Abstract
Objective
To evaluate C-reactive protein (CRP), an inflammatory marker, as a predictor of HIV-related outcomes among women and children in a resource-poor setting.
Design
We measured serum CRP concentration among 606 HIV-infected women, all of whom were not taking highly-active antiretroviral therapy, 3 to 11 months after they gave birth, and assessed relationships of CRP to HIV-related endpoints, including maternal disease progression, mother-to-child transmission of HIV, and maternal and child mortality.
Methods
We used Cox proportional hazards and logistic regression models adjusted for age, sociodemographic characteristics, anthropometric measurements, hemoglobin, CD4 cell count, HIV viral load, and, for child outcomes, breastfeeding status.
Results
Ninety-four women had a high CRP concentration (>10 mg/l). During the study follow-up, 56 women progressed to WHO stage 4 and 188 died, and a high maternal CRP concentration was significantly associated with a 2.26-fold (95% CI 1.64–3.12) greater risk of progression to stage 4 or death. Among children, 174 acquired HIV and 116 died by age 2 years, and a high maternal CRP concentration was associated with a 3.03-fold (95% CI 1.85–4.96) greater risk of child mortality. In multivariate analyses among adults, a high maternal CRP concentration was significantly associated with a 1.55-fold (95% CI 1.08–2.23) greater risk of progression to stage 4 or death. A maternal CRP concentration was not significantly associated with mother-to-child transmission of HIV.
Conclusions
A high maternal CRP concentration independently predicts HIV disease progression, maternal mortality, and child mortality in a resource-poor setting. C-reactive protein may be an important and inexpensive prognostic indicator for HIV-infected women and their children.
Keywords: C-reactive protein, HIV/AIDS, disease progression, mortality, mother-to-child transmission, resource-poor setting
Introduction
The cost of routinely monitoring CD4 T-cell lymphocytes and HIV plasma viral load are prohibitively high for many resource-poor settings, and less-expensive assays may be useful in these situations to guide HIV care and treatment [1]. C-reactive protein (CRP), an acute-phase response protein that increases in acute and chronic inflammatory disorders [2], has been shown to predict cardiovascular events among both individuals with angina and healthy physicians [3–5], and predict mortality among patients with pancreatic or colorectal cancer [6,7]. Increased CRP concentrations also predict mortality among HIV-negative immunocompromised patients with Pneumocystis carinii pneumonia [8].
Higher CRP concentrations have been associated with lower CD4 cell counts and higher HIV RNA levels among HIV-infected individuals [9], but results from studies evaluating serum CRP as a predictor of mortality in resource-rich settings are conflicting. Studies have reported significant associations between increased CRP concentrations and faster time to AIDS and greater risk of mortality, but did not use clinically established CRP cut-off concentrations [9–11]. A third study found that a clinically elevated CRP concentration was associated with a significant reduction in the risk of mortality [12]. While a prognostic indicator of HIV disease progression and mortality would be beneficial to all HIV clinicians, those working in resource-poor settings have a greater need for an inexpensive prognostic marker. Therefore, the objective of this study was to evaluate serum CRP as a predictor of adverse maternal and child HIV-related outcomes in a resource-poor setting.
Methods
Study Design
Starting in April 1995, 1,078 HIV-infected pregnant women were enrolled into a randomized trial of multivitamin supplementation in Dar es Salaam, Tanzania. The trial design has been described in detail [13]. In brief, women were provided daily multivitamins and followed, along with their child, until the end of the study in August 2003. In this sub study, we measured CRP concentration in stored frozen plasma samples obtained from 611 women. All samples had been collected within one year after the woman gave birth. None of the participants had received antiretroviral therapy during the study.
Laboratory Methods
Detailed laboratory methods of the parent study have been described [13]. In brief, we used Roche Amplicor version 1.5 assay to quantify HIV-1 RNA and Becton Dickinson FACScount or FACSCAN systems to quantify CD4 T-cell count. Plasma samples were then stored in a −70 Celsius freezer until 611, which had enough stored blood at the time of testing, were obtained for CRP testing. We used an immunoturbidimetric assay on a Hitachi 917 analyzer (Roche Diagnostics) using reagents and calibrators from Denka Seiken (Niigata, Japan) to determine the concentration of CRP in plasma. This assay had a sensitivity of 0.03 mg/l and day-to-day variability of 2.81, 1.61 and 1.1% at concentrations of 0.91, 3.07 and 13.38 mg/l, respectively. Among the 611 plasma samples tested, results were not available for four plasma samples that hemolyzed during laboratory testing.
Statistical Analyses
Among the 607 plasma samples with measured CRP concentrations, we excluded one sample for being obtained within one month of giving birth. Therefore, CRP concentrations from 606 HIV-positive women were used in the analyses. A clinically elevated CRP concentration has been established as >10 mg/l [2,14]. All analyses were conducted using SAS 9.1 [(SAS Institute, Cary, NC)].
We determined associations between high maternal CRP concentration (>10 mg/l), using those with CRP ≤10 mg/l as a reference group, and primary maternal and child HIV-related outcomes. In addition, primary outcomes were compared among quartiles of CRP concentration, using the lowest CRP quartile as the reference group. Clinical data (body mass index, mid-upper arm circumference, hemoglobin, CD4 cell count, and HIV viral load) were included in analyses if they were collected within 28 days of the CRP sample.
Methods for determining the predefined primary clinical and mortality outcomes have been described [15,16]. In brief, primary maternal outcomes were progression to WHO stage 4, mortality to AIDS-related causes, and mortality to any cause. Primary child outcomes were mortality by age 2 years and mother-to-child transmission of HIV by age 2 years.
Maternal characteristics were compared between women with normal (≤10 mg/l) and high (>10 mg/l) CRP concentrations using a two-sided t-test for continuous variables and a chi-square test for categorical variables. Kaplan-Meier curves were used to display maternal progression to stage 4 or death among women with a normal and high CRP concentration; curves were compared using the log-rank test. Relationships of CRP concentration with predefined primary HIV-related endpoints were assessed using Cox proportional hazards models for maternal outcomes and logistic regression models for child outcomes. Maternal endpoints were included only if they occurred after the CRP sample date, and child endpoints were included if they occurred before or after the CRP sample date and before the child reached age 2. Among the CRP quartiles, a test for trend was conducted using the median CRP concentration of each quartile (lowest quartile, median 0.34 mg/l, IQR 0.23–0.46 mg/l; 2nd quartile, median 1.14 mg/l, IQR 0.87–1.55 mg/l; 3rd quartile median 2.96 mg/l, IQR 2.38–3.89 mg/l; highest quartile, median 18.18 mg/l, IQR 7.53–41.90 mg/l). When combined maternal outcomes were examined, the first end point to occur was used in the model. Univariate models were adjusted for maternal age and vitamin trial regimen group. Multivariate models were adjusted for maternal age, vitamin trial regimen group, sociodemographic characteristics, anthropometric measurements, hemoglobin, CD4 cell count, HIV plasma viral load, and, for child outcomes, breastfeeding status. We used categories of CD4 count (<200, 200–399, 400–599, 600–799, ≥800 cells/µl) and viral load (<25,000, 25,000–49,999, ≥50,000 copies/ml) in multivariate models.
Results
Among this cohort of 606 HIV-positive women, the median CRP concentration was 1.95 mg/l [interquartile range (IQR) 0.63 to 5.17 mg/l]. Ninety-four (15.5%) women had a high CRP concentration. Serum samples for CRP measurement were obtained a mean (± standard deviation) of 210 (± 27) days after women gave birth, and there was no significant difference in the time from delivery to obtaining the serum sample between the two groups (Table 1). Women with a high CRP concentration were significantly less likely to be breastfeeding at the date of the CRP sample, and significantly more likely to have a body mass index <18.5 kg/m2, a mid-upper arm circumference <22 cm, a hemoglobin <11 g/dl, and a CD4 count <200 cells/µl. Plasma viral load was not significantly different between the two groups.
Table 1.
Maternal characteristics stratified by normal (≤10 mg/l) versus high (>10 mg/l) C-reactive protein concentration among 606 HIV-infected women.
| CRP ≤ 10 N (%) |
CRP > 10 N (%) |
p-value1 | |
|---|---|---|---|
| Time from delivery to C-reactive protein sample (days)2 | 210 ± 26 | 209 ± 36 | 0.72 |
| Maternal age at recruitment <25 years | 268 (52) | 46 (49) | 0.54 |
| Gestational age at randomization <20 weeks | 181 (35) | 39 (41) | 0.26 |
| Mother has ≤ primary school education | 459 (90) | 87 (93) | 0.39 |
| Spending <500 TSh on food per household member per day | 182 (40) | 42 (48) | 0.16 |
| Still breastfeeding at date of CRP sample | 426 (92) | 66 (81) | 0.003 |
| Body Mass Index <18.5 kg/m2 | 50 (10) | 25 (27) | <0.0001 |
| Mid-Upper Arm Circumference <22 cm | 30 (6) | 24 (26) | <0.0001 |
| Hemoglobin <11 g/dl | 220 (45) | 59 (67) | <0.0001 |
| CD4 <200 cells/µl | 38 (8) | 15 (17) | 0.005 |
| Viral load >50,000 copies/ml | 39 (35) | 8 (44) | 0.43 |
CRP – C-reactive protein.
Statistical analyses conducted using t-tests for continuous data and Chi-squared for categorical data.
Mean ± standard deviation.
Maternal Outcomes
Women were followed for a median of 63.2 months (IQR 49.5 to 71.5 months) from the CRP sample date, and 56 (9.5%) women progressed to WHO stage 4, 144 (26.2%) women died from an AIDS-related cause, and 188 (31.0%) women died from any cause. In Kaplan-Meier analyses, the median time to progression to stage 4 or death from any cause was 6.8 and 4.3 years among women with a normal and high CRP concentration, respectively (Figure 1). Women with a high CRP concentration experienced a significantly faster progression to stage 4 or death compared to women with a normal CRP concentration (p=0.002). The median time to progression to stage 4 or death was 6.8 and 5.2 years for women in the 2nd and highest CRP quartiles, respectively. Over 50% of the women in the lowest and 3rd CRP quartiles survived without progression to stage 4 during the follow-up period.
Figure 1.
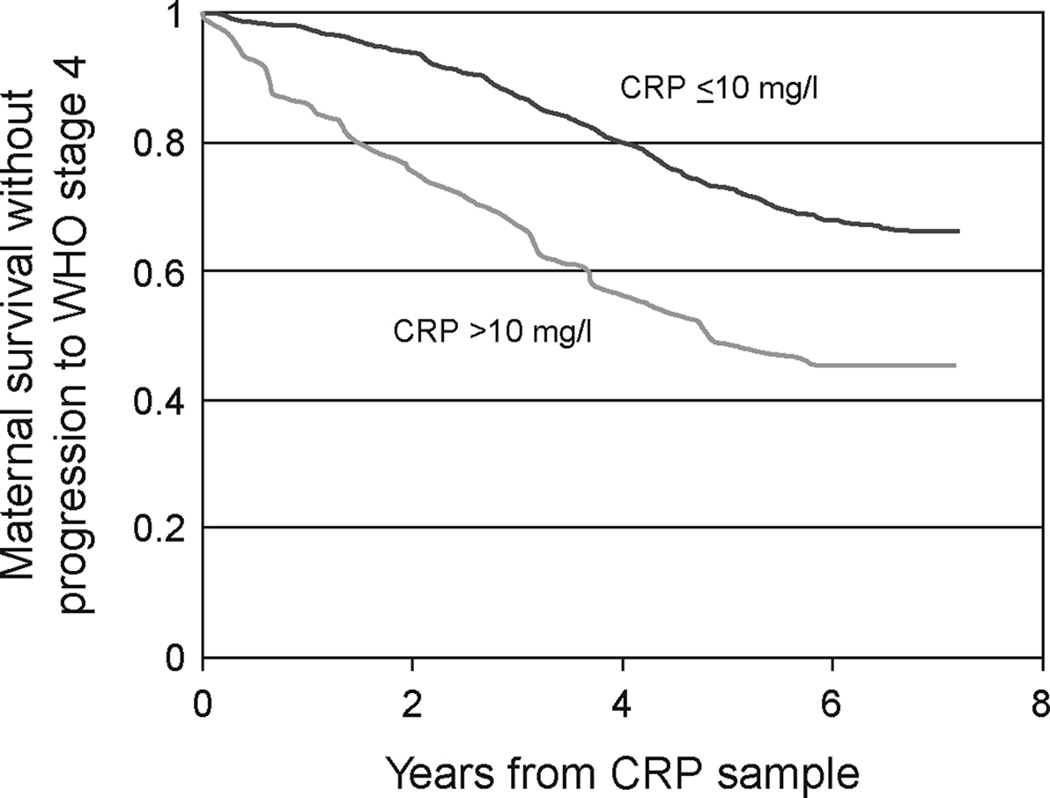
Kaplan-Meier curve of survival without progression to WHO stage 4 among 588 HIV-infected women with a normal (≤10 mg/l) or high (>10 mg/l) C-reactive protein (CRP) concentration.
In univariate proportional hazard analyses, a high CRP concentration was associated with an increased risk of progression to stage 4, mortality from AIDS-related causes, mortality from any cause, progression to stage 4 or death from AIDS-related causes, and progression to stage 4 or death from any cause (Table 2). In multivariate analyses, women with a high CRP concentration had a 1.90 (95% CI 0.94–3.81) greater risk of progression to stage 4, a 1.42-fold (95% CI 0.94–2.12) greater risk of progression to stage 4 or death from AIDS-related causes, and a 1.55-fold (95% CI 1.08–2.23) greater risk of progression to stage 4 or death from any cause after accounting for CD4 count, HIV viral load, and other prognostic variables.
Table 2.
Association between maternal C-reactive protein concentrations (modeled ≤, > 10 mg/l) and adverse maternal and child HIV-related outcomes.1
| Outcomes | Maternal CRP (mg/l) |
No. of Events/ No. at Risk (%) |
Age-adjusted Model2 | Multivariate Model3 | ||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P value | Hazard Ratio (95% CI) |
P value | |||
| Maternal Outcomes | ||||||
| Progression to stage 4 | ≤ 10 | 45/498 (9%) | 1.00 (Reference) | -- | 1.00 (Reference) | -- |
| > 10 | 11/90 (12%) | 1.99 (1.03–3.86) | 0.04 | 1.90 (0.94–3.81) | 0.07 | |
| Death from AIDS-related causes | ≤ 10 | 111/512 (22%) | 1.00 (Reference) | -- | 1.00 (Reference) | -- |
| > 10 | 31/94 (33%) | 1.88 (1.26–2.81) | 0.002 | 1.00 (0.63–1.59) | 0.99 | |
| Death from any cause | ≤ 10 | 147/512 (29%) | 1.00 (Reference) | -- | 1.00 (Reference) | -- |
| > 10 | 41/94 (44%) | 1.88 (1.33–2.66) | 0.0004 | 1.19 (0.80–1.77) | 0.38 | |
| Progression to stage 4 or death from AIDS-related causes | ≤ 10 | 136/498 (27%) | 1.00 (Reference) | -- | 1.00 (Reference) | -- |
| > 10 | 39/90 (43%) | 2.23 (1.56–3.19) | <0.0001 | 1.42 (0.94–2.12) | 0.10 | |
| Progression to stage 4 or death from any cause | ≤ 10 | 168/498 (34%) | 1.00 (Reference) | -- | 1.00 (Reference) | -- |
| > 10 | 49/90 (54%) | 2.26 (1.64–3.12) | <0.0001 | 1.55 (1.08–2.23) | 0.02 | |
| Child Outcomes | ||||||
| Mother to child transmission of HIV by age 2 | ≤ 10 | 145/470 (31%) | 1.00 (Reference) | -- | 1.00 (Reference) | -- |
| > 10 | 29/85 (34%) | 1.17 (0.71–1.91) | 0.54 | 0.84 (0.47–1.50) | 0.56 | |
| Death from any cause by age 2 | ≤ 10 | 82/475 (17%) | 1.00 (Reference) | -- | 1.00 (Reference) | -- |
| > 10 | 34/89 (38%) | 3.03 (1.85–4.96) | <0.0001 | 1.70 (0.86–3.34) | 0.13 | |
| Mother to child transmission HIV or death from any cause by age 2 | ≤ 10 | 172/475 (36%) | 1.00 (Reference) | -- | 1.00 (Reference) | -- |
| > 10 | 43/89 (48%) | 1.66 (1.05–2.62) | 0.03 | 0.94 (0.53–1.67) | 0.83 | |
CRP – C-reactive protein.
Maternal outcomes assessed using Cox proportional hazard models and child outcomes assessed using logistic regression models.
Univariate models adjusted for maternal age <25 years and vitamin trial regimen group.
Multivariate models adjusted for maternal age <25 years, vitamin trial regimen cohort, gestational age <20 weeks at enrollment, maternal education ≤ primary school, spending <500 TSh on food per day, BMI<18.5 kg/m2, middle upper arm circumference <22 cm, hemoglobin <11 g/dl, CD4 count categories (<200, 200–399, 400–599, 600–799, ≥800) cells/µl, and viral load categories (<25,000, 25,000–49,999, ≥50,000) copies/ml. Analyses for child outcomes were additionally adjusted for breastfeeding status at date of CRP sample (yes/no).
Higher quartiles of CRP concentration were also significantly associated with adverse maternal HIV-related outcomes (Table 3). In univariate analyses, the highest two CRP quartiles were significantly associated with all of the adverse maternal outcomes, except for progression to stage 4, when compared to the lowest CRP quartile. In multivariate analyses, women in the highest CRP quartile had a 2.49-fold (95% CI 1.07–5.81) greater risk of progression to stage 4, a 1.88-fold (95% CI 1.13–3.15) greater risk of progression to stage 4 or mortality from AIDS-related causes, and a 2.22-fold (95% CI 1.40–3.50) greater risk of progression to stage 4 or mortality from any cause.
Table 3.
Risk of adverse maternal and child outcomes among 606 HIV-infected women by C-reactive protein quartiles.1
| Outcome | Maternal Quartile of CRP |
No. of Events/ No. at Risk (%) |
Age-adjusted model2 | Multivariate model3 | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P value | P value for trend4 |
Hazard Ratio (95% CI) |
P value | P value for trend4 |
|||
| Maternal Outcome | ||||||||
| Progression to stage 4 | Lowest quartile | 13/146 (9%) | 1.00 (Reference) | -- | 0.14 | 1.00 (Reference) | -- | 0.15 |
| 2nd quartile | 11/150 (7%) | 0.92 (0.41–2.06) | 0.84 | 1.04 (0.42–2.62) | 0.92 | |||
| 3rd quartile | 17/146 (12%) | 1.59 (0.77–3.28) | 0.21 | 1.80 (0.81–3.96) | 0.15 | |||
| Highest quartile | 15/146 (10%) | 1.70 (0.80–3.61) | 0.17 | 2.49 (1.07–5.81) | 0.04 | |||
| Death from AIDS-related Causes | Lowest quartile | 24/150 (16%) | 1.00 (Reference) | -- | 0.013 | 1.00 (Reference) | -- | 0.67 |
| 2nd quartile | 39/153 (25%) | 1.74 (1.04–2.90) | 0.03 | 2.21 (1.26–3.88) | 0.006 | |||
| 3rd quartile | 37/151 (25%) | 1.74 (1.04–2.91) | 0.04 | 1.85 (1.04–3.27) | 0.04 | |||
| Highest quartile | 42/152 (28%) | 2.25 (1.36–3.73) | 0.002 | 1.35 (0.74–2.46) | 0.33 | |||
| Death from any cause | Lowest quartile | 33/150 (22%) | 1.00 (Reference) | -- | 0.0004 | 1.00 (Reference) | -- | 0.29 |
| 2nd quartile | 47/153 (31%) | 1.52 (0.97–2.37) | 0.07 | 1.98 (1.22–3.21) | 0.006 | |||
| 3rd quartile | 48/151 (32%) | 1.63 (1.04–2.54) | 0.03 | 1.64 (0.99–2.70) | 0.05 | |||
| Highest quartile | 60/152 (39%) | 2.32 (1.51–3.56) | 0.0001 | 1.73 (1.05–2.86) | 0.03 | |||
| Progression to stage 4 or death from AIDS-related causes | Lowest quartile | 32/146 (22%) | 1.00 (Reference) | -- | 0.0009 | 1.00 (Reference) | -- | 0.23 |
| 2nd quartile | 44/150 (29%) | 1.47 (0.93–2.32) | 0.10 | 1.85 (1.10–3.08) | 0.02 | |||
| 3rd quartile | 47/146 (32%) | 1.75 (1.11–2.75) | 0.02 | 2.01 (1.23–3.28) | 0.006 | |||
| Highest quartile | 52/146 (36%) | 2.32 (1.49–3.63) | 0.0002 | 1.88 (1.13–3.15) | 0.02 | |||
| Progression to stage 4 or death from any cause | Lowest quartile | 39/146 (27%) | 1.00 (Reference) | -- | <0.0001 | 1.00 (Reference) | -- | 0.02 |
| 2nd quartile | 51/150 (34%) | 1.39 (0.91–2.11) | 0.13 | 1.78 (1.12–2.83) | 0.02 | |||
| 3rd quartile | 58/146 (40%) | 1.74 (1.16–2.61) | 0.008 | 1.87 (1.20–2.93) | 0.006 | |||
| Highest quartile | 69/146 (47%) | 2.49 (1.68–3.71) | <0.0001 | 2.22 (1.40–3.50) | 0.0007 | |||
| Child Outcome | ||||||||
| Mother to child transmission of HIV by age 2 | Lowest quartile | 41/135 (30%) | 1.00 (Reference) | -- | 0.92 | 1.00 (Reference) | -- | 0.11 |
| 2nd quartile | 39/141 (28%) | 0.86 (0.51–1.46) | 0.58 | 0.82 (0.46–1.46) | 0.50 | |||
| 3rd quartile | 51/141 (36%) | 1.25 (0.75–2.09) | 0.39 | 1.19 (0.67–2.13) | 0.55 | |||
| Highest quartile | 43/138 (31%) | 1.07 (0.64–1.81) | 0.79 | 0.62 (0.33–1.16) | 0.14 | |||
| Death from any cause by age 2 | Lowest quartile | 25/137 (18%) | 1.00 (Reference) | -- | 0.003 | 1.00 (Reference) | -- | 0.64 |
| 2nd quartile | 23/142 (16%) | 0.84 (0.45–1.59) | 0.60 | 1.33 (0.54–3.28) | 0.54 | |||
| 3rd quartile | 26/143 (18%) | 0.99 (0.53–1.83) | 0.97 | 1.01 (0.40–2.51) | 0.99 | |||
| Highest quartile | 42/142 (30%) | 1.92 (1.08–3.41) | 0.03 | 1.26 (0.57–2.80) | 0.57 | |||
| Mother to child transmisstion of HIV or death from any cause by age 2 | Lowest quartile | 50/137 (37%) | 1.00 (Reference) | -- | 0.26 | 1.00 (Reference) | -- | 0.15 |
| 2nd quartile | 47/142 (33%) | 0.84 (0.51–1.39) | 0.50 | 0.93 (0.52–1.66) | 0.81 | |||
| 3rd quartile | 59/143 (41%) | 1.22 (0.75–1.98) | 0.44 | 1.16 (0.66–2.06) | 0.60 | |||
| Highest quartile | 59/142 (42%) | 1.28 (0.78–2.08) | 0.33 | 0.68 (0.37–1.26) | 0.22 | |||
CRP - C-reactive protein.
Range of CRP concentration (mg/l) by quartile: lowest quartile (0.02–0.62), 2nd quartile (0.63–1.94), 3rd quartile (1.95–5.16), highest quartile (5.17–351.6).
Maternal outcomes assessed using Cox proportional hazard models and child outcomes assessed using logistic regression models.
Univariate models adjusted for maternal age <25 years and vitamin trial regimen group.
Multivariate models adjusted for maternal age <25 years, vitamin trial regimen cohort, gestational age <20 weeks at enrollment, maternal education ≤ primary school, spending <500 TSh on food per day, BMI<18.5 kg/m2, middle upper arm circumference <22 cm, hemoglobin <11 g/dl, CD4 count categories (<200, 200–399, 400–599, 600799, ≥800) cells/µl, and viral load categories (<25,000, 25,000–49,999, ≥50,000) copies/ml. Analyses for child outcomes were additionally adjusted for breastfeeding status at date of CRP sample (yes/no).
P value for trend among CRP quartile medians (lowest quartile 0.335, 2nd quartile 1.14, 3rd quartile 2.96, highest quartile 18.175).
In further multivariate analyses, CRP was not a significant predictor of progression to stage 4 or mortality from any cause when establishing the follow-up cut-off at one year after the CRP sample date (hazard ratio 1.80, 95% CI 0.66–4.89). However, CRP was a significant predictor of progression to stage 4 or mortality from any cause within two years of the CRP sample date (HR 2.14, CI 1.10–4.18), and within four years of the CRP sample date (HR 1.93, CI 1.27–2.95) despite adjusting for CD4 count, HIV viral load, and other prognostic variables.
Child Outcomes
Among children, 174 (31.4%) acquired HIV and 116 (20.6%) died from any cause before 2 years of age. In univariate analyses, children born to women with a high CRP concentration had a significantly greater risk of mortality, but not mother-to-child transmission of HIV, before 2 years of age (Table 2). In multivariate analyses, children born to women with a high CRP concentration did not have a significantly greater risk of becoming infected with HIV, but had a 3.03-fold (95% CI 1.85–4.96) greater risk of death before reaching 2 years of age.
Only the highest quartile of CRP concentration was significantly associated with child mortality before age 2 when compared to the lowest CRP quartile (Table 3). In multivariate analyses, the higher CRP quartiles were not predictive of child outcomes after adjusting for maternal CD4 count, HIV viral load, and other prognostic variables.
Discussion
In this cohort of HIV-infected women in a resource-poor setting, a clinically elevated CRP concentration was predictive of HIV disease progression and mortality, independent of anthropometric measurements, CD4 cell count, and plasma viral load. In addition, a high post-partum maternal CRP concentration was predictive of child mortality, but not HIV transmission, before the age of 2 years, independent of maternal anthropometric measurements, CD4 cell count, plasma viral load, and breastfeeding status at the time of the serum CRP measurement.
Few studies have examined CRP as a predictor of AIDS or mortality in HIV-infected patients. Among 474 HIV-infected adults in Baltimore/Washington D.C., those with a CRP concentration >2.3 mg/l were significantly more likely to develop clinical AIDS than those with a CRP ≤1.2 mg/l when adjusting for CD4 cell count, plasma viral load and hemoglobin [9]. In another study, CRP concentrations ≥4 mg/l were associated with a 1.93-fold (95% CI 1.12–3.30) greater risk of AIDS-related mortality after adjusting for lean body mass (measured by bioelectrical impedance analysis and adjusted for height) and CD4 cell count, but not HIV plasma viral load, among 129 HIV-positive males in France [10]. Another study found that slightly increased CRP concentrations (4.1–6.0 versus ≤4.0 mg/l), but not a clinically significant CRP concentrations (≥13.6 versus ≤4.0 mg/l), were associated with an increased risk of mortality when adjusting for age, body mass index, serum albumin, CD4 cell count, and HIV-1 RNA among 204 HIV-infected women in New York [11]. This study was limited by the use of a low sensitivity CRP assay. A study among 62 HIV-positive homosexual men with Mycobacterium avium-intracellulare (MAC) infection in Sweden found that patients with a clinically elevated CRP concentration (>10 mg/l) had a significantly longer mean survival (365 days) compared to patients with a normal CRP concentrations (180 days) [12]. Our study expands on these previous studies by having a larger sample size, by examining a clinically established cut-off value for CRP, by extending analyses to include maternal HIV disease progression and adverse child HIV-related outcomes, by quantifying CRP with a high sensitivity assay, and by determining associations in a resource-poor setting.
The clinical significance of a high CRP concentration may be not only in detecting an underlying opportunistic infection among HIV-positive patients, but also in assisting in the diagnosis of infections, particularly among patients with inconclusive chest radiographs [12]. C-reactive protein is a component of the innate immune system and may activate the complement system during infections [17]. In HIV-infected patients, CRP concentrations increase with a range of infections [12,18,19], including pneumococcal community-acquired pneumonia [20] and pulmonary tuberculosis [21]. Patients infected with Pneumocystis carinii pneumonia (PCP), Toxoplasmosis, MAC, and pulmonary tuberculosis have significantly lower increases in CRP concentration compared to patients infected with more common bacterial pathogens [12,19]. However, one study found no significant difference in CRP concentrations between HIV-positives infected with PCP versus a common bacterial-causing pneumonia [22]. While it appears that CRP increases are greatest in those with bacterial infections [23], clinical interpretations may need to be adjusted for patient with a very low CD4 count (<50 cell/µl), since they have been observed to mount a smaller CRP response to PCP infections [22].
Although CRP could simply be a marker for opportunistic infections or underlying disease, high circulating concentrations of CRP may contribute to promoting disease progression and increasing mortality among HIV-infected patients. The basic metabolic disturbances that lead to HIV-associated weight loss and wasting, such as gastrointestinal malabsorption, altered metabolism, and changes in protein production, appear to represent an adaptive response to a generalized inflammatory state [24–27]. Weight loss and wasting are strong independent predictors of HIV-related morbidity and mortality [10,28,29]. In addition, an inflammatory process may lead to depleted vitamin and mineral concentrations, which may in turn impact HIV disease progression. High CRP concentrations have been shown to be associated with depleted stores of several vitamins and minerals among HIV-negative [30–33] and HIV-positive [34,35] adults. Deficiencies of several vitamins and minerals have also been associated with disease progression and mortality among HIV-positive adults [35–37].
Whether CRP is a marker of infectious diseases or a contributor to metabolic disturbances, resolution of acute inflammation appears to be important for survival. Among an HIV-positive cohort being treated for a MAC infection, 42 patients who normalized CRP concentrations after treatment had a significantly longer median survival than the 20 patients who did not normalize CRP concentrations after treatment (p=0.016) [12].
We noted an association between high maternal CRP concentration and increased risk of child mortality. As described, high CRP may be a marker or cause of accelerated maternal HIV disease progression, which in turn may lead to deficient transfer of passive immunity and nutrients to the child. This may thus lower immune function among children and subsequently elevate the risk of mortality [38, 39]. Poor caring practices due to maternal morbidity or death is a plausible social explanation leading to poor child outcomes. A recent study found that HIV-exposed but HIV-uninfected children had an increased risk of mortality if their HIV-infected mothers had advanced disease, and the most common cause of infant mortality was pneumonia and/or sepsis [40].
In our laboratory in Tanzania, a CD4 count and an HIV viral load cost approximately US $10 and $40 per blood sample, respectively. Meanwhile, a CRP test costs approximately $2 per blood sample. Thus, a CRP test is approximately one-fifth the cost of a CD4 count and one-twentieth the cost of a HIV viral load. In our multivariate analyses, a high CRP was better than either CD4 count <200 and viral load ≥50,000 copies for predicting maternal disease progression to Stage 4. C-reactive protein was not better than CD4 count or viral load in predicting maternal mortality outcomes, but CRP remained a very strong and almost comparable predictor. In addition, a high CRP was better than viral load ≥50,000 copies for predicting child mortality before age two. Therefore, CRP may at least be a better and less expensive marker of maternal HIV disease progression and child mortality before age two, as compared to CD4 count or HIV viral load, in this resource-poor setting.
Our study population included women and children who were not taking antiretroviral therapy, and to our knowledge no studies have assessed CRP concentrations as a predictor of adverse HIV-related outcomes among people taking highly-active antiretroviral therapy (HAART). Few studies have examined the differences and longitudinal changes of CRP concentrations among HIV-infected patients taking antiretroviral therapy. One cross-sectional study found no significant difference in CRP concentrations between HIV-positive adolescents taking and not taking antiretroviral therapy [41]. Two longitudinal studies have suggested that antiretroviral therapy may significantly increase CRP concentrations despite expected immunologic and virologic responses [42,43]. These studies indicate that an inflammatory state may persist after the introduction of antiretroviral therapy and may continue to increase the risk of HIV-associated morbidity and mortality in the post-HAART era.
In conclusion, these results demonstrate that CRP is an independent predictor of adverse maternal and child HIV-related outcomes among HIV-positive mothers not taking antiretroviral therapy in a resource-poor setting. Future studies should assess associations between intrapersonal longitudinal changes in CRP concentrations and risks of adverse HIV-related outcomes, and evaluate associations between a high CRP concentration and risks of adverse maternal and child HIV-related outcomes among patients taking HAART. Whether CRP is simply a marker of disease or part of the disease process itself, CRP may be an important and inexpensive prognostic indicator for HIV-infected patients, particularly in resource-poor settings.
Acknowledgements
We would like to express our gratitude to the women and children who participated in this study.
Supported by grants from the National Institute of Child Health and Human Development (R01 32257) and the Fogarty International Center, National Institutes of Health. PKD was supported as a National Institutes of Health Fogarty-Ellison Clinical Research Fellow.
Footnotes
Author’s Contributions
PKD and RK analyzed and interpreted the data, and PKD wrote the initial draft of the manuscript. WWF and GM are principal investigators of the Tanzania Vitamin Supplementation Trial and contributed to the study design and its implementation. FM contributed to the study implementation. All authors participated in the manuscript preparation and revision, and read and approved the final manuscript.
References
- 1.Rabkin M, El-Sadr W, Katzenstein DA, Mukherjee J, Masur H, Mugyenyi P, et al. Antiretroviral treatment in resource-poor settings: clinical research priorities. Lancet. 2002;360:1503–1505. doi: 10.1016/S0140-6736(02)11478-4. [DOI] [PubMed] [Google Scholar]
- 2.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. New Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 3.Haverkate F, Thompson SG, Pyke SDM, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. Lancet. 1997;349:462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [Erratum, N Engl J Med1997; 337:356]. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 6.Falconer JS, Fearon KCH, Ross JA, Elson R, Wigmore SJ, Garden OJ, et al. Acute phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–2082. doi: 10.1002/1097-0142(19950415)75:8<2077::aid-cncr2820750808>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, McMillan DC. Association between plasminogen activator inhibitor-1 and survival in colorectal cancer: measuring C-reactive protein may be more useful. Brit Med J. 1998;317:750. doi: 10.1136/bmj.317.7160.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roblot F, Godet C, Le Moal G, Garo B, Faouzi Souala M, Dary M, et al. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii Pneumonia in Immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2002;21:523–531. doi: 10.1007/s10096-002-0758-5. [DOI] [PubMed] [Google Scholar]
- 9.Lau B, Sharrett AR, Kingsley LA, Post W, Palella FJ, Visscher B, et al. C-reactive protein is a marker for Human Immunodeficiency Virus disease progression. Arch Intern Med. 2006;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 10.Melchior JC, Niyongabo T, Henzel D, Durack-Bown I, Henri SC, Boulier A. Malnutrition and wasting, immunodepression, and chronic inflammation as independent predictors of survival in HIV-infected patients. Nutrition. 1999;15:865–869. [PubMed] [Google Scholar]
- 11.Feldman JG, Goldwasser P, Holman S, DeHovitz J, Minkoff H. C-Reactive protein is an independent predictor of mortality in women with HIV-1 infection. J Acquir Immune Defic Syndr. 2003;32:210–214. doi: 10.1097/00126334-200302010-00014. [DOI] [PubMed] [Google Scholar]
- 12.Grutzmeier S, Sandstrom E. C-reactive protein levels in HIV complicated by opportunistic infections and infections with common bacterial pathogens. Scand J Infect Dis. 1999;31:229–234. doi: 10.1080/00365549950163491. [DOI] [PubMed] [Google Scholar]
- 13.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, Hunter DJ. Rationale and design of the Tanzania Vitamin and HIV Infection Trial. Control Clin Trials. 1999;20:75–90. doi: 10.1016/s0197-2456(98)00045-2. [DOI] [PubMed] [Google Scholar]
- 14.Young B, Gleeson M, Cripps AW. C-reactive protein: a critical review. Pathology. 1991;23:118–124. doi: 10.3109/00313029109060809. [DOI] [PubMed] [Google Scholar]
- 15.Fawzi WW, Msamanga G, Hunter D, Renjifo B, Antelan G, Bang H, et al. Randomized Trial of Vitamin Supplements in Relation to Transmission of HIV-1 Through Breastfeeding and Early Child Mortality. AIDS. 2002;16:1935–1944. doi: 10.1097/00002030-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 16.Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. New Engl J Med. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 17.Volanakis JE. Acute phase proteins in rheumatic disease. In: Koopman WJ, editor. Arthritis and allied conditions: a textbook of rheumatology. 13th ed. Baltimore: Lippincott Williams & Wilkins; 1997. pp. 505–514. [Google Scholar]
- 18.Noursadeghi M, Miller RF. Clinical value of C-reactive protein measurements in HIV-positive patients. Int J STD & AIDS. 2005;16:438–441. doi: 10.1258/0956462054094006. [DOI] [PubMed] [Google Scholar]
- 19.Schleicher GK, Herbert V, Brink A, Martin S, Maraj R, Galpin JS, et al. Procalcitonin and C-reactive protein levels in HIV-positive subjects with tuberculosis and pneumonia. Eur Respir J. 2005;25:688–692. doi: 10.1183/09031936.05.00067604. [DOI] [PubMed] [Google Scholar]
- 20.Lala SG, Madhi SA, Pettifor JM. The discriminative value of C-reactive protein levels in distinguishing between community-acquired bacteraemic and respiratory virus-associated lower respiratory tract infections in HIV-1-infected and –uninfected children. Ann Trop Paediatr. 2002;22:271–279. doi: 10.1179/027249302125001570. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Wiktor S, Coulibaly D, Ackah AN, Lal RB. Serum C-reactive protein and detection of tuberculosis in persons co-infected with the human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2001;95:41–42. doi: 10.1016/s0035-9203(01)90328-1. [DOI] [PubMed] [Google Scholar]
- 22.Storgaard M, Laursen AL, Andersen PL. The C-reactive protein responses in HIV-infected patients with pneumonia. Scand J Infect Dis. 1993;25:305–309. doi: 10.3109/00365549309008503. [DOI] [PubMed] [Google Scholar]
- 23.Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci. 1982;389:406–418. doi: 10.1111/j.1749-6632.1982.tb22153.x. [DOI] [PubMed] [Google Scholar]
- 24.Grunfeld C, Pang M, Shimizu L, Shigenaga JK, Jensen P, Feingold KR. Resting energy expenditure, caloric intake, and short-term weight change in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Am J Clin Nutr. 1992;55:455–460. doi: 10.1093/ajcn/55.2.455. [DOI] [PubMed] [Google Scholar]
- 25.Godfried MH, van der Poll T, Jansen J, Romijin JA, Schattenkerk JK, Endert E, et al. Soluble receptors for tumour necrosis factor: a putative marker of disease progression in HIV infection. AIDS. 1993;7:33–36. [PubMed] [Google Scholar]
- 26.Belec L, Meillet D, Hernvann A, Gresenguet G, Gherardi R. Differential elevation of circulating interleukin-1β, tumor necrosis factor alpha, and interleukin-6 in AIDS-associated cachectic states. Clin Diag Lab Immun. 1994;1:117–120. doi: 10.1128/cdli.1.1.117-120.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimaniol AC, Zylberberg H, Zavala F, Viard JP. Inflammatory cytokines and inhibitors in HIV infection: correlation between interleukin-1 receptor antagonist and weight loss. AIDS. 1996;10:1349–1356. doi: 10.1097/00002030-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler DA, Gibert CL, Launer CA, Muurahainen N, Elion RA, Abrams DI, et al. Weight loss as a predictor of survival and disease progression in HIV infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:80–85. doi: 10.1097/00042560-199805010-00012. [DOI] [PubMed] [Google Scholar]
- 29.Jones CY, Hogan JW, Snyder B, Klein RS, Rompalo A, Schuman P, et al. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis. 2003;37(Suppl 2):S69–S80. doi: 10.1086/375889. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES, Liu S, Mannino DM, Giles WH, Smith SJ. C-reactive protein concentrations and concentrations of blood vitamins, carotenoids, and selenium among United States adults. Eur J Clin Nutr. 2003;57:1157–1163. doi: 10.1038/sj.ejcn.1601667. [DOI] [PubMed] [Google Scholar]
- 31.Maehira F, Luyo GA, Miyagi I, Oshiro M, Yamane N, Kuba M, et al. Alterations of serum selenium concentrations in the acute phase of pathological conditions. Clin Chim Acta. 2002;316:137–146. doi: 10.1016/s0009-8981(01)00744-6. [DOI] [PubMed] [Google Scholar]
- 32.Ghayour-Mobarhan M, Taylor A, New SA, Lamb DJ, Ferns GA. Determinants of serum copper, zinc, and selenium in healthy subjects. Ann Clin Biochem. 2005;42:364–375. doi: 10.1258/0004563054889990. [DOI] [PubMed] [Google Scholar]
- 33.Koyanagi A, Kuffo D, Gresely L, Shenkin A, Cuevas LE. Relationships between serum concentrations of C-reactive protein and micronutrients, in patients with tuberculosis. Ann Trop Med Parasitol. 2004;98:391–399. doi: 10.1179/000349804225003424. [DOI] [PubMed] [Google Scholar]
- 34.Baeten JM, McClelland RS, Richardson BA, Bankson DD, Lavreys L, Wener MH, et al. Vitamin A deficiency and the acute phase response among HIV-1-infected and – uninfected women in Kenya. J Acquir Immune Syndr. 2002;31:243–249. doi: 10.1097/00126334-200210010-00016. [DOI] [PubMed] [Google Scholar]
- 35.Look MP, Rockstroch JK, Rao GS, Kreuzer KA, Barton S, Lemoch H, et al. Serum selenium versus lymphocyte subsets and markers of disease progression and inflammatory response in human immunodeficiency virus-infection. Biol Trace Elem Res. 1997;56:31–41. doi: 10.1007/BF02778982. [DOI] [PubMed] [Google Scholar]
- 36.Semba RD, Tang AM. Micronutrients and the pathogenesis of human immunodeficiency virus infection. Br J Nutr. 1999;81:181–189. doi: 10.1017/s0007114599000379. [DOI] [PubMed] [Google Scholar]
- 37.Kupka R, Fawzi W. Zinc nutrition and HIV infection. Nutr Rev. 2002;60:69–79. doi: 10.1301/00296640260042739. [DOI] [PubMed] [Google Scholar]
- 38.Pitt J, Henrard D, FitzGerald G, Mofenson L, Lew J, Hillyer G, et al. Human immunodeficiency virus (HIV) type 1 antibodies in perinatal HIV-1 infection: Association with human HIV-1 transmission, infection, and disease progression. For the Women and Infants Transmission Study. J Infect Dis. 2000;182:1243–1246. doi: 10.1086/315809. [DOI] [PubMed] [Google Scholar]
- 39.Fawzi WW, Msamanga GI, Wei R, Spiegelman D, Antelman G, Villamor E, et al. Effect of providing vitamin supplements to human immunodeficiency virus-infected, lactating mothers on the child’s morbility and CD4+ cell counts. Clin Infect Dis. 2003;36 doi: 10.1086/374223. 1053-1-62. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Scott N, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005;41:1654–1661. doi: 10.1086/498029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephensen CB, Marquis GS, Douglas SD, Wilson CM. Immune activation and oxidative damage in HIV-positive and HIV-negative adolescents. J Acquir Immune Defic Syndr. 2005;38:180–190. doi: 10.1097/00126334-200502010-00009. [DOI] [PubMed] [Google Scholar]
- 42.Henry K, Kitch D, Dube M, Zackin R, Parker RA, Sprecher D, et al. C-reactive protein levels over time and cardiovascular risk in HIV-infected individuals on an indinivir-based regimen: AIDS Clinical Trials Group 5056s. AIDS. 2004;18:2434–2437. [PubMed] [Google Scholar]
- 43.Heggelund L, Mollnes TE, Ueland T, Christophersen B, Aukrust P, Froland SS. Mannose-binding lectin in HIV infection: relation to disease progression and highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;32:354–361. doi: 10.1097/00126334-200304010-00002. [DOI] [PubMed] [Google Scholar]


