Abstract
Objective
The US Food and Drug Administration–approved trial, “A Phase 1, Open-Label, First-in-Human, Feasibility and Safety Study of Human Spinal Cord-Derived Neural Stem Cell Transplantation for the Treatment of Amyotrophic Lateral Sclerosis, Protocol Number: NS2008-1,” is complete. Our overall objective was to assess the safety and feasibility of stem cell transplantation into lumbar and/or cervical spinal cord regions in amyotrophic lateral sclerosis (ALS) subjects.
Methods
Preliminary results have been reported on the initial trial cohort of 12 ALS subjects. Here, we describe the safety and functional outcome monitoring results for the final trial cohort, consisting of 6 ALS subjects receiving 5 unilateral cervical intraspinal neural stem cell injections. Three of these subjects previously received 10 total bilateral lumbar injections as part of the earlier trial cohort. All injections utilized a novel spinal-mounted stabilization and injection device to deliver 100,000 neural stem cells per injection, for a dosing range up to 1.5 million cells. Subject assessments included detailed pre- and postsurgical neurological outcome measures.
Results
The cervical injection procedure was well tolerated and disease progression did not accelerate in any subject, verifying the safety and feasibility of cervical and dual-targeting approaches. Analyses on outcome data revealed preliminary insight into potential windows of stem cell biological activity and identified clinical assessment measures that closely correlate with ALS Functional Rating Scale-Revised scores, a standard assessment for ALS clinical trials.
Interpretation
This is the first report of cervical and dual-targeted intraspinal transplantation of neural stem cells in ALS subjects. This approach is feasible and well-tolerated, supporting future trial phases examining therapeutic dosing and efficacy.
There is growing interest in the use of stem cells1–10 as a therapy in amyotrophic lateral sclerosis (ALS), a lethal neurological disorder characterized by the degeneration of motor neurons. Stem cells offer a means to replace lost cells, provide neurotrophic support, and improve the diseased microenvironment.1,7–10 Preclinical in vitro and in vivo evidence supports the therapeutic translation of stem cells,9 and studies by our group and others demonstrate that human spinal stem cells (HSSCs) produce protective growth factor profiles, differentiate into neurons, form synapses with host motor neurons, and have beneficial effects after intraspinal transplantation in G93A-SOD1 rats, an established model of ALS.2–6
In 2009, the US Food and Drug Administration (FDA) approved a phase 1 clinical trial examining the safety and feasibility of HSSC injections into the spinal cords of 18 ALS subjects. HSSCs were delivered using a novel intraspinal stabilization and injection device developed by our group.11–14 The first 12 trial subjects, representing Cohorts A to C, received HSSC transplants into the L2–L4 lumbar segments of the spinal cord. Group A subjects were nonambulatory and received 5 unilateral (A1, n = 3) or 10 total bilateral (A2, n = 3) lumbar injections. Subjects in Groups B and C were ambulatory and received 5 unilateral (n = 3) or 10 total bilateral (n = 3) lumbar injections, respectively. As previously described, interim results from these first 12 subjects demonstrated no serious adverse events associated with HSSC transplantation.15,16
Those encouraging results along with the critical need to maintain respiratory function in ALS subjects enabled FDA approval to complete HSSC injections into C3–C5 cervical segments of the spinal cord, the region where motor neurons involved in diaphragmatic function reside. To support these injections, the lumbar stabilization and injection device was adapted and optimized for cervical intraspinal HSSC delivery.12–14 Ambulatory subjects in Groups D (n = 3) and E (n = 3) received 5 unilateral cervical injections. Based on previous preclinical data demonstrating enhanced therapeutic efficacy of HSSC transplantation when injections were targeted to multiple spinal cord segments,2,4 subjects in Group E were the same subjects who previously received bilateral lumbar injections as part of Group C. This cohort represents the first examination of the feasibility of targeting both lumbar and cervical spinal cord segments in ALS subjects in separate surgeries.
The phase 1 trial consisting of 18 intraspinal transplantation surgeries in 15 ALS subjects was completed in May 2013. Here, we present the functional outcome data from the 6 subjects undergoing cervical stem cell transplantation surgery, including 3 subjects receiving both bilateral lumbar and unilateral cervical HSSC transplants. Data are also presented from the continued follow-up of the first 12 subjects receiving lumbar intraspinal HSSC transplants. Overall, results demonstrate that HSSCs can be safely transplanted into both lumbar and/or cervical human spinal cord segments, warranting future trial phases focused on cellular dosing and therapeutic efficacy.
Patients and Methods
Trial Design and Subject Selection
The goals of this phase 1 trial were to assess the safety and tolerability of the surgical procedure and the presence of neural stem cells in the spinal cord, and to examine the use of immunosuppression in ALS subjects, using a risk escalation study design consisting of 5 subject cohorts.7,15,16 Subject selection criteria, demographics, and inclusion and enrollment criteria for Groups A to C have been previously described.15,16 For Groups D and E, inclusion criteria were the same as for Group B with the additional requirement of demonstrable arm weakness with an ALS Functional Rating Scale-Revised (ALSFRS-R) arm subscore between 1 and 3; all Group E subjects were recruited from Group C and had received prior lumbar intraspinal stem cell injections.17 Detailed inclusion and exclusion criteria are available at http://www.clinicaltrials.gov/ct2/show/NCT0134 8451.
Neural Stem Cell Selection
The NSI-566RSC HSSC line used in the trial has been previously described.6,18,19 The cells are stored under Current Good Manufacturing Practice (CGMP) conditions and delivered to the surgery site at a concentration of 10,000 cells/μl.15,16 Cell viability was assessed prior to each surgery to ensure the required viability of at least 70% to proceed with transplantation.15,16
Cervical Stem Cell Transplantation Approach
For Cohorts D and E, adaptations were made to the lumbar stabilization and injection device and surgical procedure11–16 to accommodate cervical injections, including redesign of the mounting platform to adhere the device caudally to the C7 vertebrae and rostrally at the base of the skull.12–14 Briefly, standard anesthetic and monitoring techniques were adhered to similar to those for lumbar injections,15,16 and the surgical procedure for Groups D and E involved a C3–C5 laminectomy. Subjects received 5 unilateral injections spaced 4mm apart. Ten microliters was delivered at a rate of 5μl/min over 2 minutes, for a total of 500,000 cells in the 5 injections. Following completion of all injections, the dura and tissue incisions were closed and postoperative subject care was managed as previously described.15,16 A conservative lifelong, multiagent immunosuppression approach was employed for the phase 1 trial.15,16 For additional details of the cervical microinjection device, surgical procedure, and immunosuppression regimen for subjects in Groups D and E, refer to our technical approach and safety outcome report.17
Subject Assessments
All subjects received magnetic resonance imaging (MRI) during screening to calculate precise injection positioning and to serve as a baseline for the assessment of postoperative MRI scans, which will be analyzed and reported separately. To determine progression of disease status, subjects regularly underwent standard clinical evaluations as well as regular functional assessments, including ALSFRS-R, seated forced vital capacity (FVC), grip strength assessment (GST), hand-held dynamometry (HHD), electrical impedance myography (EIM), and bladder ultrasounds.15–17 Group A subjects were not ambulatory; these subjects were evaluated once preoperatively and regularly following transplantation. All remaining subjects in Groups B, C, and D were evaluated monthly for 3 months before surgery to establish a standard slope of disease progression and regularly following transplantation. Group E subjects previously received lumbar stem cell transplants as Group C; therefore, functional assessment schedules were already underway prior to surgery and were continued regularly following cervical transplantation. The schedule of all pre- and postoperative assessments is summarized in Supplementary Table 1.
Although this was a phase 1 trial and functional outcome data were collected for the purpose of assessing safety, secondary analyses of these data were performed as a means to gain insight into how cellular transplantation affected disease progression rates and to inform outcome assessment approaches in future trial phases. Presurgical disease progression rates for the various functional outcome measures were first calculated using linear regression analyses for subjects with multiple available presurgical outcome assessment data points. These slopes were utilized to determine whether postsurgical assessment data points at 6, 9, 12, and 15 months were improved relative to predicted points extrapolated from the presurgical progression rates. In addition, Pearson correlation analyses were performed using available data points for the various functional measures to determine which outcome assessments most closely correlated with ALSFRS-R scores. Finally, we calculated progression rate slopes for ALSFRS-R scores and GST outcomes based on data points across 9-month sliding windows to determine whether there were periods where progression rates were attenuated or improved relative to the presurgical progression rate. These analyses were performed for Group E subjects (individuals who received both lumbar and cervical transplantation), as they had the largest amount of available assessment data. Plotted values represent slopes generated from the available data points within each 9-month window. Best-fit curves were then generated for each subject using fourth-order polynomial analyses. All statistical analyses and curve fitting utilized R version 3.0.1 (http://cran.r-project.org/) and Prism 6 for Windows (GraphPad, SanDiego, CA).
Results
Subject Selection and General Surgical Outcomes
Subject demographics for all cohorts are presented in Table1. Enrolled subjects included 13 males and 2 females ranging in age from 35 to 66 years old. Disease duration ranged between 1.3 and 13 years at the time of surgery. All Group E subjects, 1 of 2 trial cohorts designated to receive cervical stem cell transplants, previously received lumbar stem cell transplants as Group C. In total, 15 ALS subjects underwent 18 surgeries.15–17
Table 1.
Subject Demographics
| Group | Surgery Details | Subject Number | Surgery Number | Subject Age at Surgery, yr | Disease Duration at Surgery, yr | Gender | Death, mo Postsurgery |
|---|---|---|---|---|---|---|---|
| A1 | Nonambulatory | 1 | 1 | 61.8 | 5.2 | M | 30 |
| unilateral | 2 | 2 | 43.4 | 12.7 | M | ||
| lumbar | 3 | 3 | 51.1 | 2.1 | M | 13 | |
| A2 | Nonambulatory | 4 | 4 | 37.5 | 2 | M | |
| bilateral | 5 | 5 | 66.3 | 2.2 | M | 19 | |
| lumbar | 6 | 6 | 55 | 2.2 | M | 9 | |
| B | Ambulatory | 7 | 7 | 59 | 1.6 | M | |
| unilateral | 8 | 8 | 41.1 | 5.6 | M | ||
| lumbar | 9 | 9 | 54.6 | 1.3 | M | 11 | |
| C/E | Ambulatory | 10 | 10 | 48.9 | 11.6 | M | |
| bilateral | 16 | 50.2 | 13 | ||||
| lumbar | 11 | 11 | 39.3 | 1.6 | M | ||
| and | 18 | 40.7 | 3 | ||||
| unilateral | 12 | 12 | 65.1 | 3 | M | ||
| cervical | 17 | 66.3 | 4.3 | ||||
| D | Ambulatory | 13 | 13 | 50.3 | 3.1a | M | 20 |
| unilateral | 14 | 14 | 54.3 | 1.8a | F | 7 | |
| cervical | 15 | 15 | 35.2 | 1.7 | F |
F = female; M = male.
Subject demonstrated features of bulbar onset amyotrophic lateral sclerosis.
Overall, the procedure was well tolerated across all cohorts, with minimal perioperative or postoperative complications. Only a nominal number of serious adverse events were observed during the course of the phase 1 trial.17 For cervical injections in Groups D and E, detailed reports on the intraoperative and the immediate postoperative surgical outcomes and morbidity data are presented in our recent technical approach and safety outcome report.17
At this point, 7 subject deaths have occurred (see Table1). As previously reported, Subject 6 died suddenly and unexpectedly 8 months postsurgery due to a congenital cardiac defect, and Subject 3 died of respiratory failure associated with disease progression 13 months postsurgery.15,16 Subjects 1, 5, 9, 13, and 14 also died of respiratory complications associated with ALS disease progression at 30, 19, 11, 20, and 7 months postsurgery, respectively. All patients underwent autopsy for analysis of tissue response to implantation and for the identification of the continued presence of the transplanted cells within the spinal cord. The detailed results of these analyses will be reported separately. Briefly, standard pathological analysis showed no evidence of hemorrhage, cyst formation, or inflammatory reaction within the sites of transplantation. A representative example of the initial postmortem morphological findings is presented for Subject 14 (Fig 1). This subject received cervical injections and died 7 months postsurgery. There were no morphological abnormalities within the sites of transplantation; however, a nest of cells likely composed of the transplanted cells was identified.
Figure 1.
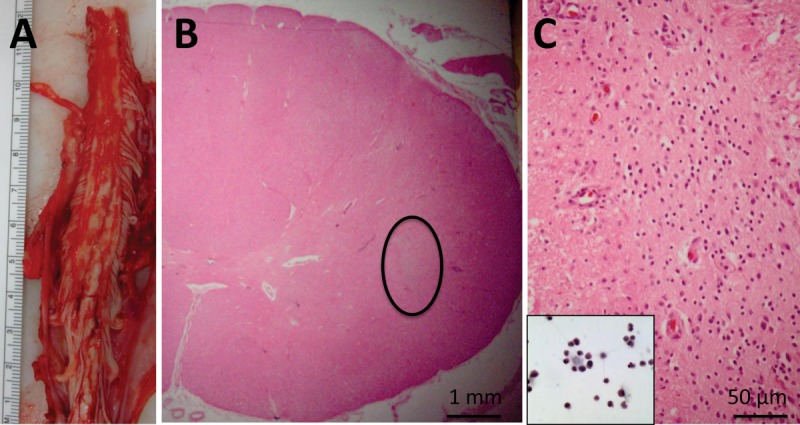
Neuropathological findings in Subject 14. (A) Gross image of cervical spinal cord at the time of autopsy. Serial sections through the region of transplantation did not demonstrate regions of cystic change, hemorrhage, or significant tissue disruption. (B) Representative cross section showing intact cord morphology using hematoxylin and eosin (H&E) staining. There is a nest of cells (circled) that are not intrinsic to the spinal cord, and do not stain with glial or neuronal markers (not shown). (C) Higher power of circled region in B showing the morphology of these cells, which is reminiscent of the morphology of the stem cells prior to transplantation (inset, H&E).
Functional Outcome Measures
Subjects regularly (see Supplementary Table 1) underwent clinical assessments. Interim results from Subjects 1 to 12 demonstrated no obvious acceleration of disease progression, and Subject 11 in Group C demonstrated modest improvements in postoperative ALSFRS-R, HHD, and EIM measurements.15 Continued functional outcome measure monitoring for Group A and B subjects is presented and discussed in Supplementary Figures 1 and 2, respectively. Overall, these subjects continued to demonstrate outcomes consistent with disease progression, but no acceleration of the disease course.
Group D: Cervical Injection
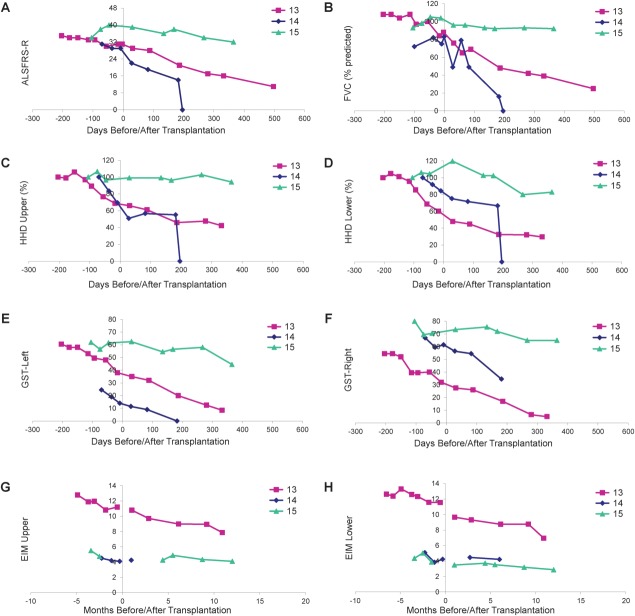
Functional outcomes are presented in Figure 2. Subjects 13 and 14 both had features of bulbar ALS. Subject 13 developed cervical kyphosis17 and died 20 months following transplantation, and Subject 14 died 200 days following transplantation. Although other clinical markers remained stable, Subject 15 demonstrated a modest decline in ALSFRS-R and HHD following transplantation, reflecting a progression that appeared slower than what is typically expected for ALS.
Figure 2.
Evaluation of disease progression in Group D subjects. Disease progression for Subjects 13 to 15 as measured by (A) Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R), (B) forced vital capacity (FVC), (C, D) hand-held dynamometry (HHD), (E, F) grip strength assessment (GST), and (G, H) electrical impedance myography (EIM). HHD is shown as a composite megascore for upper (C) or lower (D) extremities, normalized to the percentage of the score at baseline. GST data are presented for left (E) and right (F) sides. EIM is shown as 50kHz phase all muscle average for upper (G) or lower (H) extremity muscles. X-axis represents days pre- or postsurgery (day of surgery = day 0). Note that there were no precipitous declines in function after surgery for any subject. Note that a score of 0 for Subject 14 indicates subject death on the day post-transplantation indicated on the x-axis.
Group E: Dual-Targeted Injections
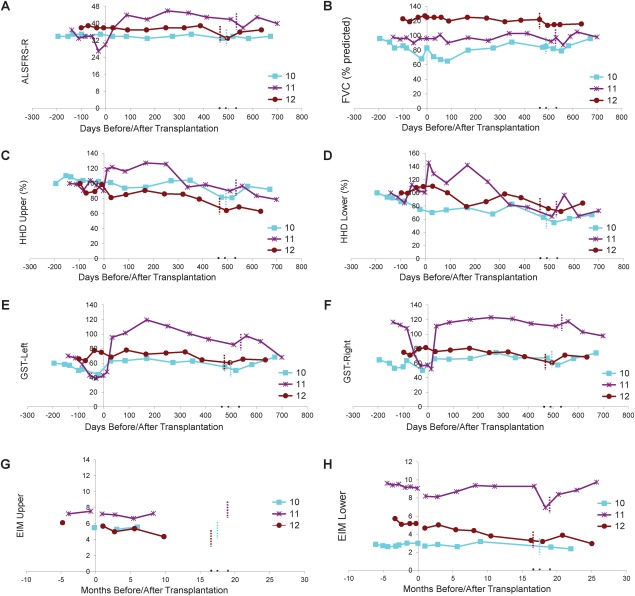
Functional outcome measures are presented in Figure 3. Subject 10 had a long disease duration and maintained a steady ALSFRS-R score accompanied by mild declines in other functional measures, suggestive of a very slowly progressive form of ALS. Subjects 11 and 12 had improved ALSFRS-R scores, steady FVC values, and modest declines in HHD megascores following HSSC transplantation, suggesting some progression of disease accompanied by multiple improved functional measures.
Figure 3.
Evaluation of disease progression in Group E subjects. Disease progression for Subjects 10 to 12 as measured by (A) Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R), (B) forced vital capacity (FVC), (C, D) hand-held dynamometry (HHD), (E, F) grip strength assessment (GST), and (G, H) electrical impedance myography (EIM). HHD is shown as a composite megascore for lower (C) or upper (D) extremities, normalized to the percentage of the score at baseline. GST data are presented for left (E) and right (F) sides. EIM is shown as 50kHz phase all muscle average for upper (G) or lower (H) extremity muscles. X-axis represents days before or after lumbar surgery (day of surgery = day 0). Note that there were no precipitous declines in function after surgery for any subject. Note that Group E subjects are subjects initially enrolled in Group C who received lumbar stem cell injections, and the short dotted vertical bars indicate the number of days after the first surgery when the second stem cell transplantations (cervical injections) were administered. Note that Subject 11 (purple lines) showed apparent improvement in ALSFRS-R and upper and lower extremity HHD.
Advanced Analyses of Functional Outcome Measures
We performed additional analyses to gain insight into the effects of the intervention on disease progression and to identify appropriate functional outcome measures for future trial phases. Comparison of postsurgical outcome data to predicted outcome points extrapolated from presurgical disease progression slopes revealed improvements in a significant number of measures at 6, 9, 12, and 15 months postsurgery (Table2). Of the 8 outcome assessments, at least 5 measures were improved in >50% of subjects at each time point relative to the predicted outcome values extrapolated from presurgical progression rates. To identify which functional assessments coordinated most closely with ALSFRS-R scores, Pearson correlations were calculated between data points for the various functional outcome measures. Results indicate that GST measures most closely reflect ALSFRS-R values throughout the study period (Fig 4), suggesting that ALSFRS-R and GST assessments will provide important outcome information in future trial phases.
Table 2.
Functional Outcomes versus Predicted Outcomes Based on Presurgical Assessments
| Time Postsurgery |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 Months |
9 Months |
12 Months |
15 Months |
|||||||||
| Assessment Measure | Subjects with Presurgical Slope Data,a No. | Subjects with Improved Outcome, No. | Ratio | Subjects with Presurgical Slope Data,a No. | Subjects with Improved Outcome, No. | Ratio | Subjects with Presurgical Slope Data,a No. | Subjects with Improved Outcome, No. | Ratio | Subjects with Presurgical Slope Data,a No. | Subjects with Improved Outcome, No. | Ratio |
| ALSFRS | 9 | 3 | 33% | 9 | 4 | 44% | 9 | 3 | 33% | 9 | 3 | 33% |
| FVC seated | 9 | 3 | 33% | 9 | 3 | 33% | 9 | 3 | 33% | 9 | 3 | 33% |
| HHD upper | 9 | 7 | 78% | 9 | 8 | 89% | 9 | 8 | 89% | 9 | 7 | 78% |
| HHD lower | 9 | 5 | 56% | 9 | 4 | 44% | 9 | 4 | 44% | 9 | 5 | 56% |
| GST left | 8 | 7 | 88% | 8 | 8 | 100% | 8 | 7 | 88% | 8 | 7 | 88% |
| GST right | 8 | 6 | 75% | 8 | 6 | 75% | 8 | 6 | 75% | 8 | 6 | 75% |
| EIM upper | 4 | 3 | 75% | 4 | 3 | 75% | 4 | 3 | 75% | 4 | 3 | 75% |
| EIM lower | 10 | 6 | 60% | 10 | 6 | 60% | 10 | 6 | 60% | 10 | 7 | 70% |
Slopes were utilized to determine whether postsurgical assessment data points at 6, 9, 12, and 15 months were improved relative to predicted outcomes extrapolated from the presurgical progression rate slopes
ALSFRS = Amyotrophic Lateral Sclerosis Functional Rating Scale; EIM = electrical impedance myography; FVC = forced vital capacity; GST = grip strength assessment; HHD = hand-held dynamometry.
Ratios indicated in bold represent outcome measures that are improved in over 50% of subjects relative to points predicted from presurgical slopes.
Figure 4.
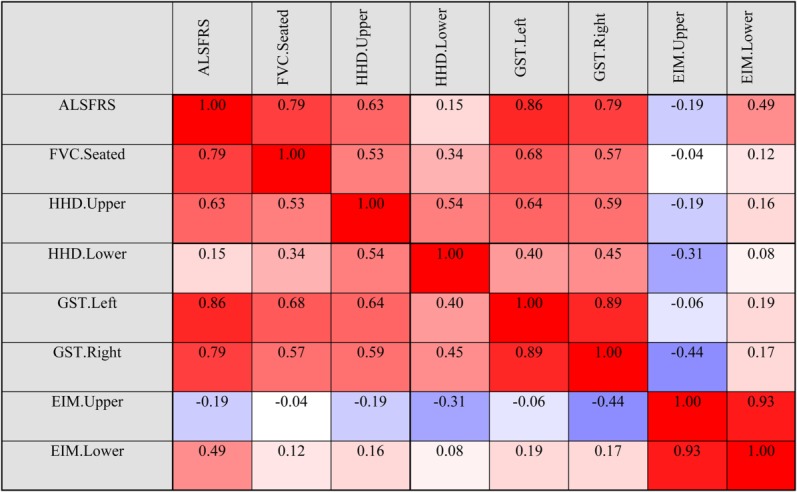
Pearson correlations for outcome assessment measures. ALSFRS = Amyotrophic Lateral Sclerosis Functional Rating Scale; EIM = electrical impedance myography; FVC = forced vital capacity; GST = grip strength assessment; HHD = hand-held dynamometry.
As shown in Figure 5, analysis of ALSFRS-R scores for Group E subjects exhibits improved outcomes (slope values higher than the presurgical slope at baseline reflect improved or attenuated progression rates during the designated window) beginning within the first month postsurgery, with slopes remaining positive for windows beginning up to 6 months postsurgery. Although the rate of benefit then decreases over time, the overall progression rate generally remains attenuated relative to the presurgical slope through the time of the second surgery. Positive slopes are again observed across treatment windows beginning at approximately 13 to 14 months postsurgery for these subjects, reflecting the second HSSC transplant in Subjects 10, 11, and 12 at 490, 532, and 464 days, respectively. This bimodal representation of HSSC benefit suggests that the biological activity of the cells shows the greatest benefits in the 6 months immediately following the surgeries (see Fig 5B), continuing to provide some benefit throughout the study evaluation period. Similar analyses of GST data for this cohort reflect comparable trends (data not shown).
Figure 5.
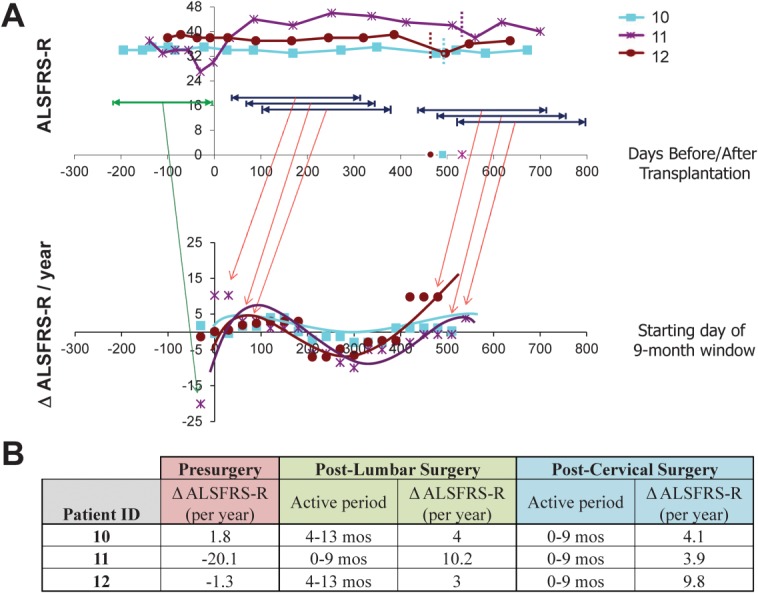
Preliminary analysis of potential windows of human spinal stem cell (HSSC) biological activity in Subjects 10 to 12. To identify the most biologically active period of the injected HSSCs, postsurgery data points for Group E subjects were divided into a series of 9-month windows, beginning each month postsurgery, and slopes were calculated across each window. Slopes were also calculated using Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) data points for the presurgical window. (A) The top panel demonstrates ALSFRS-R scores for Group E subjects during the presurgical period (green) and representative ranges associated with the various sliding postsurgical 9-month windows (dark blue). The bottom panel demonstrates the slopes obtained for each sliding window, with the x-axis corresponding to the first month for each 9-month window (ie, window 1 corresponds to months 1–10 postsurgery, window 2 corresponds to months 2–11 postsurgery, window 3 corresponds to months 3–12 postsurgery, etc). The first plotted slope for each subject corresponds to their presurgical progression rate. Slope values higher than the presurgical slope at baseline represent improved or attenuated progression rates during the designated window. Note that the starting month of the final sliding window for each patient coincides with the dates of the second surgery, which occur at 17.5, 19, and 16.6 months after the initial Cohort C surgery (time 0) for Subjects 10, 11, and 12, respectively. (B) The presurgical slope and postsurgical slopes associated with the window correlating to the peak benefit windows for both the lumbar and cervical postsurgery time frames are summarized.
Discussion
In this completed FDA-approved phase 1 trial, 18 intraspinal transplantation surgeries in 15 ALS subjects were performed following a risk escalation paradigm, progressing from nonambulatory to ambulatory subjects, lumbar to cervical spinal cord segments, and unilateral to bilateral injections across 5 cohorts. The encouraging interim results from Groups A to C,15,16 representing 12 subjects who received lumbar injections, supported the completion of the final trial with Cohorts D and E, examining cervical injections in 6 ALS subjects. Notably, the final 3 subjects receiving cervical injections previously received bilateral lumbar injections. Our study represents the first report of successful intraspinal stem cell transplantation into the cervical spinal cord and of successful repeated intraspinal stem cell transplantation into lumbar and cervical spinal cord segments in ALS subjects in an FDA-approved trial. Our ability to directly inject stem cells to target motor neurons in the region of the cervical spinal cord responsible for respiration represents a significant advance in the field of cellular therapy. In parallel, the dual-targeting approach, that is, both cervical and lumbar transplantation, has the potential to preserve respiratory function and improve motor function in ALS subjects.4 What is now required are future studies to determine whether these approaches provide sustained clinical improvement in ALS.
Of the 15 subjects in the phase 1 trial, 6 subjects died of their disease and 1 subject died of a congenital heart defect unrelated to ALS between 7 and 30 months after surgery. Of the 8 subjects who are still alive, 3 of them (Subjects 2, 8, and 10) had a long disease course prior to surgery (5.6, 11.6, and 12.7 years of known disease), likely representing atypical ALS, and have had little change in the trajectory of their disease. Subjects 7, 11, 12, and 15, who are alive with very slowly progressive or stabilized disease, had 2 clinical characteristics in common: these individuals had no bulbar features of ALS, and surgical transplantation occurred early within the course of their disease (average of 2 years and 1 month after symptom onset at the time of surgery). These preliminary results raise the possibility that intraspinal stem cell transplantation of ALS subjects with no bulbar symptoms early in the course of their disease could slow disease progression or even allow for functional improvement.
The majority of ALS trials utilize subject survival and ALSFRS-R scores for primary outcome measures.20 Our data demonstrate that GST most closely correlates with the ALSFRS-R scores. Comparisons of presurgical slopes to post-transplant data revealed that >50% of subjects demonstrated improvement across multiple clinical measures at 6, 9, 12, and 15 months postsurgery. Looking specifically at ALSFRS-R scores at the 9-month time point, the subjects who demonstrated improvements were part of Groups B, C, and E and exhibited an average disease duration <2 years prior to surgery, again suggesting that only subjects early in the disease course may experience clinical benefit. However, our experience did demonstrate a wide variation in presurgical progression rates for those individuals with multiple data points, emphasizing the importance of sufficient lead-in data to determine efficacy. Average declines of −1.1 ALSFRS-R score per month (−13.32 per year) have been reported20; however, the varied slopes we observed and the heterogeneous presentation of ALS emphasize the need for subject-specific baseline data.
We acknowledge that this study was not powered to determine efficacy and there was no control arm. In addition, some subjects exhibited a significant disease burden prior to surgery and were unlikely to show benefit, sufficient preclinical data points were unavailable for some subjects, and best-fit presurgical slopes were not always significantly powered given the number of available data points. Despite these limitations, we were able to identify potential possible therapeutic windows in our advanced evaluation of Group C/E outcome data. Of note, the 3 subjects in this cohort received the highest number of injections and demonstrated the largest effects on progression rates, suggesting that more injections are better, consistent with the neuroprotective mechanism of action hypothesized for this cell therapy.7,9 The ability to successfully administer 1.5 million HSSCs to ALS subjects over 15 total injections in Group E subjects into both lumbar and cervical spinal cord segments over the course of 2 surgeries is an important first step in evaluating the tolerance of the spinal cord for multiple HSSC transplantation procedures. The observed bimodal distribution in the 9-month sliding window slope analysis suggests there are maximal periods of benefit that correlate with the 2 surgical interventions. Furthermore, as the bell-shaped benefit curve associated with each intervention is likely due to disease progression, increasing the total cell dose and applying multiple applications of the stem cells may increase both the length and magnitude of potential benefit. These very preliminary observations on only 3 subjects provide the framework for future discussions of trial designs.
In conclusion, as we move forward, the continued assessment of data collected from subjects participating in phase 1 of the trial, evaluation of postsurgical MRI data, and characterization of the cellular grafts in deceased subjects will provide further insight into the therapeutic mechanisms and potential efficacy of intraspinal stem cell transplantation in ALS. With improved definitions for subject selection criteria, careful evaluation of clinical history prior to surgery, and utilization of the most efficient neurological assessment measures, we are primed for continued progress in future trial phases. Phase 2 of this trial commenced in September 2013 (http://ClinicalTrials.gov identifier: NCT01730716).
Acknowledgments
This phase 1 study was funded by Neuralstem; the phase 2 study is funded by the NIH National Institute of Neurological Disorders and Stroke (R01 NS077982; J.D.G., E.L.F., N.M.B., S.B.R.), the ALS Association (E.L.F.), and Neuralstem. Additional support for tissue and data analysis was provided by the NIH National Institute on Aging (5P50AG025688; J.D.G.) and the A. Alfred Taubman Medical Research Institute (E.L.F., N.M.B.).
We thank the study participants and their families for their trust and dedication to advancing the field of ALS therapeutics; the trial Data Safety Monitoring Board, chaired by Dr Z. Simmons; Dr T. G. Hazel for providing the CGMP stem cells; L. Shaw for help with subject assessments; Dr M. Gearing for assistance with neuropathology; the staff of the Emory ALS Center; and J. Duell for assistance with table preparation.
Authorship
E.L.F., N.M.B., and J.D.G. designed the study. K.J. provided critical study design input. N.M.B., T.F., M.P., J.B., and J.D.G. contributed to data acquisition. E.L.F., N.M.B., J.H., S.B.R., S.A.S., and J.D.G. were responsible for data analysis and interpretation. E.L.F. and S.A.S. drafted the manuscript. All authors critically edited the content of the article and approved the final version.
Potential Conflicts of Interest
N.M.B.: consultancy, Neuralstem; share of sale of Cleveland Clinic Foundation subsidiary IntElect to Boston Scientific; patents, licensed to Neuralstem. K.J.: stock, Neuralstem; patents, assigned to Neuralstem. S.B.R.: equity, consultancy, scientific advisor, Board of Directors, Skulpt; patents, assigned to Skulpt.
Additional supporting information can be found in the online version of this article.
References
- Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70:353–361. doi: 10.1002/ana.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefferan MP, Galik J, Kakinohana O. Human neural stem cell replacement therapy for amyotrophic lateral sclerosis by spinal transplantation. PLoS One. 2012;7:e42614. doi: 10.1371/journal.pone.0042614. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ryugo DK, Pongstaporn T. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. 2009;514:297–309. doi: 10.1002/cne.22022. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Shen P, Hazel T. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci Lett. 2011;494:222–226. doi: 10.1016/j.neulet.2011.03.017. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yan J, Chen D. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82:865–875. doi: 10.1097/01.tp.0000235532.00920.7a. , et al. [DOI] [PubMed] [Google Scholar]
- Yan J, Xu L, Welsh AM. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulis NM, Federici T, Glass JD. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;8:172–176. doi: 10.1038/nrneurol.2011.191. , et al. [DOI] [PubMed] [Google Scholar]
- Lunn JS, Hefferan MP, Marsala M, Feldman EL. Stem cells: comprehensive treatments for amyotrophic lateral sclerosis in conjunction with growth factor delivery. Growth Factors. 2009;27:133–140. doi: 10.1080/08977190902814855. [DOI] [PubMed] [Google Scholar]
- Lunn JS, Sakowski SA, Federici T. Stem cell technology for the study and treatment of motor neuron diseases. Regen Med. 2011;6:201–213. doi: 10.2217/rme.11.6. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani V, Calzarossa C, Cova L, Ticozzi N. Stem cells in amyotrophic lateral sclerosis: motor neuron protection or replacement? CNS Neurol Disord Drug Targets. 2010;9:314–324. doi: 10.2174/187152710791292666. [DOI] [PubMed] [Google Scholar]
- Riley J, Butler J, Park J. Targeted spinal cord therapeutics delivery: stabilized platform and microelectrode recording guidance validation. Stereotact Funct Neurosurg. 2007;86:67–74. doi: 10.1159/000112426. , et al. [DOI] [PubMed] [Google Scholar]
- Riley JP, Raore B, Taub JS. Platform and cannula design improvements for spinal cord therapeutics delivery. Neurosurgery. 2011;69(2 suppl):ons147–ons154. doi: 10.1227/NEU.0b013e3182195680. , et al. ; discussion ons155. [DOI] [PubMed] [Google Scholar]
- Raore B, Federici T, Taub J. Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine (Phila Pa 1976) 2011;36:E164–E171. doi: 10.1097/BRS.0b013e3181d77a47. , et al. [DOI] [PubMed] [Google Scholar]
- Riley J, Federici T, Park J. Cervical spinal cord therapeutics delivery: preclinical safety validation of a stabilized microinjection platform. Neurosurgery. 2009;65:754–761. doi: 10.1227/01.NEU.0000343524.45387.9E. , et al. ; discussion 761–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. , et al. [DOI] [PubMed] [Google Scholar]
- Riley J, Federici T, Polak M. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery. 2012;71:405–416. doi: 10.1227/NEU.0b013e31825ca05f. , et al. [DOI] [PubMed] [Google Scholar]
- Riley J, Glass J, Feldman EL. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery. 2014;74:77–87. doi: 10.1227/NEU.0000000000000156. , et al. [DOI] [PubMed] [Google Scholar]
- Guo X, Johe K, Molnar P. Characterization of a human fetal spinal cord stem cell line, NSI-566RSC, and its induction to functional motoneurons. J Tissue Eng Regen Med. 2010;4:181–193. doi: 10.1002/term.223. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johe KK, Hazel TG, Muller T. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. , et al. [DOI] [PubMed] [Google Scholar]
- Healy BC, Schoenfeld D. Comparison of analysis approaches for phase III clinical trials in amyotrophic lateral sclerosis. Muscle Nerve. 2012;46:506–511. doi: 10.1002/mus.23392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.