrial Ca2+ response at high [Na+]i impaired the ability of the mitochondria to balance NADH production to increased workload. We proposed that this mechanism could contribute to an energy supply and demand mismatch in cardiac pathologies associated with elevated resting [Na+]i, for example, in hypertrophied or failing hearts. We have recently confirmed that resting [Na+]I is elevated to a similar extent and the NADH response to pacing is defective in myocytes from failing hearts in a guinea pig aortic banding model[42].
Our recent findings, taken together with reports in the literature that rats have a higher resting [Na+]i (≥10mM) than guinea-pigs (5mM in our measurements, <7mM from other laboratories; see refs. within[40]), could explain why some investigators have failed to see fast mitochondrial Ca2+ transients, although other effectors (e.g., Mg2+, spermine, etc.) could certainly play a role as well. This speculative explanation will have to be confirmed by additional direct investigation.
Targeted molecular probes
Genetic targeting of fluorescent Ca2+ indicators has offered an alternative method to measure the kinetics of Ca2+ uptake into mitochondria. These constructs typically incorporate a peptide leader sequence taken from an electron transport protein that is recognized by the protein import machinery of mitochondria, used to direct the trafficking of Ca2+-sensitive reporters like the photoprotein aequorin or FRET-based sensors like the pericams[43]. Although this method has its own limitations, the consensus finding has been that mitochondria take up Ca2+ rapidly, roughly in synchrony with cytosolic Ca2+ release in cardiac myocytes[44, 45]. Again, even taking into account nonlinearities in the kinetic response of aequorin to Ca2+, mitochondrial Ca2+ decay is observed to be noticeably slower than that of the cytoplasm. Interestingly, in a study by Bell et al[45], transient changes in mitochondrial [ATP], reported by targeted luciferase, were observed within the first minute of rapid pacing while mitochondrial Ca2+ oscillated on a beat-to-beat basis.
Indirect methods
Direct measurements of fast mitochondrial Ca2+ transients using the methods described above are perhaps the most convincing arguments for a special spatiotemporal relationship between the sites of SR Ca2+ release and mitochondrial Ca2+ uptake; however, other indirect observations also support this notion. Several studies indicate that mitochondria are effective local Ca2+ buffers of the cytosolic Ca2+ transient [22, 37, 41, 46–49]. When blocking mitochondrial Ca2+ uptake, an increase of cytosolic Ca2+ transients is often observed[22, 49] and impairs the recovery of inactivation of the L-type Ca2+ current during a train of pulses[50].
Spatial considerations
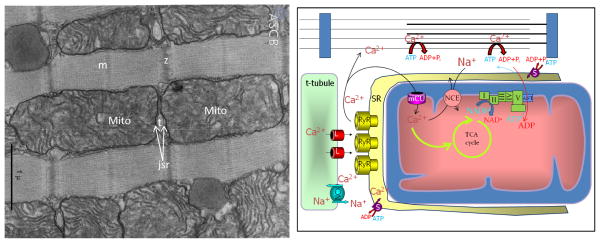
In reviewing the points in favor of rapid mitochondrial Ca2+ uptake, it is worth mentioning the common sense argument that the sites of Ca2+ release are generally located in the space between two mitochondria (Fig. 1), and spikes of Ca2+ of 50 μmolar or so are released into a narrow space roughly 40nm away from the nearest mitochondrial membrane. It is therefore reasonable to assume that at least part of the mitochondrion experiences a Ca2+ gradient exceeding that of the bulk space. How rapidly this pulse of Ca2+ might be transmitted into and distributed across the convoluted architecture of the matrix cristae is a question that will require further investigation and more detailed modelling of Ca2+ diffusion with realistic structural models to answer.
Figure 1.
Left panel: Transmission electron micrograph of bat myocardium illustrating close juxtaposition of junctional SR (jsr) Ca2+ release sites at the t-tubules (t) and the mitochondria (Mito) (Porter KR. Mitochondria and transverse tubules of bat heart. ASCB Image & Video Library. August 2006: FND-10. Available at: http://cellimages.ascb.org/u?/p4041coll12,56. All rights reserved. Reprinted under license from The American Society for Cell Biology). Right panel: Schematic representation of the key components of excitation-contraction-bioenergetic coupling. Arguing in favor of a rapid component of Ca2+ uptake, mitochondria are in close proximity to the SR junction containing L-type Ca2+ channels (L) and ryanodine receptors (RyR), which could drive fast uptake due to a large local Ca2+ gradient, despite a relatively high Km for Ca2+ uptake via the mitochondrial uniporter (mCU). Slower Ca2+ extrusion from the mitochondria via the mitochondrial Na+/Ca2+ exchanger (NCE) results in Ca2+ accumulation during rapid pacing. The fast Ca2+ signals may be required to provide rapid compensation for increased metabolic demand (and NADH oxidation) during high workloads through the activation of tricarboxylic acid (TCA) cycle dehydrogenases.
Rebuttal
The preceding article comprehensively discussed the ongoing controversy surrounding mitochondrial Ca2+ uptake kinetics. Several elegant empirical studies ([22, 41, 44]; see also reviews[11, 51]) were presented that have generated convincing evidence that mitochondria indeed can respond to rapid cytosolic Ca2+ oscillations with rapid changes of mitochondrial [Ca] ([Ca]m), or in other words, that the kinetics of the mitochondrial Ca2+ transport machinery are fast enough to allow for (nearly) beat-to-beat changes of [Ca]m in the heart. Nonetheless, as also discussed in the preceding article a number of studies that supported rapid mitochondrial Ca2+ uptake are plagued by experimental shortcomings and flaws. For example, fluorescence microscopy imaging studies attempting to measure cytosolic [Ca] and [Ca]m simultaneously are particularly prone to misinterpretation of experimental results. Limitations in spatial resolution and thus sufficient signal separation are the crux of such light microscopic approaches because mitochondria are smaller than the resolvable volume. As a consequence cytosolic and mitochondrial Ca2+ transients have (suspiciously) similar kinetics due to signal ‘contamination’. While experimental limitations may have hampered such studies, from a teleological perspective one may contemplate the question whether a beat-to-beat change in [Ca]m is desirable and/or even required for mitochondrial function. Shuttling of Ca2+ across the inner mitochondrial membrane (IMM) is energetically expensive and occurs under consumption of energy that is deprived from the cell and not available to cover other cellular energy demands. In the following COUNTERPOINT we will compile and discuss experimental evidence in support of a model of slow integration of cytosolic Ca2+ oscillations by mitochondrial Ca2+ transport and buffering, and we address the question whether from a point of view of cellular energetics this model is favorable over rapid changes of [Ca]m.
Counterpoint: Evidence against fast mitochondrial Ca2+ uptake in the heart
Historically, two different theories have evolved on how mitochondria decode rapid cardiac Ca2+ transients (for review see for example ref.[51]). In model I, originally induced by Crompton[52], Ca2+ uptake into mitochondria is slow and coupled to an even slower release of accumulated Ca2+ ions. Fast cytosolic Ca2+ oscillations are integrated by the Ca2+ transport machinery of the IMM. The response to a train of cytosolic Ca2+ transients is a frequency-dependent net accumulation of Ca2+ in the matrix compartment until a new steady-state is reached when Ca2+ uptake during a single cycle equals Ca2+ efflux. Consequently, beat-to-beat changes in [Ca]m are small, and energetic requirements of integrated mitochondrial Ca2+ transport are minimal. In contrast, model II explains how fast cytosolic Ca2+ oscillations are efficiently translated into beat-to-beat changes of [Ca]m. As a requirement, rapid mitochondrial Ca2+ uptake as well as fast Ca2+ efflux mechanisms are a mandatory prerequisite, with Ca2+ uptake being large enough to overcome the buffering capacity of both the cytosol and mitochondrial matrix. In this scenario, mitochondrial Ca2+ uptake would effectively buffer cytosolic Ca2+ transients during e-c coupling. This, in turn, would require SR Ca2+ release and reuptake to be large enough to compensate for this additional fast buffering and to still provide sufficient Ca2+ for contraction. With approximately one third of cell volume being occupied by mitochondria in cardiac cells, the additional SR Ca2+ fluxes (and linked energy consumption) would be substantial[11].
Slow integration of cytosolic Ca2+ transients by mitochondrial Ca2+ transport and buffering
As has been discussed in the preceding article the controversy surrounding beat-to-beat changes in [Ca]m in the heart is certainly (at least in part) related to experimental limitations of available methods for reliable measurements of [Ca]m. This caveat applies to many such studies (for an overview of methods and results see Table 1 in ref.[51]) irrespective whether the outcome favored model I or II (e.g. contamination of mitochondrial Ca2+ signal by fast cytosolic Ca2+ signals due to insufficient signal separation). Evidence in support of slow mitochondrial integration of cytosolic Ca2+ transients are based on studies utilizing electron probe microanalysis (EPMA) and fluorescent techniques. EPMA uses flash-frozen tissue samples and has the advantages of a resolution close to electron microscopy, however it measures total mitochondrial Ca2+ ([Ca]m,tot) instead of free [Ca]m. Studies using EPMA performed on hamster[53, 54] and rat[55] papillary muscles were not able to resolve fast changes in [Ca]m,tot, not even after β-adrenergic stimulation of hamster papillary muscle[53] or whole hearts[56].
In a majority of studies, fluorescents dyes (such as the membrane-permeable ester forms of indo-1, rhod-2 or fluo-3) known to compartmentalize in mitochondria were used to monitor [Ca]m directly. To eliminate the cytosolic component of the fluorescent signals, cells were treated with MnCl2 [28, 29, 33, 57], exposed to high temperature[32, 39] or permeabilized[58, 59]. Using indo-1 loaded rat[28, 39], hamster[29], ferret and cat[33] ventricular myocytes with subsequent Mn-quenchning of cytosolic dye, it was shown that an increase in the stimulation frequency from 0.2 to 4 Hz in the presence of β–adrenergic stimulation[28, 32] or cellular loading of Ca2+ through sarcolemmal Na/Ca2+ exchange[33] led to a slow rise of [Ca]m from ~100–200 to ~500–800 nM, however no beat-to beat changes in [Ca]m were observed. In the absence of β-adrenergic stimulation, only modest increases in [Ca]m could be achieved in rat myocytes by electrical stimulation at 2 Hz[29], which indicated that only large amplitude Ca2+ transients were sensed by mitochondria. Miyata et al.[28] demonstrated an exponential relationship between [Ca]m and [Ca]i, with the threshold for activation of mitochondrial Ca2+ uptake being at a [Ca]i of ~500 nM. Similarly, Zhou et al.[33] reported that under conditions of high cellular Ca2+ load imposed by membrane depolarization in ferret and cat myocytes, phasic increases of [Ca]m could be detected, although they were slow and only observed at diastolic [Ca]i >400 nM. The authors concluded that mitochondria of intact cells did not take up detectible amounts of Ca2+ during individual contractions. A general criticism of the experimental approach outlined above is the possible interference of Mn with Ca2+ transporters of the IMM. First, Mn can be sequestered rapidly by mitochondria since it competes with Ca2+ for the mitochondrial Ca2+ uniporter (MCU), and Mn can potentially quench the fluorescence of the dye compartmentalized inside of mitochondria. While Miyata et al.[28] provided evidence that Mn had no effect on mitochondrial Ca2+ uptake as well as cell shortening, other studies demonstrated that Mn significantly inhibits Ca2+ efflux[60]. Nonetheless, removal of cytosolic indo-1 by heat treatment gave similar results to the Mn quench technique[32, 39]. Moreover, when fura-2 loaded rat heart mitochondria were exposed to Ca2+ oscillations at 100 cycles/min, [Ca]m increased proportionally to the average rise in extramitochondrial [Ca], but independently of oscillation frequency[61]. Furthermore, Griffiths[39] revealed that there might be a species-dependent difference in the kinetics of [Ca]m. Utilizing the heat treatment protocol, the author demonstrated [Ca]m transients in indo-1 loaded quinea-pig myocytes, while no changes in [Ca]m were observed in rat cardiomyocytes.
A novel experimental approach was developed in our laboratory by Sedova et al.[58] which allowed to simulate fast cytosolic Ca2+ transients in membrane-permeabilized cells. [Ca]m was measured with fluo-3 entrapped inside mitochondria and after removal of cytosolic indicator by plasma membrane permeabilization with digitonin. Permeabilized cells have the unique advantage that the cytosolic environment can be controlled precisely while the arrangements and interaction between intracellular membranes and organelles (SR, mitochondria) remain structurally and functionally intact[62, 63]. Control experiments indicated that mitochondrial Ca2+ uptake was initiated only when extramitochondrial [Ca] was elevated to more than 0.5 μM, confirming the existence of a threshold for MCU activation[33, 59]. Ca2+ entry via the uniporter exhibited a sigmoidal extramitochondrial [Ca] dependence (K0.5=4.4 μM). To simulate cytosolic Ca2+ transients, cells were placed in the laminar flow of Ca2+ free solution containing 1 mM EGTA and exposed to rapid ejections of 100 μM Ca2+ from a micropipette positioned upstream of the cell with regard to the direction of the bulk flow. Calculations revealed that with the applied technique cells were exposed to ~5 μM extramitochondrial [Ca] during each pulse of stimulation, which is in the range of physiological cytosolic Ca2+ transients amplitude and close to the estimated K0.5 for the MCU. With this technique rapid beat-to-beat changes in [Ca]i (with Ca2+ transient durations of 100 to 500 ms) could be simulated by rapid switching (0.25 – 1 Hz) extramitochondrial [Ca] to high levels. Exposure to a train of Ca2+ transients evoked a gradual, frequency- and Ca2+ transient duration-dependent elevation of [Ca]m, but did not lead to [Ca]m oscillations. These data suggest that in cat ventricular myocytes fast cytosolic [Ca] transients are integrated by mitochondrial Ca2+ transport systems resulting in a frequency-dependent net accumulation of Ca2+ in the matrix, thus supporting model I of mitochondrial decoding of fast cytosolic Ca2+ signals.
Is Fast mitochondrial Ca2+ cycling energetically desirable?
The process of mitochondrial Ca2+ cycling involves continuous fluxes of Ca2+ and Na ions, as well as protons (H+) driven by the respiratory chain[64]. Ca2+ influx dissipates the mitochondrial membrane potential (ΔΨ), whereas efflux dissipates the mitochondrial ΔpH (indirectly via mitochondrial Na/Ca2+ and Na/H exchange)[52]. Generally, a biphasic time course of mitochondrial Ca2+ uptake is expected from its electrogenic nature. Rapid Ca2+ entry is slowed to a level at which Ca2+ influx is balanced by H+ extrusion[65]. Moreover, rapid H+ pumping by the F1F0 ATP-synthase may contribute to maintaining ΔΨ during high rates of Ca2+ import at the expense of ATP[66], therefore making fast beat-to-beat changes in [Ca]m energetically expensive. However, no beat-to-beat changes in mitochondrial ([ATP]m) or cytosolic ([ATP]i) free ATP levels were observed in beating rat ventricular myocytes in the absence or presence of isoproterenol (i.e. upon increased workload)[45]. Only when myocytes were stimulated at high frequencies (2 Hz from rest) in the presence of isoproterenol, significant (on average to 10% of the initial signal) decreases in [ATP]m were observed. This [ATP]m drop, however, lasted only 30–60 s, and was followed by a slower recovery that exceeded the initial level and resulted in a sustained rise in [ATP]m. These data are in agreement with mitochondrial NADH or NAD(P)H measurements[67–69] as indirect indicators of the energetic state of mitochondria. Similar to the results presented above, physiological increases in the workload of whole hearts did not evoke any changes in NAD(P)H[67]. Nonetheless, data obtained on isolated rat ventricular trabeculae indicate that with a sudden increase in stimulation frequency (3 Hz from rest) or extracellular [Ca], there was a transient decrease in mitochondrial NADH ([NADH]m), consistent with NADH production not keeping up with the increased ATP and NADH consumption[69]. However, this [NADH]m decline was followed by a recovery toward previous levels. This slow rise of [NADH]m was dependent on increased average [Ca]i and was paralleled by an equivalently slow increase of [Ca]m [70]. It was concluded that the increased average [Ca]i caused an increase in [Ca]m and stimulation of dehydrogenases and NADH production. The kinetics of the measured [NADH]m changes in response to increased pacing frequency did not require a rapid transmission of cytosolic Ca2+ signals into the mitochondrial matrix. In fact, the time course of the delayed [NADH]m recovery was reminiscent of the slow changes in [Ca]m reported by Miyata and co-workers[28] under comparable conditions. The same is true for the direct [ATP]m measurements reported by Bell et al.[45], where the time course of [ATP]m changes closely followed the slower changes in [Ca]m.
In conclusion, experimental data do not support an absolute requirement for a rapid (beat-to-beat) transmission of cytosolic Ca2+ signals into the mitochondrial matrix to regulate the mitochondrial response to altered cellular energy demands. While the following conclusion may be more teleological rather than empirical, it appears that a slow integration of cytosolic Ca2+ oscillations is cost-efficient and, at the same time, sufficient to fully respond to the cellular energetic requirements of the heart.
Rebuttal
Experimental limitations of measuring mitochondrial Ca2+
Certainly, it is agreed that the resolution of the central question posed in this article will be resolved eventually by improvements of the experimental methods for assessing intramitochondrial Ca2+. I would argue that great improvements have been made already with respect to the development of genetically-targeted Ca2+ sensors[43, 45] and, in our own experiments, careful attention to elimination of cytosolic Ca2+ signals and simultaneous measurement of both compartments with dual sensors[41]. Moreover, the directionally opposite effects of an inhibitor of the mitochondrial Ca2+ uniporter on the fast mitochondrial transient (inhibition) versus the cytosolic transient (enhancement), along with the inverse effects of a mitochondrial Na+/Ca2+ exchange inhibitor, provides unequivocal (in my mind) evidence that the indicators are localized as expected.
While one can always fall back on the argument that previous evidence in favor of fast mitochondrial Ca2+ uptake was artifactually due to signal contamination, it is equally easy to challenge whether inadequate experimental design or lack of control over important factors could have resulted in a failure to observe fast mitochondrial Ca2+ transients. I have already discussed the important role of intracellular Na+, and undoubtedly other modulators (e.g., Pi[41, 42]) will also prove to be important. With respect to two of the methods discussed above (the work of Zhou et al[33] and Sedova et al[58]), there is an essential limitation in how the Ca2+ gradients driving mitochondrial Ca2+ uptake are applied. In both cases, the Ca2+ is increased by first driving up bulk Ca2+ (either by activation of sarcolemmal Na+/Ca2+ exchange in the first study or by increasing bath Ca2+ in the second study) and then relying on Ca2+ diffusion to the mitochondrial membranes. This ignores the complex spatiotemporal factors associated with native SR Ca2+ release from the two juxtaposed compartments (cf. Fig. 1). Indeed, under the right conditions, when Ca2+ release is initiated by triggering the ryanodine receptor, mitochondrial Ca2+ can rise rapidly without much change in bulk Ca2+[34].
Energetic cost
The latter argument is that it would be energetically costly for the mitochondria to respond rapidly to Ca2+ on each beat. This would be true if there were no mechanistic requirement for the Ca2+ signal to be transmitted rapidly to the bioenergetic subsystem. However, as we have recently shown, matching energy supply with demand does requires a rapid mitochondrial Ca2+ response[42]. If fast mitochondrial Ca2+ transients are inhibited (e.g., by blocking the mitochondrial Ca2+ uniporter or increasing [Na+]i), an increase in workload results in net oxidation of the mitochondrial NADH pool, indicating that the stimulation of oxidative phosphorylation is very fast and requires an almost immediate increase in NADH production to remain in balance. Fast mitochondrial Ca2+ uptake is thus required to stimulate the tricarboxylic acid cycle dehydrogenases. Furthermore, impaired mitochondrial Ca2+ uptake and high [Na+]i contribute to altered NADH responses in cardiomyocytes from failing hearts[42], underscoring the pathophysiological implications of this mechanism. Hence, Ca2+ cycling across the mitochondrial membrane does result in an energetic cost, but it pays for a fast bioenergetic response. We would argue that a rapid mitochondrial effect on NADH production (mediated by fast Ca2+ uptake) is necessary to respond to frequent and nearly instantaneous changes in the metabolic demand of the heart, which accounts for the lack of an observable change in the NADH redox potential under a variety of conditions.
Fast versus slow: a straw man for both sides
Finally, while I have argued in favor of a fast component of mitochondrial Ca2+ uptake, the integrated effect on matrix Ca2+ depends on both fast and slow processes. As we have reported previously in a computational model of mitochondrial Ca2+ uptake in response to brief (~20 μM) pulses of Ca2+ to simulate local Ca2+ release[41], fast Ca2+ influx coupled with slow efflux permits the mitochondrion to respond to changes in either the frequency or amplitude of SR Ca2+ release synergistically. Because the Ca2+ pulses were brief (50 ms), their effects on bioenergetics were rapidly compensated by proton pumping, so that the drop in ΔΨm during uptake was quickly reversed and did not substantially change the average electrochemical driving force.
In the end the real argument is not whether mitochondria take up Ca2+ on each beat, I believe we both agree that some Ca2+ must enter during each transient, but is rather about the kinetics and magnitude of the uptake. The answer to the latter question requires more precise measurements of the compartmentalized Ca2+ pools and a better understanding of the influence of the ultrastructural arrangement of the organelles involved.
Biographies

Dr. Lothar A. Blatter is a Professor of Molecular Biophysics and Physiology at Rush University Medical Center Chicago. He received his M.D. degree and the degree of Doctor of Medicine from the University of Bern, Switzerland. He received postdoctoral training in physiology and pharmacology at the University of Bern, Switzerland, at the Mayo Clinic in Rochester, MN, and at the University of Maryland at Baltimore. He was recognized as an Established Investigator of the American Heart Association. He has authored over 90 peer-reviewed articles and he serves on the Editorial Board of The Journal of Physiology. Since 1993 he has maintained an active, independent and extramurally funded (NIH, AHA) research program and is actively involved in graduate and medical teaching. His research focuses on cellular calcium signaling pathways in the cardiovascular system (cardiac cells, vascular endothelial cells, vascular smooth muscle cells), using high-resolution imaging techniques, electrophysiological methods and molecular biology approaches.

Brian O’Rourke, PhD, FAHA
Professor of Medicine
Dr. Brian O’Rourke is a Professor in the Division of Cardiology, Department of Medicine of the Johns Hopkins University. Dr. O’Rourke’s laboratory uses an integrated approach to study the biophysics and physiology of cardiac cells in normal and diseased states. A major emphasis of Dr. O’Rourke’s work is to characterize the mechanisms of control and modulation of mitochondrial function. In this regard, he has elucidated how some ion channels in the mitochondrial inner membrane play an instrumental role in protecting myocytes from necrotic and apoptotic cell death (e.g., the mitochondrial KATP and KCa2+ channels), while others contribute to mitochondrial dysfunction (e.g., permeability transition pores and inner membrane anion channels). Over the course of his career, Dr. O’Rourke has been most interested in studying the dynamics of mitochondrial energetics by taking a cross-disciplinary approach, working with collaborators in the areas of computational biology, proteomics, biophysics and molecular biology to gain a comprehensive understanding of the integrative physiology of the cardiomyocyte. A main theme is to understand how failures at the subcellular level scale to produce global electrical and contractile dysfunction in the heart. Rational strategies can then be devised to correct dysfunction during the progression of disease through a comprehensive understanding of basic mechanisms. Dr. O’Rourke currently directs a Program Project on mitochondrial function in cardiac disease (P01 HL081427), holds an NIH MERIT award (R37HL54598), and participates in a Systems Biology grant on sudden cardiac death (R33 HL087338).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brian O’Rourke, Division of Cardiology, Department of Medicine, The Johns Hopkins University, Baltimore, MD 21205.
Lothar A. Blatter, Dept. Molecular Biophysics & Physiology, Rush University medical Center, Chicago, IL 60612
References
- 1.Patterson SW, Piper H, Starling EH. The regulation of the heart beat. J Physiol. 1914 Oct 23;48(6):465–513. doi: 10.1113/jphysiol.1914.sp001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans CL, Matsuoka Y. The effect of various mechanical conditions on the gaseous metabolism and efficiency of the mammalian heart. J Physiol. 1915 Jul 5;49(5):378–405. doi: 10.1113/jphysiol.1915.sp001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lardy HA, Wellman H. Oxidative phosphorylations; role of inorganic phosphate and acceptor systems in control of metabolic rates. J Biol Chem. 1952 Mar;195(1):215–24. [PubMed] [Google Scholar]
- 4.Chance B, Williams GR. A method for the localization of sites for oxidative phosphorylation. Nature. 1955 Aug 6;176(4475):250–4. doi: 10.1038/176250a0. [DOI] [PubMed] [Google Scholar]
- 5.Katz LA, Swain JA, Portman MA, Balaban RS. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol. 1989 Jan;256(1 Pt 2):H265–74. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- 6.Bose S, French S, Evans FJ, Joubert F, Balaban RS. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J Biol Chem. 2003 Oct 3;278(40):39155–65. doi: 10.1074/jbc.M306409200. [DOI] [PubMed] [Google Scholar]
- 7.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990 May;258(5 Pt 1):C755–86. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 8.Balaban RS, Bose S, French SA, Territo PR. Role of calcium in metabolic signaling between cardiac sarcoplasmic reticulum and mitochondria in vitro. Am J Physiol Cell Physiol. 2003 Feb;284(2):C285–93. doi: 10.1152/ajpcell.00129.2002. [DOI] [PubMed] [Google Scholar]
- 9.Territo PR, Mootha VK, French SA, Balaban RS. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)- ATPase. Am J Physiol Cell Physiol. 2000 Feb;278(2):C423–35. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 10.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994 Aug;267(2 Pt 1):C313–39. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 11.Huser J, Blatter LA, Sheu SS. Mitochondrial calcium in heart cells: beat-to-beat oscillations or slow integration of cytosolic transients? J Bioenerg Biomembr. 2000 Feb;32(1):27–33. doi: 10.1023/a:1005556227425. [DOI] [PubMed] [Google Scholar]
- 12.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004 Jan 22;427(6972):360–4. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 13.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J Biol Chem. 2001 Jan 26;276(4):2586–99. doi: 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar]
- 14.Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995 Nov 17;270(46):27510–5. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- 15.Buntinas L, Gunter KK, Sparagna GC, Gunter TE. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim Biophys Acta. 2001 Apr 2;1504(2–3):248–61. doi: 10.1016/s0005-2728(00)00254-1. [DOI] [PubMed] [Google Scholar]
- 16.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990 Apr;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 17.Denton RM, McCormack JG. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–54. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- 18.Baysal K, Jung DW, Gunter KK, Gunter TE, Brierley GP. Na(+)- dependent Ca2+ efflux mechanism of heart mitochondria is not a passive Ca2+/2Na+ exchanger. Am J Physiol. 1994 Mar;266(3 Pt 1):C800–8. doi: 10.1152/ajpcell.1994.266.3.C800. [DOI] [PubMed] [Google Scholar]
- 19.Jung DW, Baysal K, Brierley GP. The sodium-calcium antiport of heart mitochondria is not electroneutral. J Biol Chem. 1995 Jan 13;270(2):672–8. doi: 10.1074/jbc.270.2.672. [DOI] [PubMed] [Google Scholar]
- 20.Paucek P, Jaburek M. Kinetics and ion specificity of Na(+)/Ca(2+) exchange mediated by the reconstituted beef heart mitochondrial Na(+)/Ca(2+) antiporter. Biochim Biophys Acta. 2004 Nov 4;1659(1):83–91. doi: 10.1016/j.bbabio.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Wendt-Gallitelli MF, Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–72. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isenberg G, Han S, Schiefer A, Wendt-Gallitelli MF. Changes in mitochondrial calcium concentration during the cardiac contraction cycle. Cardiovasc Res. 1993 Oct;27(10):1800–9. doi: 10.1093/cvr/27.10.1800. [DOI] [PubMed] [Google Scholar]
- 23.Hansford RG. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- 24.Chacon E, Ohata H, Harper IS, Trollinger DR, Herman B, Lemasters JJ. Mitochondrial free calcium transients during excitation-contraction coupling in rabbit cardiac myocytes. FEBS Lett. 1996 Mar 11;382(1–2):31–6. doi: 10.1016/0014-5793(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 25.Ohata H, Chacon E, Tesfai SA, Harper IS, Herman B, Lemasters JJ. Mitochondrial Ca2+ transients in cardiac myocytes during the excitation-contraction cycle: effects of pacing and hormonal stimulation. J Bioenerg Biomembr. 1998 Jun;30(3):207–22. doi: 10.1023/a:1020588618496. [DOI] [PubMed] [Google Scholar]
- 26.Trollinger DR, Cascio WE, Lemasters JJ. Mitochondrial calcium transients in adult rabbit cardiac myocytes: inhibition by ruthenium red and artifacts caused by lysosomal loading of Ca(2+)-indicating fluorophores. Biophys J. 2000 Jul;79(1):39–50. doi: 10.1016/S0006-3495(00)76272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trollinger DR, Cascio WE, Lemasters JJ. Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem Biophys Res Commun. 1997 Jul 30;236(3):738–42. doi: 10.1006/bbrc.1997.7042. [DOI] [PubMed] [Google Scholar]
- 28.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991 Oct;261(4 Pt 2):H1123–34. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 29.Di Lisa F, Gambassi G, Spurgeon H, Hansford RG. Intramitochondrial free calcium in cardiac myocytes in relation to dehydrogenase activation. Cardiovasc Res. 1993 Oct;27(10):1840–4. doi: 10.1093/cvr/27.10.1840. [DOI] [PubMed] [Google Scholar]
- 30.Di Lisa F, Fan CZ, Gambassi G, Hogue BA, Kudryashova I, Hansford RG. Altered pyruvate dehydrogenase control and mitochondrial free Ca2+ in hearts of cardiomyopathic hamsters. Am J Physiol. 1993 Jun;264(6 Pt 2):H2188–97. doi: 10.1152/ajpheart.1993.264.6.H2188. [DOI] [PubMed] [Google Scholar]
- 31.Miyata H, Lakatta EG, Stern MD, Silverman HS. Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circ Res. 1992 Sep;71(3):605–13. doi: 10.1161/01.res.71.3.605. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths EJ, Stern MD, Silverman HS. Measurement of mitochondrial calcium in single living cardiomyocytes by selective removal of cytosolic indo 1. Am J Physiol. 1997 Jul;273(1 Pt 1):C37–44. doi: 10.1152/ajpcell.1997.273.1.C37. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, Matlib MA, Bers DM. Cytosolic and mitochondrial Ca2+ signals in patch clamped mammalian ventricular myocytes. J Physiol. 1998 Mar 1;507( Pt 2):379–403. doi: 10.1111/j.1469-7793.1998.379bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000 Feb;32(1):97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 35.Belmonte S, Morad M. “Pressure-flow”-triggered Cai-transients in rat cardiac myocytes: possible mechanisms and role of mitochondria. J Physiol. 2008 Jan 10; doi: 10.1113/jphysiol.2007.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000 May 19;275(20):15305–13. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- 37.Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2380–5. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Perez C, Hajnoczky G, Csordas G. Physical coupling supports the local Ca2+ transfer between SR subdomains and the mitochondria in heart muscle. J Biol Chem. 2008 Sep 12; doi: 10.1074/jbc.M803385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffiths EJ. Species dependence of mitochondrial calcium transients during excitation-contraction coupling in isolated cardiomyocytes. Biochem Biophys Res Commun. 1999 Sep 24;263(2):554–9. doi: 10.1006/bbrc.1999.1311. [DOI] [PubMed] [Google Scholar]
- 40.Pieske B, Houser SR. [Na+]i handling in the failing human heart. Cardiovasc Res. 2003 Mar 15;57(4):874–86. doi: 10.1016/s0008-6363(02)00841-6. [DOI] [PubMed] [Google Scholar]
- 41.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006 Jul 21;99(2):172–82. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008 Aug 1;103(3):279–88. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE. 2004 Jan;13(215):re1. doi: 10.1126/stke.2152004re1. 2004. [DOI] [PubMed] [Google Scholar]
- 44.Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, Pozzan T. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. Embo J. 2001 Sep 3;20(17):4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bell CJ, Bright NA, Rutter GA, Griffiths EJ. ATP regulation in adult rat cardiomyocytes: time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins. J Biol Chem. 2006 Sep 22;281(38):28058–67. doi: 10.1074/jbc.M604540200. [DOI] [PubMed] [Google Scholar]
- 46.Pacher P, Csordas P, Schneider T, Hajnoczky G. Quantification of calcium signal transmission from sarco-endoplasmic reticulum to the mitochondria. J Physiol. 2000 Dec 15;529(Pt 3):553–64. doi: 10.1111/j.1469-7793.2000.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duchen MR, Leyssens A, Crompton M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol. 1998 Aug 24;142(4):975–88. doi: 10.1083/jcb.142.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci. 2004 Dec 15;117(Pt 26):6327–37. doi: 10.1242/jcs.01559. [DOI] [PubMed] [Google Scholar]
- 49.Seguchi H, Ritter M, Shizukuishi M, Ishida H, Chokoh G, Nakazawa H, Spitzer KW, Barry WH. Propagation of Ca2+ release in cardiac myocytes: role of mitochondria. Cell Calcium. 2005 Jul;38(1):1–9. doi: 10.1016/j.ceca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez JA, Garcia MC, Sharma VK, Young KC, Matlib MA, Sheu SS. Mitochondria regulate inactivation of L-type Ca2+ channels in rat heart. J Physiol. 2001 Oct 15;536(Pt 2):387–96. doi: 10.1111/j.1469-7793.2001.0387c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dedkova EN, Blatter LA. Mitochondrial Ca(2+) and the heart. Cell Calcium. 2008 Jul;44(1):77–91. doi: 10.1016/j.ceca.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Crompton FM. The role of Ca2+ in the function and dysfunction of heart mitochondria. In: Langer GA, editor. Calcium and the Heart. New York: Raven Press; 1990. pp. 167–98. [Google Scholar]
- 53.Moravec CS, Bond M. Effect of inotropic stimulation on mitochondrial calcium in cardiac muscle. J Biol Chem. 1992 Mar 15;267(8):5310–6. [PubMed] [Google Scholar]
- 54.Moravec CS, Bond M. Calcium is released from the junctional sarcoplasmic reticulum during cardiac muscle contraction. Am J Physiol. 1991 Mar;260(3 Pt 2):H989–97. doi: 10.1152/ajpheart.1991.260.3.H989. [DOI] [PubMed] [Google Scholar]
- 55.Horikawa Y, Goel A, Somlyo AP, Somlyo AV. Mitochondrial calcium in relaxed and tetanized myocardium. Biophys J. 1998 Mar;74(3):1579–90. doi: 10.1016/S0006-3495(98)77869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moravec CS, Desnoyer RW, Milovanovic M, Schluchter MD, Bond M. Mitochondrial calcium content in isolated perfused heart: effects of inotropic stimulation. Am J Physiol. 1997 Sep;273(3 Pt 2):H1432–9. doi: 10.1152/ajpheart.1997.273.3.H1432. [DOI] [PubMed] [Google Scholar]
- 57.Schreur JH, Figueredo VM, Miyamae M, Shames DM, Baker AJ, Camacho SA. Cytosolic and mitochondrial [Ca2+] in whole hearts using indo-1 acetoxymethyl ester: effects of high extracellular Ca2+ Biophys J. 1996 Jun;70(6):2571–80. doi: 10.1016/S0006-3495(96)79828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sedova M, Dedkova EN, Blatter LA. Integration of rapid cytosolic Ca2+ signals by mitochondria in cat ventricular myocytes. Am J Physiol Cell Physiol. 2006 Nov;291(5):C840–50. doi: 10.1152/ajpcell.00619.2005. [DOI] [PubMed] [Google Scholar]
- 59.Fry CH, Powell T, Twist VW, Ward JP. Net calcium exchange in adult rat ventricular myocytes: an assessment of mitochondrial calcium accumulating capacity. Proceedings of the Royal Society of London Series B, Containing papers of a Biological character. 1984 Dec 22;223(1231):223– 38. doi: 10.1098/rspb.1984.0091. [DOI] [PubMed] [Google Scholar]
- 60.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem J. 1990 Mar 1;266(2):329–34. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leisey JR, Grotyohann LW, Scott DA, Scaduto RC., Jr Regulation of cardiac mitochondrial calcium by average extramitochondrial calcium. Am J Physiol. 1993 Oct;265(4 Pt 2):H1203–8. doi: 10.1152/ajpheart.1993.265.4.H1203. [DOI] [PubMed] [Google Scholar]
- 62.Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Molecular and cellular biochemistry. 1998 Jul;184(1–2):81–100. [PubMed] [Google Scholar]
- 63.Fiskum G, Kowaltowksi AJ, Andreyev AY, Kushnareva YE, Starkov AA. Apoptosis-related activities measured with isolated mitochondria and digitonin-permeabilized cells. Methods in enzymology. 2000;322:222–34. doi: 10.1016/s0076-6879(00)22023-5. [DOI] [PubMed] [Google Scholar]
- 64.Crompton M, Heid I. The cycling of calcium, sodium, and protons across the inner membrane of cardiac mitochondria. Eur J Biochem. 1978 Nov 15;91(2):599–608. doi: 10.1111/j.1432-1033.1978.tb12713.x. [DOI] [PubMed] [Google Scholar]
- 65.Kauffman RF, Lardy HA. Biphasic uptake of Ca2+ by rat liver mitochondria. J Biol Chem. 1980 May 10;255(9):4228–35. [PubMed] [Google Scholar]
- 66.Brand MD, Lehninger AL. Superstoichiometric Ca2+ uptake supported by hydrolysis of endogenous ATP in rat liver mitochondria. J Biol Chem. 1975 Oct 10;250(19):7958–60. [PubMed] [Google Scholar]
- 67.Heineman FW, Balaban RS. Effects of afterload and heart rate on NAD(P)H redox state in the isolated rabbit heart. Am J Physiol. 1993 Feb;264(2 Pt 2):H433–40. doi: 10.1152/ajpheart.1993.264.2.H433. [DOI] [PubMed] [Google Scholar]
- 68.Brandes R, Bers DM. Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J. 1996 Aug;71(2):1024–35. doi: 10.1016/S0006-3495(96)79303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brandes R, Bers DM. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ Res. 1997 Jan;80(1):82–7. doi: 10.1161/01.res.80.1.82. [DOI] [PubMed] [Google Scholar]
- 70.Brandes R, Bers DM. Simultaneous measurements of mitochondrial NADH and Ca(2+) during increased work in intact rat heart trabeculae. Biophys J. 2002 Aug;83(2):587–604. doi: 10.1016/S0006-3495(02)75194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]