Abstract
The clinical course and risk factors associated with β2-agonist therapy for asthma have not been investigated previously in patients with the Long QT Syndrome (LQTS). The risk of a first LQTS-related cardiac event due to β2-agonist therapy was examined in 3,287 patients enrolled in the International LQTS Registry with QTc≥450msec. The Cox proportional hazards model was used to assess the independent contribution of clinical factors for first cardiac events (syncope, aborted cardiac arrest, or sudden death) from birth through age 40. Time-dependent β2-agonist therapy for asthma was associated with an increased risk for cardiac events (hazard ratio (HR) = 2.00, 95% confidence interval 1.26–3.15, p = 0.003) after adjustment for relevant covariates including time-dependent β-blocker use, sex, QTc, and history of asthma. This risk was augmented within the first year after the initiation of β2-agonist therapy (HR = 3.53; p = 0.006). The combined use of β2-agonist and anti-inflammatory steroids was associated with an elevated risk for cardiac events (HR = 3.66; p < 0.01). β-blocker therapy was associated with a reduction in cardiac events in those using β2-agonists (HR = 0.14; P = 0.05). In conclusion, β2-agonist therapy was associated with an increased risk for cardiac events in asthmatic patients with LQTS, and this risk was diminished in patients receiving β-blockers.
Previous studies have indicated that asthma is associated with an increased risk for cardiac events in patients with the long QT syndrome (LQTS), and this risk is diminished with the administration of β-blockers.1 Asthma is an inflammatory disorder of the airways characterized by reversible bronchi obstruction and sporadic bronchospasm.2 One of the current treatments of asthma is the use of β2-adrenergic receptor agonists for bronchodilation.3 β2-adrenergic receptors predominate in the lungs, and approximately 25% of the β-adrenergic receptors in the heart are of the β2 type.4,5 However, current literature provides minimal information on the effects of inhaled β2-agonist treatment for asthma in LQTS. Using the International Long QT Syndrome Registry, we determined the risk associated with inhaled bronchodilator therapy for asthma in LQTS patients and the influence of β-adrenergic blockers on outcome in this LQTS population.
Methods
We studied 6,015 members of 1,275 LQTS families enrolled in the International Long QT Syndrome Registry.6 Informed consent was obtained for all patients enrolled in the Registry. At the time of enrollment, a baseline electrocardiogram was obtained and measurements were made for RR, QT, and the Bazett-corrected QTc interval.7 From the baseline QTc values, we identified 3,287 patients with QTc ≥ 450 msec, a subgroup with a high likelihood of being affected with LQTS. The Registry data included detailed information on the clinical history of patients with LQTS, the age when inhaled bronchodilator therapy was initiated for treatment of asthma, and the onset and offset of specific LQTS therapy including β-blockers as previously reported.6 Blood samples were obtained for genetic studies in 1,036 subjects with mutation identification by standard genetic tests.
Asthma therapy was initiated at the discretion of each patient’s attending physician. In 101 patients treated with inhaled β2-agonist bronchodilators, albuterol, salmeterol, or metaproterenol were utilized in 96% of the administered therapy. In 37 patients treated with inhaled steroid therapy, beclomethasone, triamcinolone, or budesonide were utilized in 95% of the administered therapy.
In 1,622 patients who received β-blocker therapy for LQTS, propranolol, atenolol, nadolol, or metoprolol were utilized in 96% of the administered therapy.
The primary end point was the first occurrence of syncope (defined as the transient loss of consciousness that was sudden in onset and offset), aborted cardiac arrest (requiring external defibrillation during resuscitation), or LQTS-related sudden cardiac death (unexpected sudden death exclusive of a known cause) from birth through age 40 years.1,8,9 The probability of cardiac events in patients using β2-agonists was compared to those not using β2-agonists in the LQTS-affected population.
The Wilcoxon test for continuous variables and the χ2 or Fisher exact tests for categorical variables were used to compare the clinical characteristics between patients with or without β2-agonist treatment among the LQTS affected patients. Graphical display of the time to event before and after starting β-agonist therapy utilized the Mantel-Byar method.10 The Cox proportional hazards model11 was used to evaluate the significant and independent contribution of time-dependent β-agonist therapy to the occurrence of cardiac events. The interaction of time-dependent β-blocker use with β2-agonist use was assessed using the Cox regression model. The statistical software used to perform the analyses was SAS version 9.1.3 (SAS, Cary, North Carolina). All tests were two-sided, and statistical significance set at 0.05 level.
Results
In the study population of 3,287 affected LQTS patients (QTc ≥ 450 msec), 101 (3.1%) patients utilized β2-agonist therapy. The clinical characteristics of the LQTS study population by use or non-use of β2-agonist therapy are presented in Table 1. There were significant gender, age at baseline electrocardiogram, baseline QTc, and heart rate differences between the 2 treatment groups. Therapy with β2-agonists was associated with a higher frequency of syncope. Over 90% of patients receiving β2-agonist therapy were categorized as having a history of asthma. β-blocker use was significantly more frequent in the β2-agonist treated patients than in those not receiving β2-agonist therapy. The dosages of three different β-blockers prescribed in patients with and without β2-agonist therapy are presented in Table 2.
Table 1.
Baseline characteristics in long QT syndrome population (QTc ≥ 450 msec) by beta-adrenergic therapy
| Parameters | Beta-adrenergic Therapy | P-value | ||
|---|---|---|---|---|
| No (N=3186) |
Yes (N=101) |
|||
| Female | 1986 (62%) | 74 (73%) | 0.03 | |
| Age at 1st ECG recording (years) | 28 ± 22 | 19 ± 13 | < 0.001 | |
| ECG data | ||||
| QTc baseline (msec) | 494 ± 47 | 507 ± 60 | 0.04 | |
| RR baseline (msec) | 829 ± 209 | 786 ± 215 | 0.02 | |
| Asthma | 198 (6%) | 93 (92%) | < 0.001 | |
| Congenital Deafness | 57 (2%) | 3 (3%) | 0.43 | |
| LQTS mutations* | (n=1005) | (n=31) | ||
| LQT1 | 522 (52%) | 19 (61%) | ||
| LQT2 | 365(36%) | 8 (26%) | ||
| LQT3 | 90 (9%) | 3 (10%) | 0.61 | |
| Other | 17 (2%) | 0 | ||
| Multiple Mutations | 11 (1%) | 1 (3%) | ||
| Medical Interventions | ||||
| β-blockers | 1555 (49%) | 67 (66) | < 0.001 | |
| Implanted defibrillator | 300 (9%) | 18 (18%) | 0.005 | |
| Pacemaker | 207 (6%) | 15 (15%) | < 0.001 | |
| Left cardiac sympathectomy | 107 (3%) | 2 (2%) | 0.77 | |
| 1st Cardiac Event | 1360 (43%) | 60 (59%) | < 0.001 | |
| Syncope | 1242 (39%) | 59 (58%) | < 0.001 | |
| Aborted cardiac arrest | 88 (3%) | 1 (1%) | 0.53 | |
| LQTS-related death | 30 (1%) | 0 | 0.33 | |
| Age at 1st cardiac event, years | 14 ± 10 | 13 ± 8 | 0.26 | |
Values are mean ± SD where indicated.
Mutation identification was available in 1036 patients, and the percentages in the specific genotypes are relative to the number of genotyped patients in each therapy group. The p-value refers to the difference in the distribution of the mutational genotypes by Chi square analysis.
Table 2.
Prescribed β-blocker dose in long QT syndrome patients by beta-adrenergic therapy
| Beta2-adrenergic Therapy | ||
|---|---|---|
| Beta-blockers | No | Yes |
| Atenolol | 50 ± 35 mg (n=594) | 57 ± 51 mg (n=23) |
| Metoprolol | 85 ± 81 mg (n=210) | 83 ± 65 mg (n=7) |
| Nadolol | 61 ± 52 mg (n=468) | 62 ± 44 mg (n=10) |
| Propranolol | 82 ± 80 mg (n=742) | 96 ± 47 mg (n=8) |
Values are mean ± SD.
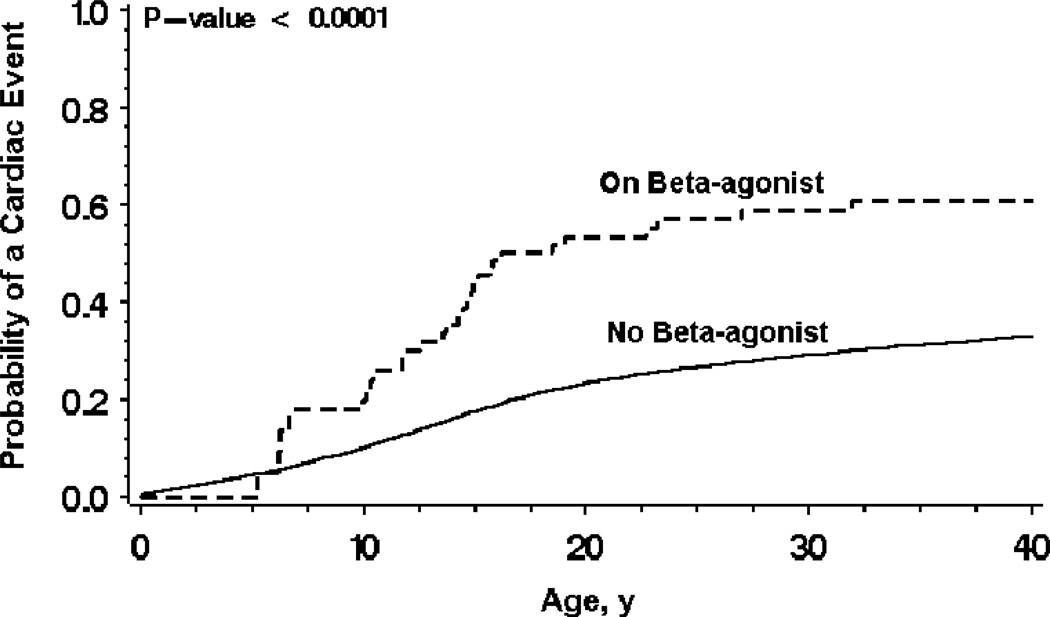
Time-dependent β2-agonist therapy was associated with an increase in cardiac events as shown in the Mantel-Byar graph (Fig. 1). The findings from the Cox proportional hazards regression analysis for time to first cardiac event are presented in Table 3. Time-dependent β2-agonist use was associated with a 2-fold increased risk for cardiac events after adjustment for relevant covariates including sex, QTc, history of asthma, and time-dependent β-blocker use. Males up to age 13 had an augmented risk compared to females of the same age, but the converse was true after age 13. The risk for LQTS-related cardiac events from time-dependent β2 agonist therapy was greater in the first year after initiation of therapy (HR = 3.53; 95% CI, 1.44–8.64; p = 0.006) than in later years (HR = 1.77; 95% CI, 1.06–2.95; P = 0.03). In comparing the long-acting versus the short-acting β2-agonist therapy, there was no significant difference on the outcome of cardiac events.
Figure 1.
Mantel-Byar graph of probability of a first cardiac event by use or non-use of beta2-agonist therapy.
Table 3.
Multivariate Cox model for cardiac events in long QT syndrome patients
| Factor | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Time-dependent β2-agonist therapy (yes:no) | 2.00 | 1.26 – 3.15 | 0.003 |
| Males:Females (≤ 13 years of age) | 1.47 | 1.28 – 1.69 | < 0.0001 |
| Females:Males (> 14 years of age) | 2.43 | 1.97 – 2.99 | < 0.0001 |
| QTc > 500msec:QTc≤ 500msec | 1.92 | 1.73 – 2.13 | < 0.0001 |
| Asthma: No asthma | 1.16 | 0.97 – 1.40 | 0.11 |
| Time-dependent β-blocker use (yes:no) | 1.03 | 0.82–1.29 | 0.81 |
Asthma was forced into the model even though it had an insignificant p-value to show the independent contribution of β2-agonist therapy.
Anti-inflammatory steroids were not associated with an augmented risk for cardiac events (HR = 1.61; 95% CI, 0.40–6.47; P = 0.50). However, the combined use of β2-agonist and anti-inflammatory treatment was associated with an elevated risk (HR = 3.66; 95% CI, 1.37–9.81; P < 0.01), possibly reflecting more severe underlying bronchial asthma.
The influence of β-blocker use on patients receiving and not receiving β2-agonists was evaluated in an interaction model. β-blockers had no evident effect in those not receiving β2-agonists (HR = 1.10; 95% CI, 0.88–1.37; P = 0.41), but a meaningful reduction in events was evident in those on β2-agonists (HR = 0.14; 95% CI, 0.02–1.04; P = 0.05), with a bidirectional interaction significant at p = 0.05.
Discussion
This study highlights four important issues regarding asthma treatment in LQTS patients. β2-agonist therapy was associated with a two-fold increased risk of cardiac events in patients using this medication, with the risk higher within the first year of starting β2-agonist treatment than thereafter. The combination of β2-agonist and anti-inflammatory steroids was associated with a higher probability of cardiac events. The utilization of β-blocker therapy in conjunction with β2-agonist treatment lowered the risk of cardiac events.
In the last 25 years, there has been an increase in the incidence and prevalence of asthma,12 and this increased frequency of asthma surely involves patients with LQTS. Management of LQTS patients with asthma is a challenge since β2-agonist therapy represents a relative contraindication because of its potential to trigger ventricular tachyarrhythmias in this repolarization disorder.13
In a previous report from the LQTS Registry, Rosero, et al. showed that asthma was associated with an increased risk for cardiac events.1 In the current study, we showed that the risk is largely related to the use of β2-agonist therapy, and this therapy is a more potent risk factor than a history of asthma (Table 3).
The currently recommended treatment for patients with LQTS is administration of β-blocker agents. These drugs diminish the risk of cardiac events by decreasing sympathetic stimulation of the heart.14 On the other hand, β2-adrenergic drugs used in the treatment of asthma work in an opposing manner and are predominately aimed at the β2-receptors located primarily in the lungs. These drugs, widely used to treat asthma, are associated with an increased likelihood of cardiac events. Even though the β2-adrenergic agonists exert a preferential activity on the adrenergic receptors in the lungs, there is also some concurrent stimulation upon the β-receptors located within the heart. This latter stimulation may trigger cardiac arrhythmias in LQTS resulting in the increased cardiac events as observed in the current study with β2-agonist anti-asthmatic therapy.
For asthma patients taking both a β-blocker and a β2-agonist, there was a significant reduction in the probability of cardiac events. Although β-blockers may exacerbate bronchospastic asthma,15 this therapy seems to outweigh the negative effect imparted by the β-agonist therapy on cardiac events. While there was an increase in syncope with β2-agonist use, we did not identify any fatal cardiac events in patients receiving asthmatic therapy. We cannot exclude the possibility that the ICD that was implanted in 18% of the β2-agonist group prevented mortality, but we think this is unlikely for several reasons. Appropriate ICD shocks for life-threatening arrhythmias in LQTS are quite infrequent.16 When we excluded patients with ICDs from the Cox analysis, or assumed that all the patients with ICDs died within three years after device implantation, the hazard ratios for β2-agonist use and relevant covariates were essentially the same as those presented in Table 3.
The combined use of β2-agonists and anti-inflammatory steroids has been shown to have complementary effects with a greater benefit at decreasing exacerbations and reducing hospitalizations for patients with bronchial asthma.17 The combined therapy may allow for improved bronchodilation and anti-inflammatory effects in-comparison to each treatment alone.18 While our study did not address the respiratory impact from combined therapies, it did identify an increased risk of cardiac events with the joint use of these medications. Anti-inflammatory steroids can increase the transcription of glucocorticoid-regulated genes,19,20 which includes β-adrenergic receptors. This up-regulation of β-adrenergic receptors along with increased receptor stimulation due to β2-agonists, may contribute to this augmented risk for cardiac events.
There are several limitations in this observational, retrospective study of patients enrolled in the LQTS Registry. Asthmatic therapy was not randomly assigned, dosage information of the β2-agonist therapy was not recorded, and objective measures of the severity of the airway disease were not available. Guidelines have used factors such as symptom control and need for medication in order to categorize the severity of asthma.21 We were unable to evaluate the influence of factors such as obesity, smoking, diet and exercise on the adverse outcome of cardiac events. The composite cardiac end point was dominated by syncope, and we could not draw any significant conclusions on the relationship between β-agonists and its propensity to cause mortality due to limited power. We were also unable to evaluate the contribution of specific LQTS genotypes to the risk of cardiac events due to a limited number of genotyped patients receiving β2-agonists who experienced cardiac events. The dose of β-blockers utilized in patients with and without asthma was similar, but we cannot draw any conclusions about the appropriate dose of β-blocker therapy that should be used in LQTS patients being treated for asthma.
Acknowledgment
The authors thank the members of the International Long QT Syndrome Investigative Group including: Michael J. Ackerman, MD, PhD (Mayo Clinic College of Medicine, Rochester, MN), Jesaia Benhorin, MD (Bikur Cholim Hospital, Jerusalem, Israel), Emanuela Locati, MD, PhD (Niguarda Hospital, Milan Italy), Carlo Napolitano, MD and Silvia Priori, MD, PhD (Fondazione S. Maugeri-University of Pavia, Pavia, Italy), Peter J. Schwartz, MD and Carla Spazzolini, MS (Policlinico S. Matteo IRCCS, University of Pavia, Pavia, Italy), Jeffrey Towbin, MD (Baylor College of Medicine, Houston, TX), G. Michael Vincent, MD and Li Zhang, MD (University of Utah Medical School, Salt Lake City, UT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosero SZ, Zareba W, Moss AJ, Robinson JL, Hajj Ali RH, Locati EH, Benhorin J, Andrews ML. Asthma and the risk of cardiac events in the Long QT syndrome. Long QT Syndrome Investigative Group. Am J Cardiol. 1999;84:1406–1411. doi: 10.1016/s0002-9149(99)00586-x. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 3.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125:2309–2321. doi: 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 5.Sears MR. Adverse effects of beta-agonists. J Allergy Clin Immunol. 2002;110:S322–S328. doi: 10.1067/mai.2002.129966. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A, Jr, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 7.Bazett JC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- 8.Hobbs JB, Peterson DR, Moss AJ, McNitt S, Zareba W, Goldenberg I, Qi M, Robinson JL, Sauer AJ, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Towbin JA, Vincent GM, Zhang L. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA. 2006;296:1249–1254. doi: 10.1001/jama.296.10.1249. [DOI] [PubMed] [Google Scholar]
- 9.Sauer AJ, Moss AJ, McNitt S, Peterson DR, Zareba W, Robinson JL, Qi M, Goldenberg I, Hobbs JB, Ackerman MJ, Benhorin J, Hall WJ, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49:329–337. doi: 10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 10.Mantel N, Byar DP. Evaluation of response-time data involving transient states: an illustration using heart transplant data. J Am Stat Association. 1974;69:81–86. [Google Scholar]
- 11.Cox DR. Regression models and life-tables. J Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 12.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 13.Ferguson GT, Funck-Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest. 2003;123:1817–1824. doi: 10.1378/chest.123.6.1817. [DOI] [PubMed] [Google Scholar]
- 14.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, Medina A, Zhang L, Robinson JL, Timothy K, Towbin JA, Andrews ML. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 15.van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest. 2005;127:818–824. doi: 10.1378/chest.127.3.818. [DOI] [PubMed] [Google Scholar]
- 16.Zareba W, Moss AJ, Daubert JP, Hall WJ, Robinson JL, Andrews M. Implantable cardioverter defibrillator in high-risk long QT syndrome patients. J Cardiovasc Electrophysiol. 2003;14:337–341. doi: 10.1046/j.1540-8167.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- 17.Greenstone IR, Ni Chroinin MN, Masse V, Danish A, Magdalinos H, Zhang X, Ducharme FM. Combination of inhaled long-acting beta2-agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database Syst Rev. 2005:CD005533. doi: 10.1002/14651858.CD005533. [DOI] [PubMed] [Google Scholar]
- 18.Kardos P, Keenan J. Tackling COPD: a multicomponent disease driven by inflammation. MedGenMed. 2006;8:54. [PMC free article] [PubMed] [Google Scholar]
- 19.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mano K, Akbarzadeh A, Townley RG. Effect of hydrocortisone on beta-adrenergic receptors in lung membranes. Life Sci. 1979;25:1925–1930. doi: 10.1016/0024-3205(79)90614-3. [DOI] [PubMed] [Google Scholar]
- 21.Boulet LP, Becker A, Berube D, Beveridge R, Ernst P. Canadian Asthma Consensus Report, 1999. Canadian Asthma Consensus Group. CMAJ. 1999;161:S1–S61. [PMC free article] [PubMed] [Google Scholar]