TO THE EDITOR
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that mediates the toxicity of 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD), polycyclic aromatic hydrocarbons, and related environmental contaminants (Abel and Haarmann-Stemmann, 2010). The unligated AhR is trapped in a cytosolic multiprotein complex, which rapidly dissociates upon ligand binding. Subsequently, the AhR shuttles into the nucleus, dimerizes with ARNT, and binds to xenobiotic-responsive elements (XREs) in the promoter of target genes, e.g., encoding cytochrome P450 (CYP) 1 monooxygenases, to enforce transcription (Abel and Haarmann-Stemmann, 2010). Furthermore, AhR-triggered activation of c-src tyrosine kinase stimulates EGFR and downstream mitogen-activated protein kinase signaling, resulting in the induction of XRE-independent genes, such as cyclooxygenase-2 (COX-2; Abel and Haarmann-Stemmann, 2010). We have previously shown that the AhR in keratinocytes is not only activated by anthropogenic chemicals but also by UVB irradiation, which leads to the intracellular formation of the tryptophan photoproduct and high-affinity AhR ligand 6-formylindolo[3,2-b]carbazole (FICZ; Rannug et al., 1995; Fritsche et al., 2007). Indeed, UVB exposure enhances AhR/XRE binding (Supplementary Figure 1 online) and accompanied CYP1A1/1B1 expression (Katiyar et al., 2000), as well as XRE-independent COX-2 expression (Fritsche et al., 2007).
Because (i) overexpression of a constitutively active AhR causes inflammatory skin lesions (Tauchi et al., 2005), (ii) an increase in CYP activity leads to reactive oxygen species formation (Puntarulo and Cederbaum, 1998), (iii) CYP1 enzymes are critical for chemical-induced skin carcinogenesis (Shimizu et al., 2000), and (iv) COX-2 is involved in UV-induced inflammation and carcinogenesis (Elmets et al., 2010), it was postulated that a transient inhibition of AhR may protect human skin against the detrimental effects of UVB irradiation (Agostinis et al., 2007; Haarmann-Stemmann et al., 2012). Moreover, we have shown that the expression of matrix metalloproteinase-1 (MMP-1), which is critically involved in extrinsic skin aging, is upregulated in an AhR-dependent manner in tobacco smoke extract–exposed keratinocytes (Ono et al., 2013). Therefore, we decided to develop an AHR antagonist that is suitable for topical UV-protection. We screened a library of compounds that possess the structural prerequisites to interact with AhR and identified E/Z-2-benzylidene-5,6-dimethoxy-3,3-dimethylindan-1-one (BDDI; Figure 1a) as the most promising candidate.
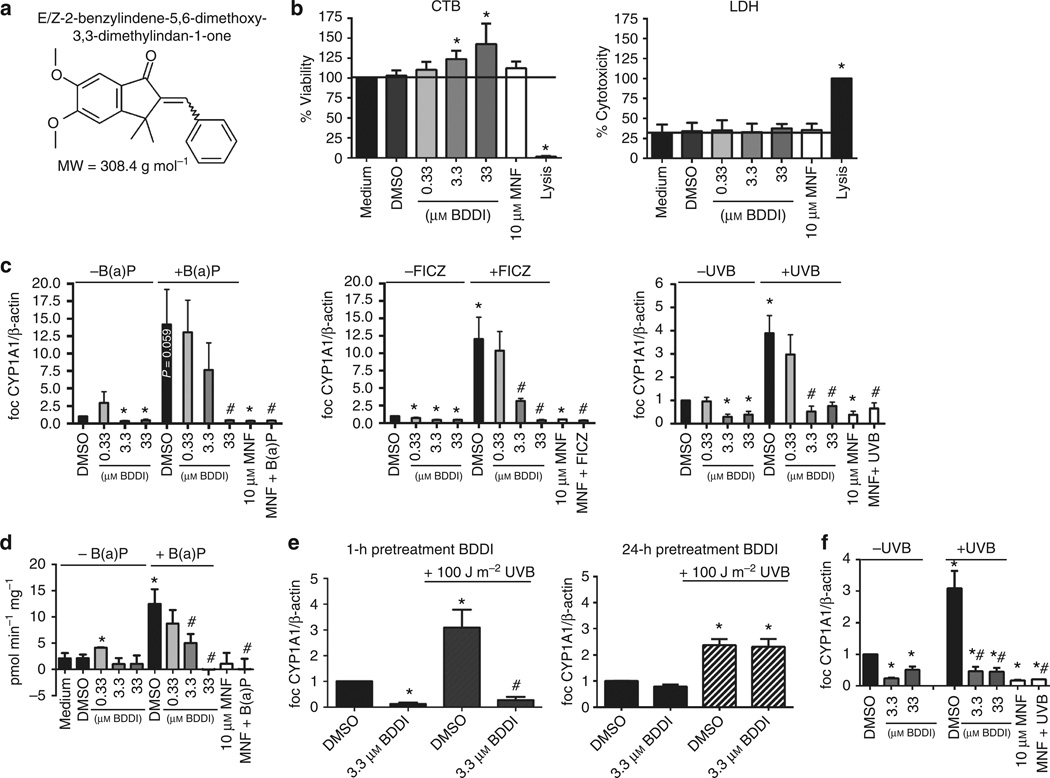
Figure 1. Characterization of antagonistic capacities of E/Z-2-benzylidene-5,6-dimethoxy-3,3-dimethylindan-1-one (BDDI) in normal human epidermal keratinocytes (NHEKs).
(a) Chemical structure of BDDI. (b) Effect of BDDI on cell viability (CellTiter Blue; CTB) and cytotoxicity (lactate dehydrogenase; LDH). The black line marks the respective control level. (c) Effect of BDDI on B(a)P (250 nM for 4 hours)-, 6-formylindolo[3,2-b]carbazole (FICZ; 100 nM for 4 hours)-, and UVB (100 Jm−2 for 8 hours)-induced CYP1A1 mRNA expression (quantitative reverse transcription-PCR data). (d) Effect of BDDI on 7-O-ethoxyresorufin-deethylase (EROD) activity induced by 24 hours B(a)P shown in activity as pmol min−1−mg−1. (e) Transient effect of BDDI pretreatment (1 and 24 hours) on CYP1A1 mRNA expression induced by 100 Jm−2 UVB. (f) NHEKs were irradiated with 100 Jm−2 UVB and directly after irradiation treated with 3.3 and 33 µM BDDI. After 8 hours, RNA was isolated, and CYP1A1 transcription was analyzed. Each graph represents mean±SEM of three independent experiments; *P<0.05 versus medium/DMSO control; #P<0.05 versus treatment (B(a)P, FICZ, or UVB, respectively).
In concentrations from 0.33 to 33 µM, BDDI did not negatively affect cell viability or cause cytotoxicity in normal human epidermal keratinocytes (NHEKs; Figure 1b; for description of methods see Supplementary Material online). It is noteworthy that exposure to higher concentrations of BDDI enhanced cell viability (Figure 1b) without increasing the proliferation rate (data not shown). Exposure of NHEKs to 0.33 to 33 µM BDDI or 10 µM of the specific AhR inhibitor 3′-methoxy-4′-nitroflavone (MNF; Lu et al., 1995) resulted in a concentration-dependent decrease of basal CYP1A1 expression (Figure 1c). AhR activation by 10nM FICZ or 250 nM benzo(a)pyrene [B(a)P] resulted in 12- to 14-fold induction of CYP1A1 transcription after 4 h, whereas irradiation with 100 Jm−2 UVB led to a fourfold increase in CYP1A1 expression after 8 hours (Figure 1c). Pretreatment with 10µM MNF or 33 µM BDDI significantly attenuated CYP1A1 induction. Interestingly, a dose of 3.3 µM BDDI was sufficient to repress UVB- and FICZ-stimulated, but not B(a)P-induced, CYP1A1 expression, which was probably due to the different half-lives of the inducing agents (Figure 1c). To confirm the inhibitory effect of BDDI on CYP1A1, we treated NHEKs for 24 h with 250 nM B(a)P alone or in combination with BDDI and measured CYP1A-mediated 7-O-ethoxyresorufin-deethylase (EROD) activities. A 1 hour pretreatment with 0.33 to 33 µM BDDI resulted in a dose-dependent decline of B(a)P-induced EROD activity (Figure 1d), thereby confirming the AhR antagonistic properties of BDDI. Importantly, BDDI only transiently represses AhR function. Whereas a BDDI pretreatment for 1 hour attenuated UVB-mediated CYP1A1 induction in NHEKs, a pretreatment for 24 hours was not effective (Figure 1e). BDDI treatment of NHEKs directly after irradiation also decreased UVB-mediated CYP1A1 induction, providing evidence that BDDI does not act as a UVB-filter (Figure 1f).
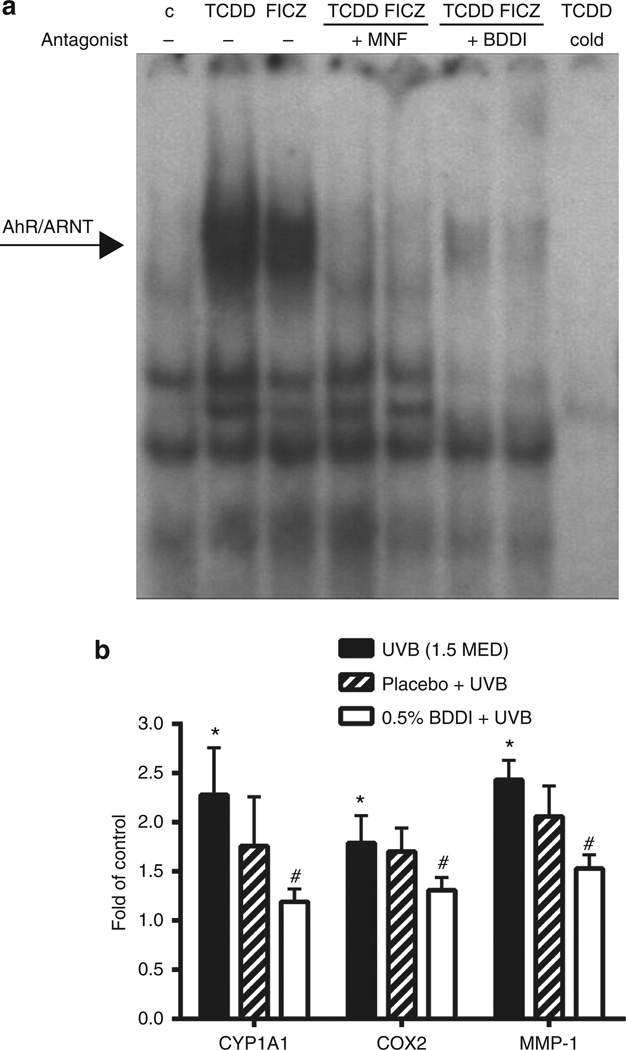
To elucidate the mode of action of BDDI, we performed an electrophoretic mobility shift assay (EMSA) that is well established to detect a direct binding of AhR/ARNT to a XRE consensus oligonucleotide (Denison et al., 1988; Vogel et al., 2004). Upon exposure of human HaCaT keratinocytes to 10 nM TCDD or 100 nM FICZ, we observed a strong binding of the AhR/ARNT complex to its DNA target motif (Figure 2a). Co-exposure of HaCaT cells to 3.3 µM BDDI or 10 µM MNF clearly blocked TCDD-and FICZ-triggered AhR/XRE binding (Figure 2a), providing evidence that BDDI acts as a true competitive AhR antagonist.
Figure 2. BDDI disturbs XRE binding of aryl hydrocarbon receptor (AhR)/ARNT and represses UVB-induced gene expression in a human in vivo study.
(a) HaCaT keratinocytes were treated with 10 nM 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 100 nM 6-formylindolo[3,2-b]carbazole (FICZ), 10 µM 3′-methoxy-4′-nitroflavone (MNF), and/or 3.3 µM BDDI for 90 minutes before isolation of nuclear extracts and were hybridized with a xenobiotic-responsive element (XRE) consensus oligonucleotide. A representative electrophoretic mobility shift assay of three independent experiments is shown. (b) Volunteers were pretreated with 0.5% BDDI or placebo for 4 days every day followed by UVB irradiation (1.5 minimal erythema dose, MED) on day 4. Twenty-four hours after irradiation, 4-mm skin biopsies were taken, RNA was isolated and reverse transcribed, and mRNA expression of CYP1A1, cyclooxygenase-2, and matrix metalloproteinase-1 was measured. Gene expression in sham-irradiated, untreated control skin were arbitrarily set as 1; n=10, mean±SEM, *P<0.001 versus unirradiated control, #P<0.001 versus UVB (1.5 MED).
To investigate whether BDDI is suitable for UV-protection of human skin, we treated defined skin areas of 10 healthy volunteers once daily on 4 consecutive days with a formulation containing 0.5% BDDI or a placebo formulation (Figure 2b). On day 4, 2 hours after the application of the substances, volunteers were irradiated with 1.5 MED (minimal erythema dose) UVB, and 24 hours later skin biopsies were taken. Quantitative expression analyses revealed a significantly increased expression of CYP1A1, COX-2, and MMP-1 in UVB-irradiated compared with sham-irradiated skin. Topical application of BDDI, but not the placebo formulation, significantly reduced the UVB-induced expression of all these genes, indicating that BDDI penetrates human skin and blocks AhR-dependent signaling. This experiment also revealed that the AhR is activated upon UVB irradiation in human skin in vivo. Importantly, the erythema response of the volunteers was not significantly affected during the study.
As CYP1A1, COX-2, and MMP-1 are critically involved in cutaneous inflammatory diseases, skin cancer, and skin aging, we propose that the topical application of this chemical inhibitor presents a promising strategy to protect human skin against UVB-induced damage. In contrast to MNF (structural safety alert), BDDI has the clinical advantage of being suitable for dermal applications in humans. Our in vitro data further indicate that BDDI may protect against the adverse effects of polycyclic aromatic hydrocarbons, which are frequently found on airborne particulate matter (Vierkotter et al., 2010). Finally, BDDI may serve as a tool to study the involvement of AhR signaling in human skin (patho)physiology.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Symrise GmbH & Co. KG (Holzminden, Germany) and the DFG (SFB 728). CV was supported by the NIEHS (1R01ES019898) and AG by the DAAD RISE program.
Abbreviations
- AhR
aryl hydrocarbon receptor
- BDDI
E/Z-2-benzylidene-5,6-dimethoxy-3,3-dimethylindan-1-one
- COX-2
cyclooxygenase-2
- CYP
cytochrome P450
- EROD
7-O-ethoxyresorufindeethylase
- FICZ
6-formylindolo[3,2-b]carbazole
- MMP-1
matrix metalloproteinase-1
- MNF
3′-methoxy-4′-nitroflavone
- NHEK
normal human epidermal keratinocyte
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- XRE
xenobiotic-responsive element
Footnotes
CONFLICT OF INTEREST
JK serves as scientific consultant for Symrise GmbH & Co. KG, Holzminden, Germany.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- Agostinis P, Garmyn M, Van Laethem A. The aryl hydrocarbon receptor: an illuminating effector of the UVB response. Sci STKE. 2007;2007:e49. doi: 10.1126/stke.4032007pe49. [DOI] [PubMed] [Google Scholar]
- Denison MS, Fisher JM, Whitlock JP., Jr The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J Biol Chem. 1988;263:17221–17224. [PubMed] [Google Scholar]
- Elmets CA, Viner JL, Pentland AP, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, doubleblind, placebo-controlled trial. J Natl Cancer Institute. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche E, Schafer C, Calles C, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarmann-Stemmann T, Abel J, Fritsche E, et al. The AhR-Nrf2 pathway in keratinocytes: on the road to chemoprevention? J Invest Dermatol. 2012;132:7–9. doi: 10.1038/jid.2011.359. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Matsui MS, Mukhtar H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol. 2000;114:328–333. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Lu YF, Santostefano M, Cunningham BD, et al. Identification of 3’-methoxy-4’-nitroflavone as a pure aryl hydrocarbon (Ah) receptor antagonist and evidence for more than one form of the nuclear Ah receptor in MCF-7 human breast cancer cells. Arch Biochem Biophys. 1995;316:470–477. doi: 10.1006/abbi.1995.1062. [DOI] [PubMed] [Google Scholar]
- Ono Y, Torii K, Fritsche E, et al. Role of the aryl hydrocarbon receptor in tobacco smoke extract-induced matrix metalloproteinase-1 expression. Exp Dermatol. 2013;22(5):349–353. doi: 10.1111/exd.12148. [DOI] [PubMed] [Google Scholar]
- Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med. 1998;24:1324–1330. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- Rannug U, Rannug A, Sjoberg U, et al. Structure elucidation of two tryptophanderived, high affinity Ah receptor ligands. Chem Biol. 1995;2:841–845. doi: 10.1016/1074-5521(95)90090-x. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Nakatsuru Y, Ichinose M, et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M, Hida A, Negishi T, et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol. 2005;25:9360–9368. doi: 10.1128/MCB.25.21.9360-9368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierkotter A, Schikowski T, Ranft U, et al. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol. 2010;130:2719–2726. doi: 10.1038/jid.2010.204. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Park S, et al. Dioxin increases C/EBPbeta transcription by activating cAMP/protein kinase A. J Biol Chem. 2004;279:8886–8894. doi: 10.1074/jbc.M310190200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.