Abstract
Purpose
To report a case of a major vascular complication during TAVR and the endovascular management thereof. Additionally, we discuss a possible correlation with long-term steroid use.
Case Report
A 79 year old woman with a history of critical aortic stenosis underwent elective transcatheter aortic valve replacement (TAVR). Her procedure was complicated by rupture of her right iliac artery, life threatening retroperitoneal hemorrhage, and thrombus extending into the distal right lower extremity. This case was emergently managed by stent placement, thrombectomy, and tPA via a percutaneous approach.
Conclusions
Peripheral vascular complications are common during percutaneous TAVR, and chronic steroid use may predispose patients. Endovascular management is often possible and may potentially save valuable time in emergent situations.
Keywords: TAVR, TAVI, Peripheral, Endovascular
Case Summary
This is a case of a 79-year-old woman with a history of highly symptomatic severe aortic stenosis with a calculated aortic valve (AV) area of 0.7 cm2 and left ventricular ejection fraction of greater than 65%. She had a history of severe asthma, for which she had used oral steroids for many years, in addition to regularly scheduled inhalers. The patient was deemed to be at high risk for death or major morbidity with surgical aortic valve replacement (EuroSCORE II 13.97%) and was therefore considered for TAVR. She underwent successful TAVR via a percutaneous femoral approach with placement of a 26 mm Sapien valve (Edwards Lifesciences, CA, USA) using a 24French (F) delivery system.
Following successful valve deployment, completion angiography of the right common iliac was performed using a 7F Destination sheath (Terumo), advanced from the contralateral femoral artery. An iliac rupture with dissection extending into the proximal superior femoral artery (SFA) was noted.
After Heparin reversal, a 9×20mm Optapro balloon (Cordis) was emergently advanced from the left femoral artery (LFA) and inflated in the proximal right common iliac artery (CIA) to occlude distal flow. The Optapro was then deflated while a 40mm Coda balloon (Cook Medical) was advanced via the right femoral artery (RFA) into the distal abdominal aorta and inflated. The patient became hypotensive but stabilized with vasopressors, blood, and crystalloid infusion. Due to active hemorrhage and hemodynamic instability, an immediate endovascular approach was elected.
The 7F LFA sheath was exchanged for a 12F Amplatzer Torque delivery system (St Jude Medical) sheath advanced into the right CIA. A Glidewire Advantage (Terumo) was advanced through the 12F sheath into the contralateral (right) SFA. After deflating and removing the Coda balloon, a 10×100mm Viabahn stent (Gore) was advanced over the Glidewire and deployed in the common iliac artery extending into the external iliac artery (EIA), spanning the ruptured segment. An 11×50mm Viabahn stent was overlapped distally and deployed extending into the CFA, covering the arteriotomy site.
Angiography showed an extensive clot in the right SFA with poor distal flow. Using Angiojet (Bayer Healthcare) thrombectomy followed by infrapopliteal tPA bolus, the blood flow to the right lower extremity was restored. Angiogram revealed improved flow down the SFA with one vessel run off to the foot via the peroneal artery. The anterior tibial and posterior tibial arteries were felt to be chronically occluded and reconstitute distally via collaterals from the peroneal artery.
Repeat angiography of the right iliac was performed showing continued contrast extravasation. Despite post dilatation of the Viabahn stents with a 10×40mm Optapro balloon, the extravasation continued. Two overlapping 10×38mm iCast Atrium stents were deployed in the iliac artery. The stents were serially postdilated with the 10mm Optapro balloon, stopping further extravasation.
Intra and post operatively, the patient required a total of 10 units of packed red blood cells and 6 units of fresh frozen plasma. Due to progressive decline of the hematocrit, the patient was taken to the vascular surgery suite several hours following TAVR. Angiography revealed no ongoing bleeding, and no surgical intervention was required. The patient was extubated on the following day and remained for a total of 3 days in the CCU. The hospital course was complicated by a seizure. Subsequent MRI showed multiple acute punctuate lesions that were felt to probably be due to her transaortic valvular procedure. The patient progressed well and was ambulating before discharge to inpatient cardiac rehab on day 11 of her hospital stay.
At one year of follow up, the patient is stable. The symptoms of heart failure have completely resolved. Clinically, there have been no untoward consequences of the vascular complication.
Discussion
This case depicts a major vascular complication of a TAVR procedure and its successful endovascular management. Transcatheter aortic valve replacement is a relatively new procedure for high-risk patients with severe aortic stenosis. The major complications of this procedure include stroke, vascular access complications, and paravalvular leak. The Valve Academic Research Consortium (VARC)1 has outlined definitions for major and minor vascular complications. According to the VARC, vascular rupture and subsequent requirement for blood transfusion, as in this case, are defined as major complications. As demonstrated in the PARTNER trial major and minor vascular complications during TAVR were associated with decreased survival and were risk factors of late mortality (OR 2.78, CI 1.58–4.82, p < 0.001).2 Further analysis of the PARTNER trial (total n=419) indicated that the incidences of major and minor vascular complications for a transfemoral approach were 15.3% and 11.9%, within 30 days of the procedure.3 This data was reported with first-generation TAVR systems using 22–24F sheaths, as was the case described here.
In the present case the chronic use of steroids could have contributed to the intraprocedural events. Steroids are well known to increase vessel fragility.4 The pathophysiologic mechanism involves impairment of collagen formation, connective tissue strength, impaired anti-inflammatory effect and wound healing.5 Several studies have investigated the role of long-term steroid use on risk of vascular complication in patients undergoing coronary angiography. In a prospective randomized trial of 503 patients who underwent a cardiac catheterization Bogart et al. studied various risk factors for intraprocedural and postprocedural access site complications.6 Of the preprocedural variables, only chronic steroid use was associated with an increased risk of complications. In a more recent study Ellis et al. assessed the potential risk of long-term steroid use in the setting of coronary angioplasty in a cohort of 12,883 patients (114 on long-term steroids). Although steroid use was not associated with increased risk of composite major ischemia events, it was associated with a threefold risk (p< 0.01) of major vascular complications and a three- to fourfold risk (p< 0.026) of coronary perforation.7 Currently there is no published literature on potential risk of chronic steroid use in TAVR procedures, however the angiography and angioplasty data presented herein could be applied. The larger bore sheaths used in currently available delivery systems would only increase the incidence of these complications, however the degree to which steroids affect the complication rate is thus far not described. Retrospective analyses of existing TAVR databases are needed to further clarify the proposed relationship.
The reversal of heparin in this case may have contributed to the development of thrombus in the distal vasculature. With the degree of life-threatening hemorrhage and hemodynamic instability, it was performed to help stabilize the situation. As our practice has evolved, including more experience with endovascular management of vascular complications, we have found that rapid inflation of occlusive balloons can often obviate the need for heparin reversal.
Conclusion
We demonstrated a dramatic case of a major vascular complication, which must be anticipated by any proceduralist during TAVR and we outlined a way to manage such a complication by percutaneous approach. This case provides an example of the value of peripheral vascular skills being available during TAVR. Using a percutaneous approach has the potential to save valuable time in comparable acute situations. We further proposed a causal relationship between chronic steroid use and an increased risk of peripheral vascular complications during TAVR, which needs to be further evaluated.
Figure 1.
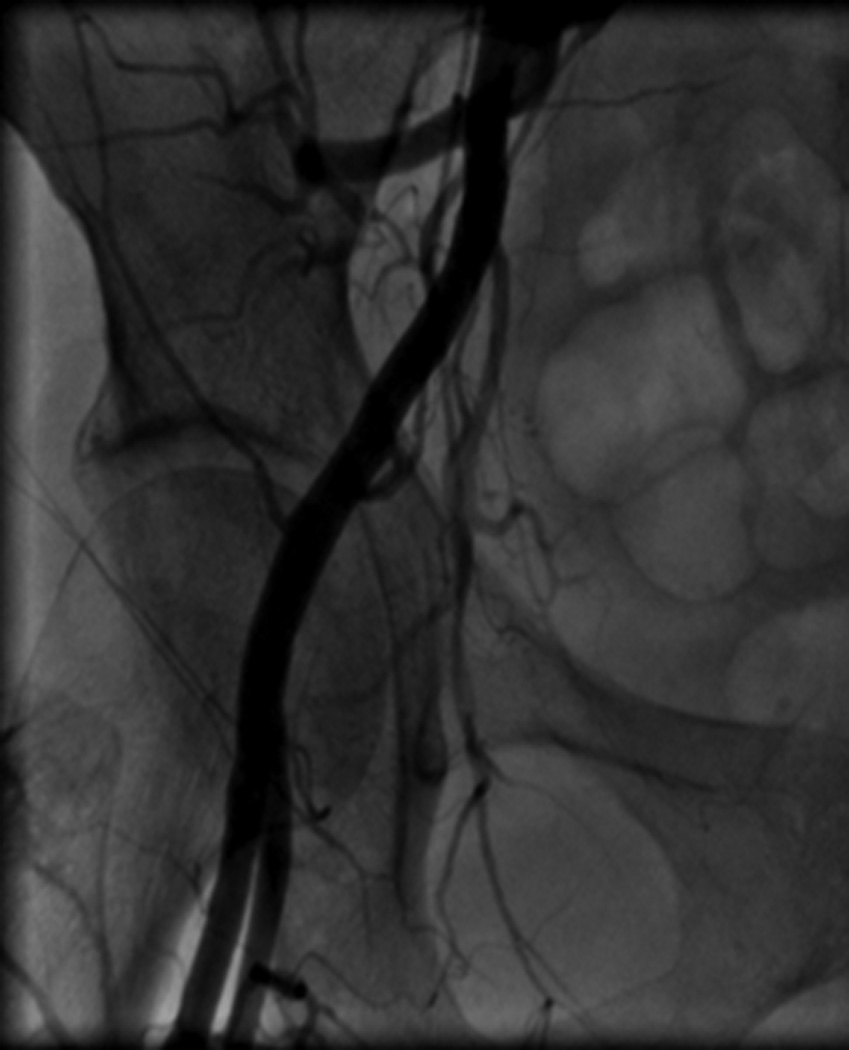
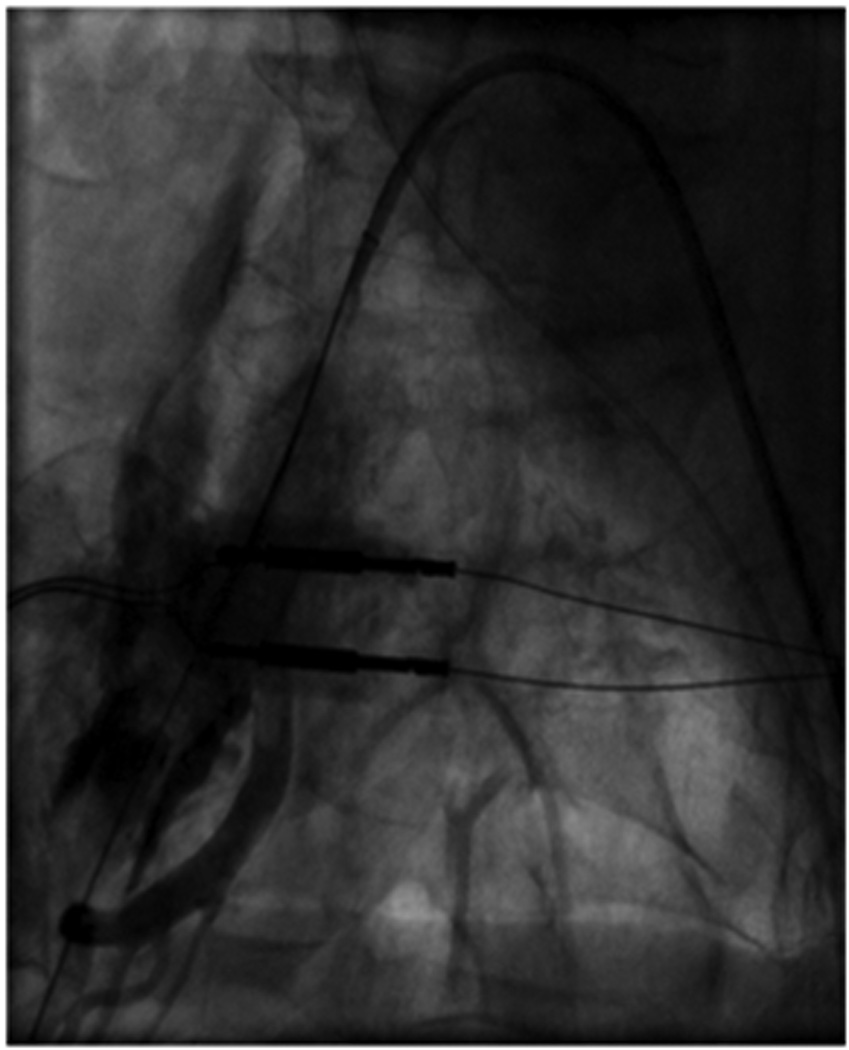
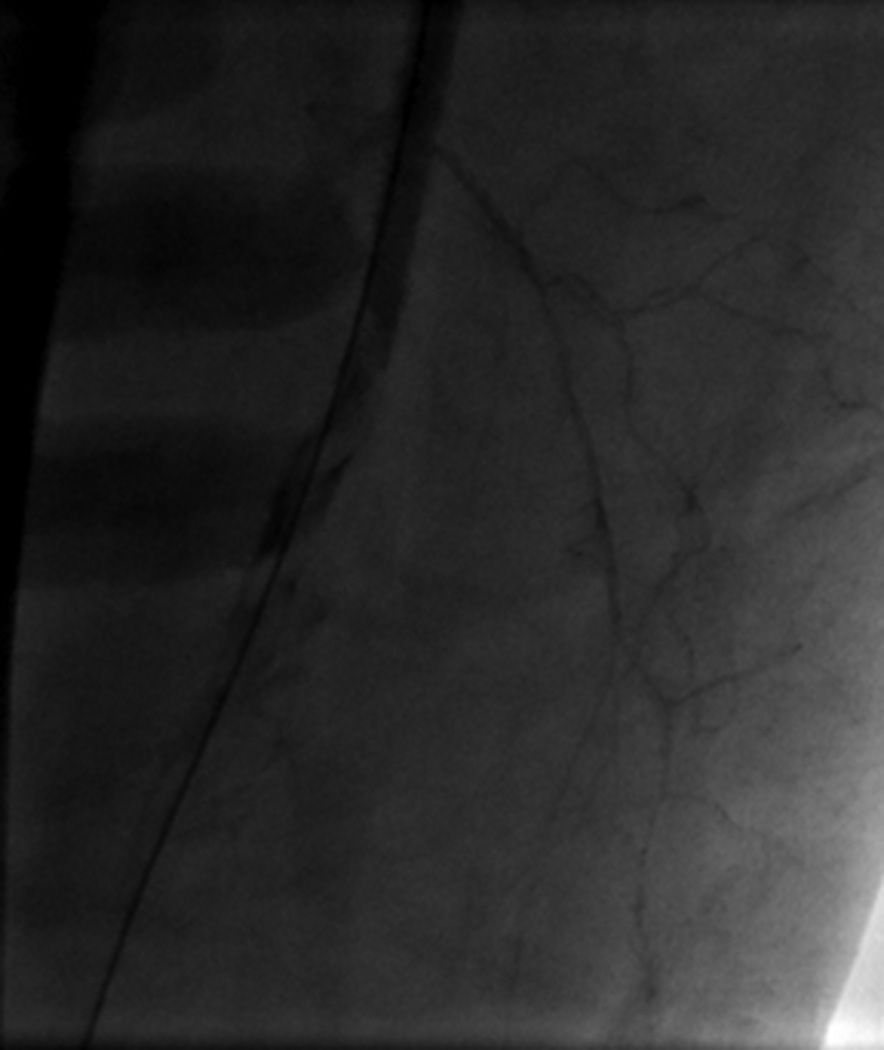
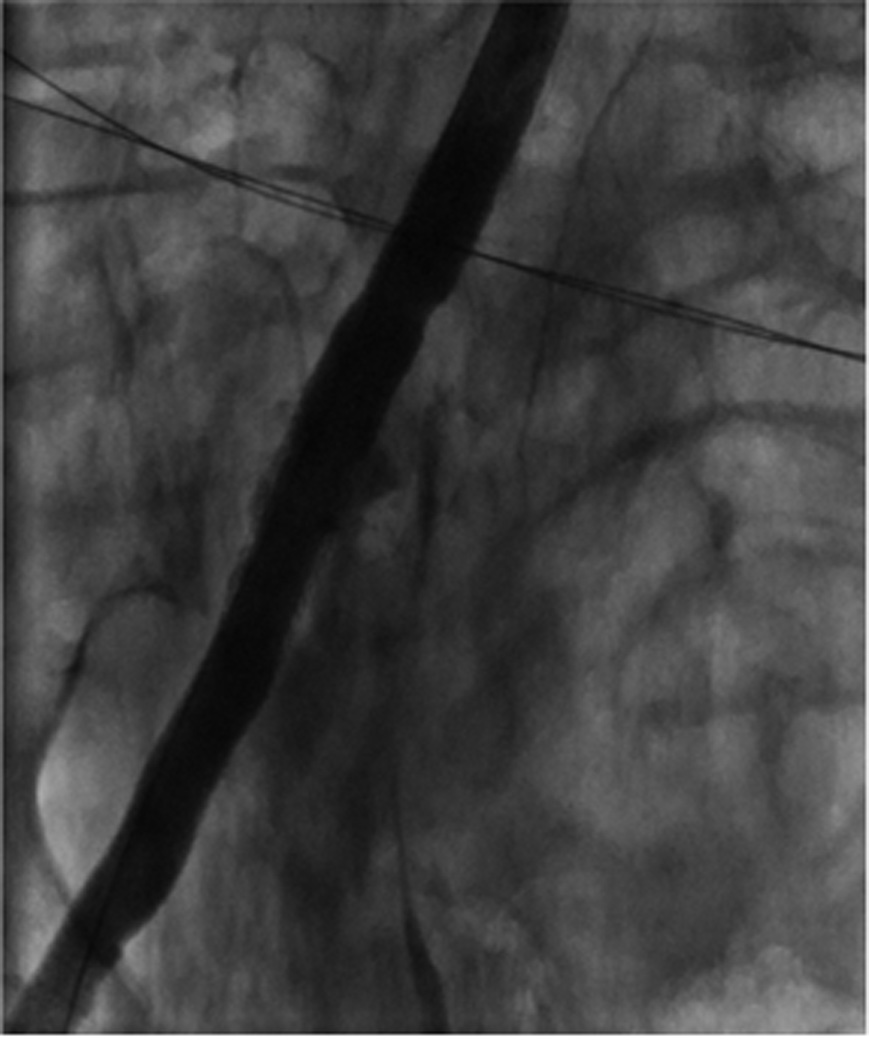
Image A shows right iliac angiography prior to insertion of the 24F delivery system. Image B shows the rupture of the right SFA. Image C shows thrombus in the SFA. Image D shows a patent CIA with no further contrast extravasation following deployment of stents
References
- 1.Leon MB, Piazza N, Nikolsky E. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the valve academic research consortium. Journal of the American College of Cardiology. 2011;57:253–269. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Kodali SK, Williams MR, Smith CR. Two-year outcomes after transcatheter or surgical aortic-valve replacement. The New England journal of medicine. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 3.Genereux P, Webb JG, Svensson LG. Vascular complications after transcatheter aortic valve replacement: Insights from the partner (placement of aortic transcatheter valve) trial. Journal of the American College of Cardiology. 2012;60:1043–1052. doi: 10.1016/j.jacc.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 4.McEvoy CE, Niewoehner DE. Adverse effects of corticosteroid therapy for copd. A critical review. Chest. 1997;111:732–743. doi: 10.1378/chest.111.3.732. [DOI] [PubMed] [Google Scholar]
- 5.Baxter JD, Forsham PH. Tissue effects of glucocorticoids. The American journal of medicine. 1972;53:573–589. doi: 10.1016/0002-9343(72)90154-4. [DOI] [PubMed] [Google Scholar]
- 6.Bogart DB, Bogart MA, Miller JT. Femoral artery catheterization complications: A study of 503 consecutive patients. Catheterization and cardiovascular diagnosis. 1995;34:8–13. doi: 10.1002/ccd.1810340304. [DOI] [PubMed] [Google Scholar]
- 7.Ellis SG, Semenec T, Lander K. Effects of long-term prednisone (>or =5 mg) use on outcomes and complications of percutaneous coronary intervention. The American journal of cardiology. 2004;93:1389–1390. A1386. doi: 10.1016/j.amjcard.2004.02.036. [DOI] [PubMed] [Google Scholar]


