Abstract
Background
β-blockers are the mainstay therapy in patients with the congenital long-QT syndrome (LQTS) types 1 and 2. However, limited data exist regarding the efficacy and limitations of this form of medical management within high-risk subsets of these populations.
Methods and Results
Multivariate analysis was carried out to identify age-related gender- and genotype- specific risk factors for cardiac events (comprising syncope, aborted cardiac arrest [ACA] or sudden cardiac death [SCD]) from birth through age 40 years among 971 LQT1 (n=549) and LQT2 (n=422) patients from the International LQTS Registry. Risk factors for cardiac events included the LQT1 genotype (HR=1.49, p=0.003) and male gender (HR=1.31, p=0.04) in the 0-14 years age-group; and the LQT2 genotype (HR=1.67, p<0.001) and female gender (HR=2.58, p<0.001) in the 15-40 years age-group. Gender-genotype subset analysis showed enhanced risk among LQT1 males (HR=1.93, p<0.001) and LQT2 females (HR=3.28, p<0.001) in the 2 respective age-groups. β-blocker therapy was associated with a significant risk-reduction in high-risk patients, including a 67% reduction (p=0.02) in LQT1 males and a 71% reduction (p<0.001) in LQT2 females. Life-threatening events (ACA/SCD) rarely occurred as a presenting symptom among β-blocker-treated patients. However, high-risk patients who experienced syncope during β-blocker therapy had a relatively high rate of subsequent ACA/SCD (>1 event per 100 patient-years).
Conclusions
The present findings suggest that β-blocker therapy should be routinely administered to all high-risk LQT1 and LQT2 patients without contraindications as a first line measure, whereas primary defibrillator therapy should be recommended for those who experience syncope during medical therapy.
Keywords: long QT syndrome, β-blockers, cardiac events, sudden cardiac death
Introduction
The congenital long QT syndrome (LQTS) is a genetic channelopathy with variable penetrance, that is associated with increased propensity for polymorphic ventricular tachyarrhythmias and sudden cardiac death (SCD) in young individuals with normal cardiac morphology.1 To date, more than 500 mutations have been identified in 12 LQTS genotypes, with the LQTS type 1 (LQT1) and LQTS type 2 (LQT2) genotypes accounting for nearly 90% of identified cases.1
LQT1 patients, who harbor mutations impairing IKs, have been shown to experience the majority of their events during exercise.2 This may be due to the fact that the repolarizing current IKs activates during increased heart rate, and is essential for QT interval adaptation during tachycardia.3 Similarly, most cardiac events in LQT2 patients, harboring mutations impairing IKr,4 are triggered by increased sympathetic activity associated with arousal episodes.2 Accordingly, β-blockers are the mainstay medical therapy in these LQTS populations. By contrast, the efficacy of β-blocker therapy appears to be more limited among LQTS type 3 patients who exhibit excessive further prolongation of the QT interval at slow heart rates.5 Despite the sympathetic mechanisms associated with the 2 main LQTS genotypes, previous studies that assessed efficacy of β-blocker therapy in LQT1 and LQT2 patients have suggested important limitations of this mode of medical therapy among treated patients.6.7 These studies, however, were limited by a relatively small sample size of genotyped patients. Furthermore, there are no data regarding the efficacy and limitations of medical therapy in age-gender risk-subsets of genotyped patients, despite the fact that the clinical course of male and female LQT1 and LQT2 patients exhibits significant age-related differences.8.9 This study was undertaken to: 1) identify high-risk subsets of LQT1 and LQT2 patients, categorized by gender and genotype; 2) evaluate the efficacy of β-blocker therapy within the identified risk-subsets; 3) assess the residual event rate during β-blocker therapy among high-risk LQTS patients; and 4) propose a possible strategy for the management of LQT1 and LQT2 patients based on our findings.
Methods
Study Population
The study comprised patients enrolled in the International Long-QT Syndrome Registry. Patients were included if they had a genetically confirmed LQT1 or LQT2 mutation. Patients with multiple mutations (identified in the LQT1-8 genes) and those with congenital deafness were excluded from the study. A total of 971 genotyped LQT1 (n=549) and LQT2 (n=422) patients were included in the study.
The LQTS genotype was determined using standard mutational analytic techniques involving 5 established genetic laboratories associated with the International LQTS Registry. Informed consent for genetic and clinical data was obtained from all patients.
Data Collection and Management
The latest update to the registry was carried out on March 2009. Upon enrollment in the registry, complete past history was obtained from birth to the patient's enrolled age, and ongoing clinical information was obtained at yearly intervals thereafter. In the present study we assessed the clinical course of study patients from birth through age 40 years (follow-up was censored after age 40 years to minimize the confounding effect of acquired cardiovascular disease on the occurrence of LQTS-related cardiac events). Thus, follow-up time for each study patient comprised clinical and medical information from birth to enrollment in the registry and prospective follow-up information from enrollment through age 40 years. For each patient, data on personal and family history, cardiac events, and therapy were systematically recorded at enrollment and at each visit or medical contact. Clinical data were recorded on prospectively designed forms and included patient and family history and demographic, ECG, therapeutic, and cardiac event information.
Follow-up data regarding β-blocker therapy included the starting date, type of β-blocker, and discontinuation date in case it occurred. Among subjects who died, the usage of a β-blocker before death was determined retrospectively.
End points
The primary end point of the study was the occurrence of a first cardiac event, comprising syncope (defined as transient loss of consciousness that was abrupt in onset and offset), an appropriate implantable carioverter defibrillator (ICD) shock, aborted cardiac arrest ([ACA] requiring external defibrillation as part of the resuscitation), or LQTS-related SCD (death abrupt in onset without evident cause, if witnessed, or death that was not explained by any other cause if it occurred in an non-witnessed setting such as sleep), whichever occurred first, from birth through age 40 years. In a secondary analysis we also assessed the rate of fatal or near fatal events (comprising ACA or SCD) by gender and genotype among patients who did- and did not- experience syncope during β-blocker therapy.
Statistical Analysis
The clinical characteristics of study patients were compared by genotype using the chi-square test for categorical variables, and the t-test for continuous variables. The Kaplan-Meier estimator was used to assess the time to a first event and the cumulative event rates by genotype and gender, and groups were compared using the log-rank test.
Multivariate Cox proportional hazards regression modeling was used to assess risk factors that independently contribute to the development of cardiac events. Data from the International LQTS Registry demonstrate that an age-interaction exists regarding the effect of gender and genotype on the occurrence of cardiac events, with a crossover effect for both genotype and gender after the onset of adolescence.8,9 Therefore, to avoid a violation of the proportional-hazards assumption, models were carried out separately within prespecified younger- (0 through 14 years) and older- (15 through 40 years) age-groups. Pre-specified covariates in the multivariate models included the LQTS genotype (LQT1 or LQT2), gender, the corrected baseline QT interval ([QTc] using Bazett's formula10) dichotomized at 500 milliseconds, and LQTS-related therapies (including β-blockers, pacemaker implantation, treatment with an ICD, and left cardiac sympathetic denervation) which were assessed as time-dependent covariates (i.e. by incorporating in the Cox model data for each patient that identify the effect of each follow-up time “on” and “off” therapy from birth through age 40 years). In a further analysis, risk-subsets were identified within each age-group by including 4 gender-genotype subcategories in the multivariate models (i.e. LQT1 males, LQT1 females, LQT2 males, and LQT2 females, with the lowest risk subset as the reference group in each model).
Interaction-term analysis was employed to assess the efficacy of time-dependent β-blocker therapy in reducing the risk of the end point by gender and by genotype in each age-group, and within the identified gender-genotype risk subsets. All models were stratified by the decade in which study patients were born to account for changes in the baseline hazard function for different calendar time-periods.
The statistical software used for the analyses is SAS version 9.20 (SAS Institute Inc, Cary, NC).
Results
The clinical characteristics of the study patients by genotype are shown in Table 1. There was no statistically significant difference between the two genotypes regarding gender and QTc duration. However, LQTS-related therapies, including β-blockers, pacemakers, and ICDs, were administered to higher proportion of LQT2 patients, whereas medical therapy with β-blockers was initiated earlier among LQT1 patients. The main β-blocker subtypes that were administered at anytime during follow-up to study patients included atenolol, nadolol, propranolol, and metoprolol (Table 1).
Table 1.
Clinical and electrocardiographic characteristics of study patients by genotype.
| LQT1 (N=549) |
LQT2 (N=422) |
P-VALUE | |
|---|---|---|---|
| Clinical and ECG characteristics | |||
| Follow-up time, yrs | 31 ± 12 | 31 ± 12 | 0.82 |
| Female, n (%) | 324 (59) | 246 (58) | 0.82 |
| QTc interval, msec | 484 ± 51 | 490 ± 57 | 0.17 |
| QTc interval ≥ 500 msec, n (%) | 181 (33) | 154 (36) | 0.25 |
| RR interval, msec | 844 ± 212 | 862 ± 239 | 0.16 |
| QRS duration, msec | 79 ± 16 | 81 ± 18 | 0.19 |
| PR interval msec. | 151 ± 29 | 149 ± 29 | 0.09 |
| β-blocker therapy | |||
| Treated at anytime (%) | 295 (54) | 261 (62) | 0.01 |
| Age β-blocker therapy initiated, yrs | 15 ± 12 | 17 ± 12 | 0.02 |
| Follow-up time on β-blockers, yrs | 5.0 ± 6.8 | 6.1 ± 7 | 0.006 |
| β-blocker subtype used anytime:* | |||
| Atenolol, n (% of β-blockers used) | 139 (47) | 104 (40) | 0.08 |
| Last recorded dose, mg (mean ± SD) | 49 ± 32 | 61 ± 43 | 0.04 |
| Nadolol, n (% of β-blockers used) | 121 (41) | 98 (38) | 0.40 |
| Last recorded, mg (mean ± SD) | 58 ± 45 | 61 ± 46 | 0.62 |
| Propranolol, n (% of β-blockers used) | 143 (48) | 134 (51) | 0.50 |
| Last recorded dose, mg (mean ± SD) | 96 ± 71 | 113 ± 96 | 0.22 |
| Metoprolol, n (% of β-blockers used) | 36 (12) | 53 (20) | 0.009 |
| Last recorded dose, mg (mean ± SD) | 67 ± 55 | 84 ± 49 | 0.03 |
| Other, n (% of β-blockers used) | 3 (1) | 0 (0) | 0.25 |
| Other LQTS-related therapies | |||
| LCSD, n (%) | 16 (3) | 15 (4) | 0.57 |
| Pacemaker, no (%) | 13 (2) | 45 (11) | <0.001 |
| ICD n (%) | 50 (9) | 79 (19) | <0.001 |
| Cardiac Events During Follow-Up | |||
| Appropriate ICD shocks | 9 (2) | 13 (3) | 0.14 |
| Syncope, n (%) | 207 (38) | 190 (45) | 0.02 |
| ACA, n (%) | 20 (4) | 25 (6) | 0.09 |
| SCD, n (%) | 8 (1) | 9 (2) | 0.43 |
| Any cardiac event (first shock, syncope, ACA, or SCD) | 213 (39) | 195 (46) | 0.02 |
Plus - minus values are means ± SD.
β-blocker subtypes were administered to each patient at any time during follow-up (i.e. some patients received different β-blockers at different time-points). Therefore, the sum of the subtype percentages per genotype may be >100%.
ACA = aborted cardiac arrest; ECG = electrocardiogram; ICD = implantable cardioverter defibrillator; LCSD = left cervical sympathetic denervation; SCD = sudden cardiac death.
Risk Factors for Cardiac Events
The primary end point of the study, comprising the first occurrence of syncope, ACA, appropriate ICD shock, or LQTS-related SCD, occurred in 213 LQT1 patients (39%) and 195 LQT2 patients (46%; Table 1). Syncope comprised the majority of first cardiac events among patients with both genotypes (89% and 86%, respectively); ACA or SCD occurred as a first presentation in 11% and 14% of LQT1 and LQT2 patients, respectively; whereas an appropriate ICD shock occurred as a first cardiac event only in 1 patient (due to the fact that most ICDs were implanted after the occurrence of a first syncope or ACA). Three patients reported missing the recommended dose of β-blockers at the day of the event (2 syncopal episodes and 1 appropriate ICD shock among 1 male and 2 females, at ages 6, 18, and 31 years, respectively) and none reported being treated with a QT prolonging drug at the time of the event.
Kaplan-Meier survival analysis (Fig. 1 and 2) showed that during childhood event rates were higher among LQT1 patients and males, whereas after the onset of adolescence the rate of both cardiac events and life-threatening events was higher among LQT2 patients and females. Consistently, multivariate analysis demonstrated that gender and genotype risk factors were different between the 2 age-groups (Table 2). In the 0 through 14-year age-group, patients with the LQT1 genotype had a significant 49% (p=0.003) increase in the risk of cardiac events as compared with patients with the LQT2 genotype, and males had a significant 31% (p=0.04) risk-increase as compared with females. In contrast, in the 15 through 40-year age-group, the risk of a first cardiac event was significantly higher among LQT2 patients (67% increase in the risk as compared with LQT1 patients; p<0.001) and among females (2.6-fold increase in the risk as compared with males; p<0.001).
Figure 1. Probability of cardiac events by genotype.
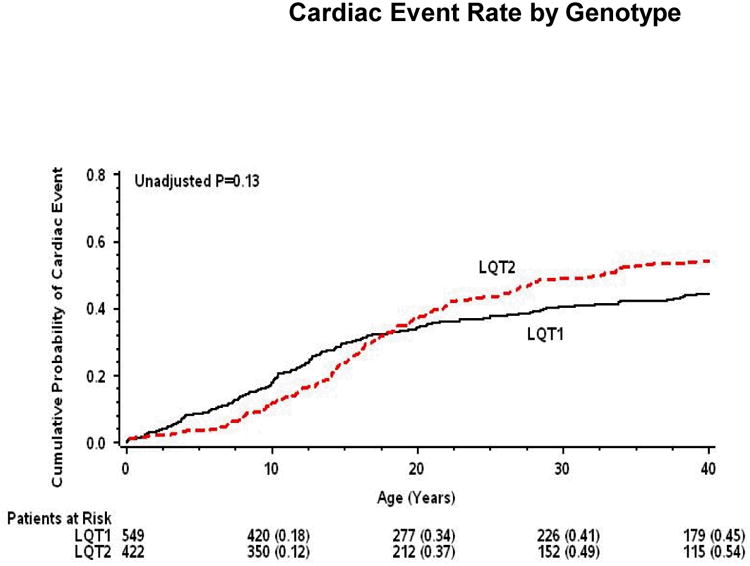
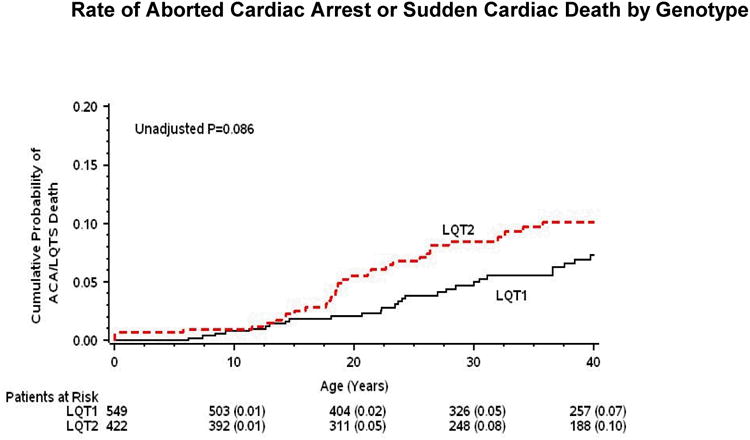
Kaplan-Meier estimates of the probability of (A) a first cardiac event of any-type; and (B) aborted cardiac arrest or sudden cardiac death, by genotype from birth through age 40 years.
Figure 2. Probability of cardiac events by gender.

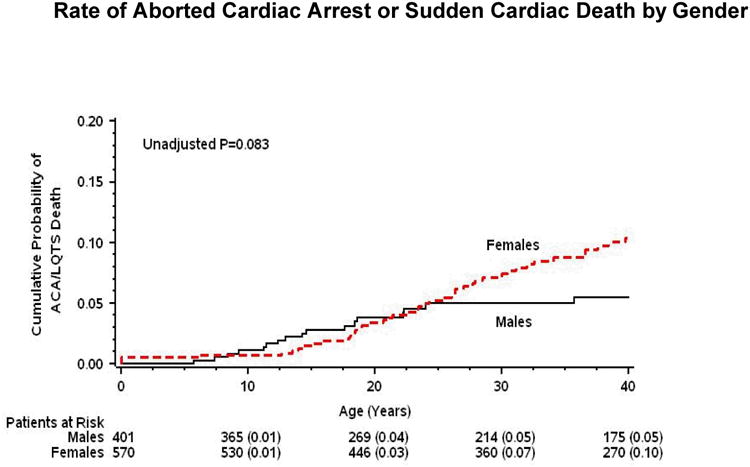
Kaplan-Meier estimates of the probability of (A) a first cardiac event of any-type; and (B) aborted cardiac arrest or sudden cardiac death, by gender from birth through age 40 years.
Table 2. High-risk genotype and gender groups and corresponding β-blocker effect*.
| HIGH-RISK GROUP | ADJUSTED RISK† | β-BLOCKER EFFECT‡ | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age-group: 0-14 yrs | ||||||
| LQT1 genotype | 1.49 | 1.14 – 1.93 | 0.003 | 0.46 | 0.23 – 0.89 | 0.02 |
| Male gender | 1.31 | 1.01 – 1.71 | 0.04 | 0.41 | 0.20 – 0.80 | 0.01 |
| Age-group: 15-40 yrs | ||||||
| LQT2 genotype | 1.67 | 1.31 – 2.13 | <0.001 | 0.36 | 0.22 – 0.62 | <0.001 |
| Female gender | 2.58 | 1.90 – 3.49 | <0.001 | 0.43 | 0.28 – 0.68 | <0.001 |
Models were carried out separately in the 0-14 years and 15-40 years age-groups; all models were adjusted for genotype, gender, baseline QTc (dichotomized at ≥500 msec) and time-dependent LQTS-related therapies (including β-blockers, left cardiac sympathetic denervation, pacemaker and ICD implantation).
The risk for each genotype and gender high-risk group is compared with the respective genotype and gender lower-risk group (i.e. the respective references groups for the LQT1 genotype, male gender, LQT2 genotype, and female gender are: the LQT2 genotype, female gender, LQT1 genotype, and male gender).
Results for β-blocker effect were obtained by including a β-blocker-by-risk-group interaction-term in the multivariate models.
We further identified gender-genotype risk subsets within the 2 prespecified age-groups (Table 3). In the 0 through 14-year age-group, LQT1 males exhibited the highest risk of cardiac events; LQT1 females had intermediate risk; and LQT2 males and females had the lowest risk. In contrast, in the 15 through 40-year age-group, risk was highest among LQT2 females; intermediate among LQT1 females; and lowest among LQT1 and LQT2 males.
Table 3. Gender-genotype risk subsets and corresponding β-blocker effect*.
| RISK SUBSET | CUMULTIAVE PROBABLITY OF CARIDAC EVENTS† | ADJUSTED RISK | β-BLOCKER EFFECT | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Age-group: 0 through 14 years | |||||||
| LQT1 males | 39% | 1.93 | 1.34 – 2.77 | <0.001 | 0.33 | 0.13 – 0.83 | 0.02 |
| LQT1 females | 23% | 1.31 | 0.93 – 1.85 | 0.13 | 0.44 | 0.17 – 1.11 | 0.08 |
| LQT2 males | 22% | 1.10 | 0.73 – 1.67 | 0.64 | 0.57 | 0.20 – 1.59 | 0.28 |
| LQT2 females | 22% | 1.00 (reference) | 0.93 | 0.30 – 2.94 | 0.90 | ||
| Age-group 15 through 40 years | |||||||
| LQT2 females | 44% | 3.28 | 2.19 – 4.92 | <0.001 | 0.29 | 0.15 – 0.58 | <0.001 |
| LQT1 females | 20% | 1.64 | 1.08 – 2.48 | 0.02 | 0.66 | 0.36 – 1.12 | 0.10 |
| LQT2 males | 8% | 0.85 | 0.50 – 1.45 | 0.85 | 0.54 | 0.27 – 1.12 | 0.09 |
| LQT1 males | 8% | 1.00 (reference) | 0.77 | 0.32 – 1.87 | 0.56 | ||
Models were carried out separately in the 0-14 years and 15-40 years age-groups; all models were adjusted for genotype, gender, baseline QTc (dichotomized at ≥500 msec) and time-dependent LQTS-related therapies (including β-blockers, left cardiac sympathetic denervation, pacemaker and ICD implantation).
Data were obtained from a Kaplan-Meier survival analysis.
Results for β-blocker effect were obtained by including a β-blocker-by-risk-subset interaction-term in the multivariate models.
Patients with a prolonged QTc duration (≥500 msec) had a higher risk for cardiac events as compared with those who exhibited lower QTc durations in both age-groups (0-14 years: HR=2.30 [p<0.001]; age 15-40 years: HR=2.22 [p<0.001]).
β-Blocker Efficacy
The benefit of medical therapy with β-blockers was shown to be pronounced among high-risk patients in both age-groups (Table 2). In the 0 through 14-year age-group, treatment with β-blockers was associated with a 54% (p=0.02) reduction in the risk of cardiac events among LQT1 patients, and a 59% (p=0.01) risk-reduction among males; and in the 15 through 40-year age-group, β-blocker therapy was associated with a 64% (p<0.001) reduction in the risk of cardiac events among LQT2 patients, and a 57% (p<0.001) risk-reduction among females.
Consistent with these findings, treatment with β-blockers was shown to be associated with a pronounced benefit within gender-genotype high-risk subsets (Table 3). Thus, LQT1 males, who were identified to be the highest risk subset in the 0 through 14-year age-group, derived a 67% (p=0.02) reduction in the risk of cardiac events from β-blocker therapy; and LQT2 females, who were identified to be the highest risk subset in the 15 through 40-year age-group, derived a 71% (p<0.001) risk-reduction. The benefit of β-blocker therapy appeared to be less pronounced among lower-risk subsets in both age-groups (Table 3), possibly due to the lower event rate in these populations.
β-blocker effect by subtype and residual events during therapy
β-blocker therapy was associated with a similar magnitude of risk reduction in both LQT1 (HR=0.45; p<0.001) and LQT2 (HR=0.42; p<0.001) patients. The efficacy of the different β-blocker subtypes in reducing the risk of cardiac events among LQT1 and LQT2 patients is shown in Table 4. Among LQT1 patients treatment with atenolol was associated with a significant 77% (p=0.008) reduction in the risk of cardiac events and treatment with nadolol was not associated with a statistically significant risk reduction (HR=0.40; p=0.09), whereas among LQT2 patients treatment with nadolol was associated with a significant 87% reduction in the risk of cardiac events (p<0.001) and atenolol was not associated with a statistically significant benefit.
Table 4.
Risk-reduction by β-blocker type in LQT1 and LQT2 patients.*
| HIGH-RISK GROUP | LQT1 PATIENTS | LQT2 PATIENTS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Any type of β-blocker | 0.45 | 0.26 – 0.80 | 0.006 | 0.42 | 0.23 – 0.76 | 0.004 |
| By beta-blocker subtype | ||||||
| Atenolol | 0.23 | 0.08 – 0.67 | 0.008 | 0.69 | 0.32 – 1.49 | 0.34 |
| Nadolol | 0.40 | 0.14 – 1.16 | 0.09 | 0.13 | 0.03 – 0.62 | 0.01 |
| Propranolol | 0.60 | 0.27 – 1.34 | 0.21 | 0.49 | 0.21 – 1.16 | 0.10 |
| Metoprolol | 0.65 | 0.32 – 1.44 | 0.27 | 0.64 | 0.08 – 5.17 | 0.67 |
Models were carried out separately for LQT1 and LQT2 patients, events were assessed from birth through age 40 years; all models were adjusted for genotype, gender, baseline QTc (dichotomized at ≥500 msec) and other time-dependent LQTS-related therapies (including, left cardiac sympathetic denervation, pacemaker and ICD implantation); patients who were treated concomitantly with more than one β-blocker were not included in the analysis.
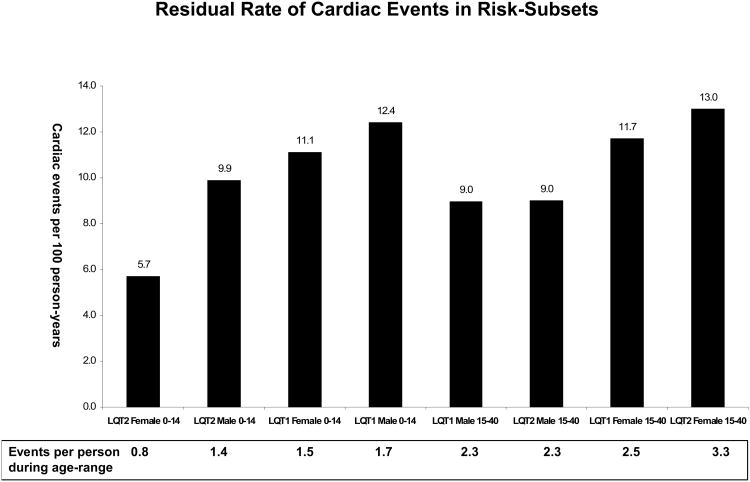
Figures 3A and B show the respective rates of residual cardiac events and life-threatening events (comprising ACA or SCD) during β-blocker therapy among patient subsets in the 2 prespecified age-groups. The results of this analysis demonstrate that despite the pronounced risk-reduction associated with β-blocker therapy, high-risk patients who were treated with β-blockers still experienced a considerable residual event rates during therapy. Notably, the highest rate of life-threatening events during β-blocker therapy was demonstrated among high-risk subsets that experienced prior syncope despite therapy (LQT1 males: 1.2 events per 100 person-years [corresponding to 0.17 events per treated patient in the 0 through 14-year age-range]; LQT2 females: 2.4 events per 100 patient-years [corresponding to 0.60 events per treated patient in the 15 through 40-year age-range]). In contrast, only a minority of residual life-threatening events were not preceded by syncope that occurred during β-blocker therapy (Fig. 3B).
Figure 3. Residual event rates during β-blocker therapy.
Residual rate of (A) cardiac events (comprising syncope, appropriate ICD shocks, aborted cardiac arrest, or sudden cardiac death); and (B) life-threatening events (comprising aborted cardiac arrest or sudden cardiac death) during β-blocker therapy in high-risk subsets of LQT1 and LQT2 patients. Data regarding the rate of residual life-threatening events in each subset are shown separately for events that were preceded by syncope during β-blocker therapy and for events that occurred without prior syncope during therapy.
Event rates were analyzed in LQT1 and LQT2 patients who were on β-blocker therapy at any time during follow-up. In each age-related subgroup event rates per 100 person-years were calculated by dividing the number of events during β-blocker therapy by the total follow-up time in years on β-blocker therapy, and multiplying the result by 100; event rates per 100 person-years are converted at the bottom of each figure to event rates per treated patient during the 14 years- (age-group: 0-14 years) and 25 years- (age-group: 15-40 years) follow-up time in each age-range.
ACS = aborted cardiac arrest; SCD = sudden cardiac death.
Discussion
The present study provides an assessment of the benefits and limitations of β-blocker therapy in risk subsets of 971 genetically-confirmed LQT1 and LQT2 patients. We have shown that: 1) risk factors for cardiac events are age, gender, and genotype dependent, with high-risk populations including LQT1 males in the younger age-group and LQT2 females in the post-adolescence age-group; 2) β-blocker therapy is associated with a substantial and significant reduction in the risk of cardiac events within the identified LQT1 and LQT2 risk subsets; 3) despite the significant reduction in the risk of cardiac events, treatment with this mode of medical therapy is associated with a residual event rate, that is more pronounced among high-risk patients; and 4) a history syncope during β-blocker therapy is present in the majority of LQT1 and LQT2 patients who experience life-threatening events despite medical therapy.
Recent studies from the International LQTS Registry have assessed the benefit of β-blocker therapy within age-groups of LQTS patients.11-13 These studies showed that treatment with β-blockers is associated with a significant 53% reduction in risk of LQTS-related life-threatening events during the childhood period;11 a significant 64% reduction in the risk of ACA or SCD during the adolescence period;12 and with an overall 60% reduction in the risk of cardiac events in the age-range of 18-40 years.13 Similar to the current report, these populations included genetically tested individuals from the International LQTS Registry. However, prior registry analyses did not assess the efficacy of medical therapy within risk subsets of genotyped LQTS patients. Thus, currently it is not known whether the benefit of β-blocker therapy is maintained in high-risk LQT1 and LQT2 patients. In the present study we have utilized updated registry information to identify high-risk subsets of LQTS patients, who were shown to derive a meaningful reduction in the risk of cardiac events with β-blocker therapy. These findings suggest that high-risk LQT1 and LQT2 patients should be routinely treated with β-blockers as a first line therapeutic measure.
A recent study by Priori et al.7 suggested inadequate protection from β-blocker therapy among LQT2 patients. The study demonstrated nearly a 3-fold increase in the risk of cardiac events during β-blocker therapy among LQT2 patients as compared with LQT1 patients, but did not evaluate risk-reduction associated with β-blocker therapy in these populations. By contrast, in the current study we evaluated β-blocker efficacy in a time-dependent manner within risk- and age-subsets, and have demonstrated a pronounced 64% reduction in the risk of cardiac events in high-risk patients with the LQT2 genotype. These findings suggest that the higher residual event rates in LQT2 patients in the current study and in the study by Priori et al.7 are most likely due to a higher overall event rate in adolescent and adult patients with this genotype, rather than to an attenuated efficacy of medical therapy in this high-risk population.
Vincent et al.14 have recently studied the effect of β-blocker therapy in LQT1 patients who were compliant to medical therapy. The study demonstrated a significant benefit of β-blocker therapy and suggested a very low residual event rate during medical therapy in this population. We have consistently shown that LQT1 males derive a significant 67% reduction in the risk of cardiac events with β-blocker therapy. However, despite the significant risk reduction, LQT1 males experienced the highest rate of residual cardiac events during the childhood period. These apparently contradictory results can be explained by the fact that the study by Vincent et al. assessed the yield of medical therapy mostly among adult LQT1 patients (median age: 26 years) who have a low rate of cardiac events during the post-adolescence period regardless of medical therapy, whereas in the current analysis we assessed β-blocker efficacy in LQT1 males during the high-risk childhood period, and have therefore identified a higher residual event rate despite medical therapy. Notably, in contrast to the study by Vincent et al.14 non-compliance did not affect our results regarding β-blocker efficacy since patients not taking β-blocker therapy on day of the event were not considered to be treated at the time of the event, in accordance with the time-dependent statistical models that were employed in the present study.
Current guidelines for device-based therapy of cardiac rhythm abnormalities provide a Class IIa recommendation for primary ICD therapy in LQTS patients who experience syncope and/or ventricular tachyarrhyrhmias while receiving β-blockers, and a Class IIb recommendation for ICD therapy for LQTS patients with risk factors for SCD (regardless of medical therapy).17 Consistent with the Class IIa recommendation, our findings demonstrate that LQTS patients who experience syncope during β-blocker therapy have a relatively high-rate of subsequent life-threatening events. In contrast, we have shown that ACA or SCD rarely occur as a presenting symptom among patients who are treated with β-blockers. These findings suggest that high-risk LQT1 and LQT2 patients should be routinely managed with β-blockers as a first line-therapy, and should be referred for primary ICD implantation if they become symptomatic during therapy or when compliance or intolerance to medical therapy is a concern.
Limitations
Eight study patients (0.8%) experienced SCD prior to genetic testing in their families, and were therefore considered to be carriers of the LQT1 (n=5) and LQT2 (n=3) genotypes based on the mutation that was identified in a first degree family member. Alternative analyses, which were carried out by excluding this small patient subset, yielded virtually identical results.
The International LQTS Registry records therapies that are prescribed at the discretion of the treating physicians to enrolled subjects, and therefore β-blocker administration was not randomized. To reduce possible bias associated with treatment of higher-risk patients, we evaluated the benefit of β-blocker therapy within risk-subsets of genotyped patients. Accordingly, we have shown that β-blocker therapy is associated with a significant reduction in the risk of cardiac events in each high-risk patient subset that was assessed in the genotyped LQT1 and LQT2 populations.
We carried out a secondary analysis that did not identify a difference in the benefit associated with each of the 4 main β-blocker subtypes when treatment dosages were above- and below- median values (not shown). However, since height and weight measurements were not consistently recorded for all registry patients, this analysis is limited by the confounding effect of age and body surface area on the dose-related effects of medical therapy in children and adult LQTS patients.
Data regarding β-blocker usage were obtained retrospectively following a fatal event, and therefore may be biased by a higher recall in this population. In addition, the younger and older age-group categories in the present study were prespecified according to prior data from the International LQTS Registry.8,9 However, it is possible that there is a more gradual change in risk categories during follow-up that was not assessed in the present study.
Conclusions and Clinical Implications
We have shown that the phenotypic expression of patients with the LQT1 and LQT2 genotypes is age- and gender-dependent, and that this information can be used to identify high-risk LQTS patients who will derive a pronounced benefit from β-blocker therapy. Additional risk factors that were identified in the present study included a prolonged QTc duration (≥500 msec), and a history of syncope during therapy with β-blockers, which was shown to be present in the majority of study patients who experienced ACA or SCD despite medical therapy.
Based on the results of the present study and recent data from the International LQTS Registy,1 we have developed a proposed strategy for the management of LQT1 and LQT2 patients. The recommendations are shown in Figure 4 and summarized as follows: 1) low-risk LQT1 and LQT2 patients (including LQT1 females and LQT2 patients in the 0-14 age-group and LQT1 patients and LQT2 males in the 15-50 age-group, without prior syncope and with a QTc < 500 msec) were not shown to derive a statistically significant benefit from β-blocker therapy in the present study. These results are consistent with recent reports from the International LQTS Registry that failed to identify a significant association between β-blocker therapy and risk-reduction among low-risk LQTS patients.11-13 Therefore, in low-risk patients we recommend administration of β-blocker therapy on an individual basis, and routine initiation of medical therapy if they become symptomatic or if follow-up ECGs show an increase in QTc duration; 2) high-risk LQT1 and LQT2 patients (including LQT1 males in the 0-14 age-group, LQT2 females in the 15-40 age-group, patients with a history of syncope and/or documented torsade de pointes [TdP] without β-blocker therapy, and patients with a prolonged QTc) were shown to derive a significant and pronounced reduction in the risk of cardiac events after the initiation of medical therapy, and to have a negligible rate of ACA or SCD as a presenting symptom during therapy. Therefore, treatment with β-blockers should be routinely recommended as a first line measure for all high-risk LQT1 and LQT2 patients without contraindications; 3) primary ICD therapy should be recommended for high-risk LQT1 and LQT2 patients if syncope and/or TdP occur during medical therapy or when compliance or intolerance to β-blocker therapy is a concern; 4) secondary prevention with an ICD should be recommended to all LQTS patients who experience ACA.
Figure 4. A proposed strategy for the management of LQT1 and LQT2 patients.
Patients are categorized as low-risk and high-risk based on the findings of the present study.
BB = β-blockers; ICD = implantable cardioverter defibrillator; LCSD = left cardiac sympathetic denervation; Rx = treatment.
*Lifestyle modifications include restriction from competitive sports and swimming in patients with both genotypes; and avoidance of unexpected auditory stimuli in the bedroom (especially during rest or sleep) and potassium supplements (to levels > 4 mEq/L) in LQT2 patients.
†Some authorities recommend different therapeutic approaches, including routine administration of β-blocker therapy to low-risk patients and primary ICD therapy to high-risk patients with ≥ 2 risk factors.
We would like to stress that the proposed management strategy reflects our recommendations, which are based on the results of the present study and recent registry data. Some authorities recommend different therapeutic approaches in LQTS patients, including routine empirical administration of β-blockers to all low-risk patients18 and primary ICD therapy to patients who have ≥ 2 risk factors regardless of medical therapy.
Our subanalysis regarding the efficacy of β-blocker subtypes suggest that atenolol therapy is associated with a pronounced risk-reduction among LQT1 patients and nadolol therapy is highly effective in LQT2 patients. More specific recommendations regarding initial and maintenance β-blocker dosages, and regarding enhancement of compliance for medical therapy among treated patients, have been recently provided by Vincent et al.14
It should be noted, however, that regardless of the management strategy in an individual patient, careful observation is warranted before and after the initiation of medical therapy in LQTS patients due to the variable phenotypic expression of this genetic disorder during follow-up.
Acknowledgments
This study was supported in part by research grants HL-33843 and HL-51618 from the National Institutes of Health, Bethesda, Md. We want to thank Drs. Michael J. Ackerman, Jesaia Benhorin, Elizabeth Kaufman, Carlo Napolitano, Silvia G. Priori, Peter J. Schwartz, and Jeffrey A. Towbin who enrolled patients in the International LQTS Registry and contributed clinical information and genetic data.
Financial support: The study was supported in part by research grants HL-33843 and HL-51618 from the National Institutes of Health, Bethesda, Md.
References
- 1.Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291–2300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 4.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG: a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantù F, Towbin JA, Keating MT, Hammoude H, Brown AM, Chen LS. Long QT syndrome patients with mutations on the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, Medina A, Zhang L, Robinson JL, Timothy K, Towbin JA, Andrews ML. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 7.Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, Nastoli J. Association of long QT syndrome loci and cardiac events among patients treated with β-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 8.Locati EH, Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Lehmann MH, Towbin JA, Priori SG, Napolitano C, Robinson JL, Andrews M, Timothy K, Hall WJ. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome. Findings from the International LQTS Registry. Circulation. 1998;97:2237–2244. doi: 10.1161/01.cir.97.22.2237. [DOI] [PubMed] [Google Scholar]
- 9.Zareba W, Moss AJ, Locati EH, Lehmann MH, Peterson DR, Hall WJ, Schwartz PJ, Vincent GM, Priori SG, Benhorin J, Towbin JA, Robinson JL, Andrews ML, Napolitano C, Timothy K, Zhang L, Medina A International Long QT Syndrome Registry. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol. 2003;42:103–109. doi: 10.1016/s0735-1097(03)00554-0. [DOI] [PubMed] [Google Scholar]
- 10.Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- 11.Goldenberg I, Moss AJ, Peterson DR, McNitt S, Zareba W, Andrews ML, Robinson JL, Locati EH, Ackerman MJ, Benhorin J, Kaufman ES, Napolitano C, Priori SG, Qi M, Schwartz PJ, Towbin JA, G Vincent M, Zhang L. Risk factors for aborted cardiac Arrest and sudden cardiac death in children with the congenital long-QT syndrome. Circulation. 2008;117:2184–2191. doi: 10.1161/CIRCULATIONAHA.107.701243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbs JB, Peterson DR, Moss AJ, McNitt S, Zareba W, Goldenberg I, Qi M, Robinson JL, Sauer AJ, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Towbin JA, Vincent GM, Zhang L. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA. 2006;296:1249–1254. doi: 10.1001/jama.296.10.1249. [DOI] [PubMed] [Google Scholar]
- 13.Sauer AJ, Moss AJ, McNitt S, Peterson DR, Zareba W, Robinson JL, Qi M, Goldenberg I, Hobbs JB, Ackerman MJ, Benhorin J, Hall WJ, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long-QT Syndrome in Adults. J Am Coll Cardiol. 2007;49:329–337. doi: 10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 14.Vincent GM, Schwartz PJ, Denjoy I, Swan H, Bithell C, Spazzolini C, Crotti L, Piippo K, Lupoglazoff JM, Villain E, Priori SG, Napolitano C, Zhang L. High efficacy of β-blockers in long-QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of β-blocker treatment “failures”. Circulation. 2009;119:215–221. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]
- 15.Boyle MB, MacLusky NJ, Naftolin F, Kaczmarek LK. Hormonal regulation of K+-channel messenger RNA in rat myometrium during oestrus cycle and in pregnancy. Nature. 1987;330:373–375. doi: 10.1038/330373a0. [DOI] [PubMed] [Google Scholar]
- 16.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–1474. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 17.ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) Circulation. 2008;117(21):e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 18.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2006 Sep 5;114(10):e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]