Abstract
Background
Antifolates are currently in clinical use for malaria preventive therapy and treatment. The drugs kill the parasites by targeting the enzymes in the de novo folate pathway. The use of antifolates has now been limited by the spread of drug-resistant mutations. GTP cyclohydrolase I (GCH1) is the first and the rate-limiting enzyme in the folate pathway. The amplification of the gch1 gene found in certain Plasmodium falciparum isolates can cause antifolate resistance and influence the course of antifolate resistance evolution. These findings showed the importance of P. falciparum GCH1 in drug resistance intervention. However, little is known about P. falciparum GCH1 in terms of kinetic parameters and functional assays, precluding the opportunity to obtain the key information on its catalytic reaction and to eventually develop this enzyme as a drug target.
Methods
Plasmodium falciparum GCH1 was cloned and expressed in bacteria. Enzymatic activity was determined by the measurement of fluorescent converted neopterin with assay validation by using mutant and GTP analogue. The genetic complementation study was performed in ∆folE bacteria to functionally identify the residues and domains of P. falciparum GCH1 required for its enzymatic activity. Plasmodial GCH1 sequences were aligned and structurally modeled to reveal conserved catalytic residues.
Results
Kinetic parameters and optimal conditions for enzymatic reactions were determined by the fluorescence-based assay. The inhibitor test against P. falciparum GCH1 is now possible as indicated by the inhibitory effect by 8-oxo-GTP. Genetic complementation was proven to be a convenient method to study the function of P. falciparum GCH1. A series of domain truncations revealed that the conserved core domain of GCH1 is responsible for its enzymatic activity. Homology modelling fits P. falciparum GCH1 into the classic Tunnelling-fold structure with well-conserved catalytic residues at the active site.
Conclusions
Functional assays for P. falciparum GCH1 based on enzymatic activity and genetic complementation were successfully developed. The assays in combination with a homology model characterized the enzymatic activity of P. falciparum GCH1 and the importance of its key amino acid residues. The potential to use the assay for inhibitor screening was validated by 8-oxo-GTP, a known GTP analogue inhibitor.
Keywords: Antifolate, Folate pathway, GTP cyclohydrolase I, Malaria
Background
The folate pathway of Plasmodium falciparum is a well-established malaria drug target with proven benefits in treatment and prophylaxis [1,2]. The combination of antifolate pyrimethamine and sulphadoxine has been included in anti-malarial drug regimens for decades [3]. These antifolate compounds target two different enzymes in the folate pathway of P. falciparum, with pyrimethamine and sulphadoxine inhibiting dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS), respectively [3]. The inhibition of the folate pathway cuts down the amount of folate derivatives that act as one-carbon carriers in nucleotide synthesis and amino acid metabolism. The malaria parasites became resistant to antifolates by gaining mutations at the dhfr and dhps genes [4-6]. The residue changes decrease the binding affinity of the drugs to the targeted enzymes [7,8]. The interest in antifolates has been renewed in recent years with the development of new lead compounds and the novel applications in malaria treatment [3,9]. Next-generation antifolates have now been developed in order to target drug-resistant folate enzymes [10]. The new P218 compound was designed to fit into the active site of pyrimethamine-resistant DHFR resulting in effective clearance of drug-resistant parasites [11]. Moreover, the existing antifolates can save the lives of infants and pregnant women at risk from malaria when administered as intermittent preventive regimens [9,12-14].
The rise in genomic analyses of malaria parasites revealed a unique role of GTP cyclohydrolase I (GCH1), the first and the rate-limiting enzyme of the folate pathway, in pyrimethamine resistance (Figure 1) [15]. Copy number polymorphism of P. falciparum gch1 is found in malaria parasites from certain endemic countries, with some isolates from Thailand containing more than ten copies of gch1[16,17]. Extra P. falciparum GCH1 from gene amplification was shown to reduce pyrimethamine sensitivity slightly, but, most important of all, the extra enzyme could reduce the cost of drug-resistant mutations to the parasite during the gain of pyrimethamine resistance [18,19]. The increase in the rate-limiting GCH1 was found to improve the folate flux by several orders of magnitude [20]. The drug-resistant mutations at dhfr, though advantageous under pyrimethamine pressure, are costly in terms of fitness due to the changes at the active site [21-23]. Having extra rate-limiting GCH1 can boost the folate flux to compensate for the loss of the products in the pathway. The role of P. falciparum GCH1 in drug resistance evolution makes it necessary to characterize this enzyme biochemically and functionally. Understanding the properties of P. falciparum GCH1 will lead to the development of a new category of inhibitors that goes beyond killing an individual parasite. The inhibition of P. falciparum GCH1 might be able to prevent drug resistance evolution of other drug-targeted folate enzymes and could become a new strategy for fighting the emerging threat of malaria drug resistance.
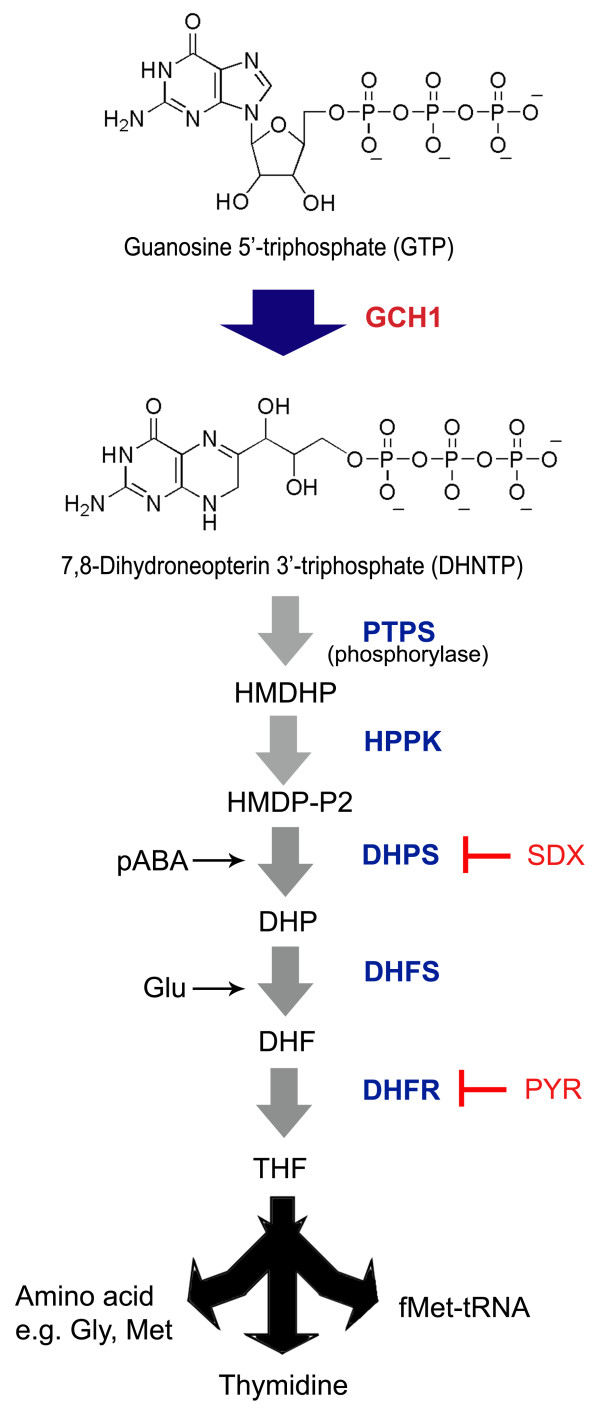
Figure 1.
GCH1 reaction in the folate pathway of Plasmodium falciparum. Malaria parasites cannot salvage folate and need their own de novo folate pathway to synthesize folate derivatives [24]. GCH1 converts GTP to 7,8-dihydroneopterin 3′-triphosphate, which will become the pterin moiety of folate derivatives. Several P. falciparum strains were found to contain multiple copies of gch1 (shown here as a large blue arrow). The next step in the folate pathway of P. falciparum is driven by 6-pyruvoyltetrahydropterin synthase (PTPS) to generate 6-hydroxymethyl-7,8 dihydroneopterin (HMDHP). It is worth noting that bacteria need an extra phosphorylase enzyme to remove the phosphate groups [25]. HMDHP is activated by the addition of two phosphate groups by hydroxymethyl dihydropteridine pyrophosphokinase (HPPK). Dihydropteroate synthase (DHPS) then combines the pterin moiety with 4-aminobenzoate (pABA) to produce 7,8-dihydropteroate (DHP). The last component to be added is glutamate via the reaction driven by dihydrofolate synthase (DHFS) to form 7,8-dihydrofolate (DHF). DHF is then reduced to 5,6,7,8-tetrahydrofolate (THF) by dihydrofolate reductase (DHFR). Anti-malarial sulphadoxine (SDX) and pyrimethamine (PYR) were combined to target two enzymes in the folate pathway of malaria parasites. For the chemical detail of the malarial folate pathway, see [2].
GCH1 is a well-conserved protein found in bacteria, protozoa, plants and animals including human [26,27]. The enzyme converts GTP into 7,8 dihydroneopterin triphosphate, the precursor of the pterin moiety in folate derivatives (Figure 1) [26]. The enzyme forms a homodecameric barrel-like structure with ten zinc-containing active sites with each of them formed between three subunits [26]. The enzyme catalyzes the breakages of the guanine and ribose rings in GTP and rearranges them to form 7,8-dihydroneopterin triphosphate (Figure 1) [26,28]. The product was further processed by subsequent enzymes in the pathway, including DHPS and DHFR. In metazoa, GCH1 is controlled by GTP cyclohydrolase I feedback regulatory protein (GFRP) which acts as a negative regulator by binding to the N-terminus of GCH1 [29,30]. This N-terminal extension does not exist in the bacterial GCH1 proteins from Escherichia coli and Thermus thermophilus.
With a series of the new findings on the significance of P. falciparum GCH1, it is important to characterize this enzyme from malaria parasites. Here the biochemical properties of recombinant P. falciparum GCH1 were reported. The roles of the key residues and domains were tested by genetic complementation assays. A homology model was built to explore the overall structure and conserved residues at the active site. The information on the GCH1 enzyme could form a basis for the development of the chemical modulators of P. falciparum GCH1.
Methods
Plasmid construction
A series of P. falciparum GCH1 truncations was constructed by PCR cloning from pET45b(+)/GCH1 with Pfu DNA polymerase (Vivantis) and confirmed by direct sequencing [19]. The genomic DNA samples from the 7G8 and RO-33 P. falciparum strains (a gift from Dr Sarah Volkman, Harvard School of Public Health, Boston, MA, USA) were used to construct N88Y and R230K, respectively. H279S was made by QuikChange II (Agilent Technologies) with the pET45b(+)/GCH1 template. Each corresponding clone was inserted into pBAD33 with a ribosomal binding site.
Functional complementation assay
Functional complementation was performed in E. coli K12 MG1655 ΔfolE (a gift from Professor Andrew Hanson, University of Florida, Gainseville, FL, USA). Construct was transformed by heat shock to E. coli K12 MG1655 ΔfolE with 300 μM thymidine supplement. Growth analysis was performed with preculture in LB broth (Bio Basic) supplemented with 300 μM thymidine (Sigma-Aldrich), 30 μg ml-1 kanamycin (Bio Basic) and 34 μg ml-1 chloramphenicol (Sigma-Aldrich). Starting culture was grown in the same media with 0.02% arabinose (Calbiochem) and without thymidine supplement at 37°C. Bacterial growth was determined using Spectrophotometer (Shimadzu UV-2501PC) at two-hour intervals. Each experiment was completed independently in at least triplicate.
Protein expression and enzymatic assay
Plasmodium falciparum Δ1-195 GCH1 or core GCH1 was cloned into pET45b(+) and expressed in E. coli BL21(DE3)RIL with 0.4 mM isopropyl β-D-1-thiogalactopyranoside at 37°C for two hours. Protein was purified by Ni2+-sepharose (GE Healthcare) at 4°C in 50 mM NaH2PO4, 100 mM NaCl and 20% glycerol, pH 8 with 20 mM, 70 mM and 300 mM imidazole for binding, washing and elution, respectively. The purified protein was dialyzed against 50 mM Tris–HCl, 100 mM KCl and 20% glycerol, pH 7.8 at 4°C for 18 hours. The assay was performed according to a published protocol with minor modification [31]. In short, the complete reaction was composed of 50 mM Tris–HCl pH 7.8, 100 mM KCl, 20% glycerol, 250 μM GTP and 2.5 μM recombinant P. falciparum GCH1. The reaction was incubated in the dark at 37°C for 90 min and stopped with 67 mM HCl. Non-fluorescent 7,8-dihydroneopterin triphosphate was oxidized by 0.067% iodine (dissolved in 2% KI) to form fluorescent neopterin at room temperature in the dark for one hour. 0.12% ascorbic acid and 55.6 mM NaOH were then added. The product was measured by SpectraMax M5 (Molecular Devices) with neopterin standard (Sigma). All experiments for obtaining kinetic parameters were done in triplicate.
Homology modelling
Plasmodium falciparum GCH1 was submitted to SWISS-MODEL homology modelling server [32]. A template model was T. thermophilus GCH1 (PDB: 1WUR) which served as a template for residue 203–383 of the conserved core domain [28]. The homology model for P. falciparum was obtained as a monomer. The decameric model was constructed in PyMol by superimposition onto T. thermophilus GCH1. The decameric model was further refined by optimizing side chain positions using Gromacs molecular dynamic package and GROMOS 43A1 force field [33]. The quality of the models was assessed by PROCHECK [34]. Secondary structure data were obtained from PDB accession number 1N3T, 1WUR, 1IS8 and 1FB1.
Results
Characteristics of Plasmodium falciparum GCH1
Plasmodium falciparum PFL1155w (PF3D7_1224000) was shown to be malarial GTP cyclohydrolase I based on enzyme kinetics and complementation studies [19,35,36]. This is consistent with the GCH1 activity previously identified from parasite extract [37]. Plasmodium falciparum GCH1 was compared with the GCH1 sequences from the organisms with known protein structures [28,38,39]. The well-conserved core subunit of GCH1, which contains the active site, is located at the C-terminus of P. falciparum GCH1 (Figure 2A). The analysis of the N-terminal sequences showed a different picture with distinctively long unique sequences even among Plasmodium species (Figure 2A). The unique N-terminal sequence might suggest a regulatory mechanism unlike those GCH1 proteins from metazoan which are regulated by GFRP via the N-terminal domain. The homologue of GFRP has not been identified in Plasmodium species.
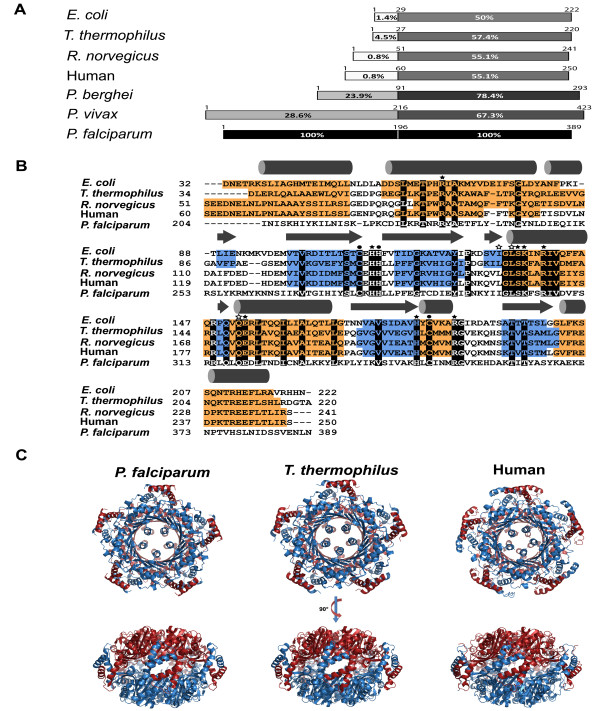
Figure 2.
Comparison of the GCH1 protein from Plasmodium falciparum to GCH1 proteins with known structures. (A) Sequence alignment of the GCH1 proteins. The GCH1 sequences were divided into the N-terminal regulatory domain and the C-terminal enzymatic core with the residue numbers on each diagram. The homology scores compared to P. falciparum GCH1were shown as percent homology and colour shade (100% and black colour to its own sequence). (B) Secondary structure diagram from known GCH1 structures and the homology model of P. falciparum GCH1 with α-helices in orange and β-strands in blue. The secondary structure diagrams of the P. falciparum GCH1 model are at the top of the alignment. The conserved amino acid residues are highlighted in black with labelled key residues (see text for detail). (C) Comparison of the overall homodecameric GCH1 structures. Two face-to-face pentameric rings are coloured in red and blue.
Comparative analysis between P. falciparum GCH1 and the GCH1 sequences from the species with structural data was performed. The key residues for coordinating with zinc are conserved with two cysteine and one histidine residues (black circles, Figure 2B) [39]. The fourth coordination with zinc was suggested to occur via a water molecule [28]. The residues that are shown by structural analysis to interact with GTP either via side chain (black stars, Figure 2B) or backbone (white stars, Figure 2B) are generally conserved. The homology model of P. falciparum GCH1 showed consistency in the overall structural component (Figure 2C). The N-terminus of P. falciparum GCH1 was excluded from the alignment and the model due to its uniqueness.
The homology model of P. falciparum GCH1 showed a similar overall structure at the core part. The core component of P. falciparum GCH1 was modelled and assembled into homodecamer based on previous structural determination [28]. The core component of GCH1 belongs to the T-fold protein family (T stands for tunnelling) [40]. Two pentameric rings are linked together by a clamp-like structure to form a face-to-face decameric barrel (Figure 2C). The tunnel in the middle of the decamer is formed by the last α-helix from every monomer. The active site is located on the external side of the barrel with ten of them formed between three subunits (the Homology model section for detail).
Enzymatic properties of Plasmodium falciparum GCH1
In order to understand the biochemical properties of P. falciparum GCH1, its kinetic parameters were determined. The core domain of GCH1 (residue 196–389) from the 3D7 strain was chosen for this work because the core GCH1 protein still retains enzymatic and complementation activities. It is consistent with the fact that the core domain of GCH1 was found to assemble into a homodecameric structure with three subunits forming one active site. The core enzyme was found to be soluble and expressed well in bacteria compared to the full-length version probably from the lack of the long repetitive amino acid stretches at the N-terminus. The kinetic assays for P. falciparum GCH1 were performed, and fits of data gave Km of 12.06 μM and kcat of 0.039 s-1. The Km values of GCH1 are in the micromolar range (4.2 μM and 31 μM for the GCH1 enzyme from T. thermophilus and human respectively) similar to the binding affinities of the SRP GTPase family [41]. It also means that the concentration of cellular GTP (~200-600 μM) exceeds the Km value of GCH1 [42].
The effects of temperature, salt and pH on the activity of P. falciparum GCH1 were then studied. The enzyme activity was found to be improved by the rise in temperature even at 50°C (Figure 3A). The finding indicates that feverish temperature in symptomatic malaria patients would not interfere with the activity of P. falciparum GCH1. High salt (1 M KCl) on the other hand could diminish the activity of the enzyme (Figure 3B). The best pH for the activity is at pH 9, but the activity peak is relative high over the broad pH range with a sudden drop when the pH reaching 12 (Figure 3C).
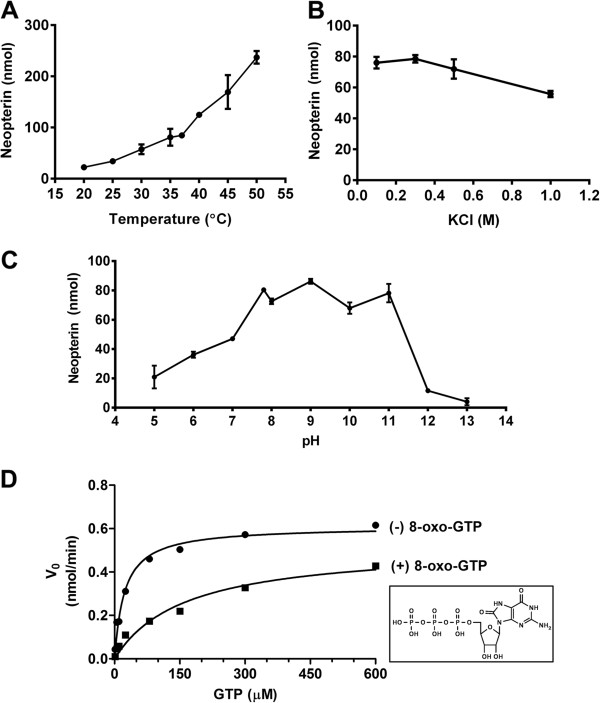
Figure 3.
Factors affecting the activities of Plasmodium falciparum GCH1. (A) Effect of temperature shift on the GCH1 activity as shown by the production of the oxidized neopterin product. (B) Effect of salt (KCl) on the GCH1 activity. (C) Effect of pH change on the activity of P. falciparum GCH1. The pH values were varied from pH 5–13 with the data point from pH 7.8, which was chosen for the enzymatic assay. Every reaction in Figure 3A-3C was performed for 90 minutes. (D) Effect of 8-oxo-GTP on the activity of P. falciparum GCH1. The inhibitory effect on initial velocity was followed under various substrate concentrations.
The enzymatic reaction of GCH1 was initiated by the attack of C8 of a guanine ring supposedly by a zinc-activated water. The modification of C8 would interfere with the enzymatic activity. 8-oxo-GTP was tested for its inhibitory effect on P. falciparum GCH1. As expected, 8-oxo-GTP could inhibit the activity of P. falciparum GCH1 with reduced fluorescent signal (Figure 3D). It shifted the Vmax to 161.8 μM, but the value of km was not changed indicating that 8-oxo-GTP acts as a competitive inhibitor of GCH1. The overall enzymatic properties of GCH1 from the P. falciparum core domain and from other organisms are in the same range, consistent with the fact that the core GCH1 sequences are relatively conserved.
Role of key residues and domains in GCH1 functional complementation
A bacterial complementation assay was developed in order to identify the components of GCH1 that are necessary for its activity. The bacterial strains without gch1 (∆folE) was used as a model for genetic complementation [43]. ∆folE requires thymidine supplement to survive. It was previously shown that the expression of P. falciparum gch1 could rescue the loss of bacterial gch1 allowing the growth in the condition without thymidine supplement [19]. The expression of wild-type P. falciparum GCH1 and mutant proteins in ∆folE bacteria was induced by arabinose. Wild-type P. falciparum GCH1 can rescue the growth of ∆folE bacteria in the condition without thymidine. Histidine 279 in P. falciparum GCH1 is a key residue for the enzymatic reaction (see below). The H279S mutant cannot rescue ∆folE bacteria, indicating that the enzymatic activity of P. falciparum GCH1 is needed for genetic complementation (Figure 4A). The natural genetic diversity in P. falciparum gch1 beside copy number polymorphism was also analyzed. Non-synonymous gch1 mutations, N88Y and R230K, were identified and cloned from South American 7G8 and African RO-33 P. falciparum strains, respectively. The mutations were confirmed by direct sequencing and cloned into inducible vectors. Both mutants can still rescue the loss of bacterial gch1 in ∆folE bacteria (Figure 4A), which suggests that these naturally occurring mutations do not compromise basal enzymatic function.
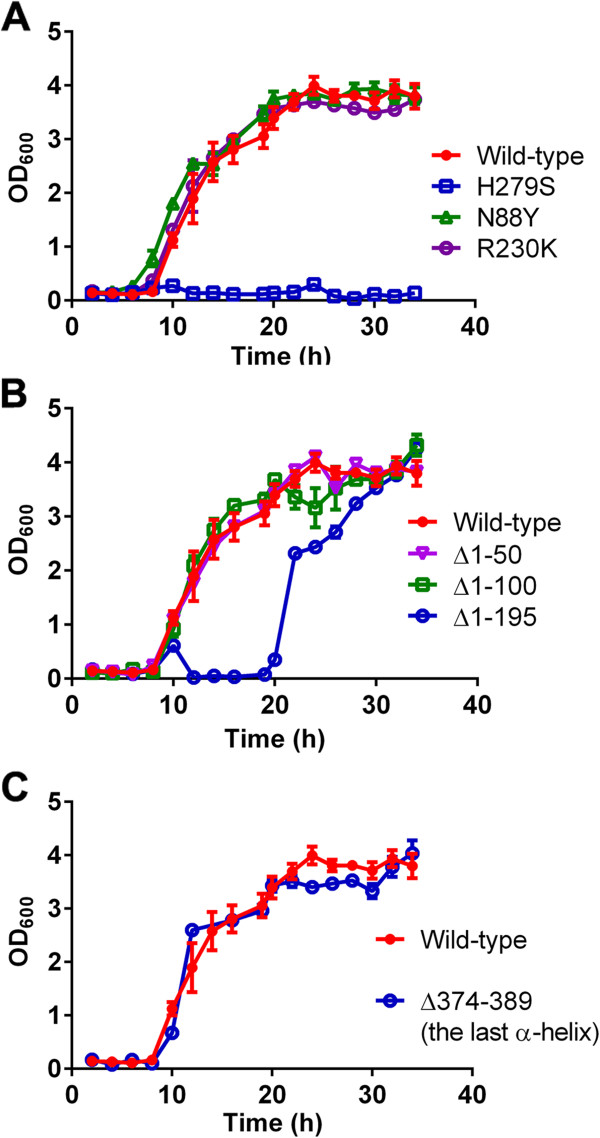
Figure 4.
Genetic complementation of Plasmodium falciparum GCH1 in bacteria. (A) Mutation effect on P. falciparum gch1 complementation. Wild-type P. falciparum GCH1 can rescue the loss of a bacterial strain without its own gch1 (folE in bacteria). The loss of functional P. falciparum GCH1 as in the H279S mutant abolishes the complementation activity. Naturally-occurring mutations (N88Y and R230K) were also tested for their genetic complementation activities. (B) Effect of the N-terminal truncation on genetic complementation. A series of the N-terminal truncates was made in order to test their effect on genetic complementation. (C) Effect of the C-terminal helix deletion on genetic complementation. The well-conserved C-terminal helix was removed and tested for the complementation activity by the mutant.
The significance of the different domains in P. falciparum GCH1 was studied starting with the N-terminal domain. A series of the N-terminal truncates was made and tested for their complementation activities. The deletions of the first 100 amino acid residues do not cause any change in genetic complementation (Figure 4B). Even the removal of the entire N-terminal domain does not completely result in the loss of complementation like in the case of H279S, but the deletion of the entire N-terminal domain cannot reach the same level of complementation observed with that of the wild-type (Figure 4B).
Interestingly, two recent phosphoproteomic analyses independently identified protein phosphorylation at P. falciparum GCH1 especially at the N-terminus of P. falciparum GCH1 [44,45]. The control of GCH1 by protein phosphorylation was reported in Drosophila melanogaster as a positive regulator for GCH1 [46]. The mutagenesis of the phosphorylation sites in D. melanogaster GCH1 attenuated the enzyme function [46]. In P. falciparum GCH1, the phosphorylation sites were located at Ser109, Ser119 and non-canonical Cys117 [44]. The deletion of this part (∆1-195) slightly compromised GCH1 complementation, but no change was observed in the ∆1-50 and ∆1-100 truncates. This observation does not exclude the importance of the N-terminal domain on the function of P. falciparum GCH1, but it suggests that the N-terminal part might play a role in enzymatic control. The regulatory mechanism, perhaps via phosphorylation, is not likely to affect bacterial complementation assay used in this study. The unique N-terminal sequence of P. falciparum GCH1 compared to that of human indicates a different regulatory partner for the malarial enzyme. It could be an alternative target for developing plasmodial GCH1 inhibitors without a significant inhibitory effect on the human counterpart. The identification of the putative GCH1 kinase in P. falciparum could reveal the role of protein phosphorylation on the regulation of this enzyme.
The last helix of GCH1 that forms the lining of the tunnel was also investigated. The tunnel at the centre of the enzyme complex is common among the T-fold proteins. Interestingly, the tunnel in GCH1 contains additional α-helices from each monomer at the center of the tunnel (Figure 2C). This conserved feature was found in all GCH1 proteins from bacteria to metazoa. Surprisingly, the deletion of this helix does not affect genetic complementation at all (Figure 4C). This finding suggests that the last helix is not directly required for the catalytic activity of P. falciparum GCH1. Nevertheless, its high degree of conservation could imply the possibility of this helix to have another role such as in enzyme regulation and protein complex assembly.
Homology model of Plasmodium falciparum GCH1
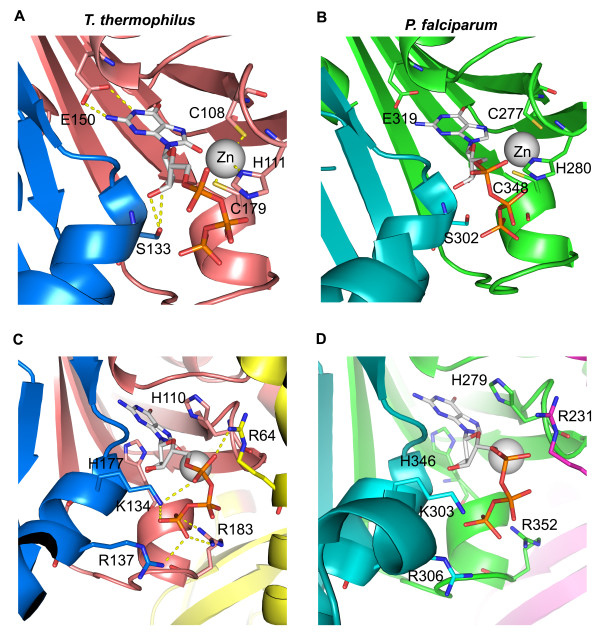
The homology model of P. falciparum GCH1 was built to observe the conserved residues with known functions based on T. thermophilus GCH1. The key residues for driving the enzymatic reactions and binding to the substrate are well conserved as expected. The active site of GCH1 is located at the interface between three subunits, two from the same pentameric ring and the other one from the opposite side. The X-ray structures of the GCH1 proteins from human, E. coli and T. thermophilus all suggested the presence of a metal ion bound to one histidine and two cysteine residues at each active site. The metal ion was found to be zinc by atomic absorption spectroscopy [39]. The zinc ion acts as Lewis base activating the water molecule to form hydroxyl nucleophile for nucleophilic attack at the guanine ring [38]. The residues in T. thermophilus GCH1 that coordinate with zinc are His 111, Cys 108 and Cys 179 (Figure 5A), which correspond to His 280, Cys 277 and Cys 348 in the P. falciparum GCH1 homology model (Figure 5B). Thermus thermophilus Glu 150 forms two hydrogen bonds with N1 and N2 of the guanine ring. This interaction could exist in P. falciparum GCH1 via conserved Glu 319. Another conserved chemical interaction is between the 2′ and 3′ hydroxyl groups of the ribose ring and Ser 133 (Ser 302 in P. falciparum GCH1) (Figure 5A and Figure 5B). Two His residues at the active site of T. thermophilus GCH1 were shown to participate in the catalytic reaction. Thermus thermophilus His177 (His 346 in P. falciparum GCH1) causes the protonation at N-7 in the guanine ring, which promotes the cleavage of N7/C8 at the guanine ring (Figures 5C and 5D) [28]. The replacement of the corresponding residue in E. coli GCH1 (His 179 in E. coli) results in the loss of enzymatic activity [47]. The second His residue is T. thermophilus His 110 (His 279 in P. falciparum GCH1) (Figures 5C and 5D). It acts as a hydrogen donor in the protonation of oxygen at the ribose moiety for the ribose ring breakage [28]. It might also participate in the C8/N9 imidazole ring cleavage to form formamidopyrimidine [28]. The triphosphate moiety of GTP is recognized by several basic residues located near the entrance of the active site pocket (Figure 5C). The structure of T. thermophilus GCH1 showed that Lys 134, Arg 137, Arg 183 and Arg 64 are responsible for the interactions with the phosphate groups. They correspond to Lys 303, Arg 306, Arg 352 and Arg 231 in the model of P. falciparum GCH1, respectively (Figures 5C and 5D).
Figure 5.
Comparison of the Plasmodium falciparum GCH1 homology model and the GCH1 structure from Thermus thermophilus. (A) and (C) the structure of T. thermophilus GCH1 showing the active site. (B) and (D) The homology model of P. falciparum GCH1 with the same views. See text for detail.
The homology model revealed strong conservation of the key residues for substrate binding and conversion in P. falciparum indicating the strong selective pressure to maintain the enzymatic activity. The H279S mutant was constructed based on the homology model as a negative control and found to lose both the enzymatic and complementation activities. Human and P. falciparum GCH1 proteins are quite diverged especially at the residues lining the active site and substrate binding pocket. The experimentally solved structure of P. falciparum GCH1 is required to validate the observation based on homology modelling.
Strategy for targeting Plasmodium falciparum GCH1
The enzymatic and complementation assays presented here have potential to be developed further for testing GCH1 inhibitors. A substrate analogue was tested to validate the capability of this assay to identify an inhibitor against plasmodial GCH1. Plasmodium falciparum GCH1 is an attractive drug target since it influences the course of drug resistance evolution [19], and it appears to be vital for erythrocytic-stage parasites as suggested by the failure to make a gch1 knockout line in P. falciparum[1]. An inhibitor specific to P. falciparum GCH1 could be combined with antifolate inhibitors against DHFR and DHPS. The next-generation anti-folates such as P218 have already shown promising results in the assays with drug-resistant strains and liver-stage parasites [11,48]. The compounds that can effectively target liver-stage parasites with small side-effects are in high demand for prophylactic and relapse treatments. Nevertheless, cross-inhibition of putative plasmodial GCH1 inhibitors with human GCH1 needs to be avoided as well since human GCH1 is an essential enzyme in the production of tetrahydrobiopterin, a coenzyme in the production of key neurotransmitters and nitric oxide [49].
The inhibition of P. falciparum GCH1 has the potential to be a new strategy for drug resistance control especially with the new antifolate compounds currently under development [11,18,19]. Malaria drug resistance is a major obstacle to malaria elimination especially with the parasites from Southeast Asia, which are prone to develop drug resistance and contain highly diverged genetic repertoires [50,51]. Target inhibition of a factor contributing to drug resistance can be a novel strategy for overcoming malaria drug resistance.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KK, NK, PJ and TC carried out protein expression, purification and enzymatic assay. PC and KK performed complementation assay. PJ, TK and PC carried out plasmid constructions. NK and TK performed molecular modelling. YY and TC conceived of the study and coordination. NK, KK, PJA, YY and TC carried out data analysis. TC prepared manuscript. All authors read and approved the final manuscript.
Contributor Information
Krittikorn Kümpornsin, Email: kkornphet@gmail.com.
Namfon Kotanan, Email: namfon.kotanan@gmail.com.
Pornpimol Chobson, Email: ppchobson@gmail.com.
Theerarat Kochakarn, Email: faith1114@gmail.com.
Piyaporn Jirawatcharadech, Email: ma_me_ow@hotmail.com.
Peera Jaru-ampornpan, Email: peera.jar@biotec.or.th.
Yongyuth Yuthavong, Email: yongyuth@biotec.or.th.
Thanat Chookajorn, Email: thanat.cho@mahidol.edu.
Acknowledgements
We would like to thank P. Wilairat and J Krungkrai for critical reading of the manuscript. This work was supported by the Commission of Higher Education-Thailand Research Fund-Mahidol University (RMU5380054) and the CPMO-National Science and Technology Development Agency. KK and TK were supported by The Thailand Research Fund through the Royal Golden Jubilee PhD Program (PHD/0044/2554 for KK and PHD/0204/2552 for TK). The authors acknowledge National e-Science Infrastructure Consortium for providing computing resources that have contributed to the research results reported within this paper.
References
- Muller IB, Hyde JE. Folate metabolism in human malaria parasites–75 years on. Mol Biochem Parasitol. 2013;188:63–77. doi: 10.1016/j.molbiopara.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Salcedo-Sora JE, Ward SA. The folate metabolic network of Falciparum malaria. Mol Biochem Parasitol. 2013;188:51–62. doi: 10.1016/j.molbiopara.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Yuthavong Y, Yuvaniyama J, Chitnumsub P, Vanichtanankul J, Chusacultanachai S, Tarnchompoo B, Vilaivan T, Kamchonwongpaisan S. Malarial (Plasmodium falciparum) dihydrofolate reductase-thymidylate synthase: structural basis for antifolate resistance and development of effective inhibitors. Parasitology. 2005;130:249–259. doi: 10.1017/s003118200400664x. [DOI] [PubMed] [Google Scholar]
- Peterson DS, Walliker D, Wellems TE. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci U S A. 1988;85:9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SJ, Galatis D, Cowman AF. Amino acids in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum involved in cycloguanil resistance differ from those involved in pyrimethamine resistance. Proc Natl Acad Sci U S A. 1990;87:3014–3017. doi: 10.1073/pnas.87.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowe CV, Cortese JF, Djimde A, Nwanyanwu OC, Watkins WM, Winstanley PA, Estrada-Franco JG, Mollinedo RE, Avila JC, Cespedes JL, Carter D, Doumbo OK. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J Infect Dis. 1997;176:1590–1596. doi: 10.1086/514159. [DOI] [PubMed] [Google Scholar]
- Yuvaniyama J, Chitnumsub P, Kamchonwongpaisan S, Vanichtanankul J, Sirawaraporn W, Taylor P, Walkinshaw MD, Yuthavong Y. Insights into antifolate resistance from malarial DHFR-TS structures. Nat Struct Biol. 2003;10:357–365. doi: 10.1038/nsb921. [DOI] [PubMed] [Google Scholar]
- Sirawaraporn W, Sathitkul T, Sirawaraporn R, Yuthavong Y, Santi DV. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Proc Natl Acad Sci U S A. 1997;94:1124–1129. doi: 10.1073/pnas.94.4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardaji A, Bassat Q, Alonso PL, Menendez C. Intermittent preventive treatment of malaria in pregnant women and infants: making best use of the available evidence. Expert Opin Pharmacother. 2012;13:1719–1736. doi: 10.1517/14656566.2012.703651. [DOI] [PubMed] [Google Scholar]
- Anthony MP, Burrows JN, Duparc S, Moehrle JJ, Wells TN. The global pipeline of new medicines for the control and elimination of malaria. Malar J. 2012;11:316. doi: 10.1186/1475-2875-11-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012;109:16823–16828. doi: 10.1073/pnas.1204556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayentao K, Garner P, van Eijk AM, Naidoo I, Roper C, Mulokozi A, MacArthur JR, Luntamo M, Ashorn P, Doumbo OK, ter Kuile FO. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. 2013;309:594–604. doi: 10.1001/jama.2012.216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, Critchley J, Danquah I, Dodoo A, Kobbe R, Lell B, May J, Premji Z, Sanz S, Sevene E, Soulaymani-Becheikh R, Winstanley P, Adjei S, Anemana S, Chandramohan D, Issifou S, Mockenhaupt F, Owusu-Agyei S, Greenwood B, Grobusch MP, Kremsner PG, Macete E, Mshinda H, Newman RD, Slutsker L, Tanner M. et al. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009;374:1533–1542. doi: 10.1016/S0140-6736(09)61258-7. [DOI] [PubMed] [Google Scholar]
- Senn N, Rarau P, Stanisic DI, Robinson L, Barnadas C, Manong D, Salib M, Iga J, Tarongka N, Ley S, Rosanas-Urgell A, Aponte JJ, Zimmerman PA, Beeson JG, Schofield L, Siba P, Rogerson SJ, Reeder JC, Mueller I. Intermittent preventive treatment for malaria in Papua New Guinean infants exposed to Plasmodium falciparum and P. vivax: a randomized controlled trial. PLoS Med. 2012;9:e1001195. doi: 10.1371/journal.pmed.1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AD, Gregory JF 3rd. Folate biosynthesis, turnover, and transport in plants. Annu Rev Plant Biol. 2011;62:105–125. doi: 10.1146/annurev-arplant-042110-103819. [DOI] [PubMed] [Google Scholar]
- Kidgell C, Volkman SK, Daily J, Borevitz JO, Plouffe D, Zhou Y, Johnson JR, Le Roch K, Sarr O, Ndir O, Mboup S, Batalov S, Wirth DF, Winzeler EA. A systematic map of genetic variation in Plasmodium falciparum. PLoS Pathog. 2006;2:e57. doi: 10.1371/journal.ppat.0020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Miller B, Barends M, Jaidee A, Patel J, Mayxay M, Newton P, Nosten F, Ferdig MT, Anderson TJ. Adaptive copy number evolution in malaria parasites. PLoS Genet. 2008;4:e1000243. doi: 10.1371/journal.pgen.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinberg A, Siu E, Stern C, Lawrence EA, Ferdig MT, Deitsch KW, Kirkman LA. Direct evidence for the adaptive role of copy number variation on antifolate susceptibility in Plasmodium falciparum. Mol Microbiol. 2013;88:702–712. doi: 10.1111/mmi.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpornsin K, Modchang C, Heinberg A, Ekland EH, Jirawatcharadech P, Chobson P, Suwanakitti N, Chaotheing S, Wilairat P, Deitsch KW, Kamchonwongpaisan S, Fidock DA, Kirkman LA, Yuthavong Y, Chookajorn T. Origin of Robustness in Generating Drug-Resistant Malaria Parasites. in press. [DOI] [PMC free article] [PubMed]
- Hossain T, Rosenberg I, Selhub J, Kishore G, Beachy R, Schubert K. Enhancement of folates in plants through metabolic engineering. Proc Natl Acad Sci U S A. 2004;101:5158–5163. doi: 10.1073/pnas.0401342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovsky ER, Chookajorn T, Brown KM, Imwong M, Shaw PJ, Kamchonwongpaisan S, Neafsey DE, Weinreich DM, Hartl DL. Stepwise acquisition of pyrimethamine resistance in the malaria parasite. Proc Natl Acad Sci U S A. 2009;106:12025–12030. doi: 10.1073/pnas.0905922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KM, Costanzo MS, Xu W, Roy S, Lozovsky ER, Hartl DL. Compensatory mutations restore fitness during the evolution of dihydrofolate reductase. Mol Biol Evol. 2010;27:2682–2690. doi: 10.1093/molbev/msq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chookajorn T, Kumpornsin K. 'Snakes and Ladders' of drug resistance evolution. Virulence. 2011;2:244–247. doi: 10.4161/viru.2.3.16194. [DOI] [PubMed] [Google Scholar]
- Nzila A, Ward SA, Marsh K, Sims PF, Hyde JE. Comparative folate metabolism in humans and malaria parasites (part I): pointers for malaria treatment from cancer chemotherapy. Trends Parasitol. 2005;21:292–298. doi: 10.1016/j.pt.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus SM, Wegkamp A, Sybesma W, Hugenholtz J, Gregory JF 3rd, Hanson AD. A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of bacteria and plants. J Biol Chem. 2005;280:5274–5280. doi: 10.1074/jbc.M413759200. [DOI] [PubMed] [Google Scholar]
- Grawert T, Fischer M, Bacher A. Structures and reaction mechanisms of GTP cyclohydrolases. IUBMB Life. 2013;65:310–322. doi: 10.1002/iub.1153. [DOI] [PubMed] [Google Scholar]
- Basset G, Quinlivan EP, Ziemak MJ, Diaz De La Garza R, Fischer M, Schiffmann S, Bacher A, Gregory JF, Hanson AD 3rd. Folate synthesis in plants: the first step of the pterin branch is mediated by a unique bimodular GTP cyclohydrolase I. Proc Natl Acad Sci U S A. 2002;99:12489–12494. doi: 10.1073/pnas.192278499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Nakagawa N, Kuramitsu S, Yokoyama S, Masui R. Novel reaction mechanism of GTP cyclohydrolase I. High-resolution X-ray crystallography of Thermus thermophilus HB8 enzyme complexed with a transition state analogue, the 8-oxoguanine derivative. J Biochem. 2005;138:263–275. doi: 10.1093/jb/mvi120. [DOI] [PubMed] [Google Scholar]
- Harada T, Kagamiyama H, Hatakeyama K. Feedback regulation mechanisms for the control of GTP cyclohydrolase I activity. Science. 1993;260:1507–1510. doi: 10.1126/science.8502995. [DOI] [PubMed] [Google Scholar]
- Higgins CE, Gross SS. The N-terminal peptide of mammalian GTP cyclohydrolase I is an autoinhibitory control element and contributes to binding the allosteric regulatory protein GFRP. J Biol Chem. 2011;286:11919–11928. doi: 10.1074/jbc.M110.196204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ER, Wachter H, Werner-Felmayer G. Determination of tetrahydrobiopterin biosynthetic activities by high-performance liquid chromatography with fluorescence detection. Methods Enzymol. 1997;281:53–61. doi: 10.1016/s0076-6879(97)81008-7. [DOI] [PubMed] [Google Scholar]
- Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc. 2009;4:1–13. doi: 10.1038/nprot.2008.197. [DOI] [PubMed] [Google Scholar]
- Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- Stephens LL, Shonhai A, Blatch GL. Co-expression of the Plasmodium falciparum molecular chaperone, PfHsp70, improves the heterologous production of the antimalarial drug target GTP cyclohydrolase I, PfGCHI. Protein Expr Purif. 2011;77:159–165. doi: 10.1016/j.pep.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Maita N, Okada K, Hatakeyama K, Hakoshima T. Crystal structure of the stimulatory complex of GTP cyclohydrolase I and its feedback regulatory protein GFRP. Proc Natl Acad Sci U S A. 2002;99:1212–1217. doi: 10.1073/pnas.022646999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krungkrai J, Yuthavong Y, Webster HK. Guanosine triphosphate cyclohydrolase in Plasmodium falciparum and other Plasmodium species. Mol Biochem Parasitol. 1985;17:265–276. doi: 10.1016/0166-6851(85)90001-5. [DOI] [PubMed] [Google Scholar]
- Rebelo J, Auerbach G, Bader G, Bracher A, Nar H, Hosl C, Schramek N, Kaiser J, Bacher A, Huber R, Fischer M. Biosynthesis of pteridines. Reaction mechanism of GTP cyclohydrolase I. J Mol Biol. 2003;326:503–516. doi: 10.1016/s0022-2836(02)01303-7. [DOI] [PubMed] [Google Scholar]
- Auerbach G, Herrmann A, Bracher A, Bader G, Gutlich M, Fischer M, Neukamm M, Garrido-Franco M, Richardson J, Nar H, Huber R, Bacher A. Zinc plays a key role in human and bacterial GTP cyclohydrolase I. Proc Natl Acad Sci U S A. 2000;97:13567–13572. doi: 10.1073/pnas.240463497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloc'h N, Poupon A, Mornon J-P. Sequence and structural features of the T-fold, an original tunnelling building unit. Proteins Struct Funct Bioinformatics. 2000;39:142–154. doi: 10.1002/(sici)1097-0134(20000501)39:2<142::aid-prot4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Shan SO, Schmid SL, Zhang X. Signal recognition particle (SRP) and SRP receptor: a new paradigm for multistate regulatory GTPases. Biochemistry. 2009;48:6696–6704. doi: 10.1021/bi9006989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- Klaus SM, Kunji ER, Bozzo GG, Noiriel A, de la Garza RD, Basset GJ, Ravanel S, Rebeille F, Gregory JF 3rd, Hanson AD. Higher plant plastids and cyanobacteria have folate carriers related to those of trypanosomatids. J Biol Chem. 2005;280:38457–38463. doi: 10.1074/jbc.M507432200. [DOI] [PubMed] [Google Scholar]
- Solyakov L, Halbert J, Alam MM, Semblat JP, Dorin-Semblat D, Reininger L, Bottrill AR, Mistry S, Abdi A, Fennell C, Holland Z, Demarta C, Bouza Y, Sicard A, Nivez MP, Eschenlauer S, Lama T, Thomas DC, Sharma P, Agarwal S, Kern S, Pradel G, Graciotti M, Tobin AB, Doerig C. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat Commun. 2011;2:565. doi: 10.1038/ncomms1558. [DOI] [PubMed] [Google Scholar]
- Treeck M, Sanders JL, Elias JE, Boothroyd JC. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites' boundaries. Cell Host Microbe. 2011;10:410–419. doi: 10.1016/j.chom.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburk CD, Bowling KM, Xu D, Huang Z, O'Donnell JM. A typical N-terminal extensions confer novel regulatory properties on GTP cyclohydrolase isoforms in Drosophila melanogaster. J Biol Chem. 2006;281:33302–33312. doi: 10.1074/jbc.M602196200. [DOI] [PubMed] [Google Scholar]
- Nar H, Huber R, Auerbach G, Fischer M, Hosl C, Ritz H, Bracher A, Meining W, Eberhardt S, Bacher A. Active site topology and reaction mechanism of GTP cyclohydrolase I. Proc Natl Acad Sci U S A. 1995;92:12120–12125. doi: 10.1073/pnas.92.26.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 2012;9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapatos G. The neurobiology of tetrahydrobiopterin biosynthesis: a model for regulation of GTP cyclohydrolase I gene transcription within nigrostriatal dopamine neurons. IUBMB Life. 2013;65:323–333. doi: 10.1002/iub.1140. [DOI] [PubMed] [Google Scholar]
- Rathod PK, McErlean T, Lee PC. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, Maslen G, O'Brien J, Djimde A, Doumbo O, Zongo I, Ouedraogo JB, Michon P, Mueller I, Siba P, Nzila A, Borrmann S, Kiara SM, Marsh K, Jiang H, Su XZ, Amaratunga C, Fairhurst R, Socheat D, Nosten F, Imwong M, White NJ, Sanders M, Anastasi E, Alcock D, Drury E. et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]