Abstract
Intracranial atherosclerosis is one of the most common causes of stroke worldwide and is associated with a high risk of recurrent stroke. New therapeutic approaches to treat this high-risk disease include dual antiplatelet treatment, intensive management of risk factors, and endovascular therapy. Early data from randomised trials indicate that aggressive medical therapy is better than stenting for prevention of recurrent stroke in high-risk patients with atherosclerotic stenosis of a major intracranial artery. Nevertheless, there are subgroups of patients who remain at high risk of stroke despite aggressive medical therapy. Further research is needed to identify these high-risk subgroups and to develop more effective treatments. Non-invasive vascular imaging methods that could be used to identify high-risk patients include fractional flow on magnetic resonance angiography (MRA), quantitative MRA, and high-resolution MRI of the atherosclerotic plaque. Alternative therapies to consider for future clinical trials include angioplasty alone, indirect surgical bypass procedures, ischaemic preconditioning, and new anticoagulants (direct thrombin or Xa inhibitors).
Introduction
Intracranial atherosclerotic stenosis (ICAS) of a major intracranial artery is one of the most common causes of stroke worldwide and is associated with a high risk of recurrent stroke compared with other stroke subtypes. ICAS is particularly prevalent in black, Asian, Hispanic, and Indian populations, and in some Arabic countries, which suggests that the global burden of stroke from ICAS is likely to grow as populations continue to expand in regions most affected by the disease.1
Recent clinical trials have improved understanding of risk factors associated with stroke recurrence, imaging characteristics that are associated with prognosis, and treatments that significantly reduce stroke recurrence in patients with ICAS. Although the results of these trials are changing the standard of care for patients with ICAS, they also emphasise the need for further research into identification of patients at highest risk of stroke from ICAS and development of new therapies to lower the risk of stroke in these patients. In this Review, we discuss the findings from these studies, focusing on randomised therapeutic trials. We also discuss novel imaging techniques that are being developed to identify the patients at highest risk of stroke and new therapeutic strategies that might improve the prognosis of these high-risk patients.
Epidemiology and risk factors for recurrent stroke
ICAS causes about 5–10% of strokes in white people, 15–29% of transient ischaemic attacks or strokes in black people, and up to 30–50% of strokes in Asian people.1–6 The frequency of ICAS as a cause of stroke also seems to be higher in northern India and Egypt than in white populations.7,8 Potential explanations for racial and ethnic differences in the prevalence of ICAS include genetic susceptibility of some racial and ethnic groups, and differences in lifestyle and risk factor profiles between races.2,9–12
Traditional risk factors associated with ICAS include hypertension, smoking, diabetes mellitus, and hyperlipidaemia. 5,13–16 In the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial, the most important modifiable risk factors for an increased risk of recurrent stroke and vascular events associated with ICAS were raised blood pressure and cholesterol concentrations.17 In the trial, which enrolled 569 patients with 50–99% symptomatic ICAS, the risk factors most strongly associated with recurrent ischaemic stroke and other major vascular events were mean systolic blood pressure greater than 140 mm Hg and mean cholesterol concentration greater than 5·20 mmol/L (200 mg/dl) during follow-up.17–19
In a substudy of the Trial of cilOstazol in Symptomatic intracranial Stenosis 2 (TOSS-2) investigators examined potential lipoprotein predictors of ICAS progression in 230 patients with symptomatic stenosis of the middle cerebral artery and the basilar artery. Increased apolipoprotein B/A-I from baseline was associated with progression of stenosis on magnetic resonance angiography (MRA), whereas increased HDL concentrations were associated with stable stenosis.20 Other risk factors or biomarkers associated with an increased risk of recurrent stroke or progression of intracranial atherosclerosis include the metabolic syndrome,21,22 reduced adiponectin,23 increased lipoprotein-associated phospholipase A2,24 and increased C-reactive protein, E-selectin, plasminogen activator inhibitor-1, and lipoprotein (a).25
Some imaging characteristics, such as degree of stenosis and collateral circulation, affect risk of recurrent events and outcome in patients with ICAS. In the WASID trial, patients with at least 70% stenosis of a major intracranial artery had an increased risk of recurrent stroke in the territory of the stenosis compared with patients with 50–69% stenosis.26 However, the presence of robust collaterals in patients with greater than or equal to 70% stenosis mitigated the risk of recurrent stroke.27 In another study of 69 patients with symptomatic ICAS, Lau and colleagues28 showed that robust antegrade flow and good collaterals were protective against stroke.
The risk of recurrent stroke in patients presenting with transient ischaemic attacks or stroke also depends on how recently the ischaemic events occurred. In the WASID trial, patients whose qualifying event for the trial occurred 17 days or fewer before enrolment (the median time to enrolment in the trial) had a significantly higher risk of recurrent stroke during follow-up than did patients whose qualifying event for the trial occurred more than 17 days (up to 90 days) before enrolment.26
Mechanisms of stroke associated with ICAS
There are three main hypothesised mechanisms of stroke related to ICAS: hypoperfusion, artery-to-artery embolism, and plaque extension over small penetrating artery ostia (also known as branch atheromatous disease).27,29 Combinations of these ischaemic mechanisms can also occur—eg, when hypoperfusion prevents clearing of a distal embolus.30–33
The underlying mechanism of stroke is typically inferred by characteristics on neuroimaging. For example, ischaemic infarcts in a watershed distribution on brain imaging suggest hypoperfusion through a highly stenotic artery, whereas a distal wedge-shaped territorial infarct might indicate artery–artery embolism. High-resolution MRI, an emerging imaging method, can be used to identify plaque extension over small penetrating artery ostia, which can result in lacunar infarctions. Importantly, the initial stroke mechanism in ICAS might be a predictor of the mechanism of subsequent stroke or the risk of recurrent stroke. Khan and colleagues34 analysed patients in the WASID trial with lacunar strokes at study entry to establish whether they were more likely to have subsequent lacunar strokes during follow-up. In that post-hoc analysis, the rates of recurrent stroke were similar in patients whose qualifying events for the trial were lacunar versus non-lacunar strokes. In patients whose qualifying strokes were lacunar, recurrent strokes within the same vascular territory were usually non-lacunar and distal to the stenotic intracranial artery.34
Diagnostic imaging
Diagnostic methods used to identify ICAS include transcranial Doppler (TCD) ultrasound, MRA, CT angiography (CTA), conventional cerebral angiography, and high-resolution MRI. TCD, MRA, CTA, and high-resolution MRI are non-invasive methods that provide safer and less expensive ways to assess the intracranial arteries than conventional cerebral angiography; however, the accuracy of these methods is less clearly established. The Stroke Outcomes and Neuroimaging of Intracranial Atheroclerosis (SONIA) trial assessed the accuracy of TCD and MRA compared with catheter cerebral angiography35 and showed that TCD and MRA had high negative predictive values (86–91%) but low positive predictive values (36–59%). These data suggest that TCD and MRA are useful screening tests for exclusion of intracranial arterial stenosis, but are unreliable to establish diagnosis of ICAS and estimate the severity of stenosis (figure 1). Other studies in which catheter angiography was used as the standard reference suggest that CTA is more accurate than MRA for the diagnosis of ICAS36 and that CTA has a high sensitivity and specificity for detection of 50% or higher ICAS.37 However, neither CTA nor any of the other non-invasive diagnostic tests accurately measure the degree (or percentage) of intracranial stenosis. Conventional cerebral angiography is therefore the gold standard for diagnosis and quantification of luminal stenosis of the intracranial circulation,38 which is a key prognostic indicator (figure 2).
Figure 1. Magnetic resonance angiography showing a flow gap in the right middle cerebral artery in a patient with a recent right hemisphere infarct.
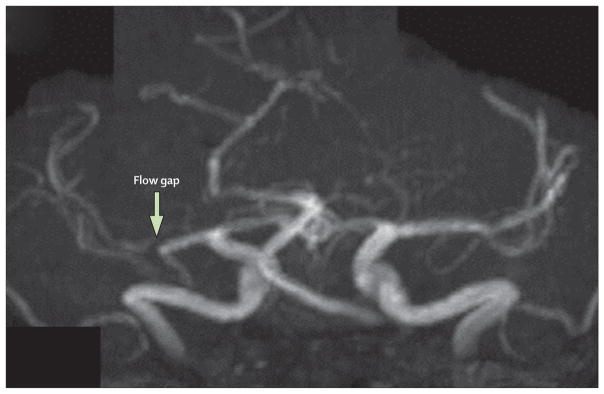
This gap suggests a flow-limiting stenosis, but the degree of stenosis cannot be accurately measured.
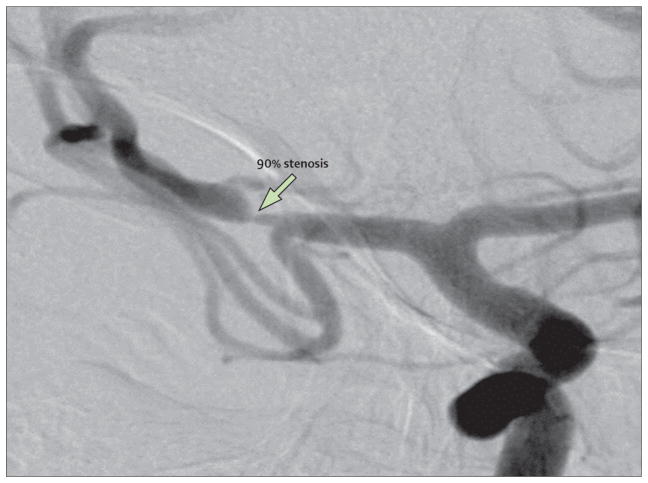
Figure 2. Catheter angiogram showing 90% stenosis of the right middle cerebral artery in the patient whose magnetic resonance angiography is shown in figure 1.
If the patient’s stroke occurred within 30 days, this degree of stenosis is associated with a particularly high risk of recurrent stroke (as high as 23% at 1 year, based on data from the WASID trial).19
Treatment
Antithrombotic therapy
Anticoagulation was first reported as a treatment for symptomatic ICAS in 1955.39 Subsequently, data from a retrospective study suggested that warfarin was more effective than aspirin for stroke prevention in patients with symptomatic ICAS.40 However, data from WASID (a double-blinded, randomised trial comparing aspirin [1300 mg per day] with warfarin [target international normalised ratio (INR) 2–3]) showed no benefit of warfarin over aspirin for prevention of stroke and vascular death in patients with ICAS. Aspirin was also shown to be safer than warfarin, with a lower rate of death and major haemorrhage than warfarin.19 Some subgroups of patients with symptomatic ICAS, such as those with severe (70–99%) stenosis, vertebrobasilar stenosis, or previous stroke symptoms on antithrombotic therapy (so-called medical failures),41 were previously thought to benefit from anticoagulation therapy; however, findings from the WASID study showed that none of these subgroups has a significant benefit from warfarin.41,42
The use of short-term dual antiplatelet therapy (aspirin and clopidogrel) could be particularly effective at lowering the early risk of stroke recurrence in patients with ICAS. In the CLopidogrel plus Aspirin for Infarction Reduction (CLAIR) study,43 patients with recently (≤7 days) symptomatic ICAS who were given clopidogrel (300 mg for the first day, then 75 mg per day) plus aspirin (75–160 mg per day) had significantly lower rates of microembolic signals detected by use of TCD on day 2 and day 7 after randomisation than did those given aspirin (75–160 mg per day) alone. Additionally, when the recurrent stroke events in the CLAIR study were combined in a weighted analysis with the events from the similarly designed Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial44 (limited to patients with recently [within 3 months] symptomatic >50% extracranial carotid stenosis), patients given aspirin alone had significantly more recurrent stroke events than did those given aspirin and clopidogrel combined.45
The use of short-term combination aspirin and clopidogrel followed by aspirin alone is also supported by the early results of the NIH-funded multicentre randomised Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial.45 In SAMMPRIS, patients with 70–99% ICAS who had had a stroke or transient ischaemic attack within the preceding 30 days were randomly assigned to aggressive medical management plus angioplasty and stenting (PTAS) or aggressive medical management alone for stroke prevention. Aggressive medical management included protocol-driven intensive management of blood pressure and LDL concentration, with target levels of less than 140 mm Hg (<130 mm Hg in patients with diabetes) for systolic blood pressure and LDL lower than 1·81 mmol/L, use of a lifestyle modification programme, and the combination of aspirin (325 mg per day) and clopidogrel (75 mg per day) for 90 days followed by aspirin (325 mg per day) alone for the remainder of the trial. Enrolment in SAMMPRIS was halted early in 2011 because of the high rate of periprocedural stroke in the stenting arm, although follow-up of enrolled patients will end in 2013. Patients in the aggressive medical management alone group had a 30-day rate of stroke or death of 5·8%, which is substantially lower than the 10·7% rate at 30 days in patients in the WASID trial (with the same entry criteria as SAMMPRIS) who were given aspirin (1300 mg per day) or warfarin (target INR 2–3) and usual blood pressure and LDL management.46 Because the effect of intensive risk factor management and a lifestyle modification programme on stroke recurrence within the first 30 days would be expected to be modest, the lower rate of stroke at 30 days in SAMMPRIS than in WASID might be largely driven by early use of dual antiplatelet treatment in SAMMPRIS.
The antiplatelet agent cilostazol, a phosphodiesterase inhibitor, might decrease progression of atherosclerosis in patients with symptomatic middle cerebral and basilar artery stenosis. Kwon and colleagues47 randomly assigned 135 individuals to either daily cilostazol (200 mg per day) plus aspirin (100 mg per day) or placebo plus aspirin (100 mg per day). Disease progression of symptomatic ICAS, as measured by MRA and TCD at 6 months, was significantly lower in the cilostazol group than in the placebo group. No individuals in either group had strokes or transient ischaemic attacks. In a follow-up trial, 457 patients with symptomatic middle cerebral or basilar artery stenosis were randomly assigned to cilostazol (100 mg twice daily) plus aspirin (75–150 mg per day) or clopidigrel (75 mg per day) plus aspirin (75–150 mg per day) to establish the number of new ischaemic lesions on MRI at 7 months. New ischaemic lesions (18·7% vs 12·0%; p=0·078) or haemorrhagic events (0·9% vs 2·6%; p=0·163) did not differ significantly between the cilostazol and clopidogrel groups.48 No data have been published for the superiority or equivalence of other antiplatelet regimens such as monotherapy with clopidigrel, cilostazol, or extended release dipyridamole, or the combination of dipyridamole and aspirin for stroke prevention in patients with symptomatic ICAS.
Risk factor modification
Results of secondary stroke prevention trials focusing on lowering of LDL concentrations or blood pressure showed significant reductions in recurrent stroke risk with a statin49 and angiotensin-converting-enzyme (ACE) inhibitor.50 However, these trials were undertaken in patients with heterogeneous causes of stroke. Data for the specific effect of risk factor control on risk of recurrent stroke in patients with ICAS are based on post-hoc analyses of the WASID and SAMMPRIS trials. The results of WASID suggested that patients with poorly controlled systolic blood pressure (>140 mm Hg) and cholesterol (>5·20 mmol/L) during follow-up had the highest rates of major vascular events, including recurrent stroke.17 By contrast with the common practice of maintenance of slightly raised blood pressure in patients with ICAS to reduce the risk of stroke from distal hypoperfusion, increased mean systolic blood pressures during follow-up in WASID did not lower the risk of stroke in the territory of the stenotic artery, and actually increased the risk of recurrent stroke overall.18
Additional supporting evidence for the role of risk factor control in stroke prevention in patients with ICAS comes from the SAMMPRIS trial. Patients in the aggressive medical management alone group of the SAMMPRIS trial had a much lower rate of stroke than expected (5·8% at 30 days and 12·2% at 1 year) compared with patients in the WASID trial who met the SAMMPRIS entry criteria and received aspirin or warfarin and usual management of vascular risk factors (10·7% at 30 days and 25% at 1 year).46 The lower risk in patients in the SAMMPRIS trial from 90 days after enrolment (when clopidogrel was stopped) to 1 year compared with risk in patients in the WASID trial suggests that intensive risk factor management in SAMMPRIS had an important role, but this effect will only become clearer at the end of the trial when analyses correlating risk factor control with outcomes will be done.
Surgical treatment
Extracranial to intracranial bypass surgery was the most commonly used and most thoroughly studied surgical technique for stroke prevention in patients with symptomatic ICAS. The extracranial to intracranial bypass trial,51 undertaken in the 1980s, was a prospective, international, multicentre, randomised trial comparing extracranial to intracranial bypass (superficial temporal artery to the middle cerebral artery) and medical therapy in 1377 patients with extracranial carotid occlusion or intracranial carotid or middle cerebral artery stenosis. The procedure did not lower the rate of stroke compared with aspirin in the group as a whole, and was associated with a worse outcome than was aspirin alone in patients with middle cerebral artery stenosis.51,52 On the basis of these findings, extracranial to intracranial bypass has been abandoned as a treatment for the prevention of stroke in patients with symptomatic anterior circulation ICAS. Bypass has been done for vertebrobasilar insufficiency, but available data from case series suggest a high complication rate.53
Endovascular treatment
Endovascular treatment emerged as a potential option for stroke prevention for ICAS in the 1980s. Angioplasty alone was typically used to treat severe ICAS in patients with recurrent ischaemic events on medical therapy. The outcome data with angioplasty have largely been limited to single-centre, observational, retrospective reports that show periprocedural stroke rates of 4–50%.54–65 Some of the variability in the outcome data is attributable to the heterogeneity of the patients treated. Generally, lower complication rates were reported in less acute cases whereas higher rates of stroke and mortality were recorded in patients with unstable symptoms. Device development and improvements in the technical aspects of the procedure, such as the application of undersized angioplasty balloons and a slow balloon inflation technique, have shown more promising results.59,64,65 Nevertheless, there are no prospective, multicentre, externally adjudicated studies on angioplasty alone to treat ICAS and no data comparing patients treated with angioplasty alone versus concurrent medically treated controls. Angioplasty alone is often associated with immediate elastic recoil of treated arteries, residual post-procedure stenosis in as many as 50% of treated patients, restenosis, and dissection.
Because of these limitations of angioplasty and the success of stenting in the coronary circulation, stenting became the preferred endovascular treatment for ICAS for most interventionists. Initial experience with percutaneous angioplasty and stenting (PTAS) with coronary balloon expandable stents showed improved post-treatment luminal diameters compared with angioplasty alone; however, difficulty in navigation of the intracranial vessels, and trauma during balloon inflation and deployment of the stents, resulted in high morbidity and mortality rates.66–68 Drug-eluting stents are commonly used to prevent restenosis after stenting in the coronary circulation and have been used for this purpose for intracranial stenosis in a few small, single-centre studies, which show conflicting results for the incidence of restenosis.69,70 Moreover, the US Food and Drug Administration (FDA) did not approve an investigational device exemption to use these stents in a pilot study of patients with intracranial stenosis because the safety of drug elution in the cerebral circulation has not been established.
In 2005, the FDA approved the self-expanding Wingspan stent (Stryker Neurovascular, Freemont, CA, USA) for use under the humanitarian device exception in medically refractory patients with transient ischaemic attack or stroke secondary to 50–99% stenosis of a major intracranial artery. This approval was based on findings from a European and Asian study of 45 patients.71 Subsequently, the results of two multicentre registry studies in the USA (the National Institutes of Health [NIH]-sponsored Wingspan registry and the US Wingspan registry) suggested that intracranial PTAS with the Wingspan stent could be done with high technical success rates and with 30-day stroke rates of 6–9%.72,73
As noted earlier, the SAMMPRIS trial, which began enrolment in 2008, assessed high-risk patients with 70–99% ICAS who had had a stroke or transient ischaemic attack within the preceding 30 days to establish whether aggressive medical management plus angioplasty and stenting with the Wingspan system was superior to aggressive medical management alone for stroke prevention. SAMMPRIS had enrolled 451 patients before enrolment was stopped in April, 2011, because of the high risk of periprocedural stroke and death in the PTAS group. The 30-day rate of stroke or death was 14·7% in the PTAS group and 5·8% in the medical group. The 1-year primary endpoint (30-day rate of stroke and death and ischaemic stroke after 30 days) rates were 20·0% in the PTAS group and 12·2% in the medical group. Contrary to the investigators’ hypothesis, findings from SAMMPRIS showed that aggressive medical management was superior to PTAS in the treatment of patients with high-risk symptomatic ICAS.46
In an attempt to better understand the mechanism of periprocedural complications associated with PTAS in the SAMMPRIS trial, investigators undertook a detailed review of the PTAS patients who had had intracerebral haemorrhage, subarachnoid haemorrhage, ischaemic stroke, or cerebral infarct with temporary signs within 30 days of treatment.74 The results of these analyses showed that three patients had ischaemic stroke during the diagnostic angiogram (although two were deemed to be unconnected with the procedure); 21 patients had either an ischaemic stroke (n=19) or cerebral infarct with temporary signs (n=2) within 30 days of PTAS (15 of the ischaemic strokes occurred in the perforator territories of the stenotic arteries after successful angioplasty and stenting, and many of these occurred in the basilar artery [n=8]); six patients had subarachnoid haemorrhage (three from wire perforation), and seven patients had a delayed intraparenchymal haemorrhage. Risk factors that were significantly associated with periprocedural ischaemic events were non-smoking (possibly because smoking increases the conversion of clopidogrel to its active metabolite),75 basilar artery stenosis, diabetes, and older age, whereas risk factors associated with periprocedural intracranial haemorrhages included high percentage of stenosis and clopidogrel load associated with an activated clotting time above the target range.74
Although these periprocedural complication rates in SAMMPRIS were higher than previously reported in the Wingspan humanitarian device exception study and Wingspan registries,71–73 the increased periprocedural risk was not due to operator inexperience.76 Possible explanations for the higher periprocedural events in SAMMPRIS than in the previous registries include the higher severity of stenosis needed for enrolment in SAMMPRIS and the earlier treatment in SAMMPRIS (within 30 days of the qualifying event), which might have increased the risk of PTAS. Additionally, SAMMPRIS had a more rigorous adjudication process, with local evaluation by study neurologists (not required in the registries) followed by external blinded adjudication by a committee of expert neurologists. Researchers in other studies have subsequently reported higher rates of periprocedural events with the Wingspan Stenting System than in the Humanitarian Device Exception Study and registries.77 Periprocedural complications might not be limited to the Wingspan stent—enrolment in an industry-sponsored randomised trial of intracranial stenting with the balloon expandable Pharos Vitesse Neurovascular Stent System (Codman Neurovascular, Raynham, MA, USA versus medical management was also prematurely terminated in January, 2012.78 The final results of this trial are expected in October, 2013.
Treatment recommendations
On the basis of current data from randomised trials, we recommend the following treatment for patients with symptomatic ICAS. Patients with moderate stenosis (<70%) or patients whose transient ischaemic attack or stroke occurred more than 30 days ago (even if they have >70% stenosis) should be treated with aspirin (325 mg/day) and intensive risk factor management, primarily to achieve systolic blood pressure of less than 140 mm Hg and LDL concentration greater than 1·81 mmol/L (figure 3). In WASID, such patients made up 75% of the study cohort and had a stroke rate of 3–9% with usual risk factor management,26 which suggests that their stroke rate would be even lower with intensive risk factor management. For patients with 70–99% stenosis and events within the past 30 days, a combination of clopidogrel (75 mg per day) with aspirin (325 mg per day) for 90 days seems warranted, on the basis of the results of SAMMPRIS46 and CLAIR,43 followed by aspirin (325 mg per day) alone plus intensive risk factor management (figure 3). We do not recommend the use of clopidogrel in addition to aspirin for more than 90 days after initiation of treatment because of the risk of major haemorrhage from more prolonged use of dual antiplatelet therapy that was established in the MATCH and CHARISMA trials.79,80 However, the benefit of prolonged use of dual antiplatelet therapy for high-risk patients with intracranial stenosis might outweigh the risk of major haemorrhage, but this effect will need to be proven in future trials before the treatment can be recommended. Patients in the SAMMPRIS study who were managed with dual antiplatelet therapy for 90 days followed by aspirin alone plus intensive risk factor management had a primary endpoint rate of 12·2% at 1 year (as of April, 2011, data analysis),46 which implies that a subgroup of these patients still had a risk of stroke at 1 year that exceeds 12·2%.
Figure 3. Treatment recommendations for patients with symptomatic 50–99% intracranial arterial stenosis, based on the results of the WASID and SAMMPRIS trials19,46.
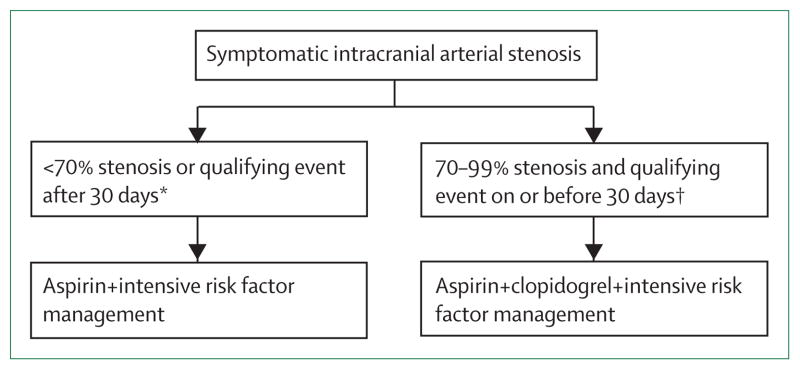
*75% of patients with symptomatic intracranial arterial stenosis in the WASID trial were in this category.19 †25% of patients in the WASID trial19 and 100% of patients in the SAMMPRIS trial45 were in this category.
Directions for future research
Future research should focus on identification of this particularly high-risk subgroup and testing of alternative therapies in these patients. Novel non-invasive imaging techniques that could have a role in identification of high-risk patients include quantitative MRA,81 fractional flow reserve on MRA,82 high-resolution MRI,83–87 and vasomotor reactivity and emboli detection on TCD.43 Quantitative MRA is a technique that combines time-of-flight (TOF) and phase-contrast MRA techniques to derive vessel-specific volumetric flow rates. This technique is being assessed in a multicentre trial to establish whether patients with compromised flow distal to a symptomatic vertebral or basilar artery stenosis is predictive of an increased risk of stroke.81
Fractional flow reserve is an index that uses a pressure gradient across a stenosis to identify lesions of haemodynamic significance. In the coronary circulation, results of studies have shown that fractional flow reserve measured during angiography is useful for identification of patients with haemodynamically significant stenosis who should be treated with endovascular therapy.88 TOF MRA signal intensity correlates with blood flow velocity, which implies that a comparison of the signal intensity on TOF MRA just distal and proximal to a symptomatic intracranial stenosis could be a reasonable measure of fractional flow reserve associated with the stenosis. Results of a post-hoc analysis of patients in the WASID and SONIA trials suggests that patients with distal to proximal signal ratios of less than 0·9 on TOF MRA are at a higher risk of stroke than are those with ratios of 0·9 or greater.82
High-resolution MRI is a promising new method that typically uses 3 Tesla or higher magnets to assess ICAS. Traditional imaging tests (MRA, CTA, TCD, and conventional diagnostic angiogram) estimate luminal patency or severity of stenosis either through direct visualisation (MRA, CTA, or conventional angiogram) or indirectly (TCD) by measurement of flow velocity.90 High-resolution MRI enables visualisation of both the lumen and the vessel wall, which might aid assessment of the underlying pathological abnormality of the stenosis (atherosclerosis, inflammation, or vasospasm)83 (figure 4). High-resolution MRI could prove useful to establish the particular stroke mechanism associated with ICAS and provide detailed information about atherosclerotic plaque formation, which might ultimately drive secondary prevention strategies.84–87
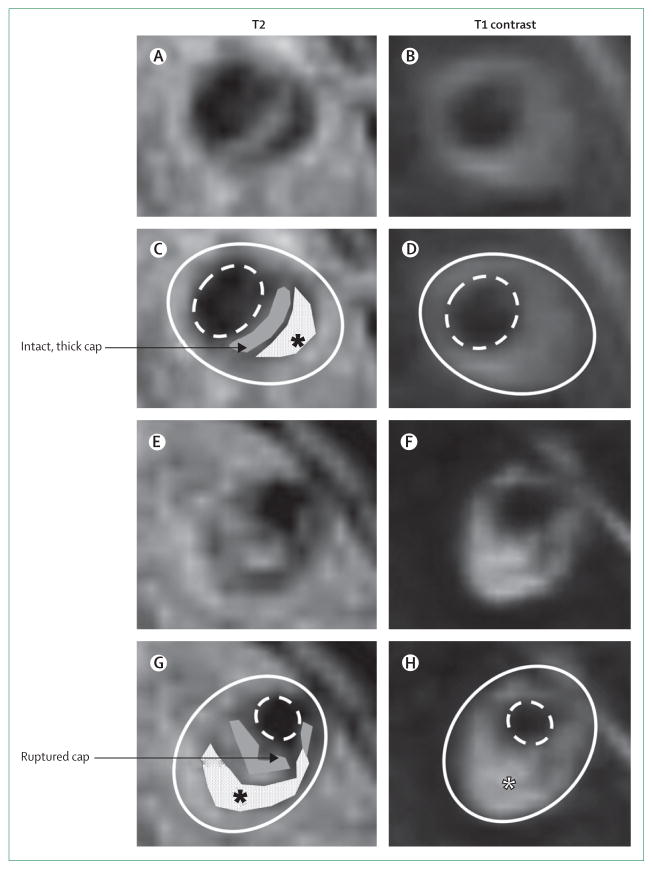
Figure 4. High-resolution MRI of vertebral artery stenoses with plaque components.
Panels A–D show T2-weighted and T1 post-contrast images (panels C and D have plaque components marked) of a cross-section of a vertebral artery plaque with a thick, intact, fibrous cap (grey) and lipid core (white with black asterisk). Panels E–H show T2-weighted and T1 post-contrast images (panels G and H have plaque components marked) of a cross-section of a vertebral artery plaque with a ruptured fibrous cap (grey) and lipid core (white with black asterisk), which enhances with contrast (white asterisk) and is also indicative of plaque rupture. The solid white line shows the outside vessel wall and the dashed white line the lumen.
When reliable non-invasive tests have been developed and validated for identification of patients with ICAS who are at high risk of stroke despite aggressive medical therapy, alternative therapies will need to be compared with aggressive medical therapy in these patients. Promising preliminary data have emerged for some alternative therapies: angioplasty alone,59,64 an indirect surgical bypass procedure called encephaloduroarteriosynangiosis, 91 and ischaemic preconditioning.92 Encephaloduroarteriosynangiosis is a neurosurgical indirect revascularisation procedure that has been used for many years in patients with moyamoya disease. During the procedure, the donor arteries (superficial temporal artery and middle meningeal arteries) are placed in close proximity to the superficial brain arteries distal to the intracranial stenosis, and subsequently a network of collaterals forms between the donor artery and the adjacent superficial brain vessels without a surgical anastomosis. In one study, 13 patients with symptomatic atherosclerotic intracranial arterial stenosis, in whom medical therapy was not effective, underwent encephaloduroarterio synangiosis.91 Follow-up angiography showed that the donor blood vessels increased in size in all but one patient and all patients showed evidence of a vascular blush or new branches from the donor arteries to the superficial vessels. Of the 13 patients followed up for a median of 54 months, two had recurrent ischaemic events.91
Ischaemic preconditioning is another new potential treatment to prevent stroke in patients with ICAS. Meng and colleagues91 undertook a small trial, in which 68 patients were randomly assigned to upper limb ischaemic preconditioning versus usual care. Patients in the ischaemic preconditioning group underwent five brief cycles consisting of occlusion of both brachial arteries with a blood pressure cuff twice daily for 300 days. Remarkably, the stroke rate was significantly lower at 300 days in the upper limb ischaemic preconditioning group than in the usual care group (7·9% vs 26·7%, p<0·01),91 which suggests that this treatment might become an important option for patients with ICAS if these findings can be duplicated in a larger multicentre randomised trial.
As an alternative pharmacological treatment, the direct thrombin and Xa inhibitors, now approved for atrial fibrillation,92 should be compared with antiplatelet therapy for patients with ICAS, in view of the results of the WASID trial, which suggested that when the INR was kept between 2 and 3 in patients given warfarin, the ischaemic stroke and myocardial infarct rates were low and the major haemorrhages few.19
Conclusions
In summary, substantial progress has been made in the treatment of patients with ICAS over the past decade, which has resulted in a better prognosis for patients with this high-risk disease. Multifaceted medical management that incorporates short-term dual antiplatelet treatment (for 90 days) followed by aspirin monotherapy, coupled with intensive management of vascular risk factors is the treatment of choice for stroke prevention in these patients. Despite this aggressive medical management, a large subgroup of patients are still at high risk of recurrent stroke. Further research should focus on identification and treatment of this high-risk subgroup to lower their risk of recurrent stroke.
Search strategy and selection criteria.
We searched PubMed between 2000 and 2013 with the search terms “intercranial atherosclerosis”, “stroke”, “angioplasty”, “stenting”, “antiplatelet therapy”, “vascular imaging”, and “epidemiology”. Additionally, we searched references from relevant articles and those from a personal library. We included only references relevant to the topics covered in the Review. There were no language restrictions.
Footnotes
Conflicts of interest
CAH has consulted for Covidien LP, Boehringer Ingelheim, and CE Outcomes LLP, and has served as an expert witness in non-corporate medical malpractice cases involving stroke. MIC was the principal investigator of the SAMMPRIS trial, funded by NIH; Stryker Neurovascular provided stents for the SAMMPRIS trial and paid for some of the third party monitoring of sites in that trial; AstraZeneca Corporation provided rosuvastatin for patients in the SAMMPRIS trial. MIC was the principal investigator of the WASID trial and NIH Wingspan registry, both of which were also funded by NIH; Bayer provided aspirin and Bristol-Myers Squibb provided warfarin for the WASID trial. MIC has received personal fees from GORE Associates, Merck/Ponexel, and Medtronic for participating as a stroke adjudicator or data safety board monitoring member on clinical trials unrelated to the submitted work. MIC has also been an expert witness in non-corporate medical malpractice cases involving stroke. TNT was an investigator on the SAMMPRIS trial, funded by NIH. She was an investigator on the CHIASM (Characterization of Intracranial Atherosclerotic Stenosis using HR MRI) study, funded by NIH. She was on the clinical event adjudication committee for the VERITAS study, funded by NIH.
References
- 1.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–99. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Safdar K, Patel M, Janssen RS, Frankel MR. Stroke in young black patients. Risk factors, subtypes, and prognosis. Stroke. 1995;26:1995–98. doi: 10.1161/01.str.26.11.1995. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg LA. Clinical characteristics of transient ischemic attacks in black patients. Neurology. 1991;41:1410–14. doi: 10.1212/wnl.41.9.1410. [DOI] [PubMed] [Google Scholar]
- 5.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974–80. doi: 10.1161/01.str.27.11.1974. [DOI] [PubMed] [Google Scholar]
- 6.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–59. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumar G, Kalita J, Kumar B, Bansal V, Jain SK, Misra U. Magnetic resonance angiography findings in patients with ischemic stroke from north India. J Stroke Cerebrovasc Dis. 2010;19:146–52. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Moustafa RR, Moneim AA, Salem HH, Shalash AS, Azmy HA. Intracranial steno-occlusive arterial disease and its associations in Egyptian ischemic stroke patients. Stroke. 2013;44:538–41. doi: 10.1161/STROKEAHA.112.679050. [DOI] [PubMed] [Google Scholar]
- 9.Feldmann E, Daneault N, Kwan E, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40:1541–45. doi: 10.1212/wnl.40.10.1540. [DOI] [PubMed] [Google Scholar]
- 10.Gorelick PB, Caplan LR, Langenberg P, et al. Clinical and angiographic comparison of asymptomatic occlusive cerebrovascular disease. Neurology. 1988;38:852–58. doi: 10.1212/wnl.38.6.852. [DOI] [PubMed] [Google Scholar]
- 11.Williams AO, Resch JA, Loewenson RB. Cerebral atherosclerosis—a comparative autopsy study between Nigerian Negroes and American Negroes and Caucasians. Neurology. 1969;19:205–10. doi: 10.1212/wnl.19.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Arenillas JF, Molina CA, Chacon P, et al. High lipoprotein (a), diabetes, and the extent of symptomatic intracranial atherosclerosis. Neurology. 2004;63:27–32. doi: 10.1212/01.wnl.0000132637.30287.b4. [DOI] [PubMed] [Google Scholar]
- 13.Resch JA, Baker AB. Etiologic mechanisms in cerebral atherosclerosis. Preliminary study of 3,839 cases. Arch Neurol. 1964;10:617–28. doi: 10.1001/archneur.1964.00460180083008. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995;45:659–63. doi: 10.1212/wnl.45.4.659. [DOI] [PubMed] [Google Scholar]
- 15.Ingall TJ, Homer D, Baker HL, Jr, Kottke BA, O’Fallon WM, Whisnant JP. Predictors of intracranial carotid artery atherosclerosis. Duration of cigarette smoking and hypertension are more powerful than serum lipid levels. Arch Neurol. 1991;48:687–91. doi: 10.1001/archneur.1991.00530190033011. [DOI] [PubMed] [Google Scholar]
- 16.Rincon F, Sacco RL, Kranwinkel G, et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovasc Dis. 2009;28:65–71. doi: 10.1159/000219299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi S, Turan TN, Lynn MJ, et al. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063–68. doi: 10.1212/01.wnl.0000279338.18776.26. [DOI] [PubMed] [Google Scholar]
- 18.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007;115:2969–75. doi: 10.1161/CIRCULATIONAHA.106.622464. [DOI] [PubMed] [Google Scholar]
- 19.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–16. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 20.Kim DE, Kim JY, Jeong SW, et al. Association between changes in lipid profiles and progression of symptomatic intracranial atherosclerotic stenosis: a prospective multicenter study. Stroke. 2012;43:1824–30. doi: 10.1161/STROKEAHA.112.653659. [DOI] [PubMed] [Google Scholar]
- 21.Ovbiagele B, Saver JL, Lynn MJ, Chimowitz M. Impact of metabolic syndrome on prognosis of symptomatic intracranial atherostenosis. Neurology. 2006;66:1344–49. doi: 10.1212/01.wnl.0000210530.46058.5c. [DOI] [PubMed] [Google Scholar]
- 22.Bang OY, Kim JW, Lee JH, et al. Association of the metabolic syndrome with intracranial atherosclerotic stroke. Neurology. 2005;65:296–98. doi: 10.1212/01.wnl.0000168862.09764.9f. [DOI] [PubMed] [Google Scholar]
- 23.Bang OY, Saver JL, Ovbiagele B, Choi YJ, Yoon SR, Lee KH. Adiponectin levels in patients with intracranial atherosclerosis. Neurology. 2007;68:1931–37. doi: 10.1212/01.wnl.0000263186.20988.9f. [DOI] [PubMed] [Google Scholar]
- 24.Massot A, Pelegri D, Penalba A, et al. Lipoprotein-associated phospholipase A2 testing usefulness among patients with symptomatic intracranial atherosclerotic disease. Atherosclerosis. 2011;218:181–87. doi: 10.1016/j.atherosclerosis.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Arenillas JF, Alvarez-Sabin J, Molina CA, et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. 2008;39:1456–63. doi: 10.1161/STROKEAHA.107.498600. [DOI] [PubMed] [Google Scholar]
- 26.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 27.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–74. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau AY, Wong EH, Wong A, Mok VC, Leung TW, Wong KS. Significance of good collateral compensation in symptomatic intracranial atherosclerosis. Cerebrovasc Dis. 2012;33:517–24. doi: 10.1159/000337332. [DOI] [PubMed] [Google Scholar]
- 29.Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989;39:1246–50. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- 30.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–82. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 31.Lee DK, Kim JS, Kwon SU, Yoo SH, Kang DW. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: early diffusion-weighted imaging study. Stroke. 2005;36:2583–88. doi: 10.1161/01.STR.0000189999.19948.14. [DOI] [PubMed] [Google Scholar]
- 32.Schreiber S, Serdaroglu M, Schreiber F, Skalej M, Heinze HJ, Goertler M. Simultaneous occurrence and interaction of hypoperfusion and embolism in a patient with severe middle cerebral artery stenosis. Stroke. 2009;40:e478–80. doi: 10.1161/STROKEAHA.109.549378. [DOI] [PubMed] [Google Scholar]
- 33.Caplan LR, Wong KS, Gao S, Hennerici MG. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc Dis. 2006;21:145–53. doi: 10.1159/000090791. [DOI] [PubMed] [Google Scholar]
- 34.Khan A, Kasner SE, Lynn MJ, Chimowitz MI. Risk factors and outcome of patients with symptomatic intracranial stenosis presenting with lacunar stroke. Stroke. 2012;43:1230–33. doi: 10.1161/STROKEAHA.111.641696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldmann E, Wilterdink JL, Kosinski A, et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68:2099–106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 36.Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26:1012–21. [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen-Huynh MN, Wintermark M, English J, et al. How accurate is CT angiography in evaluating intracranial atherosclerotic disease? Stroke. 2008;39:1184–88. doi: 10.1161/STROKEAHA.107.502906. [DOI] [PubMed] [Google Scholar]
- 38.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–46. [PMC free article] [PubMed] [Google Scholar]
- 39.Millikan CH, Siekert RG, Shick RM. Studies in cerebrovascular disease. III. The use of anticoagulant drugs in the treatment of insufficiency or thrombosis within the basilar arterial system. Proc Staff Meet Mayo Clin. 1955;30:116–26. [PubMed] [Google Scholar]
- 40.Chimowitz MI, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488–93. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- 41.Turan TN, Maidan L, Cotsonis G, et al. Failure of antithrombotic therapy and risk of stroke in patients with symptomatic intracranial stenosis. Stroke. 2009;40:505–09. doi: 10.1161/STROKEAHA.108.528281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasner SE, Lynn MJ, Chimowitz MI, et al. Warfarin vs aspirin for symptomatic intracranial stenosis: subgroup analyses from WASID. Neurology. 2006;67:1275–78. doi: 10.1212/01.wnl.0000238506.76873.2f. [DOI] [PubMed] [Google Scholar]
- 43.The CLAIR study investigators. The effectiveness of dual antiplatelet treatment in acute ischemic stroke patients with intracranial arterial stenosis: a subgroup analysis of CLAIR study. Int J Stroke. 2012 doi: 10.1111/j.1747-4949.2012.00828.x. published online Aug 7. [DOI] [PubMed] [Google Scholar]
- 44.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic carotid Stenosis (CARESS) trial. Circulation. 2005;111:2233–40. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 45.Wong KSL, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:489–97. doi: 10.1016/S1474-4422(10)70060-0. [DOI] [PubMed] [Google Scholar]
- 46.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. New Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon SU, Cho YJ, Koo JS, et al. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–6. doi: 10.1161/01.STR.0000157667.06542.b7. [DOI] [PubMed] [Google Scholar]
- 48.Kwon SU, Hong KS, Kang DW, et al. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke. 2011;42:2883–90. doi: 10.1161/STROKEAHA.110.609370. [DOI] [PubMed] [Google Scholar]
- 49.Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 50.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 51.Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group . N Engl J Med. 1985;313:1191–200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 52.Bogousslavsky J, Barnett HJ, Fox AJ, Hachinski VC, Taylor W. Atherosclerotic disease of the middle cerebral artery. Stroke. 1986;17:1112–20. doi: 10.1161/01.str.17.6.1112. [DOI] [PubMed] [Google Scholar]
- 53.Hopkins LN, Budny JL. Complications of intracranial bypass for vertebrobasilar insufficiency. J Neurosurg. 1989;70:207–11. doi: 10.3171/jns.1989.70.2.0207. [DOI] [PubMed] [Google Scholar]
- 54.Higashida RT, Tsai FY, Halbach VV, et al. Transluminal angioplasty for atherosclerotic disease of the vertebral and basilar arteries. J Neurosurg. 1993;78:192–98. doi: 10.3171/jns.1993.78.2.0192. [DOI] [PubMed] [Google Scholar]
- 55.Clark WM, Barnwell SL, Nesbit G, O’Neill OR, Wynn ML, Coull BM. Safety and efficacy of percutaneous transluminal angioplasty for intracranial atherosclerotic stenosis. Stroke. 1995;26:1200–04. doi: 10.1161/01.str.26.7.1200. [DOI] [PubMed] [Google Scholar]
- 56.Touho H. Percutaneous transluminal angioplasty in the treatment of atherosclerotic disease of the anterior cerebral circulation and hemodynamic evaluation. J Neurosurg. 1995;82:953–60. doi: 10.3171/jns.1995.82.6.0953. [DOI] [PubMed] [Google Scholar]
- 57.Takis C, Kwan ES, Pessin MS, Jacobs DH, Caplan LR. Intracranial angioplasty: experience and complications. AJNR Am J Neuroradiol. 1997;18:1661–68. [PMC free article] [PubMed] [Google Scholar]
- 58.Marks MP, Marcellus M, Norbash AM, Steinberg GK, Tong D, Albers GW. Outcome of angioplasty for atherosclerotic intracranial stenosis. Stroke. 1999;30:1065–69. doi: 10.1161/01.str.30.5.1065. [DOI] [PubMed] [Google Scholar]
- 59.Connors JJ, 3rd, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg. 1999;91:415–23. doi: 10.3171/jns.1999.91.3.0415. [DOI] [PubMed] [Google Scholar]
- 60.Nahser HC, Henkes H, Weber W, Berg-Dammer E, Yousry TA, Kuhne D. Intracranial vertebrobasilar stenosis: angioplasty and follow-up. AJNR Am J Neuroradiol. 2000;21:1293–301. [PMC free article] [PubMed] [Google Scholar]
- 61.Alazzaz A, Thornton J, Aletich VA, Debrun GM, Ausman JI, Charbel F. Intracranial percutaneous transluminal angioplasty for arteriosclerotic stenosis. Arch Neurol. 2000;57:1625–30. doi: 10.1001/archneur.57.11.1625. [DOI] [PubMed] [Google Scholar]
- 62.Gress DR, Smith WS, Dowd CF, Van Halbach V, Finley RJ, Higashida RT. Angioplasty for intracranial symptomatic vertebrobasilar ischemia. Neurosurgery. 2002;51:23–27. doi: 10.1097/00006123-200207000-00004. discussion 27–29. [DOI] [PubMed] [Google Scholar]
- 63.Gupta R, Schumacher HC, Mangla S, et al. Urgent endovascular revascularization for symptomatic intracranial atherosclerotic stenosis. Neurology. 2003;61:1729–35. doi: 10.1212/01.wnl.0000103900.65021.5b. [DOI] [PubMed] [Google Scholar]
- 64.Marks MP, Wojak JC, Al-Ali F, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006;37:1016–20. doi: 10.1161/01.STR.0000206142.03677.c2. [DOI] [PubMed] [Google Scholar]
- 65.Marks MP, Marcellus ML, Do HM, et al. Intracranial angioplasty without stenting for symptomatic atherosclerotic stenosis: long-term follow-up. AJNR Am J Neuroradiol. 2005;26:525–30. [PMC free article] [PubMed] [Google Scholar]
- 66.Fiorella D, Turan TN, Derdeyn CP, Chimowitz MI. Current status of the management of symptomatic intracranial atherosclerotic disease: the rationale for a randomized trial of medical therapy and intracranial stenting. J Neurointerv Surg. 2009;1:35–39. doi: 10.1136/jnis.2009.000125. [DOI] [PubMed] [Google Scholar]
- 67.Kessler IM, Mounayer C, Piotin M, Spelle L, Vanzin JR, Moret J. The use of balloon-expandable stents in the management of intracranial arterial diseases: a 5-year single-center experience. AJNR Am J Neuroradiol. 2005;26:2342–48. [PMC free article] [PubMed] [Google Scholar]
- 68.Abruzzo TA, Tong FC, Waldrop AS, Workman MJ, Cloft HJ, Dion JE. Basilar artery stent angioplasty for symptomatic intracranial athero-occlusive disease: complications and late midterm clinical outcomes. AJNR Am J Neuroradiol. 2007;28:808–15. [PMC free article] [PubMed] [Google Scholar]
- 69.Fields JD, Petersen BD, Lutsep HL, et al. Drug eluting stents for symptomatic intracranial and vertebral artery stenosis. Interv Neuroradiol. 2011;17:241–47. doi: 10.1177/159101991101700217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park S, Lee DG, Chung WJ, Lee DH, Suh DC. Long-term outcomes of drug-eluting stents in symptomatic intracranial stenosis. Neurointervention. 2013;8:9–14. doi: 10.5469/neuroint.2013.8.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bose A, Hartmann M, Henkes H, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38:1531–37. doi: 10.1161/STROKEAHA.106.477711. [DOI] [PubMed] [Google Scholar]
- 72.Zaidat OO, Klucznik R, Alexander MJ, et al. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology. 2008;70:1518–24. doi: 10.1212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fiorella D, Levy EI, Turk AS, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38:881–87. doi: 10.1161/01.STR.0000257963.65728.e8. [DOI] [PubMed] [Google Scholar]
- 74.Fiorella D, Derdeyn CP, Lynn MJ, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) Stroke. 2012;43:2682–88. doi: 10.1161/STROKEAHA.112.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bliden KP, Dichiara J, Lawal L, et al. The association of cigarette smoking with enhanced platelet inhibition by clopidogrel. J Am Coll Cardiol. 2008;52:531–33. doi: 10.1016/j.jacc.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 76.Derdeyn CP, Fiorella D, Lynn MJ, et al. Impact of operator and site experience on outcomes after angioplasty and stenting in the SAMMPRIS trial. J Neurointerv Surg. 2012;0:1–6. doi: 10.1136/neurintsurg-2012-010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.StrykerNeurovascular; Wingspan Stent System, FDANeurologicalDevices Advisory Committee Meeting; March 23, 2012; [accessed Sept 11, 2013]. http://www.fda/gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicaDevices/MedicalDevicesAdvisoryCommittee/NeurologicalDevicesPanel/UCM297341.pdf. [Google Scholar]
- 78.Zaidat OO, Castonguay AC, Fitzsimmons BF, et al. Design of the Vitesse Intracranial Stent Study for Ischemic Therapy (VISSIT) trial in symptomatic intracranial stenosis. J Stroke Cerebrovasc Dis. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.10.021. published online Dec 21. [DOI] [PubMed] [Google Scholar]
- 79.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–37. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 80.Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 81.Amin-Hanjani S, Rose-Finnell L, Richardson D, et al. Vertebrobasilar flow Evaluation and Risk of Transient ischaemic Attack and Stroke study (VERiTAS): rationale and design. Int J Stroke. 2010;5:499–505. doi: 10.1111/j.1747-4949.2010.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liebeskind DS, Kosinski AS, Lynn MJ, et al. for the SONIA and WASID Investigators. Noninvasive fractional flow on MRA predicts stroke risk of intracranial stenosis in SONIA/WASID. Stroke. 2013:44. doi: 10.1111/jon.12101. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-Tesla contrast-enhanced MRI. Neurology. 2009;72:627–34. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 84.Ma N, Jiang WJ, Lou X, et al. Arterial remodeling of advanced basilar atherosclerosis: a 3-Tesla MRI study. Neurology. 2010;75:253–58. doi: 10.1212/WNL.0b013e3181e8e714. [DOI] [PubMed] [Google Scholar]
- 85.Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. 2010;212:507–11. doi: 10.1016/j.atherosclerosis.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 86.Park JK, Kim SH, Kim BS, Choi G, Jeong SY, Choi JC. Imaging of intracranial plaques with black-blood double inversion recovery MR imaging and CT. J Neuroimag. 2011;21:e64–68. doi: 10.1111/j.1552-6569.2010.00499.x. [DOI] [PubMed] [Google Scholar]
- 87.Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. 2013;44:287–92. doi: 10.1161/STROKEAHA.112.664680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 89.Newell DW, Aaslid R. Transcranial Doppler: clinical and experimental uses. Cerebrovasc Brain Metab Rev. 1992;4:122–43. [PubMed] [Google Scholar]
- 90.Dusick JR, Liebeskind DS, Saver JL, Martin NA, Gonzalez NR. Indirect revascularization for nonmoyamoya intracranial arterial stenoses: clinical and angiographic outcomes. J Neurosurg. 2012;117:94–102. doi: 10.3171/2012.4.JNS111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–61. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 92.Contractor T, Levin V, Martinez MW, Marchlinski FE. Novel oral anticoagulants for stroke prevention in patients with atrial fibrillation: dawn of a new era. Postgrad Med. 2013;125:34–44. doi: 10.3810/pgm.2013.01.2622. [DOI] [PubMed] [Google Scholar]