Abstract
A liposomal formulation capable of encapsulating 76 to 92% of the antimycobacterial drug ethambutol and showing prolonged in vitro release kinetics is described. In vitro efficacy is equivalent to that of the free drug, suggesting that encapsulation of ethambutol has the potential to shorten the current regimens for tuberculosis.
Treatment of tuberculosis may be improved by significantly decreasing therapy length (5). Current treatment involves combination therapy (often including ethambutol as the third drug) administered over 6 to 12 months. Long antibiotic-exposure periods are required due in part to their inability to effectively permeate macrophages (2) or sites of necrosis and disease. Therefore, although blood levels of drugs may reach the MIC the bacteria are not exposed to sufficiently high concentrations for periods approaching the time required to divide. Since ethambutol has a relatively low (2.5 h) half-life value in serum (11), liposome encapsulation could improve drug pharmacokinetics (by sustaining drug release from the carrier) and pharmacodynamics (by enhancing delivery to disease sites) (1, 8).
Ethambutol liposomes were prepared (essentially as described by Jia et al.) (7) by injecting solubilized lipid (sphingomyelin-cholesterol at 63/37 [mol/mol]) into 0.5 M H2SO4 and extruding with a LIPEX extruder (Northern Lipids Inc., Vancouver, Canada). Vesicles were diluted in 115 mM NaSO4-50 mM NaH2PO4, and the external pH was adjusted to 7.5. Ethambutol was added at a drug/lipid ratio of 1:3 (mol/mol) and loaded at 60°C. The formulation was then diafiltered (Midgee ultrafiltration column; Amersham, Piscataway, N.J.) to remove unencapsulated drug and solvent and concentrated to 100 mg of lipid/ml.
Drug levels were quantitated using a Pierce-modified Lowry protein assay (MJS Biolynx, Brockville, Ontario, Canada) and an ethambutol standard curve. Drug encapsulation results were determined using microcon-30 filtration devices (Millipore, Billerica, Mass.) by separation of liposome-associated drug from free drug. In vitro drug release was quantified following incubation at 37°C after dilution in phosphate-buffered saline (pH 7.4) or mouse serum. Vesicle size was determined using a Nicomp 300ZS apparatus (Particle Sizing Systems, Santa Barbara, Calif.). Vesicle size and drug encapsulation were determined at 5°C, the ambient temperature, and 37°C for stability studies.
Disk diffusion assays were carried out using mid-log-phase Mycobacterium tuberculosis var. bovis BCG containing green fluorescent protein (4) swabbed onto agar plates with 7H10-oleic acid-albumin-dextrose-catalase (OADC)-50 μg of hygromycin/ml. Ethambutol at 0 to 10 μg was tested for both free and encapsulated drug and incubated for 18 days at 37°C. MIC determinations were carried out at 0 to 8 μg/ml in 7H9 broth-10% OADC incubated for 3 weeks at 37°C with shaking. Intracellular assays were conducted with THP-1 cells grown in RPMI 1640 medium with 10% fetal bovine serum-1% l-glutamine-1% penicillin-streptomycin, differentiated with phorbol myristate acetate, and plated at 0.75 × 106 cells per well in 24-well culture plates. Following overnight incubation, cells were left to rest for 2 days and washed to remove antibiotics; fresh medium was added before a 3-h infection in the presence of 10% human serum at a ratio of 5:1 was performed. After removal of bacterial cells and lysing of one control well, liposomal or free antibiotic solutions at peak serum levels of 6 and 12 μg/ml (11) in fresh medium were added. Empty liposomes or control medium solutions were added to appropriate wells. All treatments were incubated for 48 h and washed; fresh medium without antibiotics was added and incubated (using the method of Majumdar et al.) (9) for another 5 days. Following incubation, adherent cells were lysed with 1% Triton X-100 in phosphate-buffered saline for 10 min. Serial dilutions were prepared in 7H9 medium, and the appropriate dilutions were plated onto 7H10-OADC-50 μg of hygromycin/ml. Viable bacteria were counted after an 18- to 22-day incubation at 37°C. GraphPad Prism was used for statistical analysis by Student's paired t test.
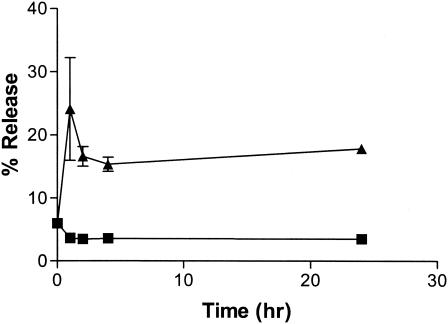
This ethambutol liposome formulation encapsulates 76 to 92% of available drug in 125- to 145-nm-diameter vesicles, following optimization of loading conditions. Liposomal drug release in vitro was characterized over 24 h at 37°C (Fig. 1). In the absence of serum, >90% of encapsulated ethambutol remained liposome associated (since no agent was present to dissipate the transmembrane pH gradient). This served as a control for spontaneous drug release, illustrating that the sphingomyelin-cholesterol bilayer remains tightly packed at 37°C, maintaining the pH gradient and preventing drug transport across the bilayer (10). In the presence of serum, however, 15 to 25% of drug was released (Fig. 1). Release therefore appears to be at least partially dependent on protein-lipid interactions (3, 12). These results illustrate the potential for these systems to sustain release at the disease site following intrapulmonary aerosol administration. Our study also shows that this formulation is stable for at least 4 months at 5°C and for at least 1 month at ambient temperature or 37°C. Under these conditions there was no change in vesicle size (131 to 132 nm) or drug encapsulation efficiency (99 to 100%).
FIG. 1.
In vitro drug release at 37°C. In vitro release assays were conducted at 37°C over 24 h with M. bovis BCG incubated without serum (squares) or with 50% serum (triangles). Results are reported as mean percentages of release ± standard errors of the means (n = 3).
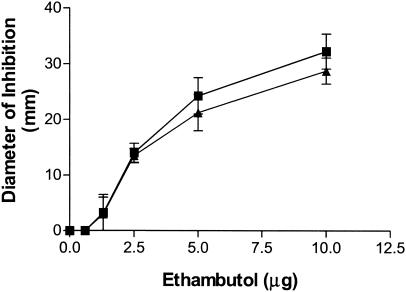
The disk diffusion assay using M. tuberculosis var. bovis BCG showed no significant differences between the activities of encapsulated and free ethambutol (Fig. 2). These results were confirmed by the broth macrodilution assay, with which the MIC of both forms of ethambutol was measured as 2 to 4 μg/ml. Since the medium used for both assays contains ammonia at sufficient concentrations to dissipate the pH gradient across the bilayer and since the process of drying the formulations on filter disks may disrupt the vesicles, these experiments demonstrate that the bioavailability of ethambutol is maintained following release.
FIG. 2.
In vitro efficacy against M. bovis BCG measured by the disk diffusion assay. Liposome-encapsulated ethambutol levels (closed triangles) were compared to free ethambutol levels (closed squares). Empty vesicle control results showed no activity (results not shown). Results are reported as the means of the zones of inhibition ± standard errors of the means (n ≥ 3).
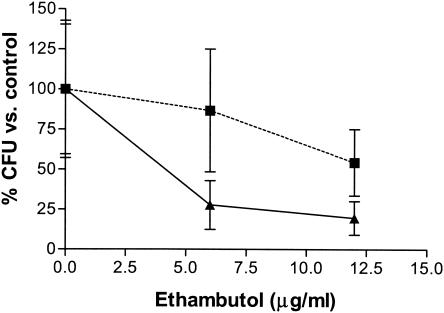
In vitro intracellular efficacy was tested over 1 week to allow liposomal drug release and to ensure that M. tuberculosis var. bovis BCG underwent several rounds of replication, conditions suitable to quantitate drug activity. Analysis of CFU numbers compared to the carrier results (that is, comparisons of free drug to medium and encapsulated drug to empty liposome levels) showed no significant differences (P = 0.15 to 0.25) between the two forms of ethambutol (Fig. 3).
FIG. 3.
In vitro efficacy against intracellular M. bovis BCG after 1 week. The levels of efficacy of encapsulated ethambutol (squares) and free ethambutol (triangles) against intracellular M. bovis BCG were compared over 1 week. Results show mean percentages ± standard errors of the means (n = 3).
This study presents a formulation of liposome-encapsulated ethambutol that reaches a drug concentration of 18 mg/ml at 100 mg of lipid/ml; this concentration is 1,000 times greater than ethambutol's MIC. This is the first step to achieving our goal of developing an inhalation therapy for the treatment of tuberculosis. As such, the ethambutol formulation was designed to optimize drug potency since small volumes (approximately 30%) (6) reach the lung upon administration. The next step will be to evaluate this formulation in an animal disease model.
Acknowledgments
T.W. was supported by a B.C. Science Council GREAT scholarship. Y.A. is a CIHR-B.C. Lung Association Scholar. General funding was provided by the CBDN and the T.B. Veterans Association.
REFERENCES
- 1.Bermudez, L. E. 1994. Use of liposome preparations to treat mycobacterial infections. Immunobiology 191:578-583. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez, L. E., M. Wu, and L. S. Young. 1987. Intracellular killing of Mycobacterium avium complex by rifapentine and liposome-encapsulated amikacin. J. Infect. Dis. 156:510-513. [DOI] [PubMed] [Google Scholar]
- 3.Bonte, F., and R. L. Juliano. 1986. Interactions of liposomes with serum proteins. Chem. Phys. Lipids 40:359-372. [DOI] [PubMed] [Google Scholar]
- 4.Cowley, S. C., and Y. Av-Gay. 2001. Monitoring promoter activity and protein localization in Mycobacterium spp. using green fluorescent protein. Gene 264:225-231. [DOI] [PubMed] [Google Scholar]
- 5.Ellner, J., P. Brennan, and D. Young (ed.). 2001. Tuberculosis: scientific blueprint for TB drug development. Harcourt Publishers, Edinburgh, United Kingdom.
- 6.Hickey, A. J., and S. Saurez. 1998. Drug properties affecting aerosol behaviour. Resp. Care 45:652-666. [PubMed] [Google Scholar]
- 7.Jia, L., M. Garza, H. Wong, D. Reimer, T. Redelmeier, J. B. Camden, and S. D. Weitman.. 2002. Pharmacokinetic comparison of intravenous carbendazim and remote loaded carbendazim liposomes in nude mice. J. Pharm. Biomed. Anal. 28:65-72. [DOI] [PubMed] [Google Scholar]
- 8.Kamath, M. P., B. D. Shenoy, S. B. Tiwari, R. Karki, N. Udupa, and M. Kotian. 2000. Prolonged release of biodegradable vesicular carriers for rifampicin—formulation and kinetics of release. Indian J. Exp. Biol. 38:113-118. [PubMed] [Google Scholar]
- 9.Majumdar, S., D. Flasher, D. S. Friend, P. Nassos, D. Yajko, W. K. Hadley, and N. Duzgunes. 1992. Efficacies of liposome-encapsulated streptomycin and ciprofloxacin against Mycobacterium avium-M. intracellulare complex infections in human peripheral blood monocytes/macrophages. Antimicrob. Agents Chem. 36:2808-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer, L. D., M. B. Bally, and P. R. Cullis. 1986. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim. Biophys. Acta 857:123-136. [DOI] [PubMed] [Google Scholar]
- 11.Peloquin, C. A., A. E. Bulpitt, G. S. Jaresko, R. W. Jelliffe, J. M. Childs, and D. E. Nix. 1999. Pharmacokinetics of ethambutol under fasting conditions, with food, and with antacids. Antimicrob. Agents Chem. 43:568-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semple, S. C., A. Chonn, and P. R. Cullis. 1996. Interactions of liposome and lipid-based carrier systems with blood proteins: relation to clearance behaviour in vivo. Adv. Drug Delivery Rev. 32:3-17. [DOI] [PubMed] [Google Scholar]