Introduction
In this chapter we review issues related to identifying the appropriate patient to test for celiac disease (CD), the performance characteristics of serological testing, the role of gene testing for Human Leukocyte Antigen DQ2 and DQ8 haplotypes, and issues related to the performance of small intestinal biopsy. The chapter concludes with a review of special diagnostic considerations in pediatric patients.
Identifying the Appropriate Patient to Test for Celiac Disease
Diagnoses of CD are increasing in the United States and worldwide. In a population-based study of individuals in Olmsted County, MN, the annual incidence of CD increased dramatically from 0.9 per 100,000 individuals in the years prior to the availability of serological tests (1950–1989) to 9.1 per 100,000 in the years 2000–2001.1 Analysis of claims data from a national insurance company found that diagnoses of CD continued to increase through the year 2003, the last year of the analysis.2
Despite evidence of increasing rates of diagnosis, the majority of patients in the United States remain undiagnosed. Population based data are sparse, but inferences on the ratio of undiagnosed-to-diagnosed individuals can be made based on what is known regarding the seroprevalence of CD in the general population (0.8–1%).3–5 In 2001, the point prevalence of diagnosed CD in Olmsted County was 0.04%.1 If the seroprevalence of CD is 0.8%, then approximately 95% of patients with CD were undiagnosed at that time. While diagnosis rates are increasing, the fact that the seroprevalence of CD is also increasing4, 6 may result in a persistently high undiagnosed-to-diagnosed ratio. The high fraction of undiagnosed patients in the United States stands in contrast to parts of Europe, including Italy and Finland, in which the threshold to test for CD is lower and so the fraction of diagnosed patients is substantially higher.7–8
One approach to address the relatively low rates of CD diagnosis in the United States is to institute a program of population screening, in which all individuals regardless of symptoms undergo serological testing for CD, and those who screen positive subsequently undergo esophagogastroduodenoscopy (EGD) with small intestinal biopsy. Advocates for this approach note that CD meets World Health Organization criteria for diseases that warrant mass screening: early clinical detection is difficult; the condition is common; screening tests are highly sensitive and specific; effective treatment is available; and untreated disease can lead to complications.9 Given the reduction in mortality risk that occurs in the years after diagnosis and institution of the gluten-free diet10 and the reduced health care expenditures after diagnosis of CD,2 screening for CD may be cost effective, and was found to be so in to three quantitative analyses.11–13
Despite calls for general population screening, problems with this approach have led to targeted case finding as the preferred method of increasing diagnosis rates. Apart from unresolved questions regarding the logistics of screening (such as deciding on the appropriate age and interval of screening) limitations of the currently available serological tests pose a significant problem. Given that the prevalence of CD in the general population is 1%, any test with an imperfect specificity will result in a large number of false-positives. Assuming that the specificity of tissue transglutaminase (TTG) IgA is 98%,14 its positive predictive value when employed in the general population is only 34%; as a result, two thirds of screened individuals who have a positive result will undergo EGD with biopsy and not be diagnosed with CD; this false-positive rate may be reduced by only performing a biopsy on patients with dual positive serologies of TTG endomysial antibody (EMA), but difficulties with the latter serology (see below, Serological and Genetic Testing) makes this approach less than ideal.
In addition to the technical limitations of serological screening and its attendant false positive rate, one objection to routine screening for CD is based on the persistent uncertainty regarding the long-term prognosis of asymptomatic, undiagnosed CD. A major argument for screening is that CD is associated with an increased mortality risk, which declines in the years following diagnosis,10 a decline that is attributed to the protective effects of the gluten-free diet. The evidence for a mortality risk in undiagnosed CD is less consistent. In an analysis of thawed serum, Rubio-Tapia, et al identified individuals with positive CD serologies (both TTG and EMA) in 14 out of 9,133 (0.2%) participants in the Warren Air Force cohort.4 With a follow-up period of 45 years, the patients with seropositivity (who all remained undiagnosed) had a nearly four-fold risk of death compared to seronegative individuals (HR 3.9; 95% CI 2.0–7.5). In a second cohort study, healthy volunteers with a positive TTG had an increased mortality compared to seronegative subjects (HR 2.53; 95% CI 1.50–4.25).15 But in four other studies in England,16 Finland,17 Ireland,18 and individuals older than 50 in Olmsted county,19 no increase in mortality was noted in undiagnosed seropositive individuals as compared to their seronegative counterparts. A recent meta-analysis found a modestly increased mortality risk in patients with CD based on serology alone (OR 1.16; 95% CI 1.02–1.31),20 but this pooled analysis included seropositive patients who underwent small intestinal biopsy that was normal,10 raising the possibility of confounding by indication.
The conflicting data with regard to mortality risk in undiagnosed CD are likely due to differences in age, definitions of seropositivity, and follow-up time, but given this residual uncertainty in magnitude of risk, if any, these data do not justify population screening. Although enteropathy-associated T cell lymphoma appears to be rising in incidence in the United States, possibly as a result of the increased number of patients with undiagnosed CD,21 given the rarity of this condition it would not justify population screening for CD based on this consideration alone. On the other end of the spectrum of clinical severity, apparently asymptomatic patients may report improved quality of life after screen-detected diagnosis of CD,22 but data on this topic are insufficient to establish that widespread screening of the population is cost-effective.
The favored alternative to population screening at this time is a case finding approach, in which health care providers order serological testing for CD in patients who exhibit one or more of the symptoms, signs, or other diseases closely associated with CD. In this approach, the problem of high false positive rates of serological tests is reduced, since the underlying prevalence of CD in a symptomatic or high-risk group is likely to be higher than that of the general population. The feasibility and effectiveness of the case finding approach was demonstrated in a multi-center study in which adult patients attending a primary care office were given a questionnaire soliciting symptoms (such as diarrhea, abdominal pain, chronic fatigue, and infertility), abnormal laboratory values (including anemia and abnormal liver tests), or associated diseases (such as irritable bowel syndrome, any autoimmune disease, Down syndrome, and Turner’s syndrome).23 Individuals responding affirmatively to one or more of these items were offered serological testing for CD, and if positive, EGD with small intestinal biopsy. During the three year period, 976 of 2,568 eligible patients (38%) responded affirmatively and agreed to serological testing. Of these 2,568 patients, 22 (2.3%) were ultimately diagnosed with CD based on serology and biopsy. Of note, the overall diagnosis rate markedly increased. As compared to the 12 month period preceding the case finding initiative, the diagnosis rate increased from 0.27 cases per 1,000 visits to 8.6 cases per 1,000 visits. Such an approach, while increasing diagnosis rates, may still leave the majority of patients undiagnosed.
Although the case finding approach is the favored strategy, it remains a matter of controversy as to which symptoms and associated diseases should prompt evaluation for CD. Given the protean clinical manifestations of CD and the expanding list of associated conditions,24 the strategy of testing for CD for one associated symptom or condition may approach that of screening the general population, since nearly 100% of respondents may respond affirmatively to at least one item. For example, in the case finding study by Catassi, et al, 64% of all participants were eligible for CD testing, and this questionnaire did not include additional items that may be justifiably included in a CD symptom checklist, such as peripheral neuropathy, 25 migraines,26 gastroesophageal reflux, 27 low bone density28 and low levels of High-density lipoprotein.29
A recent study set at a health fair in Caspar, Wyoming sheds light on the fine line between case finding and screening the general population.5 In this study, 3,850 individuals attending the health fair submitted a blood sample that was tested for TTG, and serum with positive results then underwent confirmatory EMA testing. These individuals also completed a questionnaire, querying respondents for gastrointestinal symptoms including bloating, abdominal pain, heartburn, nausea, diarrhea, and constipation. Of the 3,850 subjects, 34 had a positive TTG and EMA, yielding a prevalence of 0.8%. (31 out these 34 had not been previously diagnosed with CD, yielding an undiagnosed-to-diagnosed ratio of 10 to 1.) When comparing seropositive to seronegative individuals with regard to gastrointestinal symptoms, none of these symptoms were predictive of seropositivity. This null finding was due in part to the fact that these symptoms are quite common; more than 80% of all respondents had at least one such symptom. Thus, an aggressive case finding strategy may closely resemble a de facto mass screening approach.
At this time, there is no universally accepted threshold for testing for CD among physicians who have adopted this recommended strategy of case finding. Consensus statements from the United States and Europe broadly agree with the need to test for CD in scenarios such as chronic diarrhea and unexplained iron deficiency.30–33 But there is less agreement on whether screening asymptomatic patients in high-prevalence groups (such as first degree relatives or patients with autoimmune thyroiditis) should be recommended 31–33 or merely offered with the caveat that the benefits of diagnosing asymptomatic patients are unclear.30 Since a low threshold (i.e. a long list of symptoms that would prompt testing) may result in a testing a large proportion of patients seeking health care, it is imperative that physicians employing this strategy have a solid understanding of the performance characteristics of serological tests.
Serological and Genetic Testing
Serum Antibody Tests
CD is characterized by the presence of diverse antibodies in the serum that are made against 1) gliadin (conventional gliadin antibodies and deamidated gliadin peptide antibodies), a component of gluten, and 2) connective tissue components (tissue transglutaminase antibodies and endomysial antibodies). Overall, these tests are useful for the diagnosis of CD, although the diagnostic performance may be different for each test.
Antigliadin antibodies
Conventional gliadin antibodies are no longer recommended because of the lower sensitivity and specificity compared with other available serologic tests. However, there is considerable interest on the use of new generation deamidated gliadin peptide antibodies because these novel tests have improved diagnostic accuracy as compared to conventional gliadin antibodies.34 In a recent review, the pooled sensitivity for IgA tissue deamidated gliadin peptide antibodies was 88% and specificity of 95%.14 The role of deamidated gliadin peptide antibodies in diagnosing young children is discussed below (Diagnosis in Children).
Tissue transglutaminase antibodies
The enzyme tissue transglutaminase was recognized as the CD autoantigen. 35 This enzyme has many functions, including deamidation of gliadin peptides. 36 A wide range of kits with different characteristics measure tissue transglutaminase antibodies most often by quantitative enzyme-linked immunosorbent assay.37 The substrates could be guinea pig liver (first generation assays), human red-cell derived and human recombinant. In general, specificity tends to be higher with human-based assays compared to first-generation assays.38 The pooled sensitivity and specificity for human-based IgA tissue transglutaminase antibodies (TTG) are both 98%.14 However, sensitivity (and to a lesser degree specificity) may vary among laboratories.37 Because of its simplicity and overall good diagnostic accuracy, detection of IgA tissue transglutaminase antibodies is the serologic test of choice for the diagnosis of CD.31, 39 False positive tests are unusual with human substrates, especially at high titers.33
Endomysial antibodies
Endomysial antibodies (EMA) have been available for diagnosis of CD for almost 30 years.40 The antibodies could be measured using an indirect immunofluorescence technique using monkey esophagus, human jejunum, or human umbilical cord as substrate. 41,42,43 The target antigen is tissue transglutaminase. The pooled sensitivity and specificity for IgA EMA were found to be 95% and 99%, respectively.14 Specificity of IgA-EMA is similar for tests using either monkey esophagus or human umbilical cord substrates.42 Despite the high specificity of this antibody, there are several test-related issues that may limit its use in clinical practice. It is semi-quantitative, time consuming, operator-dependent, and expensive. However, IgA EMA testing could be clinically useful if the result of the IgA TTG test is equivocal.31 A positive IgA EMA test is strong evidence for celiac disease in patients with non-atrophic intestinal lesions (Marsh I–II).44 An emerging indication for IgA EMA testing is to support a non-biopsy based diagnosis of celiac disease in symptomatic children with high titer of IgA TTG (see below, Diagnosis in Children).33
Clinical use of serologic tests
Serologic tests are useful to evaluate patients with suspected CD and may be helpful to monitor adherence to the gluten free diet (GFD). 39 Among adult patients with chronic abdominal symptoms, IgA TTG and IgA EMA have high accuracy for the diagnosis of CD.45 The initial serologic test of choice for CD diagnosis is IgA TTG. Sequential testing (IgA TTG positive followed by IgA EMA) has been an effective strategy for detection of CD in large epidemiological studies but its accuracy in clinical practice may require further study, especially if the intention is to avoid an intestinal biopsy for confirmation of CD.46 Testing for celiac disease is accurate only if the patient continues to follow a gluten-containing diet; therefore, it is important to inform patients that they should not start a gluten-free diet until the diagnostic process is completed.39 Indeed, all serological tests could become negative after gluten withdrawal.47 False negative serologic testing should be strongly considered in patients with selective IgA deficiency. In this scenario, cascade testing or alternatively concurrent measurement of total IgA should be considered. IgA deficient patients should be evaluated by measurement of IgG TTG, IgG DGP, and/or IgG EMA.14 In addition, a false negative test result is more likely in young children (less than 2 years of age) and among patients with non-atrophic lesions.39 Further assessment is needed when serology tests are negative but clinical suspicion of celiac disease is high; approximately 10% of patients with celiac disease are seronegative. Intestinal biopsy to confirm or exclude celiac disease is indicated in people with 1) a positive serologic result from any TTG, deamidated gliadin antibodies, or EMA test and 2) seronegative patients if celiac disease is highly suspected and genetic testing is positive. A cascade testing algorithm used at Mayo Clinic for the diagnosis of celiac disease is summarized in Figure 1.
FIGURE 1.
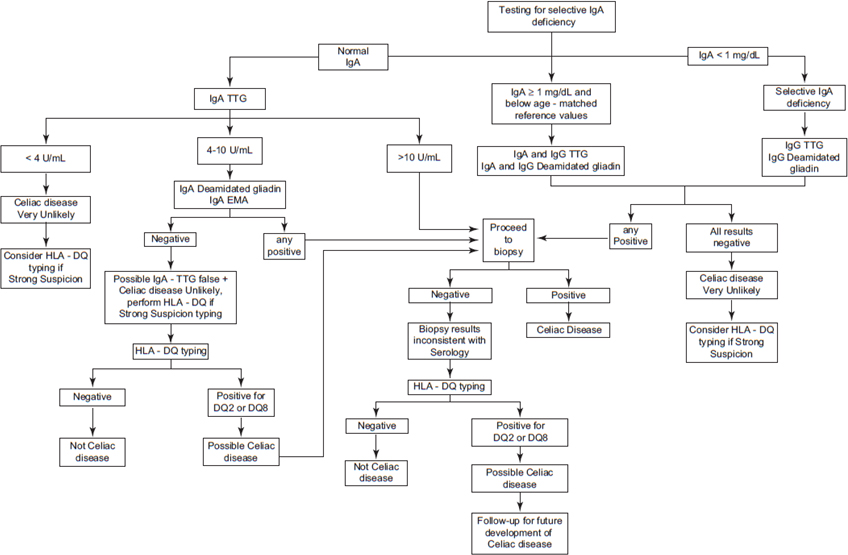
Mayo Clinic Celiac Disease Diagnostic Testing Algorithm. Serology and genetic testing are part of an automated laboratory cascade testing, meaning that a sequence of tests driven by real time results is used to diagnose celiac disease using a single blood draw. This cascade testing is intended for adults on a gluten-containing diet. (Modified and used by permission of Mayo Foundation for Medical Education and Research. All rights reserved).
Genetic Testing
Almost all patients with CD are positive for either human leukocyte antigen (HLA) DQ2 (heterodimer DQA1*05/DQB1*02) or DQ8 (heterodimer DQA1*03/DQB1*0302).48 HLA-DQ2 is carried in ~95% of patients with CD. Thus, the absence of these heterodimers has a high negative predictive value. Approximately 25%–30% of persons of European ancestry have one of these genotypes. 49–50 Thus, a positive result is of little diagnostic value. Of 1,008 European patients with CD, 61 were identified who neither carry the DQ2 nor DQ8 heterodimers but 57 encoded half of the DQ2 heterodimer.51 Other non-HLA risk factors have been used to improve identification of high-risk individuals for CD but its role on clinical evaluation of patients with suspicion of celiac disease will require further study.52 Routine addition of genetic testing (HLA-DQ testing) to TTG and EMA (or vise versa) does not increase diagnostic performance as either testing strategy alone.53 Thus, HLA-DQ genotyping is not indicated in the initial evaluation of CD. Genetic testing could be useful to exclude the diagnosis of CD (negative genetic testing) in selected cases when diagnosis is uncertain (i.e., equivocal small bowel histology).50 Genetic testing could be especially useful to exclude CD in patients already on a GFD because this test is not affected by gluten exclusion.
Small Intestinal Biopsy
Despite the diagnostic advances afforded by the availability of serological testing, the histological finding on small intestinal biopsy remains the gold standard for the diagnosis of CD, though in certain scenarios this may no longer be the case in pediatric patients (see below). Duodenal biopsy for the diagnosis of CD is most commonly performed after a patient is found to have a positive serologic test. But diagnostic biopsy may also be performed in the seronegative individual with signs and symptoms highly suspicious for CD, as none of the available serological tests has a sensitivity of 100%.
Biopsy can also be useful in the common (but less than ideal) scenario in which a patient already commenced a gluten-free diet prior to seeking medical care. The serologies in most patients with CD normalize after six to twelve months of adherence to the gluten-free diet, but the histopathological changes can persist for far longer; a recent study of patients with CD who underwent serial biopsy found that the median time to mucosal healing was 3.8 years.54 Moreover, even those patients with confirmed mucosal healing often have persistent intraepithelial lymphocytosis in the context of a normal crypt-to-villous ratio.55 Therefore, a patient with CD who is already adherent to the diet may have normal serologies but persistent histopathologic evidence of CD. However, it should be recommended not to start a GFD before completion of diagnostic investigations.
Endoscopic findings such as scalloping, fissures, a mosaic appearance, and decreased folds are often seen, but are neither sensitive nor specific findings in CD. In one study of 13 patients with scalloping of the duodenum but who ultimately were not found to have CD, alternative causes included Human Immunodeficiency Virus infection, eosinophilic enteritis, and giardiasis.56 A study of 129 patients with newly diagnosed CD found that approximately one third of such patients had an endoscopic appearance that was entirely normal, despite histopathologic evidence of intraepithelial lymphocytosis and villous atrophy.57
Despite the imperfect sensitivities of serological testing, and the poor predictive value of a normal endoscopic appearance, many patients with symptoms consistent with CD undergo EGD but do not have a duodenal biopsy during the procedure. In an analysis of the Clincal Outcomes Research Initiative (CORI) national endoscopic database, Harewood, et al identified 3,992 patients who underwent EGD for the indications of diarrhea, iron deficiency, anemia, and weight loss during the years spanning 2000–2003. Of these 3,992 patients, all of whom had a normal appearing duodenum, biopsy was performed in only 438 (11%). 58 In a follow-up study of the CORI database spanning the years 2004–2009, the rate of duodenal biopsy during examinations with the same indication improved but remained low at 43%.59 In this study, men were less likely to undergo biopsy than women (OR 0.81 95%CI 0.75–0.88), despite equal seroprevalence rates on screening,3 and older individuals were less likely to undergo biopsy compared to younger patients (OR for ≥70 compared to 20–49 0.51 95%CI 0.46–0.57), despite the fact that CD can present at any age, including in elderly individuals.60 The overall low rates of biopsy during EGD for symptoms that may be manifestations of CD indicate that endoscopist-related factors may be contributing in part to the under-diagnosis of CD.
Since the pathological findings in CD can be patchy and can affect areas of the duodenum with varying degrees of severity, multiple specimens from the duodenum should be submitted during biopsy to determine whether CD is present. Guidelines issued by the American Gastroenterological Association state that 4–6 specimens be submitted during duodenal biopsy.39 This recommendation is based on the authors’ understanding of the patchy nature of the disease, but was subsequently supported by the results of a study of CD patients, in which the sensitivity of biopsy for the diagnosis of CD declined when fewer than 4 specimens were submitted.61
In clinical practice in the United States, adherence to the standard of submitting 4–6 specimens during duodenal biopsy appears to be poor. An analysis of a national pathology database identified 132,352 patients who underwent duodenal biopsy during EGD’s performed in the years spanning 2006–2009.62 Of these 132,352 patients, ≥4 specimens were submitted during duodenal biopsy in only 45,995 (35%). Older patients were less likely to have ≥4 specimens submitted than younger patients (OR for age ≥ 80 versus <30 0.67; 95% CI 0.57–0.78), and patients with a procedure indication of diarrhea were more likely to have ≥4 specimens submitted than those with anemia (OR 1.20; 95% CI 1.10–1.30). The incremental diagnostic yield of adhering to this standard was high. The proportion of patients with a histological diagnosis of CD was only 0.7% when <4 specimens were submitted, as compared to 1.8% when ≥4 specimens were submitted. Even when the clinical indication was explicitly noted as suspected CD, adherence to this biopsy standard only occurred in 38.5% of all submissions. As the diagnostic yield for CD more than doubled regardless of the indication for the procedure, the submission of ≥4 specimens during duodenal biopsy would likely substantially increase the rate of CD diagnosis.
Other factors that may impact the diagnosis rate of CD during EGD include failure to biopsy the duodenal bulb63 and misinterpretation of subtle histopathology.64 Issues related to histopathological interpretation of duodenal bulb specimens and of interobserver variability in the histopathological diagnosis of CD are covered in further detail in chapter 5, “Histopathology of Celiac Disease”.
Thus, we propose that adequate sampling for CD diagnosis should include ≥4 specimens of the duodenum and include the duodenal bulb.
Because of the limitations associated with duodenal biopsy, some have advocated for diagnostic criteria that include, but do not entirely depend upon, biopsy results. Catassi and Fasano proposed a 5-point scoring system that incorporates 1) symptoms of CD (such as diarrhea, weight loss and iron deficiency anemia); 2) positive CD serologies at high titer; 3) the presence of a DQ2 or DQ8 haplotype; 4) characteristic histopathologic findings; and 5) a serological or histological response to the gluten-free diet.65 The presence of four out of the five criteria (or three out of four, if gene testing is not performed) would meet diagnostic criteria for CD according to this proposed system, which has not yet been validated prospectively.
This algorithm would allow for patients who have signs and symptoms of CD but have borderline histology, or who refuse biopsy, to be classified as having CD. If this approach is widely adopted, patients meeting the four non-histological criteria for CD would not require a biopsy. But any gain in sensitivity in a non-invasive scoring system is done at the cost of specificity. The algorithm would consider patients to have CD if they are not HLA-tested but have symptoms and serologies that improve on a gluten-free diet. Given the imperfect specificity of currently available serologies and the known phenomenon of gluten sensitivity in the absence of CD,66 a significant proportion of such patients may not have a positive HLA DQ2 or DQ8 and thus will likely not have CD as currently understood. Thus, an algorithm, as described above, that allows for a diagnosis of CD in adults without histologic evidence is not likely to be widely adopted by clinicians and investigators in the near future.
Diagnosis in Children
It is worth mentioning that the realm of diagnosing celiac disease historically has been solidly in the hands of pediatric gastroenterologists. It was in fact a meeting of the then just-created European Society for Pediatric Gastroenterology that established in 1969 the first diagnostic criteria, subsequently widely followed by adult as well as pediatric gastroenterologists worldwide. Essentially born in Europe with mostly biochemical research around the description of several new congenital disorders of digestion and absorption, pediatric gastroenterology soon emerged and became a powerful force in defining CD and in indicating how to diagnose it.
In the mid to late 60’s, it had become clear that CD could be diagnosed with the peroral jejunal biopsy showing atrophy of the villi, but since there were many causes of that lesion (and at that time especially chronic intestinal infections and milk protein allergy), a strong word of caution was exerted by the medical community not to diagnose CD until it could be proven that gluten was indeed the cause of the mucosal atrophy. Thus, not only was a clinical complete remission on gluten-free diet considered necessary, but this had to be followed by the documentation of the normalization of the lesion, and finally by its recurrence once gluten was reintroduced into the diet. These criteria were formalized in 1969 by a panel of experts in the then newly born European Society for Pediatric Gastroenterology (ESPGA, today ESPGHAN: European Society for Pediatric Gastroenterology, Hepatology and Nutrition).
These so-called “Interlaken criteria” (named for the area in Switzerland where this expert-only gathering took place) did not take into account another important discovery that had been made several years earlier: CD children possessed antibodies caused by the ingestion of gluten. The first category to be discovered were the anti-gliadin antibodies, detected and reported by Berger in 1964.67 Seven years later, Seah et al. identified for the first time not an anti-food protein, but an actual auto-antibody in the serum of celiac children: the anti-reticulins. 68 It took, however, several years before their diagnostic utility was fully appreciated, and the real leap forward occurred in 1984 when the anti-endomysium antibodies (EMA) were described 69 that soon dominated the scene for their high specificity.
In the late 1980’s, a large multicenter Italian study could demonstrate that by relying on strict clinical and laboratory criteria, and now also supported by EMA, a correct diagnosis of CD could be reached in 95% of cases by limiting to the one initial biopsy. 70 This plea for a change was soon followed by new diagnostic guidelines published the following year by ESPGHAN.71 These guidelines have been widely followed worldwide, not only in pediatric, but also in adult gastroenterology. Even though they were not “evidence-based” in the strictest sense of the word as used today,72 such recommendations still stemmed largely from the cited experience 70 and proved very useful not only in clinical practice but also as a reference for research.
The discovery in 1997 that tissue transglutaminase was the auto-antigen in CD led soon to the identification of a very sensitive marker: the tissue transglutaminase antibodies. Initially available as derived from guinea pigs (and hence with a good but not perfect sensitivity and specificity), the TTG antibodies were later developed from human origin and soon were shown to have extremely high sensitivity and good specificity. 73–75 North American pediatric gastroenterologists, in a plea to improve the poor diagnosis rates and long diagnostic delay (estimated to be higher than 10 years), were quick to understand the potential of TTG in the diagnosis, and in 2005 a panel of experts from NASPGHAN published evidence-based diagnostic recommendations directed at all physicians dealing with children who should be considered as possible CD patients.32 This guideline provides algorithms that are essentially based on performing first the TTG assay in children at risk (groups at risk are defined on clinical presentations and/or belonging to categories where the prevalence of CD is known to be higher), and then proceeding with further work-up only on those who have a positive TTG titer, regardless of its value. It is unclear how widely followed these recommendations have been; but certainly we have witnessed a major advance in the diagnosis rates in children over the past few years, in part due to an improving awareness of this condition, that has hit the media with a great impact.
Anti-deamidated gliadin peptides (DGP)
In pediatric age groups, there is evidence for a special role of the relatively new antibodies: the deamidated gliadin peptides. Antibodies from celiac disease patients have enhanced binding to deamidated gliadin peptides compared to controls.76 The deamidation of gliadin peptides occurs via a reaction catalyzed by tissue transglutaminase. In 2004, antibodies against the deamidated gliadin peptides (DGP) were shown to be accurate indicators for celiac disease. 77 Thus, in recent years, many studies have been done to figure out what role these antibodies can play in the disease diagnosis and follow up in the pediatric population. In IgA-deficient subjects (i.e. patients having less than 20mg/dl of total IgA in their serum) DGP IgG has sensitivity and specificity similar to TTG IgG, so they have been proposed as a useful test in such patients. 78–79
Even in patients with normal total IgA levels, TTG IgA and EMA are often negative in children with CD who are younger than two years. In the past, anti-gliadin antibodies (AGA) were used as non-specific marker of increased intestinal permeability to food proteins in young children but because of the low specificity for celiac disease (<80%) their use has been abandoned. However, the newer DGP do not suffer from such poor specificity, and--most importantly for pediatricians dealing with very young children--DGP has a high sensitivity in this patient population. Barbato, et al showed this in a study of 11 children under the age of 2 who had normal values of TTG and EMA but positive levels of DGP and subsequent histologic findings of celiac disease on endoscopy.80 To further support the use of DGP in young children, Monzani, et al showed that DGP IgA and IgG had sensitivity levels of 100% in children younger than three years.81 Similar results were also recently obtained by Mubarak et al82 in an investigation in 212 children with suspected CD: when the analysis was restricted to the 41 children below 2 years of age, the DGP-IgG had a diagnostic accuracy of 100%.
But the peculiar place that DGP has in pediatric ages goes beyond their important role in screening: in fact, DGP appears to be especially useful in monitoring dietary compliance. In 2007 Liu, et al showed that after initiation of the GFD, these antibodies became undetectable sooner than TTG,83 thus opening the way to further investigate their usefulness in this regard. Monzani, et al followed a population of 28 children and showed that DGP had a higher sensitivity than TTG IgA for monitoring compliance to the GFD;81 in 106 children on GFD for more than 1 year, sensitivity to detect dietary lapses was 60% for DGP-IgA and 76% for DGP IgA+G, while TTG IgA sensitivity was much lower at 24%. Thus, while both IgA and IgG DGP’s possessed higher sensitivity than TTG IgA, the combination of DGP IgA and DGP IgG performed better. Nachman, et al used a long-term prospective study to evaluate the predictive value of antibodies in monitoring compliance to the GFD in adults. 84 The study was on 53 adults recently diagnosed with CD and compared a complete set of antibodies including TTG IgA, EMA, DGP IgA and assays combining up to four different antibodies. The results showed that DGP IgA and TTG IgA were the most appropriate and consistent for monitoring compliance.
In addition to screening young children and to monitoring dietary compliance both in children and in adults, DGP may play a role in detection of “early cases” of celiac disease, defined as individuals with positive celiac autoantibodies who show mucosal changes of only Marsh 1 or 2 (i.e. no villous atrophy) but who subsequently develop villous atrophy (Marsh 3) when continuing to eat gluten. In a study on 42 adults proven to have early-stage CD despite normal small-bowel mucosal morphology (Marsh 1–2), and in 20 subjects with villous atrophy (Marsh 3), Kurppa et al. 85 found that sensitivity to detect early-stage CD was 79% for DGP vs. 64% for TTG.
In summary, in children the present evidence shows that TTG IgA and DGP IgA and IgG have superb and similar abilities to detect celiac disease, and that DGP IgG is also as good as TTG IgG in detecting IgA-deficient CD patients. In addition, DGP appears to have a unique and superior role in screening for celiac very young children, and may be superior to TTG in carefully monitoring dietetary compliance in diagnosed patients.
Diagnosis without a biopsy
Increased awareness and the 2005 NASPGHAN guidelines certainly allowed an improved diagnosis rate in North America, in spite of recent evidence of still inadequate utilization of a correct diagnostic approach by practicing gastroenterologists.86 Could then these guidelines be simplified? Studies from both sides of the Atlantic began raising the possibility that an accurate selection of patients might allow avoiding altogether the biopsy. In fact, it was shown that a good correlation existed between intensity of the intestinal damage and levels of serum TTG 87 and that selecting patients with elevated titers of TTG could result in an extremely high positive predictive value, hence predicting the possibility of avoiding the intestinal biopsy.88–91
In addition, great emphasis has been placed by investigators on the diagnostic dilemma for patients presenting only minor or no changes at histological analysis of duodenal mucosa.92–93 Do these patients have CD or not? Can we consider them as potential CD patients carrying a risk of developing the full-blown disease at a later stage? In essence: do they need to adhere to a GFD? Numerous speculations and proposed algorithms and scores have appeared,65, 94 and certainly the issue is still evolving as advances are made. The rapid evolution of diagnostic criteria for CD is best exemplified by a simple analysis of papers listed in Medline (Pubmed.org): a search for “celiac disease diagnosis” in a 180 day period in 2011–2012 revealed 176 entries: nearly 1 paper published every day on the subject!
For these reasons, ESPGHAN convened a panel that was charged with the task of providing new, strictly evidence-based recommendations for the diagnosis of CD in children. Their conclusions have been recently published,33 22 years after the historical paper of 1990, and are expected to set the new, world-wide standard for such diagnosis. In brief, a panel of 17 experts defined CD and developed new diagnostic criteria based on the Delphi process. Two groups of patients were defined with different diagnostic approaches to diagnose CD: children with symptoms suggestive of CD (group 1) and asymptomatic children at increased risk for CD (group 2). In group 1, the guideline suggests that diagnosis of CD is based on symptoms, positive serology, and histology that is consistent with CD. If TTG IgA is high (this is defined as more than 10 times the upper limit of normal), and only when there is also additional evidence of a compatible HLA haplotype and of a positive titer of serum EMA, then CD can be diagnosed without a duodenal biopsy. In group 2, the diagnosis of CD would be based on positive serology and histology, hence in all cases with the documentation of a positive biopsy.
Within weeks after publication of these guidelines a study whose results criticize them already appeared.95 These authors analyzed retrospectively 145 consecutive celiac disease patients with positive TTG who had an intestinal biopsy. The positive predictive value (PPV) for different cut-off points of TTG levels for the diagnosis of celiac disease was assessed, and the authors performed a simulation in a setting of routine clinical practice so to calculate the post-test probability of celiac disease. They found that no cut-off level was associated with a PPV of 100%. The highest PPV value (98.6%) was associated to a cut-off of 80U/mL (11.4×upper normal limit). Furthermore, in the frequent clinical situations carrying a pre-test probability of less than 10%, the post-test probability after was not superior to 90% even with the highest levels of TTG. Thus, the need for a confirmatory intestinal biopsy may still be present.
In conclusion, it is important to note that the new ESPGHAN evidence-based guideline does not recommend skipping the biopsy in the selected cases mentioned above, but simply allows the physician to do so at his/her discretion. As in all cases in medicine, the diagnosis is essentially a contract that must be stipulated in each individual case between the doctor and his/her patient, and is based on one side on the physician’s experience, knowledge of the literature and diagnostic acumen; and on the other on the patient’s family understanding and fully informed consent, given without reservations. No guidelines, no matter how evidence-based, can possibly ever replace such mutual trust.
Diagnoses of CD are increasing in the United States and worldwide.
Despite evidence of increasing rates of diagnosis, the majority of patients in the United States remain undiagnosed.
One approach to address the relatively low rates of CD diagnosis in the United States is to institute a program of population screening, in which all individuals regardless of symptoms undergo serological testing for CD, and those who screen positive subsequently undergo esophagogastroduodenoscopy (EGD) with small intestinal biopsy.
Despite calls for general population screening, problems with this approach have led to targeted case finding as the preferred method of increasing diagnosis rates.
The conflicting data with regard to mortality risk in undiagnosed CD are likely due to differences in age, definitions of seropositivity, and follow-up time, but given this residual uncertainty in magnitude of risk, if any, these data do not justify population screening.
Acknowledgments
Funding: BL: the National Center for Research Resources, a component of the National Institutes of Health (KL2 RR024157).
ART: American College of Gastroenterology Junior Faculty Development Award
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 2.Green PH, Neugut AI, Naiyer AJ, Edwards ZC, Gabinelle S, Chinburapa V. Economic benefits of increased diagnosis of celiac disease in a national managed care population in the United States. J Insur Med. 2008;40:218–28. [PubMed] [Google Scholar]
- 3.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 4.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz KD, Rashtak S, Lahr BD, et al. Screening for celiac disease in a North American population: sequential serology and gastrointestinal symptoms. Am J Gastroenterol. 2011;106:1333–9. doi: 10.1038/ajg.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42:530–8. doi: 10.3109/07853890.2010.514285. [DOI] [PubMed] [Google Scholar]
- 7.Catassi C, Fabiani E, Ratsch IM, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 8.Virta LJ, Kaukinen K, Collin P. Incidence and prevalence of diagnosed coeliac disease in Finland: results of effective case finding in adults. Scand J Gastroenterol. 2009;44:933–8. doi: 10.1080/00365520903030795. [DOI] [PubMed] [Google Scholar]
- 9.Fasano A. Should we screen for coeliac disease? Yes BMJ. 2009;339:b3592. doi: 10.1136/bmj.b3592. [DOI] [PubMed] [Google Scholar]
- 10.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302:1171–8. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 11.Hershcovici T, Leshno M, Goldin E, Shamir R, Israeli E. Cost effectiveness of mass screening for coeliac disease is determined by time-delay to diagnosis and quality of life on a gluten-free diet. Aliment Pharmacol Ther. 2010;31:901–10. doi: 10.1111/j.1365-2036.2010.04242.x. [DOI] [PubMed] [Google Scholar]
- 12.Shamir R, Hernell O, Leshno M. Cost-effectiveness analysis of screening for celiac disease in the adult population. Med Decis Making. 2006;26:282–93. doi: 10.1177/0272989X06289012. [DOI] [PubMed] [Google Scholar]
- 13.Long KH, Rubio-Tapia A, Wagie AE, et al. The economics of coeliac disease: a population-based study. Aliment Pharmacol Ther. 2010;32:261–9. doi: 10.1111/j.1365-2036.2010.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010;105:2520–4. doi: 10.1038/ajg.2010.276. [DOI] [PubMed] [Google Scholar]
- 15.Metzger MH, Heier M, Maki M, et al. Mortality excess in individuals with elevated IgA anti-transglutaminase antibodies: the KORA/MONICA Augsburg cohort study 1989–1998. Eur J Epidemiol. 2006;21:359–65. doi: 10.1007/s10654-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 16.Canavan C, Logan RF, Khaw KT, West J. No difference in mortality in undetected coeliac disease compared with the general population: a UK cohort study. Aliment Pharmacol Ther. 2011;34:1012–9. doi: 10.1111/j.1365-2036.2011.04811.x. [DOI] [PubMed] [Google Scholar]
- 17.Lohi S, Maki M, Rissanen H, Knekt P, Reunanen A, Kaukinen K. Prognosis of unrecognized coeliac disease as regards mortality: a population-based cohort study. Ann Med. 2009;41:508–15. doi: 10.1080/07853890903036199. [DOI] [PubMed] [Google Scholar]
- 18.Johnston SD, Watson RG, McMillan SA, Sloan J, Love AH. Coeliac disease detected by screening is not silent--simply unrecognized. QJM. 1998;91:853–60. doi: 10.1093/qjmed/91.12.853. [DOI] [PubMed] [Google Scholar]
- 19.Godfrey JD, Brantner TL, Brinjikji W, et al. Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology. 2010;139:763–9. doi: 10.1053/j.gastro.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther. 2012;35:540–51. doi: 10.1111/j.1365-2036.2011.04972.x. [DOI] [PubMed] [Google Scholar]
- 21.Sharaiha RZ, Lebwohl B, Reimers L, Bhagat G, Green PH, Neugut AI. Increasing incidence of enteropathy-associated T-cell lymphoma in the United States, 1973–2008. Cancer. 2011 doi: 10.1002/cncr.26700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilppula A, Kaukinen K, Luostarinen L, et al. Clinical benefit of gluten-free diet in screen-detected older celiac disease patients. BMC Gastroenterol. 2011;11:136. doi: 10.1186/1471-230X-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catassi C, Kryszak D, Louis-Jacques O, et al. Detection of Celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol. 2007;102:1454–60. doi: 10.1111/j.1572-0241.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 24.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 25.Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology. 2003;60:1581–5. doi: 10.1212/01.wnl.0000063307.84039.c7. [DOI] [PubMed] [Google Scholar]
- 26.Gabrielli M, Cremonini F, Fiore G, et al. Association between migraine and Celiac disease: results from a preliminary case-control and therapeutic study. Am J Gastroenterol. 2003;98:625–9. doi: 10.1111/j.1572-0241.2003.07300.x. [DOI] [PubMed] [Google Scholar]
- 27.Nachman F, Vazquez H, Gonzalez A, et al. Gastroesophageal reflux symptoms in patients with celiac disease and the effects of a gluten-free diet. Clin Gastroenterol Hepatol. 2011;9:214–9. doi: 10.1016/j.cgh.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Meyer D, Stavropolous S, Diamond B, Shane E, Green PH. Osteoporosis in a north american adult population with celiac disease. Am J Gastroenterol. 2001;96:112–9. doi: 10.1111/j.1572-0241.2001.03507.x. [DOI] [PubMed] [Google Scholar]
- 29.Brar P, Kwon GY, Holleran S, et al. Change in lipid profile in celiac disease: beneficial effect of gluten-free diet. Am J Med. 2006;119:786–90. doi: 10.1016/j.amjmed.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 30.NIH Consensus Development Conference on Celiac Disease. NIH Consens State Sci Statements. 2004;21:1–23. [PubMed] [Google Scholar]
- 31.NICE clinical guideline CG86: Coeliac disease: recognition and assessment of coeliac disease. doi: 10.1136/adc.2009.173849. (Accessed at www.nice.org.uk/CG86.) [DOI] [PubMed]
- 32.Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 34.Rashtak S, Ettore MW, Homburger HA, Murray JA. Comparative usefulness of deamidated gliadin antibodies in the diagnosis of celiac disease. Clin Gastroenterol Hepatol. 2008;6:426–32. doi: 10.1016/j.cgh.2007.12.030. quiz 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 36.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–33. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Yu L, Tiberti C, et al. A report on the International Transglutaminase Autoantibody Workshop for Celiac Disease. Am J Gastroenterol. 2009;104:154–63. doi: 10.1038/ajg.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sblattero D, Berti I, Trevisiol C, et al. Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease. Am J Gastroenterol. 2000;95:1253–7. doi: 10.1111/j.1572-0241.2000.02018.x. [DOI] [PubMed] [Google Scholar]
- 39.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Chorzelski TP, Sulej J, Tchorzewska H, Jablonska S, Beutner EH, Kumar V. IgA class endomysium antibodies in dermatitis herpetiformis and coeliac disease. Ann N Y Acad Sci. 1983;420:325–34. doi: 10.1111/j.1749-6632.1983.tb22220.x. [DOI] [PubMed] [Google Scholar]
- 41.Karpati S, Stolz W, Meurer M, Krieg T, Braun-Falco O. Extracellular binding sites of IgA anti-jejunal antibodies on normal small bowel detected by indirect immunoelectronmicroscopy. J Invest Dermatol. 1991;96:228–33. doi: 10.1111/1523-1747.ep12462097. [DOI] [PubMed] [Google Scholar]
- 42.Ladinser B, Rossipal E, Pittschieler K. Endomysium antibodies in coeliac disease: an improved method. Gut. 1994;35:776–8. doi: 10.1136/gut.35.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volta U, Molinaro N, Fratangelo D, Bianchi FB. IgA antibodies to jejunum. Specific immunity directed against target organ of gluten-sensitive enteropathy. Dig Dis Sci. 1994;39:1924–9. doi: 10.1007/BF02088126. [DOI] [PubMed] [Google Scholar]
- 44.Kurppa K, Collin P, Viljamaa M, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136:816–23. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 45.van der Windt DA, Jellema P, Mulder CJ, Kneepkens CM, van der Horst HE. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. JAMA. 2010;303:1738–46. doi: 10.1001/jama.2010.549. [DOI] [PubMed] [Google Scholar]
- 46.Walker MM, Murray JA, Ronkainen J, et al. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology. 2010;139:112–9. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Midhagen G, Aberg AK, Olcen P, et al. Antibody levels in adult patients with coeliac disease during gluten-free diet: a rapid initial decrease of clinical importance. J Intern Med. 2004;256:519–24. doi: 10.1111/j.1365-2796.2004.01406.x. [DOI] [PubMed] [Google Scholar]
- 48.Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81. doi: 10.1146/annurev.immunol.18.1.53. [DOI] [PubMed] [Google Scholar]
- 49.Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989;169:345–50. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaukinen K, Partanen J, Maki M, Collin P. HLA-DQ typing in the diagnosis of celiac disease. Am J Gastroenterol. 2002;97:695–9. doi: 10.1111/j.1572-0241.2002.05471.x. [DOI] [PubMed] [Google Scholar]
- 51.Karell K, Louka AS, Moodie SJ, et al. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003;64:469–77. doi: 10.1016/s0198-8859(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 52.Romanos J, van Diemen CC, Nolte IM, et al. Analysis of HLA and non-HLA alleles can identify individuals at high risk for celiac disease. Gastroenterology. 2009;137:834–40. 40 e1–3. doi: 10.1053/j.gastro.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 53.Hadithi M, von Blomberg BM, Crusius JB, et al. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann Intern Med. 2007;147:294–302. doi: 10.7326/0003-4819-147-5-200709040-00003. [DOI] [PubMed] [Google Scholar]
- 54.Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412–20. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iltanen S, Holm K, Partanen J, Laippala P, Maki M. Increased density of jejunal gammadelta+ T cells in patients having normal mucosa--marker of operative autoimmune mechanisms? Autoimmunity. 1999;29:179–87. doi: 10.3109/08916939908998533. [DOI] [PubMed] [Google Scholar]
- 56.Shah VH, Rotterdam H, Kotler DP, Fasano A, Green PH. All that scallops is not celiac disease. Gastrointest Endosc. 2000;51:717–20. doi: 10.1067/mge.2000.104977. [DOI] [PubMed] [Google Scholar]
- 57.Dickey W, Hughes D. Disappointing sensitivity of endoscopic markers for villous atrophy in a high-risk population: implications for celiac disease diagnosis during routine endoscopy. Am J Gastroenterol. 2001;96:2126–8. doi: 10.1111/j.1572-0241.2001.03947.x. [DOI] [PubMed] [Google Scholar]
- 58.Harewood GC, Holub JL, Lieberman DA. Variation in small bowel biopsy performance among diverse endoscopy settings: results from a national endoscopic database. Am J Gastroenterol. 2004;99:1790–4. doi: 10.1111/j.1572-0241.2004.40176.x. [DOI] [PubMed] [Google Scholar]
- 59.Lebwohl B, Tennyson C, Holub JL, Lieberman DA, Neugut AI, Green PH. Gender and racial disparities in duodenal biopsy for the diagnosis of celiac disease. Am J Gastroenterol. 2011;106:S82–S3. doi: 10.1016/j.gie.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee R, Egbuna I, Brar P, et al. Celiac disease: similar presentations in the elderly and young adults. Dig Dis Sci. 2010;55:3147–53. doi: 10.1007/s10620-010-1142-4. [DOI] [PubMed] [Google Scholar]
- 61.Pais WP, Duerksen DR, Pettigrew NM, Bernstein CN. How many duodenal biopsy specimens are required to make a diagnosis of celiac disease? Gastrointest Endosc. 2008;67:1082–7. doi: 10.1016/j.gie.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Lebwohl B, Kapel RC, Neugut AI, Green PH, Genta RM. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74:103–9. doi: 10.1016/j.gie.2011.03.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez S, Gupta A, Cheng J, et al. Prospective study of the role of duodenal bulb biopsies in the diagnosis of celiac disease. Gastrointest Endosc. 2010;72:758–65. doi: 10.1016/j.gie.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 64.Arguelles-Grande C, Tennyson CA, Lewis SK, Green PH, Bhagat G. Variability in small bowel histopathology reporting between different pathology practice settings: impact on the diagnosis of coeliac disease. J Clin Pathol. 2011 doi: 10.1136/jclinpath-2011-200372. [DOI] [PubMed] [Google Scholar]
- 65.Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691–3. doi: 10.1016/j.amjmed.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 66.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–94. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berger E, Buergin-Wolff A, Freudenberg E. [Diagnostic Value of the Demonstration of Gliadin Antibodies in Celiac Disease] Klin Wochenschr. 1964;42:788–90. doi: 10.1007/BF01479129. [DOI] [PubMed] [Google Scholar]
- 68.Seah PP, Fry L, Rossiter MA, Hoffbrand AV, Holborow EJ. Anti-reticulin antibodies in childhood coeliac disease. Lancet. 1971;2:681–2. doi: 10.1016/s0140-6736(71)92248-3. [DOI] [PubMed] [Google Scholar]
- 69.Chorzelski TP, Beutner EH, Sulej J, et al. IgA anti-endomysium antibody. A new immunological marker of dermatitis herpetiformis and coeliac disease. Br J Dermatol. 1984;111:395–402. doi: 10.1111/j.1365-2133.1984.tb06601.x. [DOI] [PubMed] [Google Scholar]
- 70.Guandalini S, Ventura A, Ansaldi N, et al. Diagnosis of coeliac disease: time for a change? Arch Dis Child. 1989;64:1320–4. doi: 10.1136/adc.64.9.1320. discussion 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker-Smith JA, SG, JS, DS, JV Revised criteria for diagnosis of coeliac disease. Report of a Working Group of ESPGAN. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoenfeld P, Guyatt G, Hamilton F, et al. An evidence-based approach to gastroenterology diagnosis. Gastroenterology. 1999;116:1230–7. doi: 10.1016/s0016-5085(99)70026-0. [DOI] [PubMed] [Google Scholar]
- 73.Wong RC, Wilson RJ, Steele RH, Radford-Smith G, Adelstein S. A comparison of 13 guinea pig and human anti-tissue transglutaminase antibody ELISA kits. J Clin Pathol. 2002;55:488–94. doi: 10.1136/jcp.55.7.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill PG, McMillan SA. Anti-tissue transglutaminase antibodies and their role in the investigation of coeliac disease. Ann Clin Biochem. 2006;43:105–17. doi: 10.1258/000456306776021599. [DOI] [PubMed] [Google Scholar]
- 75.Collin P, Kaukinen K, Vogelsang H, et al. Antiendomysial and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of coeliac disease: a biopsy-proven European multicentre study. Eur J Gastroenterol Hepatol. 2005;17:85–91. doi: 10.1097/00042737-200501000-00017. [DOI] [PubMed] [Google Scholar]
- 76.Aleanzi M, Demonte AM, Esper C, Garcilazo S, Waggener M. Celiac disease: antibody recognition against native and selectively deamidated gliadin peptides. Clin Chem. 2001;47:2023–8. [PubMed] [Google Scholar]
- 77.Schwertz E, Kahlenberg F, Sack U, et al. Serologic assay based on gliadin-related nonapeptides as a highly sensitive and specific diagnostic aid in celiac disease. Clin Chem. 2004;50:2370–5. doi: 10.1373/clinchem.2004.036111. [DOI] [PubMed] [Google Scholar]
- 78.Vermeersch P, Geboes K, Marien G, Hoffman I, Hiele M, Bossuyt X. Diagnostic performance of IgG anti-deamidated gliadin peptide antibody assays is comparable to IgA anti-TTG in celiac disease. Clin Chim Acta. 2010;411:931–5. doi: 10.1016/j.cca.2010.02.060. [DOI] [PubMed] [Google Scholar]
- 79.Villalta D, Tonutti E, Prause C, et al. IgG antibodies against deamidated gliadin peptides for diagnosis of celiac disease in patients with IgA deficiency. Clin Chem. 2010;56:464–8. doi: 10.1373/clinchem.2009.128132. [DOI] [PubMed] [Google Scholar]
- 80.Barbato M, Maiella G, Di Camillo C, et al. The anti-deamidated gliadin peptide antibodies unmask celiac disease in small children with chronic diarrhoea. Dig Liver Dis. 2011;43:465–9. doi: 10.1016/j.dld.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 81.Monzani A, Rapa A, Fonio P, Tognato E, Panigati L, Oderda G. Use of deamidated gliadin peptide antibodies to monitor diet compliance in childhood celiac disease. J Pediatr Gastroenterol Nutr. 2011;53:55–60. doi: 10.1097/MPG.0b013e3182145511. [DOI] [PubMed] [Google Scholar]
- 82.Mubarak A, Gmelig-Meyling FH, Wolters VM, Ten Kate FJ, Houwen RH. Immunoglobulin G antibodies against deamidated-gliadin-peptides outperform anti-endomysium and tissue transglutaminase antibodies in children <2 years age. APMIS. 2011;119:894–900. doi: 10.1111/j.1600-0463.2011.02817.x. [DOI] [PubMed] [Google Scholar]
- 83.Liu E, Li M, Emery L, et al. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J Pediatr Gastroenterol Nutr. 2007;45:293–300. doi: 10.1097/MPG.0b013e31806c7b34. [DOI] [PubMed] [Google Scholar]
- 84.Nachman F, Sugai E, Vazquez H, et al. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur J Gastroenterol Hepatol. 2011;23:473–80. doi: 10.1097/MEG.0b013e328346e0f1. [DOI] [PubMed] [Google Scholar]
- 85.Kurppa K, Lindfors K, Collin P, et al. Antibodies against deamidated gliadin peptides in early-stage celiac disease. J Clin Gastroenterol. 2011;45:673–8. doi: 10.1097/MCG.0b013e3181fbdfa6. [DOI] [PubMed] [Google Scholar]
- 86.Parakkal D, Du H, Semer R, Ehrenpreis ED, Guandalini S. Do gastroenterologists adhere to diagnostic and treatment guidelines for celiac disease? J Clin Gastroenterol. 2012;46:e12–20. doi: 10.1097/MCG.0b013e31822f0da0. [DOI] [PubMed] [Google Scholar]
- 87.Diamanti A, Colistro F, Calce A, et al. Clinical value of immunoglobulin A antitransglutaminase assay in the diagnosis of celiac disease. Pediatrics. 2006;118:e1696–700. doi: 10.1542/peds.2006-0604. [DOI] [PubMed] [Google Scholar]
- 88.Barker CC, Mitton C, Jevon G, Mock T. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics. 2005;115:1341–6. doi: 10.1542/peds.2004-1392. [DOI] [PubMed] [Google Scholar]
- 89.Alessio M, Tonutti E, Brusca I, et al. Correlation Between IgA Tissue Transglutaminase Antibody Ratio And Histological Finding In Celiac Disease: A Multicentre Study. J Pediatr Gastroenterol Nutr. 2011 doi: 10.1097/MPG.0b013e3182470249. [DOI] [PubMed] [Google Scholar]
- 90.Basso D, Guariso G, Bozzato D, et al. New screening tests enrich anti-transglutaminase results and support a highly sensitive two-test based strategy for celiac disease diagnosis. Clin Chim Acta. 2011;412:1662–7. doi: 10.1016/j.cca.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 91.Sugai E, Moreno ML, Hwang HJ, et al. Celiac disease serology in patients with different pretest probabilities: is biopsy avoidable? World J Gastroenterol. 2010;16:3144–52. doi: 10.3748/wjg.v16.i25.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kurppa K, Ashorn M, Iltanen S, et al. Celiac disease without villous atrophy in children: a prospective study. J Pediatr. 2010;157:373–80. 80 e1. doi: 10.1016/j.jpeds.2010.02.070. [DOI] [PubMed] [Google Scholar]
- 93.Tosco A, Salvati VM, Auricchio R, et al. Natural history of potential celiac disease in children. Clin Gastroenterol Hepatol. 2011;9:320–5. doi: 10.1016/j.cgh.2010.09.006. quiz e36. [DOI] [PubMed] [Google Scholar]
- 94.Rozenberg O, Lerner A, Pacht A, et al. A new algorithm for the diagnosis of celiac disease. Cell Mol Immunol. 2011;8:146–9. doi: 10.1038/cmi.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fernandez-Banares F, Alsina M, Modolell I, et al. Are positive serum-IgA-tissue-transglutaminase antibodies enough to diagnose coeliac disease without a small bowel biopsy? Post-test probability of coeliac disease. J Crohns Colitis. 2012 doi: 10.1016/j.crohns.2012.01.016. [DOI] [PubMed] [Google Scholar]


