Abstract
AMP-activated protein kinase (AMPK) is a stress signaling enzyme that orchestrates the regulation of energy-generating and -consuming pathways. Intrinsic AMPK activation protects the heart against ischemic injury and apoptosis, but whether pharmacologic AMPK stimulation mitigates ischemia-reperfusion damage is unknown. The aims of this study were to determine whether direct stimulation of AMPK using a small molecule activator, A-769662, attenuates myocardial ischemia-reperfusion injury, and to examine its cardioprotective mechanisms. Isolated mouse hearts pre-treated with A-769662 had better recovery of left ventricular contractile function (55% vs. 29% of baseline rate-pressure product; p=0.03) and less myocardial necrosis (56% reduction in infarct size; p<0.01) during post-ischemic reperfusion compared to control hearts. Pre-treatment with A-769662 in vivo attenuated infarct size in C57Bl/6 mice undergoing left coronary artery occlusion and reperfusion compared to vehicle (36% vs. 18%, p=0.025). Mouse hearts with genetically inactivated AMPK were not protected by A-769662, indicating the specificity of this compound. Pre-treatment with A-769662 increased the phosphorylation and inactivation of eukaryotic elongation factor 2 (eEF2), preserved energy charge during ischemia, delayed the development of ischemic contracture, and reduced myocardial apoptosis and necrosis. A-769662 also augmented endothelial nitric oxide synthase (eNOS) activation during ischemia, which partially attenuated myocardial stunning, but did not prevent necrosis. AMPK is a therapeutic target that can be stimulated by a direct-acting small molecule in order to prevent injury during ischemia-reperfusion. The use of AMPK activators may represent a novel strategy to protect the heart and other solid organs against ischemia.
Keywords: AMPK, cardioprotection, reperfusion injury, signal transduction, ischemic preconditioning
1. Introduction
AMP-activated protein kinase (AMPK) plays a critical role in the cellular response to diverse physiologic and pathologic stresses. The classic function of AMPK is to maintain energy homeostasis by modulating metabolic pathways. Recent studies have revealed an expanded role for AMPK in regulating cell growth and proliferation, cell membrane polarity, and mitochondrial biogenesis (reviewed in [1]). AMPK is emerging as a potential therapeutic target for the treatment of diabetes, obesity, cancer, and cardiovascular disease.
In the heart, intrinsic AMPK activation plays a pivotal role in the stress response to ischemia [2] and hypertrophy [3]. AMPK promotes energy production by activating glucose transport and glycolysis [4, 5], as well as mitochondrial fatty acid uptake and oxidation [6, 7]. Activated AMPK also safeguards the cellular energy charge by inhibiting ATP-consuming processes, including the biosynthesis of large macromolecules, such as glycogen, triglycerides, and proteins [8]. In addition, AMPK activation may directly alter cell survival by regulating apoptosis [2, 9], autophagy [10], endoplasmic reticulum stress [11], and reactive oxygen species generation [12]. Genetic mouse models with impaired cardiac AMPK activation sustain increased injury during ischemia-reperfusion [2, 13, 14], indicating an important endogenous protective role for AMPK in the heart.
Whether direct activation of AMPK protects the heart or other solid organs against ischemia-reperfusion injury is uncertain. Studies to date have used indirect and nonspecific activators of AMPK. AICAR (5-aminoinidazole-4-carboxamide 1-β-D-ribofuranoside) [15] and metformin [13] both activate AMPK but also have pleiotropic AMPK-independent effects. AICAR is metabolized to an AMP mimetic, which activates other AMP-activated enzymes. It also blocks cellular adenosine reuptake [16], leading to increased extracellular concentrations of adenosine, which has an important preconditioning effect. The anti-diabetic drug metformin activates increases the cellular AMP:ATP ratio by inhibiting complex I of the mitochondrial electron transport chain [17] and has AMPK-independent effects [18-20].
Interest in activating AMPK directly has led to the identification of A-769662, a non-nucleoside thienopyridone [21]. AMPK is a heterotrimeric complex containing a catalytic α and regulatory β and γ subunits. The γ subunit contains nucleotide binding domains [22]. AMPK activity is governed primarily by the phosphorylation state of Thr172 in the catalytic domain of the α subunit. This site is phosphorylated by LKB1 and calcium-calmodulin dependent protein kinase kinase (CaMKK) and dephosphorylated by protein phosphatases, including PP2C. A-769662 directly binds to the β subunit and causes allosteric stimulation of AMPK activity [23, 24]. Under selected conditions, the binding of A-769662 to the β subunit renders the phosphorylated Thr172 residue resistant to protein phosphatases, thereby greatly promoting AMPK activation [23, 24]. Thus, A-769662 has gained significant interest as a novel approach to directly activate AMPK without increasing the cellular AMP:ATP ratio. The purpose of the current experiments was to test whether direct activation of AMPK by a small molecule represents an effective strategy to protect the heart against acute ischemia-reperfusion injury.
2. Materials and methods
2.1. Animals
All animal experiments were approved by the Yale Institutional Animal Care and Use Committee. Wild type (WT) C57Bl/6 male mice were obtained from Charles River Laboratory. Transgenic mice that express a kinase dead (KD) rat α2 isoform (K45R mutation), driven in heart and skeletal muscle by the muscle creatine kinase promoter, were generated as previously described [2]. These mice were backcrossed >7 generations into the C57Bl/6 background. eNOS knockout mice (Nos3−/−, C57Bl/6; strain name: B6.129P2-Nos3tm1Unc/J; stock number: 002684) were obtained from Jackson Laboratory. Male mice were studied at 12-16 weeks of age (weight of 25-30g).
2.2. Antibodies and reagents
Western blotting was performed as previously described [4] with the following antibodies: rabbit anti-phospho-AMPKα (Thr172), rabbit anti-AMPKα, rabbit anti-ACC, rabbit anti-phospho-eNOS (Ser1177), rabbit anti-eNOS, rabbit anti-phospho-eEF2 (Thr56), rabbit anti-eEF2, rabbit anti-phospho-Akt (Ser473), and rabbit anti-Akt. All of the above were purchased from Cell Signaling. Rabbit anti-phospho-ACC (Ser79) was obtained from Millipore. A-769662 was synthesized at the University of Dundee [23]. A-769662 (MW 360.39) was initially dissolved in DMSO (278 mmol/L) and diluted in Krebs-Henseleit buffer (0.036% DMSO final concentration) for perfused heart experiments and diluted in 0.9% saline (1% DMSO final concentration) for intraperitoneal injections.
2.3. Isolated mouse heart perfusions
Isolated mouse hearts were perfused in the Langendorff mode with modified Krebs-Henseleit buffer containing glucose (7 mmol/L), oleate (0.4 mmol/L), BSA (1%), and a low-fasting concentration of insulin (10 μU/ml) at 37°C, as previously described [2, 25]. A fluid-filled latex balloon connected to a solid-state pressure transducer (Millar Instruments) was inserted into the left ventricle through a left atriotomy to measure pressure. LV developed pressure (LVDP), the first derivative of LVDP (dP/dt), LV end-diastolic pressure, and heart rate were recorded using a digital acquisition system (ADInstruments) at a balloon volume that resulted in baseline LV end-diastolic pressure of 5mmHg [2, 25]. Hearts were initially perfused at a constant aortic pressure of 80cm H2O for 5 minutes to flush out residual blood from the heart and allow stabilization. Constant flow perfusion was then achieved using a peristaltic pump with a baseline coronary flow rate of 4 ml/min. “No-flow” ischemia was induced, by terminating flow, and reperfusion was restored at a flow rate of 4 ml/min [25].
During A-769662 studies, isolated hearts were first stabilized on the perfusion apparatus for 15 minutes, then perfused for 30 minutes with Krebs-Henseleit buffer containing A-769662 (100 μmol/L) or vehicle (DMSO 0.036%). Global no-flow ischemia was induced for 25 minutes and then the hearts were reperfused (without compound) for 30 minutes. In these experiments, all hearts were paced at a heart rate of 450 min−1 during the pre-ischemic period and perfused at 4 ml/min during the non-ischemic periods.
2.4. Metabolic analyses
Lactate production was determined by measuring the concentration of lactate released in the coronary effluent during baseline perfusion, ischemia (collected during the first minute of reperfusion), and after 10 minutes of reperfusion. Serum glucose and lactate, and perfusate lactate were measured using an automated analyzer STAT plus 2300; Yellow Springs Instrument Co. [2]. AMP, ADP, and ATP levels were determined in neutralized perchloric acid extracts of heart homogenates using high-performance liquid chromatography, as previously described [2].
2.5. In vivo coronary occlusion/reperfusion
Mice were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneal injection), intubated, and ventilated (Harvard Rodent Ventilator model 683) as previously described [25, 26]. Their core temperature was maintained at 37°C. Limb lead electrocardiogram was recorded using a bioamplifier (ADInstruments) interfaced with Chart v5.2.2 software. Mice underwent left lateral thoracotomy and the proximal left coronary artery (LCA) was occluded for 20 minutes, as previously described [25, 26]. The occlusion was released after 20 minutes of ischemia, and the heart was reperfused for 4 hours.
For in vivo A-769662 experiments, WT C57Bl/6 and KD mice were injected with A-769662 (6 mg/kg intraperitoneal) 30 minutes prior to coronary artery occlusion and reperfusion. The proximal LCA was occluded for 20 minutes, and then the heart was reperfused for 4 hours [26].
For the ischemic preconditioning experiments, AMPK KD mice and WT littermates were anesthetized, ventilated and subjected to 6 cycles of 4 min ischemia (induced by transient ligation of the LCA) interspersed by 4 min reperfusion prior to 20 minutes of sustained ischemia, followed by 4 hours of reperfusion. At the completion of each protocol, hearts were perfused for vital staining, or freeze-clamped and stored in liquid nitrogen until the time of assay.
2.6. Analysis of myocyte necrosis and apoptosis
For in vitro experiments, hearts were perfused with 1% triphenyltetrazolium chloride (TTC) through the aorta at 37°C and fixed in 4% formalin, sectioned into 1mm slices, photographed using a Leica microscope, and analyzed using NIH ImageJ software [26]. For in vivo experiments, hearts were dual stained: first they were perfused with TTC and then the LCA was re-occluded and the heart was perfused with 0.5% Evan’s blue dye to define the non-ischemic region. This technique provides a measurement of the extent of myocardial necrosis as a percent of the ischemic area at risk (AAR) distal to the coronary occlusion [26]. Myocyte necrosis was also assessed by determining the release of creatine kinase into the perfusate using commercially available (Catachem) spectrophotometric assay [2]. In addition, hearts were fixed in 4% formalin and embedded in paraffin for TUNEL staining using fluorescein-labeled dUTP (Roche Diagnostics) and counterstaining with DAPI. Apoptotic nuclei were quantified by fluorescence microscopy.
2.7. Statistical analysis
Data are reported as the mean ± SEM. The number of experiments in each group is presented in the text, figure, or figure legend. Differences between A-769662 and vehicle control groups were assessed by Student two-tail t test. Changes in metabolic parameters or LV contractile function over time were determined by two-way repeated measures ANOVA using Tukey’s test for post hoc comparisons using GraphPad Prism 4.02. Differences were considered significant at P < 0.05.
3. Results
3.1. A-769662 protects isolated hearts against ischemia-reperfusion injury
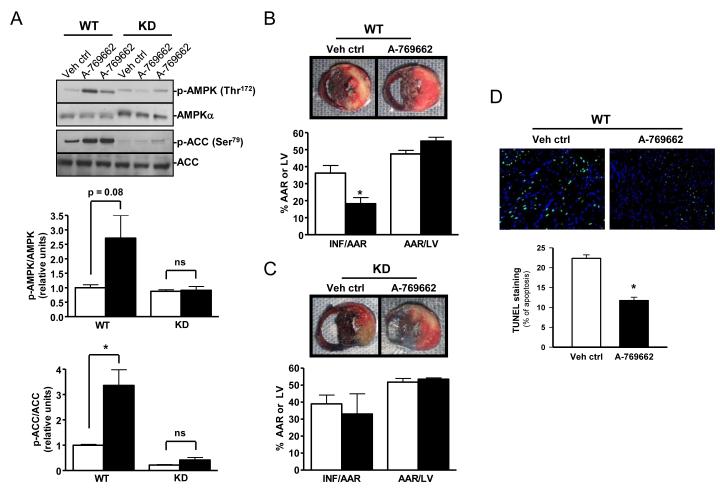
We initially examined whether A-769662 activates the AMPK pathway in isolated perfused mouse hearts. Perfusion with A-769662 (100 μmol/L) for 30 minutes activated the AMPK pathway, as evidenced by significantly increased phosphorylation of the AMPK target site on acetyl-CoA carboxylase (ACC) Ser79 (Figure 1A). The compound did not significantly increase AMPK Thr172 phosphorylation, indicating a primary allosteric mechanism of AMPK activation [23, 24]. Perfusion with A-769662 did not affect myocardial ATP content, AMP:ATP ratio, or the adenylate energy charge (Figure 1B), indicating that AMPK was not indirectly activated by a perturbation in cardiac energetics.
Figure 1. A-769662 activates the AMPK pathway and protects the heart against ischemia-reperfusion injury in vitro.
(A) Isolated C57Bl/6 mouse hearts were treated with A-769662 (100 μmol/L) or vehicle for 30 min of aerobic perfusion. Heart homogenates were immunoblotted for p-ACC (Ser79), ACC, p-AMPK (Thr172), and AMPKα. Representative blots and the ratios of phosphorylated to total protein content are shown (n=5-6/group). (B) Myocardial ATP content, AMP:ATP ratio, and adenylate charge following aerobic perfusion with A-769662 or vehicle (n=7-8/group). Adenylate charge = (ATP+½(ADP))/ (ATP+ADP+AMP). (C) Isolated C57Bl/6 hearts were treated with A-769662 (100 μmol/L) or vehicle for 30 min prior to global no-flow ischemia (25 min) and reperfusion (30 min). LV developed pressure (LVDP) and cardiac rate-pressure product (RPP = LV systolic pressure x heart rate) are shown (n=8-10/group). (D) Myocardial necrosis was quantified by TTC staining and release of creatine kinase (CK) into the coronary effluent (n=8-10/group). White bar, vehicle. Black bar, A-769662. Values are expressed as mean + SEM. *P<0.05; **P<0.01.
To determine whether A-769662 prevents ischemic injury, isolated hearts were perfused with A-769662 (100 μmol/L) or vehicle for 30 minutes prior to no-flow ischemia (25 min) and reperfusion (30 min). Hearts were reperfused without compound after ischemia. A-769662 did not affect pre-ischemic left ventricular (LV) developed pressure or the heart rate x LV systolic pressure product (Figure 1C). Hearts pre-treated with A-769662 had better recovery of LV contractile function during reperfusion compared to vehicle-treated hearts (55% vs. 29% of baseline rate-pressure product; p=0.03) (Figure 1C). Recovery of function can be delayed after ischemia-reperfusion due to myocardial stunning [27]. However, A-769662 also decreased myocardial necrosis with a 56% reduction in infarct size by TTC staining (p<0.01) and a similar 57% reduction in creatine kinase release during reperfusion (p<0.05) (Figure 1D).
3.2. Genetic inactivation of AMPK abrogates the A-769662 cardioprotective effect
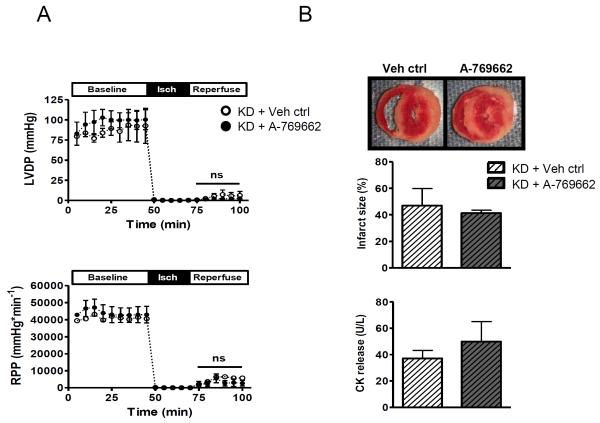
In order to determine whether cardioprotection was specifically mediated by AMPK activation, we tested the effect of A-769662 on hearts from AMPK kinase dead (KD) mice. KD hearts have absent activation of α2 AMPK complexes and markedly blunted activation of α1 complexes compared to WT hearts during ischemia (Supplementary Figure 1). In contrast to WT hearts, A-769662 did not improve the recovery of cardiac function (Figure 2A) or reduce infarct size (Figure 2B) after ischemia in the KD hearts.
Figure 2. AMPK inactivation abrogates the cardioprotective effects of A-769662 pre-treatment.
Isolated AMPK inactivated kinase dead (KD) hearts were treated with A-769662 (100 μmol/L) or vehicle for 30 min during baseline aerobic perfusion, prior to global no-flow ischemia (25 min) and subsequent reperfusion (30 min) without compound. (A) LV developed pressure (LVDP) and cardiac rate-pressure product (RPP = LV systolic pressure x heart rate) are shown (n=5/group). (B) Myocardial necrosis was quantified by TTC staining and release of CK into the coronary effluent (n=5/group). Values are expressed as mean + SEM.
3.3. A-769662 pre-treatment promotes ATP preservation during ischemia
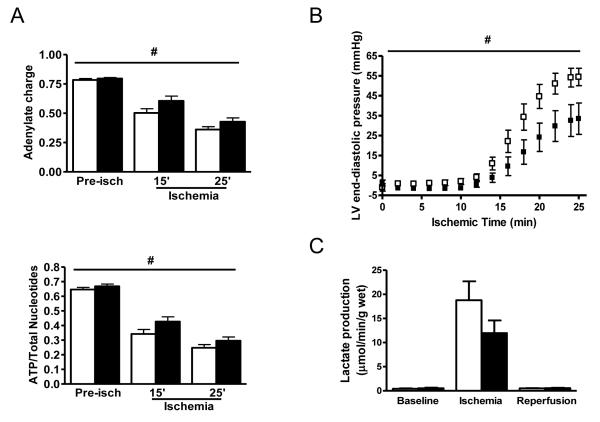
In order to delineate the mechanisms responsible for cardioprotection, we measured myocardial nucleotide content to assess whether the AMPK activator prevented ATP depletion during ischemia. The rate of decline in both ATP content and the adenylate energy charge during ischemia was slower in the hearts pre-treated with A-769662 compared to vehicle (p<0.05, 2-way ANOVA) (Figure 3A). This reduction in ATP depletion was associated with delayed development of LV ischemic contracture in hearts pre-treated with the AMPK activator (Figure 3B), consistent with improved energetics during ischemia.
Figure 3. A-769662 promotes ATP preservation and delays ischemic contracture.
(A) C57Bl/6 hearts were treated with A-769662 (100 μmol/L) or vehicle for 30 min prior to either 15 or 25 min of no-flow ischemia. Myocardial adenylate charge and ATP content (expressed as a proportion of total adenine nucleotide pool) were determined (n=5-9/group). (B) Isolated C57Bl/6 hearts were treated with A-769662 (100 μmol/L) or vehicle for 30 min prior to global no-flow ischemia (25 min). LV end-diastolic pressure during ischemia is shown (n=8/group). (C) Myocardial lactate production during baseline perfusion, ischemia, and reperfusion (n=5-7/group). White bar, vehicle. Black bar, A-769662. Values are expressed as mean + SEM. #P<0.05, 2-way ANOVA.
One potential molecular mechanism that might contribute to ATP preservation during ischemia is inhibition of energy-requiring protein synthesis. Activated AMPK is known to phosphorylate and activate eukaryotic elongation factor (eEF2)-kinase, which phosphorylates and inactivates eEF2 [8, 28]. We hypothesized that an AMPK activator might augment intrinsic AMPK pathway activation during ischemia and further inhibit eEF2. We found that A-769662 treatment did indeed augment phosphorylation of both AMPK Thr172 and ACC Ser79 during ischemia (Figure 4). In addition, A-769662 enhanced eEF2 phosphorylation during ischemia (Figure 4), consistent with the hypothesis that amplification of the eEF2 kinase response might contribute to the improvement in energy homeostasis during ischemia.
Figure 4. A-769662 pre-treatment augments AMPK activation and eEF2 phosphorylation during ischemia.
C57Bl/6 hearts were treated with A-769662 (100 μmol/L) or vehicle for 30 min during baseline aerobic perfusion prior to 25 min of global no-flow ischemia. Heart homogenates were immunoblotted for p-AMPK (Thr172), AMPKα, p-ACC (Ser79), ACC, p-eEF2 (Thr56) and eEF2. Representative blots and the ratios of phosphorylated to total protein content are shown (n=5-9/group). White bar, vehicle. Black bar, A-769662. Values are expressed as mean + SEM. *P<0.05; **P<0.01.
Another possibility is that the AMPK activator augments energy generation during ischemia. During no-flow ischemia, energy generation depends on anaerobic glycolysis, which leads to lactate production. However, A-769662 pre-treatment did not increase lactate production during ischemia (Figure 3C), suggesting that ATP preservation rather than ATP generation is a more important component of its action.
3.4. eNOS activation contributes to the effect of A-769662 to prevent myocardial stunning but does not reduce necrosis
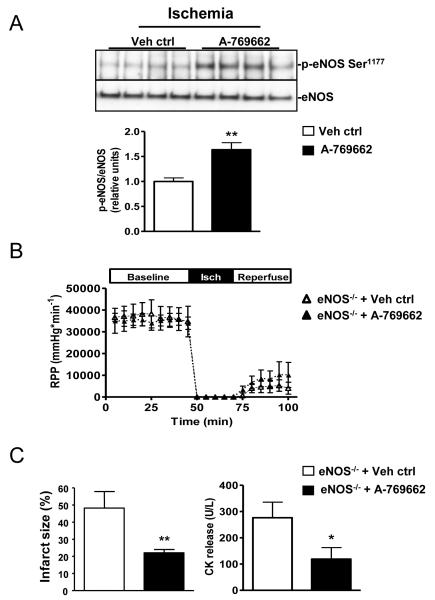
Endothelial nitric oxide synthase (eNOS) is a direct target of AMPK [29] and has cardioprotective effects during ischemia [30]. A-769662 pre-treated hearts exhibited enhanced eNOS phosphorylation at the AMPK target site (Ser1177) during ischemia (Figure 5A). Since Ser1177 is also an Akt phosphorylation site, we evaluated whether A-769662 treatment influences Akt activation; however, A-769662 did not change Akt Ser473 phosphorylation during either baseline or ischemic periods (data not shown).
Figure 5. eNOS activation contributes to the effect of A-769662 to prevent myocardial stunning but does not reduce necrosis.
(A) Isolated C57Bl/6 mouse hearts were stabilized for 15 min, then treated with A-769662 (100 μmol/L) or vehicle for 30 min during baseline aerobic perfusion prior to global no-flow ischemia (25 min). Heart homogenates were immunoblotted for p-eNOS (Ser1177) and eNOS. Representative blots and quantification of the ratio of phosphorylated to total eNOS are shown (n=5-9/group). (B and C) Isolated hearts from eNOS−/− mice (C57Bl/6 background) were treated with A-769662 (100 μmol/L) or vehicle for 30 min during baseline aerobic perfusion, prior to global no-flow ischemia (25 min) and subsequent reperfusion (30 min) without compound. Cardiac rate-pressure product (RPP) and creatine kinase (CK) release into the coronary effluent during reperfusion are shown (n=5-7/group). Values are expressed as mean + SEM. *P<0.05; **P<0.01.
In order to determine the extent to which A-769662-induced cardioprotection is mediated by eNOS activation, we investigated whether genetic eNOS deficiency abrogates the compound’s protective effect. Consistent with previous reports [30], eNOS−/− hearts were more sensitive to ischemia and exhibited greater contractile dysfunction and necrosis during ischemia-reperfusion compared to WT hearts. Nonetheless, A-769662 reduced infarct size to a comparable degree in both eNOS−/− and WT hearts (Figures 5C and 1D), indicating that augmented eNOS activation does not mediate the action of A-769662 to prevent necrosis. However, its ability to prevent LV contractile dysfunction after ischemia was blunted in eNOS−/− hearts (Figure 5B), suggesting that eNOS activation might play a role in the attenuation of post-ischemic myocardial stunning by A-7696662 treatment.
3.5. A-769662 reduces myocardial infarct size in vivo
We next utilized the coronary artery ligation model to address whether a small molecule AMPK activator also protects against ischemic injury in vivo. In pharmacokinetic studies, we found that an intra-peritoneal injection of A-769662, at the previously described dose of 6 mg/kg [21], activated the heart AMPK pathway after 20 minutes, as evidenced by both increased AMPK and downstream ACC phosphorylation in WT mice (Figure 6A and Supplementary Figure 2). Of note, there was no downstream ACC phosphorylation after A-769662 treatment in AMPK-inactivated KD mice (Figure 6A), excluding an AMPK-independent mechanism of ACC activation. Based on these results, we administered A-769662 to WT mice 30 minutes prior to coronary artery occlusion and reperfusion. Mice pre-treated with A-769662 had reduced infarct size compared to vehicle-treated controls (36% to 18%, p=0.025) (Figure 6B). A-769662 did not decrease the ischemic area at risk during coronary artery occlusion (Figure 6B), excluding the possibility that it enhanced collateral blood supply during ischemia. In addition, we observed a reduced number of TUNEL-positive nuclei in the central ischemic region following in vivo ischemia and reperfusion in A-769662 pre-treated hearts compared to control (Figure 6D). Taken together, these data suggest an important role for the AMPK activator in myocyte protection against ischemia/reperfusion–mediated myocardial necrosis and apoptosis.
Figure 6. A-769662 pre-treatment activates AMPK and reduces myocardial infarct size in vivo.
(A) Anesthetized WT and AMPK KD mice were treated with A-769662 (6 mg/kg IP) or vehicle, and hearts were excised after 20 min. Heart homogenates were immunoblotted for p-AMPK (Thr172), AMPKα, p-ACC (Ser79), and ACC. Representative blots and quantification of the ratios of phosphorylated to total protein content are shown (n=4-6/group). (B and C) Anesthetized WT and AMPK KD mice were treated with A-769662 (6 mg/kg IP) or vehicle 30 min prior to left coronary artery ligation (20 min) and reperfusion (4 hours). Representative dual stained heart sections and bar graphs quantifying the ratio of infarcted area (INF) to the area at risk (AAR) and the ratio of AAR to total left ventricular area (LV) are shown (n=5-7/group). (D) Additional hearts were fixed and apoptotic nuclei in the ischemic-reperfused region were identified by TUNEL staining (original magnification, x40) (n=4/group). White bar, vehicle. Black bar, A-769662. Values are expressed as mean + SEM. *P<0.05.
To exclude the possibility that systemic administration of A-769662 might protect the heart via a non-AMPK pathway, we also performed coronary artery ligations in KD mice. In contrast to WT mice, the KD mice demonstrated no protection with A-769662 treatment (Figure 6C). To exclude the possibility that this drug might affect hemodynamic parameters that could impact AMPK signaling or ischemic tolerance, we assessed its effect on heart rate and blood pressure and found no significant change in these parameters in vivo (Supplementary Figure 3A).
In addition, the in vivo administration of A-769662 did not affect baseline adenine nucleotide content in the hearts (Supplementary Figure 3A). Furthermore, the in vivo dose did not affect serum glucose or lactate levels in the pre-ischemic (baseline) state (Supplementary Figures 3B and 3C). Measurements were made 30 minutes after injection of compound in anesthetized non-ischemic animals. Interestingly, the A-769662 pre-treated mice had less slightly lower serum glucose concentrations at the end of ischemia (50 minutes after injection), compared to control (p=0.03) (Supplementary Figure 3B). This finding substantiates a systemic metabolic action of A-769662 to activate AMPK. However, there was no evidence for hypoglycemia in any of the mice, and serum glucose concentrations were comparable in the two groups during reperfusion.
3.6. AMPK activation and ischemic preconditioning
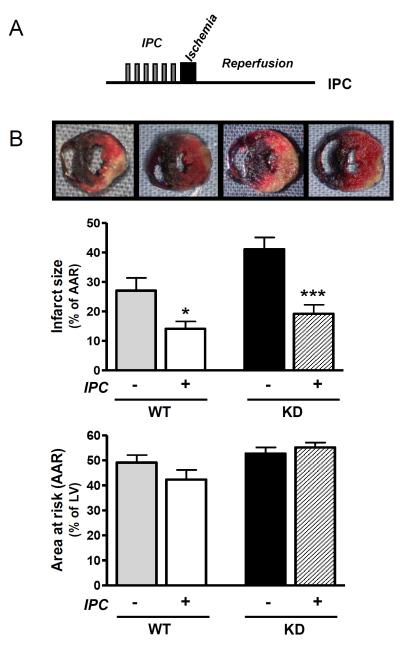
Ischemic preconditioning is a potent means to protect the heart against injury during more prolonged and potentially lethal episodes of ischemia [31]. Short periods of ischemia activate the AMPK pathway [12, 32]; however, it is not known whether AMPK activation is necessary or sufficient for ischemic preconditioning. Thus, we subjected KD mice and WT littermates to an established ischemic preconditioning protocol [33], prior to 20 minutes of sustained ischemia and 4 hours of reperfusion (Figure 7A). KD mice sustained greater cardiac injury during ischemia-reperfusion, however, ischemic preconditioning reduced necrosis to a similar extent in WT (48%) and KD (53%) mice (Figure 6B), indicating that ischemic preconditioning does not require AMPK activation in the intact mouse model. The effective preconditioning of KD hearts is also noteworthy in that it indicates that the inability of A-769662 to protect the KD hearts was not simply the consequence of greater injury during ischemia-reperfusion, but rather due to the dependence of A-769662 on AMPK activation. In addition, the reduction in necrosis by ischemic preconditioning was similar to that seen with A-769662 treatment in WT mice (Figure 7B), demonstrating that AMPK activation is sufficient to replicate the protection induced by ischemic preconditioning.
Figure 7. AMPK activity is not required for the myocardial protection induced by ischemic preconditioning.
(A) In vivo ischemic preconditioning protocol (IPC): 6 cycles of 4 min ischemia interspersed by 4 min reperfusion prior to sustained ischemia (20 min) followed by reperfusion (4 hours). Ischemia was induced by ligation of the left coronary artery in anesthetized AMPK KD mice and WT littermates in vivo. (B) Representative heart sections following dual staining and bar graphs quantifying the ratio of infarcted area to the area at risk (AAR) and the ratio of AAR to total left ventricular area (LV) (n=7-9/group). Values are expressed as mean + SEM. *P<0.05; ***P<0.001.
4. Discussion
These results provide the first evidence that treatment with a direct AMPK-activating small molecule represents an effective strategy to protect the heart against ischemia-reperfusion injury. A-769662 attenuated post-ischemic cardiac contractile dysfunction and necrosis. The cardioprotective effect of this compound was abrogated in KD hearts, indicating that its action was mediated through an AMPK-dependent mechanism. In addition to activating the AMPK pathway prior to ischemia, A-769662 pre-treatment augmented intrinsic AMPK activation during ischemia. These actions preserved myocardial energy charge during ischemia, possibly by limiting energy expenditure via inactivation of downstream eEF2, a key regulator of protein synthesis. A-769662 also increased the activation of downstream eNOS, which appeared to play a role in reducing myocardial stunning following ischemia, but did not mediate its action to decrease necrosis. Although AMPK was not required for ischemic preconditioning, consistent with the existence of other redundant pathways, AMPK activation with the small molecule replicated the potent protection seen with ischemic preconditioning.
A-769662 has a unique mode of action, activating AMPK complexes by interacting with regulatory β1 subunits [34], which are expressed in cardiac myocytes, along with β2 subunits [35]. In our experiments, A-769662 increased downstream ACC phosphorylation at Ser79, the prototypical AMPK target site, both in isolated perfused hearts and in intact hearts in vivo, but significantly increased AMPK Thr172 phosphorylation only in the in vivo setting. The observation that ACC, but not AMPK, was phosphorylated in perfused hearts indicates a primary allosteric effect of the compound on the AMPK complex under non-ischemic conditions in vitro, similar to that observed in cultured hepatocytes [21, 23]. We confirmed that phosphorylation of ACC in perfused hearts was specifically mediated by AMPK activation, by demonstrating that A-769662 had no effect on ACC phosphorylation in KD hearts. A-769662 can also increase Thr172 phosphorylation by inhibiting protein phosphatase action on this site [23, 24], and this mechanism appeared to be operative in the heart in vivo. Thus, it appears that A-769662 activates the AMPK pathway in the low workload isolated perfused heart primarily via an allosteric mechanism, but increases Thr172 phosphorylation under higher workload conditions in vivo or during ischemia when AMPK is already partially activated in the heart.
This study suggests potential downstream molecular mechanisms that might play a role in the cardioprotection afforded by the small molecule AMPK activator. A-769662 preserved the high-energy adenylate charge during ischemia, indicating a more favorable balance between energy generation and utilization pathways. A-769662 also delayed the development of ischemic contracture, consistent with improvement in myocardial energetics during ischemia. When contractile activity diminishes during ischemia, protein synthesis is an important component of residual myocardial energy expenditure. AMPK activation can inhibit protein synthesis by activating eEF2 kinase, which phosphorylates and inactivates eEF2, an important regulator of protein translation [3]. Inhibition of protein synthesis conserves ATP for more vital cellular functions, such as ion transport, that promote short-term survival during ischemia. Consistent with this hypothesis, A-769662 pre-treatment augmented ischemia-induced eEF2 phosphorylation in the heart. Since A-769662 did not augment glycolytic lactate production during ischemia, and fatty acid oxidation does not contribute to energy generation during global no-flow ischemia, our findings suggest that ATP preservation, rather than ATP generation, is likely the more important mechanism of energy conservation during ischemia. It is possible that A-769662 also briefly increased mitochondrial beta-oxidation early during reperfusion, since ACC phosphorylation was augmented at the end of ischemia. However, AMPK was deactivated by 30 minutes of reperfusion (Supplementary Figure 4), indicating that A-769662 likely did not have prolonged metabolic effects during reperfusion.
We also investigated whether AMPK-eNOS signaling pathway mediates A-769662-induced cardioprotection. AMPK phosphorylates and activates eNOS [29], which is expressed in both endothelial cells and cardiomyocytes [36]. In cardiomyocytes, nitric oxide exerts several protective effects, including activation of mitochondrial KATP channels [37] and inhibition of mitochondrial permeability transition pore opening [38]. We observed that A-769662 augmented eNOS Ser1177 phosphorylation during ischemia, consistent with hyperactivation of AMPK. A-769662 was not effective in reducing post-ischemic contractile dysfunction in eNOS−/− hearts, indicating that eNOS activation might partially mediate the compound’s effect to reduce myocardial stunning. However, A-769662 did reduce myocardial necrosis to a similar degree in eNOS−/− and WT hearts, suggesting that nitric oxide independent mechanisms mediate the cardioprotective effects of this compound. In contrast, a prior study indicated that metformin did not prevent necrosis during ischemia-reperfusion in eNOS−/− hearts [13], perhaps due to the confounding influence of additional non-AMPK effects of metformin treatment [18].
Our findings are novel in that they are the first to demonstrate a protective effect of a direct, AMP-independent AMPK activator in solid organ ischemia-reperfusion. Previous studies have used treatments that activate AMPK indirectly and have known AMPK-independent pleiotropic effects. Metformin, for example, impairs ATP production by inhibiting complex I of the respiratory chain [17]. Caloric restriction also activates AMPK, but has pleiotropic physiologic effects [39]. AICAR blocks the reuptake of adenosine, which has potent preconditioning effects [16]. Our results indicate that A-769662 directly activates AMPK without altering cellular energetics or causing cellular stress. Pharmacologic studies have also shown that A-769662 has good specificity with respect to other protein kinases [21, 23]. There is limited evidence to suggest the possibility of off-target effects, including inhibition of proteasomal [40] and sodium potassium ATPase [41] activities. In this regard, our observation that A-769662 had no effect in AMPK-inactivated KD hearts is a critical finding and points to the AMPK-dependence of this agent. In this regard, it is worth noting that the KD mouse is a replacement model, whereby AMPK α2 KD subunits replace the native α2 subunits, and there are not excess AMPK complexes that might act as a molecular sink to account for the lack of effect of A-769662 in the KD model.
Ischemic preconditioning is triggered by short bursts of ischemia interspersed by reperfusion, and short periods of ischemia are known to activate AMPK [32]. In addition, both activated AMPK and ischemic preconditioning regulate eNOS [29], p38 MAPK, and ATP-sensitive K channels [42], raising the possibility that AMPK might be required for ischemic preconditioning. However, in the current study, we found that ischemic preconditioning effectively induced cardioprotection in KD mice, indicating that AMPK activation is not necessary for ischemic preconditioning in vivo. Our findings contrast with those in isolated mouse cardiomyocytes that require AMPK for preconditioning during hypoxia-reoxygenation [42], perhaps reflecting differences in the models or preconditioning stimuli. Thus, in the intact heart, ischemic preconditioning triggers multiple, redundant signaling pathways, and does not appear to require AMPK for cardioprotection.
The current findings also demonstrate that a small molecule AMPK activator replicates the cardioprotective effect of ischemic preconditioning. Pharmacologic preconditioning is a potentially attractive therapeutic strategy in clinical situations where organ ischemia-reperfusion is anticipated. Common examples include off-pump cardiac surgery, high-risk percutaneous or surgical coronary revascularization, or heart transplantation. Preconditioning also has a potential clinical role in the treatment of patients presenting with unstable angina who are at risk for acute myocardial infarction. Although the current studies are focused on the role of AMPK activators in protecting the ischemic heart, they have similar implications for the pharmacologic protection of other solid organs (i.e. liver, kidney) against acute ischemia-reperfusion injury. Thus, the results of this study provide an initial stepping stone for future experiments to better understand the clinical potential of small molecule direct-acting AMPK agonists in protecting against ischemic injury.
Supplementary Material
Research highlights.
Pretreatment with a small molecule AMPK activator attenuates myocardial IR injury.
This protection is abrogated in AMPK inactivated transgenic mouse hearts.
A-769662 augments AMPK activation and promotes ATP preservation during ischemia.
eNOS knockout mouse hearts are also protected by A-769662.
AMPK activity is not required for ischemic preconditioning.
Acknowledgments
This work was supported by NIH [RO1 HL63811 to LY, T32 HL007950 to AK, U24 DK76169 to Yale MMPC]; and KS was supported by Diabetes UK (07/0003529), UK Medical Research Council, and British Heart Foundation.
Footnotes
Disclosures: none declared
Appendix A. Supplementary data
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–78. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- [2].Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–9. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- [4].Li J, Hu X, Selvakumar P, Russell RR, 3rd, Cushman SW, Holman GD, et al. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab. 2004;287:E834–41. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- [5].Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–55. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- [6].Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, et al. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 2003;52:1627–34. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- [7].Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–20. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- [8].Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, et al. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–23. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- [9].Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- [11].Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, et al. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–75. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khaliulin I, Clarke SJ, Lin H, Parker J, Suleiman MS, Halestrap AP. Temperature preconditioning of isolated rat hearts--a potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. J Physiol. 2007;581:1147–61. doi: 10.1113/jphysiol.2007.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- [14].Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, et al. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835–44. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tsuchida A, Yang XM, Burckhartt B, Mullane KM, Cohen MV, Downey JM. Acadesine extends the window of protection afforded by ischaemic preconditioning. Cardiovasc Res. 1994;28:379–83. doi: 10.1093/cvr/28.3.379. [DOI] [PubMed] [Google Scholar]
- [16].Gruber HE, Hoffer ME, McAllister DR, Laikind PK, Lane TA, Schmid-Schoenbein GW, et al. Increased adenosine concentration in blood from ischemic myocardium by AICA riboside. Effects on flow, granulocytes, and injury. Circulation. 1989;80:1400–11. doi: 10.1161/01.cir.80.5.1400. [DOI] [PubMed] [Google Scholar]
- [17].Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- [18].Saeedi R, Parsons HL, Wambolt RB, Paulson K, Sharma V, Dyck JR, et al. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol Heart Circ Physiol. 2008;294:H2497–506. doi: 10.1152/ajpheart.00873.2007. [DOI] [PubMed] [Google Scholar]
- [19].Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–16. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- [22].Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–69. [PMC free article] [PubMed] [Google Scholar]
- [23].Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007 doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the Mechanism of Activation of AMP-activated Protein Kinase by the Small Molecule A-769662, a Member of the Thienopyridone Family. J Biol Chem. 2007;282:32539–48. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- [25].Qi D, Hu X, Wu X, Merk M, Leng L, Bucala R, et al. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J Clin Invest. 2009;119:3807–16. doi: 10.1172/JCI39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–82. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- [27].Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117:103–14. doi: 10.1161/CIRCULATIONAHA.107.702993. [DOI] [PubMed] [Google Scholar]
- [28].Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–31. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- [29].Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–9. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- [30].Sumeray MS, Rees DD, Yellon DM. Infarct size and nitric oxide synthase in murine myocardium. J Mol Cell Cardiol. 2000;32:35–42. doi: 10.1006/jmcc.1999.1050. [DOI] [PubMed] [Google Scholar]
- [31].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- [32].Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, et al. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004;61:610–9. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- [33].Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–87. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Scott JW, van Denderen BJ, Jorgensen SB, Honeyman JE, Steinberg GR, Oakhill JS, et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol. 2008;15:1220–30. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [35].Li J, Coven DL, Miller EJ, Hu X, Young ME, Carling D, et al. Activation of AMPK alpha- and gamma-isoform complexes in the intact ischemic rat heart. Am J Physiol Heart Circ Physiol. 2006;291:H1927–34. doi: 10.1152/ajpheart.00251.2006. [DOI] [PubMed] [Google Scholar]
- [36].Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–4. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- [37].Han J, Kim N, Joo H, Kim E, Earm YE. ATP-sensitive K(+) channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H1545–54. doi: 10.1152/ajpheart.01052.2001. [DOI] [PubMed] [Google Scholar]
- [38].Wang G, Liem DA, Vondriska TM, Honda HM, Korge P, Pantaleon DM, et al. Nitric oxide donors protect murine myocardium against infarction via modulation of mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2005;288:H1290–5. doi: 10.1152/ajpheart.00796.2004. [DOI] [PubMed] [Google Scholar]
- [39].Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–96. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [40].Moreno D, Knecht E, Viollet B, Sanz P. A769662, a novel activator of AMP-activated protein kinase, inhibits non-proteolytic components of the 26S proteasome by an AMPK-independent mechanism. FEBS Lett. 2008;582:2650–4. doi: 10.1016/j.febslet.2008.06.044. [DOI] [PubMed] [Google Scholar]
- [41].Benziane B, Bjornholm M, Lantier L, Viollet B, Zierath JR, Chibalin AV. AMP-activated protein kinase activator A-769662 is an inhibitor of the Na(+)-K(+)-ATPase. Am J Physiol Cell Physiol. 2009;297:C1554–66. doi: 10.1152/ajpcell.00010.2009. [DOI] [PubMed] [Google Scholar]
- [42].Sukhodub A, Jovanovic S, Du Q, Budas G, Clelland AK, Shen M, et al. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K(+) channels. J Cell Physiol. 2007;210:224–36. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.