Abstract
Self-monitoring of actions, critical for guiding goal-directed behavior, is deficient in schizophrenia. Defective error-monitoring may reflect more general self-monitoring deficiencies. The error-related negativity (ERN) component of the event-related potential (ERP) is smaller in patients with schizophrenia, and anterior cingulate cortex (ACC) and dorsolateral pre-frontal cortex (DLPFC), both critical for error detection, are less responsive to errors in patients with schizophrenia, as revealed with functional magnetic resonance imaging (fMRI).
ERP and fMRI data were collected while 11 patients with schizophrenia and 10 healthy controls performed a Go-NoGo task requiring a button press to Xs (p=.88) while withholding responses to Ks (p=.12). We measured the ERN and ACC and DLPFC activations to false alarms.
The task elicited a robust ERN and modest activations in ACC and DLPFC to false alarms. As expected, ERN was larger in controls than patients. However, ACC and DLPFC activations were not greater in controls than patients. Surprisingly, DLPFC was more activated by errors in patients than controls.
ERPs may be superior for assessing error processing because (1) ERNs can be measured precisely without needing to control for the multiple comparisons of fMRI, and (2) ERPs have the temporal precision to detect transient activity necessary for error detection and on-the-fly behavioral adjustments.
Keywords: Error detection, schizophrenia, NoGo task, ERN, ERPs, fMRI
INTRODUCTION
Self-monitoring of thoughts and actions is critical for distinguishing self-initiated from externally generated stimuli and for guiding goal-directed behavior. Defective self-monitoring may be a core feature of schizophrenia(Feinberg 1978; Frith and Done 1989). Indeed, self-monitoring deficits have been demonstrated in schizophrenia, particularly in patients with Schneiderian first-rank symptoms performing tasks calling for self-willed actions (Mlakar et al 1994). Defective error-monitoring may reflect this more general self-monitoring deficiency. We (Turken et al 2003) and others (Frith and Done 1989; Malenka et al 1982; Malenka et al 1986) have shown that patients with schizophrenia show error-correction deficits when exteroceptive feedback is withheld such that internal monitoring of action is needed.
While behavioral studies highlight the deficits in self-monitoring in schizophrenia, brain imaging provides important details about the temporal course of error monitoring and its precise neuroanatomical underpinnings. Two in-vivo, non-invasive brain-imaging methods have been used to understand neural responses to errors, electrophysiological and hemodynamic. Electroencephalogram (EEG) based methods are relatively direct ways of measuring neuronal activity with millisecond temporal resolution. Individual EEG trials are averaged to produce an event-related potential (ERP), whose components develop and resolve within tens or hundreds of milliseconds. A less direct measure of neural activity is hemodynamic brain imaging, the most common of which is functional magnetic resonance imaging (fMRI). While fMRI operates on a much more delayed time scale than EEG, taking about 4 to 6 seconds to develop and another 8 to 10 seconds to resolve (Buckner 1998), it has superior spatial resolution, allowing a more precise delineation of brain structures and circuits involved in specific sensory and cognitive processes.
ERP studies of error monitoring
Almost simultaneously, two laboratories reported a unique brain response to errors, referred to as the error-related negativity (ERN) (Gehring et al 1993) and the negativity associated with errors (Ne) (Falkenstein et al 1991). This is a negative component of the event-related potential (ERP) starting at the onset of error responses, peaking at about 100 ms after the error, and being maximal at frontocentral midline scalp sites. Although ERN is typically elicited in situations where the subject knows the correct response but fails to execute it (Dehaene et al 1994), it can also be elicited in the absence of error awareness (Nieuwenhuis et al 2001). Also, it is independent of corrective motor responses, occurring after errors of commission in Go/NoGo tasks even though no corrective actions are possible (e.g.\ (Ford et al 2004b; Mathalon et al 2003) and occurring in response to external error feedback (Badgaiyan and Posner 1998; Luu et al 2000; Miltner et al 1997). It has been described as part of the feed forward system in which awareness of the error occurs before the error is executed; such an error monitoring system was proposed by Rabbitt to enable us to correct our errors “in flight” before they are complete (Rabbitt 1966).
ERP studies of error monitoring in schizophrenia
Using a flanker task, Kopp and Rist (1999) were the first to report the ERN was smaller in patients with schizophrenia, a finding that we and others replicated using a variety of tasks (Alain et al 2002; Bates et al 2002; Bates et al 2004; Laurens et al 2003; Mathalon et al 2002; Morris et al 2008; Morris et al 2006). In addition to abnormally small ERNs on error trials in patients, some(Alain et al 2002; Kopp and Rist 1999; Mathalon et al 2002), but not all(Bates et al 2002) investigators have reported abnormally large ERNs on correct trials, sometimes referred to as the correct-response negativity (CRN), in patients with schizophrenia.
Hemodynamic studies of error monitoring
While most researchers have interpreted the ERN as a reflection of an early error detection system involving the anterior cingulate cortex (ACC) (Coles et al 1995; Dehaene et al 1994; Falkenstein et al 1995; Falkenstein et al 1991; Falkenstein et al 2000; Gehring et al 1995; Gehring et al 1993; Holroyd et al 1998; Leuthold and Sommer 1999; Scheffers and Coles 2000), some have pointed to the contribution of conflict-related processing to the error-related responses (Carter et al 1998). Although Carter et al. (1998) showed the same region of the ACC was activated by conflict and errors, others have shown distinct areas of the ACC to be activated by conflict and by errors, suggesting potentially dissociable processes(Braver et al 2001; Kiehl et al 2000; Ullsperger and von Cramon 2001).
Hemodynamic studies of error monitoring in schizophrenia
Functional magnetic resonance imaging (fMRI) studies provide evidence of a diminished ACC response to errors in patients with schizophrenia compared to controls (Carter et al 2001; Kerns et al 2005; Laurens et al 2003), although Weiss et al(Weiss et al 2003) reported increased ACC activation to errors in patients. Involvement of ACC with error monitoring is consistent with dipole localization analyses of the ERN (Badgaiyan and Posner 1998; Dehaene et al 1994; Holroyd et al 1998; Luu et al 2000; Miltner et al 1997; Van Veen and Carter 2002) as well as with a literature showing patients with ACC lesions to have reduced error awareness (Turken and Swick 1999) and diminished or absent ERNs following errors (Stemmers et al 2000). Although these studies focus on ACC, ACC is large and different sub-regions within the ACC show group differences across studies. For example, Manoach and colleagues recently distinguished between dorsal ACC (dACC) and rostral ACC (rACC), with hypoactivity in the dACC reflecting deficient updating of context in response to errors, and hypoactivity in the rACC network reflecting diminished concern regarding behavioral outcomes (Polli et al 2008).
Dopamine, reward processing, and the ERN
Holroyd and Coles (Holroyd and Coles 2002) formulated a model of the ERN based on the neurobiology of reward processing. Midbrain dopamine neurons project to the ventral striatum, prefrontal cortex, and ACC. Reward anticipation is associated with an increase in dopamine release in the ventral striatum, and reward prediction errors (i.e., outcomes worse than expected) are associated with transient inhibition of dopamine release in the ventral striatum(Schultz 2007). Holroyd and Coles theorized that the ACC is subject to similar regulation by midbrain dopaminergic input, with ACC neurons being tonically inhibited by midbrain dopaminergic input, and with phasic inhibition of dopamine release following reward prediction errors leading to transient increases in ACC neuronal activity that give rise to the ERN. This ACC signal is thought to recruit greater input from other brain regions, including the DLPFC, to enhance performance, facilitate learning, and maximize rewards.
Abnormal error-processing and the pathophysiology of schizophrenia
Based on the Holroyd and Coles model(Holroyd and Coles 2002), abnormalities in dopaminergic neurotransmission associated with schizophrenia, either as part of its primary pathophysiology (Davis et al 1991; Laruelle and Abi-Dargham 1999) (Moore et al 1999) or secondary to its treatment with dopamine-blocking anti-psychotic medications (Laruelle et al 2005), would be expected to disrupt reward processing signals, including the ERN and possibly the CRN. However, exactly which mechanisms are disrupted by the illness, and how these disruptions account for the pattern of ERN/CRN abnormalities observed in schizophrenia, have yet to be elucidated. Importantly, although dopamine D2-receptor antagonists have been shown to reduce ERN amplitudes acutely in healthy volunteers (Zirnheld et al 2004), ERN amplitude reduction has been shown to be more pronounced in acutely hospitalized, variably medicated, schizophrenia patients than when they were clinically stabilized after 6 weeks of optimized antipsychotic treatment (Bates et al 2004) Thus, it is unlikely that antipsychotic effects alone could fully account for the ERN abnormalities in schizophrenia.
While neuropathological (Benes 2000; Honer et al 1997; Suhara et al 2002) and functional (Carter et al 1997; Holcomb et al 1996; Holcomb et al 2000; Mulert et al 2001) abnormalities of the ACC have been reported in schizophrenia, suggesting that local pathology of the ACC itself could contribute to ERN and CRN abnormalities in the disorder, other regions have also been implicated. In particular, the dorsolateral prefrontal cortex (DLPFC), which has rich connections with the ACC (Bush et al 2000), has been implicated in both error processing(Carter et al 1998) and in the pathophysiology of schizophrenia (e.g., Goldman-Rakic and Selemon 1997; Weinberger and Berman 1996). Of note, the DLPFC appears to play an important role in modulating the ACC’s differential response to errors and correct responses, as indicated by a study showing patients with DLPFC lesions to have normal amplitude ERNs but equally (and abnormally) large CRN amplitudes following correct responses(Gehring and Knight 2000). This similarity between ERN and CRN amplitudes has sometimes been observed in patients with schizophrenia (Kopp and Rist 1999; Mathalon et al 2002), consistent with compromised DLPFC function. Based on these findings, it is likely that with normal input from DLPFC, the CRN will be minimal.1
Goals of this experiment
In order to compare the sensitivity of neurophysiologic and hemodynamic measures of brain responses to errors, the same subjects were subjected to identical Go-NoGo paradigms with both imaging modalities. To increase the likelihood of errors, we attempted to establish a strong prepotent bias to respond to Go stimuli. To build up expectancy for Go stimuli, we skewed stimulus probabilities (Go stimuli=88%, NoGo stimuli=12%. In addition, we pre-trained subjects to respond to the stimulus that subsequently became the NoGo stimulus, and we emphasized speed over accuracy.
METHODS
Participants
In separate sessions, we recorded ERPs and fMRI while 11 patients with DSM-IV schizophrenia and 10 healthy comparison subjects performed a Go-NoGo task2. All gave written informed consent after procedures had been fully described. Demographic and clinical data are included in Table 1.
Table 1.
Demographic Data
| Variable | Normal Control Subjects | Schizophrenic Patients | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | SD | min | max | Mean | SD | min | max | |
| Age (years) | 37.7 | 10.3 | 26 | 55 | 37.9 | 12.7 | 23 | 68 |
| Education (years) | 18.9 | 2.5 | 16.0 | 22.0 | 12.8 | 3.4 | 5.0 | 19.0 |
| Parental Socioeconomic Status | 31.3 | 10.8 | 12.0 | 44.0 | 37.0 | 15.5 | 16.0 | 62.0 |
| BPRS Total (ERP session) | 39 | 9.6 | 19.0 | 51.5 | ||||
| BPRS Total (fMRI session) | 41 | 9.5 | 20.0 | 51.5 | ||||
| Handedness | 10 right handed | 9 right handed, 1 left handed 1 ambidextrous |
||||||
| Gender | 7 men | 8 men | ||||||
| Diagnostic Subtype | 6 Undifferentiated 4 Paranoid 1 Residual |
|||||||
| Medication | 10 atypical, 1 typical | |||||||
Patients were recruited from community mental health centers, as well as from inpatient and outpatient services of the Palo Alto Veterans Affairs Healthcare System. All patients met DSM-IV (American Psychiatric Association 1994) criteria for schizophrenia based either on the diagnosis from a SCID interview conducted by a psychiatrist or psychologist, or by consensus of a SCID interview conducted by a trained research assistant and a clinical interview by a psychiatrist or psychologist. In one case, a psychiatrist made the diagnosis by patient chart review. Prospective patient and control participants were excluded if they met DSM-IV criteria for alcohol or drug abuse within 30 days prior to study. In addition, patient and control participants were excluded for significant head injury, neurological or other medical illnesses compromising the central nervous system. Patient symptoms were assessed using the 18 item Brief Psychiatric Rating Scale (BPRS) (Faustman and Overall 1999; Overall et al 1967).
Comparison subjects were recruited by newspaper advertisements and word-of-mouth, screened by telephone using questions from the Structured Clinical Interview for DSM-IV (SCID) (First et al 1995), and excluded for any significant history of Axis I psychiatric illness.
Task
Subjects viewed an irregular sequence of K (12%) and X (88%) stimuli, presented for 100 ms each. The stimulus onset asynchrony (SOA) was 1, 2 or 3 sec, with each SOA occurring with equal probability. The interval between two K stimuli (the event of interest) varied between 7 and 24 sec (Kiehl et al 2000). Participants lifted a lever attached to the index finger of their response hand each time an X was presented, and withheld responding to K. There were 42 K stimuli and 288 X stimuli. To increase the pre-potent tendency to respond to K, we pre-trained subjects to respond to K and not X in an “oddball” target detection task. Subjects were told to go as fast as possible and if they made errors, to keep going and not slow down.
Behavioral Data Acquisition, Processing, and Analysis
A pressure-sensitive piezo-electric transducer that produced a continuous measure of response activity, sensitive to vigor or acceleration of the response, was used to record motor responses. Thus, a slow and weak, but erroneous response to a K might register as a response. Similarly, a very brisk, but partial response might also register as a response, as could small finger twitches and any pressure changes against the device. Inclusion of these “partial” responses may contribute to the rather high false alarm rate we observed in this study.
ERP Data Acquisition, Processing and Analysis
ERP Acquisition
Participants were seated in a sound attenuating, electrically shielded booth. Electroencephalogram (EEG) data recorded from Fz and Cz are reported here. Vertical electro-oculogram (EOG) was recorded from electrodes placed above and below the right eye, and horizontal EOG was recorded from electrodes placed at the outer canthus of each eye. Data were sampled at 500 Hz and band pass filtered at .05–30 Hz.
ERP Processing and Analysis
Single-trials were corrected for the effects of eye blinks and eye movements based on correlations of the VEOG and HEOG with the EEG recorded at each electrode site (Gratton et al 1983) before baseline correction. Trials exceeding ±100 μV were then rejected. Data were low pass filtered at 12 Hz before peaks were identified and measured. ERN was measured as the maximum negativity between 24 and 200 ms post-response in the response-synchronized ERPs to unsuccessful response inhibitions (i.e., “false alarms”) following NoGo stimuli. CRN was similarly measured in response-locked ERPs from correct Go trials (i.e., “hits”). Both were measured relative to a −100 to 0ms baseline. Univariate repeated measures analyses of variance (ANOVA) of peak ERN/CRN amplitudes were performed with Group (Healthy controls, Schizophrenia patients) as the between-subjects factor and within-subjects factors of Accuracy (Errors, Correct responses) and Site (Fz, Cz).
FMRI Data Acquisition, Processing and Analysis
fMRI Acquisition
Images were acquired on a General Electric 3 Tesla MRI scanner using a custom-made head coil. Subjects’ heads were stabilized with a bite bar made from their dental impression. After shimming (Kim et al 2000), functional images were acquired with a spiral gradient-echo sequence (Glover and Lai 1998) in the axial plane oriented parallel to the anterior commissure - posterior commissure (AC-PC) line prescribed from the midsagittal slice of a previously acquired SPGR anatomic sequence. Twenty-four axial slices (6 mm thick, 0 mm gap) were acquired with each 1.5 s TR (TE = 30 ms, NSA=1, FOV=24 cm; Flip angle = 70°, bandwidth=100 kHz; matrix =64 x 64). Voxel dimensions were 3.75 x 3.75 x 6mm. Images corresponding to the first 4 TRs were discarded from further analysis to eliminate non-equilibrium effects.
fMRI Processing
Image processing was performed with Statistical Parametric Mapping (SPM5) (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). Raw data were reconstructed and de-spiked using AFNI’s 3dDespike (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dDespike.html) to remove spikes in the raw fMRI time series signal. Functional images were motion corrected by realigning all scans to the first scan. Then the functional images were slice time corrected. The mean functional image was normalized to the MNI echo-planar image (EPI) template using a 12-parameter affine transformation, and the resulting parameters were used to anatomically normalize all individual functional images in the time series. The images were subsequently resliced to 2x2x2 mm3 using tri-linear interpolation and then spatially smoothed with a Gaussian filter, 8mm FWHM. Smoothing facilitated inter-subject averaging by minimizing differences in functional and gyral anatomy, enhanced the signal-to-noise ratio, and satisfied the assumptions of random Gaussian field theory implemented in SPM5. During model estimation, low frequency noise was removed with a temporal high pass filter (cut-off 128 seconds based on SPM defaults), and grand mean scaling was implemented to adjust images for global differences in image intensity across subjects.
fMRI Analysis
For individual subject (first-level) analyses, a fixed effects event-related design was implemented using multiple linear regression time series analyses (Holmes et al 1996) to determine the location and extent of brain activations. The hemodynamic responses were modeled using the SPM canonical hemodynamic response function (two gamma functions) with a temporal derivative term. For most subjects, 4 event types were modeled: hits and errors of omission (to X stimuli), and correct rejections and false alarms (to K stimuli). However, some subjects had no errors of omission, so these events could not be modeled for those subjects. Scans collected during the Rest period before the task were part of the implicit baseline but were not modeled in this analysis. Also, trials associated with a Psyscope buffer error were excluded3, as were the subsequent scans from the Rest period at the end of the session.
For group (second-level) analyses, a random-effects model was applied to individual subject contrast images derived during first-level analyses separately for healthy subjects and patients. False Alarm responses were contrasted against Hits. Before forming these contrast images, beta images for each task condition were adjusted by incorporating their associated temporal derivative beta images(Calhoun et al 2004).
In addition to the whole brain voxel-wise analysis implemented in SPM5, we adopted a region of interest (ROI) analysis to capitalize on the possibility that errors might activate different voxels within an ROI in different subjects. The voxel-wise approach will not capture consistent activation within a region if the exact voxels activated are not consistent across subjects. The ROI approach focused on two anatomical regions believed to be involved in error monitoring, DLPFC (Brodmann’s Areas 9 and 46) and ACC (BA 24 and 32). The union of the thresholded activations (p<.05, uncorrected) from patients and controls was intersected with anatomical ROI masks, generated using the WFUPickatlas (http://fmri.wfubmc.edu/cms/software) and based on the Talairach Daemon Database (Lancaster et al 2000). Mean contrast values within each ROI were extracted for each subject.
Because of our a priori focus on ACC activation on error trials, we also specifically interrogated ACC centroids published by others using a Go-NoGo task (Kiehl et al 2000; Laurens et al 2003; Menon et al 2001), by Carter et al. (Carter et al 1998) using a degraded AX-CPT paradigm where errors were less immediately obvious to the subjects, and by Ullsperger and vonCramon (Ullsperger and von Cramon 2001; Ullsperger and von Cramon 2003; Ullsperger and von Cramon 2004) using a flankers task. Table 2 lists the centroids that we interrogated.
Table 2.
Anterior Cingulate Cortex Coordinates for Centroids of Interest
| Authors | Talairach Coordinates (x,y,z) | ACC Region |
|---|---|---|
| Ullsperger & vonCramon (2001) | 7, 19, 30 | Cingulate Motor Area |
| Ullsperger and von Cramon 2003 | 4, 8, 35 | Rostral Cingulate Zone |
| Ullsperger and von Cramon 2004 | 4,14,50 | Posterior Medial frontal Cortex |
| Kiehl et al. (2000) | 4, 22, 40 | Caudal |
| Menon et al (2001) | 10,34,22 | Medial PreFrontal Cortex |
| Carter et al (1998) | 4, 25, 43 | Caudal |
| Laurens et al (2005) | −8, 52, 16 | Rostral |
RESULTS
Behavioral Data
In the ERP environment, controls made more errors (39%) than the patients (17%), F(1,19)=5.75, p=.027). The same pattern was observed in the MR environment, with controls making significantly more errors (53.5%) than patients (51.8%), (F(1,19)=6.68, p=.018). Performance in the MR environment was poorer than in the ERP environment, perhaps due to the fact that, for most subjects, MR scanning preceded ERP testing, and due to the awkward posture, restriction of movement, ambient noise, and anxiety associated with MR scanning.
ERPs
As can be seen in Figure 1, where response-synchronized ERPs to hits (correct responses to “X” stimuli) and false alarms (failure to inhibit responses to “K” stimuli) are overlaid. False alarms elicited an ERN that peaked about 125 ms post-response that was largest at Cz. The ANOVA revealed a significant effect of Group (F(1,19)=6.20, p=.022), Accuracy (F(1,19)=43.66, p<.0001), an interaction of Accuracy x Site (F(2,38)=23.43, p<.0001), and an interaction of Group x Accuracy x Site (F(1,19)=6.31, p=.021). The effects were inspected separately for errors and correct responses. For errors, there was a main effect of Group (F(1,19)=4.93, p=.039) with controls having larger ERNs than patients, a main effect of Site (F(1,19)=8.84, p=.009) with ERNs being larger at Cz than Fz, and a trend for an Group x Site interaction (F(1,19)=3.00, p=.10) with controls having a larger Site effect than patients. For correct responses, neither the Group effect (p=.21), nor the Group x Site interaction (p=.50) were significant, but the effect of Site was significant (F(1,19)=4.43, p=.049), with CRNs being larger at Fz than Cz..
Figure 1.
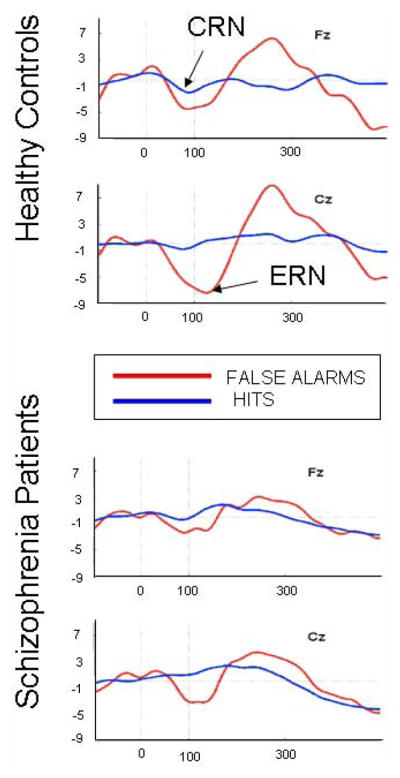
Response-synchronized ERPs overlaid to False Alarms and Hits. Response occurred at 0 ms. Negative voltage is plotted down.
fMRI
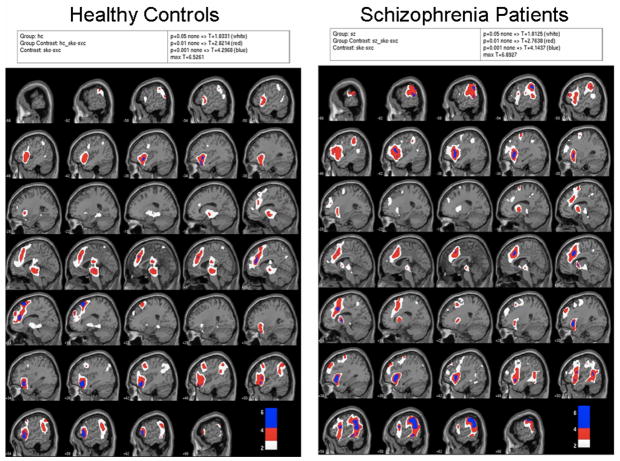
In Figure 2 the statistical probability maps (SPMs) are displayed for controls and patients separately for the contrast False Alarms – Hits. The color scheme reveals the significance levels of the activations; white indicates p=.05, red indicates p=.01, and blue indicates p=.001, all uncorrected for multiple comparisons. There was modest activation in both groups in expected regions, including the medial frontal cortex (Ridderinkhof et al 2004) that subsumes the ACC and premotor-supplementary motor areas. Also apparent is activation in the midbrain area, which is especially apparent in the images for the controls (see arrow) in Figure 2. Although we did not have the adequate spatial precision to identify the ventral tegmentum, this midbrain structure is known to be involved in reward prediction errors as described by Holroyd and Coles (2002).
Figure 2.
SPMs for False Alarms-Hits for healthy controls (left) and patients (right). White indicates p=.05, red indicates p=.01, and blue indicates p=.001, all uncorrected for multiple comparisons.
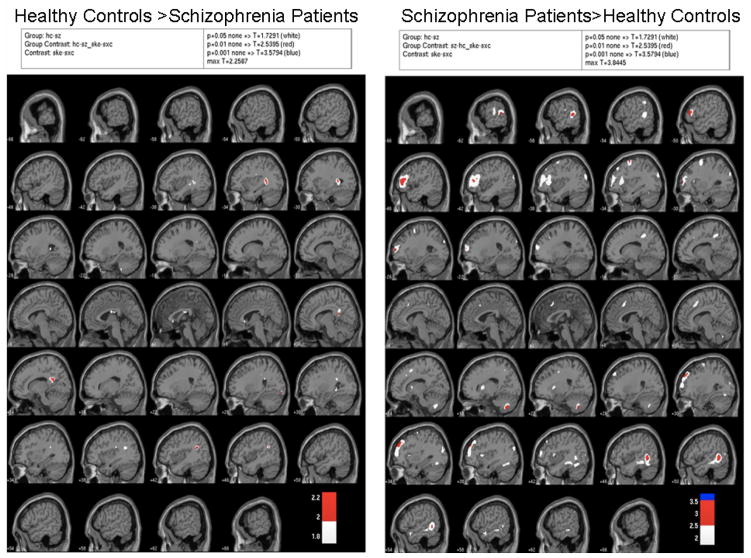
The group contrasts seen in Figure 3 reveal few differences between the groups, in spite of the very liberal thresholds used. The ROI analysis reflected the data shown in the SPMs; groups were not different for median activations extracted from BA24 (left: p=.67; right: p=.47), BA32 (left: p=.47; right: p=.29), or BA9 (left: p=.71 right: p=.37). However, patients showed greater activation in BA46 on the left than did controls (F(1,19)=7.41, p=.014), but not the right (p=.55). None of the group comparisons for the prior literature-derived centroids approached significance.
Figure 3.
SPM group contrasts showing areas more active in healthy controls than patients for False Alarms-Hits on the left and areas more active in patients than healthy controls for False Alarms-Hits on the right. White indicates p=.05, red indicates p=.01, and blue indicates p=.001, all uncorrected for multiple comparisons.
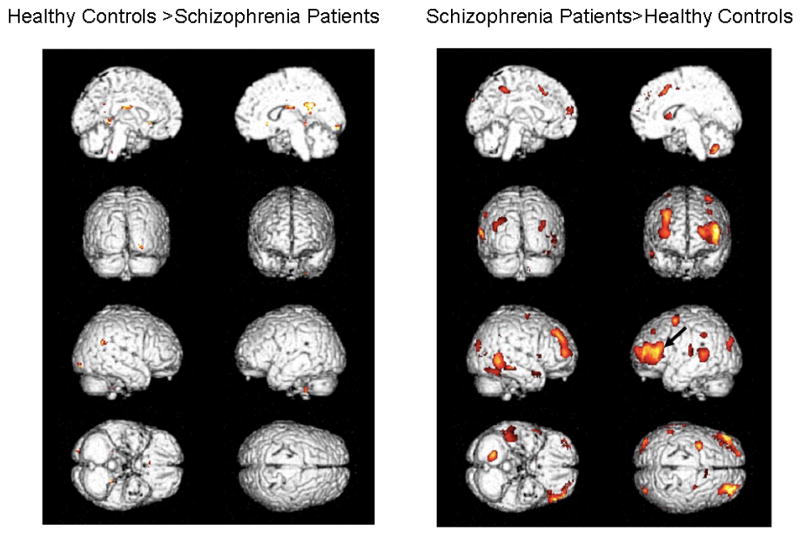
In Figures 4 and 5, we show the same activations seen in Figures 2 and 3, but only for p<.05 (uncorrected) and with activations projected on the rendered brains to enable better visualization of the lateral surfaces.
Figure 4.
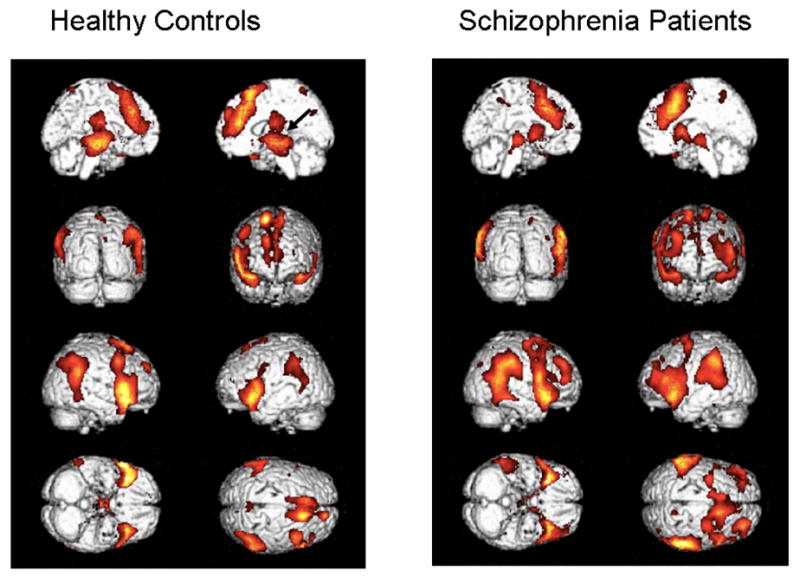
Contrasts (p<.05, uncorrected) projected onto rendered brains to enable better identification of lateral activity. Healthy controls appear on the left and patients on the right. The left and right mid-sagittal views are seen in the 1st row, backward and forward facing views are seen in the 2nd row, right and left sides are seen in the 3rd row, and bottom and top views are seen in the 4th row.
Figure 5.
Same as Figure 4, but images show the contrasts between patients and controls.
DISCUSSION
Behavioral data
Behaviorally, patients performed better than controls, making fewer false alarm errors in both the ERP and MR environments. Previously, this type of pattern suggested to us that patients did not establish as strong a prepotent tendency to respond to the Go stimulus as healthy subjects(Ford et al 2004a), and although we were not able to record reaction times in this experiment with good precision, it is likely that patients responded slowly to avoid errors. Bates et al (Bates et al 2002; Bates et al 2004) and Laurens et al (Laurens et al 2003), who used a similar paradigm, also found fewer false alarms in patients, although false alarm rates were not significantly different across groups. Our finding of significantly more false alarms in the controls may be due to the lengths we went to establish a strong prepotent response: We preceded the NoGo run with a Go run, and our NoGo stimulus probability (.12) was lower than that used by either Bates et al (.20) or Laurens et al(Laurens et al 2003) (.16).
ERP data
In spite of the small numbers of subjects in each of the groups, we were able to demonstrate the expected ERN reduction in amplitude in the patients compared to the controls (Alain et al 2002; Bates et al 2002; Bates et al 2004; Laurens et al 2003; Mathalon et al 2002; Morris et al 2008; Morris et al 2006). ERN reductions in schizophrenia have been associated with failures to inhibit pre-potent, over-learned stimulus-response pairings in Stroop tasks (Alain et al 2002), flanker tasks (Kopp and Rist 1999), and in Go-NoGo tasks like ours (Bates et al 2002)(Bates et al, 2004). However, even when a stimulus-response pairing is not over-learned but acquired during the course of the experiment, ERNs are reduced in patients(Morris et al 2008). Moreover, the feedback negativity is reduced in schizophrenia patients (Morris et al 2008). Thus, the ERN reduction in patients with schizophrenia is robust to the specific parameters of stimuli and tasks.
While ERN effects are robust to parametric variations, CRN effects are not. Both our study and the studies of Bates et al (Bates et al 2002; Bates et al 2004) using similar Go-NoGo tasks failed to find the CRN increases in schizophrenia that we and others have reported in more cognitively complex tasks involving picture naming(Mathalon et al 2002), color naming (Alain et al 2002) and response selection(Kopp and Rist 1999; Morris et al 2006). Bates et al (Bates et al 2002) suggested that CRN increases in schizophrenia in these tasks may be related to the relative uncertainty patients experience associated with their correct responses in the more complex tasks. Indeed, Coles et al (Coles et al 2000) suggested CRNs were associated with partial errors.
FMRI data
Although not significant when corrected for multiple comparisons, our data confirmed the involvement of the ACC in error detection at the p<.001, uncorrected, significance threshold. In both groups, ACC activation was associated with error responses. This confirms our earlier analyses of these data from healthy controls in which we found that dorsal and rostral ACC activity was directly related to ERN amplitude, after controlling for conflict associated with inhibiting a prepotent response(Ford et al 2004b; Mathalon et al 2004). Similarly, Ullsperger and von Cramon (Ullsperger and von Cramon 2001) also recorded ERP and fMRI data in separate sessions from the same subjects, and they found that the ACC activation matched the dipole source of the ERN. More recently, Ullsperger and colleagues (Debener et al 2005) recorded ERP s in the MR scanner and reported that the ERN amplitude correlated with fMRI activity in the dorsal ACC.
Unexpected was our failure to find group differences in our ROI-defined ACC activation to errors, in contrast to findings reported by others(Carter et al 2001; Kerns et al 2005; Laurens et al 2003). This may partially be due to the Go-NoGo paradigm: Laurens et al (Laurens et al 2003) using a similar Go-NoGo paradigm also failed to find ACC activation differences between patients and controls in the voxel-wise analysis, after controlling for multiple comparisons. They did however find that patients had less activation using a small volume correction for an ROI centered in the rostral ACC (x, y, z = −8, 52, 16). The same was not found for a more caudal centroid identified by Kiehl et al. (Kiehl et al 2000) as being preferentially active during errors of commission compared with correct rejections in healthy participants. While subdivision of the ACC into rostral and dorsal areas has illuminated different aspects of error processing deficits in patients with schizophrenia (e.g.,(Polli et al 2008), our voxel-wise activation maps suggest that, at a p<.05 uncorrected threshold, a small particle in the rostral sub-genual ACC is more active in controls than patients, while a small area of the caudal ACC is more active in patients than controls. In addition, like Laurens et al (2003), we found patients to have significantly greater activation than the controls in other brain regions. While Laurens et al (Laurens et al 2003)reported these effects in non-frontal lobe regions, we found patients, relative to controls, to have greater activation to errors in left BA46. Small sample sizes for the Laurens et al study (10 patients with schizophrenia) and ours (11 patients with schizophrenia) may have worked against both of us in finding significant reductions in ACC activation in the patients. Both Kerns et al (2005) and Carter et al (2001) studied 17 patients, and both were able to find reductions in activation in patients in the ACC (Kerns et al 2005) with the Stroop task, or in nearby medial frontal cortex regions (Carter et al, 2001) with the AX-CPT.
Comparing ERP and fMRI imaging modalities
Why are hemodynamic and neurophysiologic responses so different in their sensitivity to errors? This differential sensitivity largely results from the fact that the ERN can be precisely measured in subject ERP plots and subjected to group analyses without the need for corrections for multiple comparisons, whereas voxel-wise fMRI analyses require such corrections. Even the ROI approach in fMRI has pitfalls, since anatomically defined ROIs seldom capture the full extent of major activation clusters of avoid inclusion of unactivated voxels. Moreover, variability in the specific region of the ACC activated by errors (Ridderinkhof et al 2004) precludes a precise a priori interrogation of a single well defined region or voxel that the field agrees is the site of error processing. Thus, there is more imprecision regarding which brain region to interrogate in fMRI data than there is about which peak to identify or which electrodes to examine in ERP data.
Beyond the statistical considerations related to the multiple comparisons problem in fMRI, hemodynamic responses are slow, and signals associated with errors will necessarily include activity associated many other processes, including sensation, perception, response selection, response monitoring, self-evaluation, planning for the next trial, and any number of other processes happening in the 6 seconds it takes for the hemodynamic response to peak and the subsequent 6–12 seconds it takes to return to its baseline state. As such, the fMRI signal reflects activity averaged over time and may include processes that are not necessarily affected by schizophrenia (such as the postivity following errors or Pe), thereby diluting the effect of interest. But, if neuroanatomical precision is important, then fMRI is the method of choice. On the other hand, neurophysiologic (ERP) responses are as fast as the neural activity generating them and offer a more temporally precise estimate of activity associated with error responses themselves, but are poor at spatial localization. As such, the ERP signal is essentially averaged over space. If error monitoring is the only process one wants to assess, the neural activity can be precisely synchronized to the motor response, allowing inspection of millisecond-by-millisecond neural activity associated with initiating, making and adjusting the response, “on the fly” (Coles 1989).
As we navigate through our environment, whether consciously or not, we are constantly monitoring our actions and making both gross and minute adjustments, as needed. To hit a golf ball or simply walk across the street, our self-monitoring system needs to have millisecond resolution to make on-going, self-titrating, tiny adjustments. When the outcome of an action was not what was intended, an error is detected and immediate adjustments must be made. While fMRI data have been essential in confirming the involvement of the DLPFC and ACC in this self-monitoring system, ERP data permit an evaluation of error-related activity with precise temporal resolution, capturing the well-characterized ERN component of the ERP, without the loss of power associated with correction for multiple comparisons in voxel-wise analyses of fMRI data or the spatial imprecision associated with the application of a priori, anatomically defined, regions of interest, that may poorly represent the actual brain regions activated by errors.
Acknowledgments
This work was supported by grants from National Institute of Mental Health (MH40052, MH 58262) the Department of Veterans Affairs and the National Alliance for Research on Affective Disorders and Schizophrenia (NARSAD).
We would like to thank Max Gray for assistance in data processing and Gary Glover for imaging expertise.
Footnotes
There is also a postivity following errors, the Pe. Because of its insensitivity to schizophrenia (Mathalon et al, 2002; Bates et al, 2002; Alain et al, 2002), the Pe data were not analyzed for this report.
Data from these 10 healthy controls were part of a data set published previously (Ford et al, 2004a; Mathalon et al, 2004).
Because of a Psyscope buffer error, the last third of the trials in the fMRI environment were presented at a constant 2 sec ISI. These trials were omitted from the analysis of the fMRI data. The same error was not present in the ERP environment where the stimulus presentation was controlled by STIM software in Neuroscan.
This paper was presented, in part, at the 14th World Congress of Psychophysiology - the Olympics of the Brain - IOP2008, September 8 – 13, 2008, in St. Petersburg, Russia.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alain C, McNeely HE, He Y, Christensen BK, West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cereb Cortex. 2002;12:840–6. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder (DSM-IV) Washington: American Psychiatric Association; 1994. [Google Scholar]
- Badgaiyan R, Posner M. Mapping the cingulate cortex in response selection and monitoring. Neuroimage. 1998;7:255–260. doi: 10.1006/nimg.1998.0326. [DOI] [PubMed] [Google Scholar]
- Bates AT, Kiehl KA, Laurens KR, Liddle PF. Error-related negativity and correct response negativity in schizophrenia. Clin Neurophysiol. 2002;113:1454–63. doi: 10.1016/s1388-2457(02)00154-2. [DOI] [PubMed] [Google Scholar]
- Bates AT, Liddle PF, Kiehl KA, Ngan ET. State dependent changes in error monitoring in schizophrenia. J Psychiatr Res. 2004;38:347–56. doi: 10.1016/j.jpsychires.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–69. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Event-related fMRI and the hemodynamic response. Hum Brain Mapp. 1998;6:373–377. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<373::AID-HBM8>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Stevens MC, Pearlson GD, Kiehl KA. fMRI analysis with the general linear model: removal of latency-induced amplitude bias by incorporation of hemodynamic derivative terms. Neuroimage. 2004;22:252–7. doi: 10.1016/j.neuroimage.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Carter C, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter C, MacDonald A, Ross L, Stenger V. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: An event-related fMRI study. Am J Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintum M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: 15-O H20 PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154:1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- Coles M, Scheffers MK, Holroyd C. Why is there an ERN or NE on correct trials? (abs) Psychophysiology. 2000;37:S9. [Google Scholar]
- Coles MGH. Modern mind-brain reading: Psychophysiology, physiology, and cognition. Psychophysiology. 1989;26:251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Bernstein PS, Fournier L. Where did you go wrong? Errors, partial errors, and the nature of human information processing. Acta Psychol (Amst) 1995;90:129–144. doi: 10.1016/0001-6918(95)00020-u. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia - A review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–7. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Event-related potential correlates of errors in reaction tasks. In: Karmos G, Molmar M, Csepe V, Czigler I, Desmedt JE, editors. Perspectives of Event-Related Potential Research (EEG Suppl 44) Amsterdam: Elsevier Science BV; 1995. [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components .2. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Faustman WO, Overall JE. The Brief Psychiatric Rating Scale. In: Maruish M, editor. The Use of Psychological Testing For Treatment, Planning and Outcome Assessment, Second Edition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1999. pp. 791–830. [Google Scholar]
- Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Ford JM, Gray EM, Whitfield SL, Turken AU, Glover G, Faustman WO, Mathalon DH. Acquiring and inhibiting pre-potent responses in schizophrenia: event related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2004a;61:119–129. doi: 10.1001/archpsyc.61.2.119. [DOI] [PubMed] [Google Scholar]
- Ford JM, Whitfield SL, Mathalon DH. The neuroanatomy of conflict and error: ERP and fMRI. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts and the Brain Current Opinions on Performance Monitoring. Leipzig: MPI of Cognitive Neuroscience; 2004b. pp. 42–48. [Google Scholar]
- Frith CD, Done DJ. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol Med. 1989;19:359–363. doi: 10.1017/s003329170001240x. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Coles MGH, Meyer DE, Donchin E. A brain potential manifestation of error-related processing. In: Karnos G, Molmar M, Csepe V, Czigler I, Desmedt JE, editors. Perspectives of Event-Related Potential Research (EEG Suppl 44) Amsterdam: Elsevier Science BV; 1995. pp. 261–272. [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Knight RT. Pre-frontal cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: Interleaved versus single-shot. Magn Reson Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA. Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry. 1996;153:41–9. doi: 10.1176/ajp.153.1.41. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Weiler M, Dannals RF, Tamminga CA. Brain activation patterns in schizophrenic and comparison volunteers during a matched-performance auditory recognition task. Am J Psychiatry. 2000;157:1634–45. doi: 10.1176/appi.ajp.157.10.1634. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JDG, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. Journal of Cerebral Blood Flow and Metabolism. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MG. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neurosci Lett. 1998;242:65–68. doi: 10.1016/s0304-3940(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Honer WG, Falkai P, Young C, Wang T, Xie J, Bonner J, Hu L, Boulianne GL, Luo Z, Trimble WS. Cingulate cortex synaptic terminal proteins and neural cell adhesion molecule in schizophrenia. Neuroscience. 1997;78:99–110. doi: 10.1016/s0306-4522(96)00489-7. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162:1833–9. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–23. [PubMed] [Google Scholar]
- Kim DH, Adalsteinsson E, Glover GE, Spielman DM. SVD regularization algorithm for improved high-order shimming (abs) International Society for Magnetic Resonance in Medicine. 2000;3:1685. [Google Scholar]
- Kopp B, Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J Abnorm Psychol. 1999;108:337–346. doi: 10.1037//0021-843x.108.2.337. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–71. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Frankle WG, Narendran R, Kegeles LS, Abi-Dargham A. Mechanism of action of antipsychotic drugs: from dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin Ther. 2005;27(Suppl A):S16–24. doi: 10.1016/j.clinthera.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Sommer W. ERP correlates of error processing in spatial S-R compatibility tasks. Clin Neurophysiol. 1999;110:342–357. doi: 10.1016/s1388-2457(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. J Neurosci. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Angel RW, Hampton B, Berger P. Impaired central error correcting behavior in schizophrenia. Arch Gen Psychiatry. 1982;39:101–107. doi: 10.1001/archpsyc.1982.04290010073013. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Angel RW, Thiemann S, Weitz C, Berger PA. Central error-correcting behavior in schizophrenia. Biol Psychiatry. 1986;21:263–273. doi: 10.1016/0006-3223(86)90047-8. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Colrain I, Gray EM, Ford JM. It’s not my fault: ERPs to induced errors. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts and the Brain Current Opinions on Performance Monitoring. Leipzig: MPI of Cognitive Neuroscience; 2004. pp. 27–35. [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 2002;111:22–41. [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biol Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–43. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-production task: Evidence for a “generic” neural system for error detection. J Cogn Neurosci. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Mlakar J, Jensterle J, Frith CD. Central monitoring deficiency and schizophrenic symptoms. Psychol Med. 1994;24:557–564. doi: 10.1017/s0033291700027719. [DOI] [PubMed] [Google Scholar]
- Moore H, West AR, Grace AA. The regulation of forebrain dopamine transmission: relevance to the pathophysiology and psychopathology of schizophrenia. Biol Psychiatry. 1999;46:40–55. doi: 10.1016/s0006-3223(99)00078-5. [DOI] [PubMed] [Google Scholar]
- Morris SE, Heerey EA, Gold JM, Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr Res. 2008;99:274–85. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Yee CM, Nuechterlein KH. Electrophysiological analysis of error monitoring in schizophrenia. J Abnorm Psychol. 2006;115:239–50. doi: 10.1037/0021-843X.115.2.239. [DOI] [PubMed] [Google Scholar]
- Mulert C, Gallinat J, Pascual-Marqui R, Dorn H, Frick K, Schlattmann P, Mientus S, Herrmann WM, Winterer G. Reduced event-related current density in the anterior cingulate cortex in schizophrenia. Neuroimage. 2001;13:589–600. doi: 10.1006/nimg.2000.0727. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Overall JE, Hollister L, Pichot R. Major psychiatric disorders. A four-dimensional model. Arch Gen Psychiatry. 1967;16:146–151. doi: 10.1001/archpsyc.1967.01730200014003. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–86. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Error-correction time without external signals. Nature. 1966;212:438. doi: 10.1038/212438a0. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MGH. Performance monitoring in a confusing world: Error-related brain activity, judgements of response accuracy, and types of error. J Exp Psychol. 2000;26:141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–10. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Stemmers B, Segalowitz SJ, Witzke W, Lacher S, Schönle PW. Do patients with damage to the anterior cingulate and adjacent regions produce an Error-Related Negativity(ERN)? (abs) Psychophysiology. 2000;37:S95. [Google Scholar]
- Suhara T, Okubo Y, Yasuno F, Sudo Y, Inoue M, Ichimiya T, Nakashima Y, Nakayama K, Tanada S, Suzuki K, Halldin C, Farde L. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch Gen Psychiatry. 2002;59:25–30. doi: 10.1001/archpsyc.59.1.25. [DOI] [PubMed] [Google Scholar]
- Turken AU, Swick D. Response selection in the human anterior cingulate cortex. Nat Neurosci. 1999;2:920–924. doi: 10.1038/13224. [DOI] [PubMed] [Google Scholar]
- Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM. Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? Am J Psychiatry. 2003;160:1881–3. doi: 10.1176/appi.ajp.160.10.1881. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23:4308–14. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Neuroimaging of performance monitoring: error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences. 1996;351:1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Golaszewski S, Mottaghy FM, Hofer A, Hausmann A, Kemmler G, Kremser C, Brinkhoff C, Felber SR, Fleischhacker WW. Brain activation patterns during a selective attention test-a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res. 2003;123:1–15. doi: 10.1016/s0925-4927(03)00019-2. [DOI] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. J Cogn Neurosci. 2004;16:1098–112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]