Abstract
The incidence of obesity in pregnancy has increased over the past two decades, with nearly 50% of U.S. women aged 15–49 are classified as overweight or obese. Obesity (independent of diabetes) among gravidae poses unique risks which extend towards the fetus, with several large population-based analyses demonstrating independent increased risks for fetal malformations including neural tube defects, cardiac anomalies, and orofacial clefts as well as stillbirth and macrosomia. Unfortunately, several lines of evidence also suggest that the quality of the prenatal fetal anatomic survey and certain aspects of prenatal diagnostic screening programs are significantly limited. The net effect is that among obese gravidae, the increased risk of fetal anomalies is further offset by a concomitant diminished ability to sonographically detect such malformation in the prenatal interval. The purpose of this summary review is to systematically examine the evidence suggesting an increased risk of fetal malformations in obese gravidae, the contributing role of diabetes, and the limitations of prenatal diagnostic and sonographic screening among this at-risk population.
Introduction
Obesity is currently a worldwide epidemic, with its highest prevalence in developed nations. Overweight and obese adults now outnumber those with a normal BMI, with a notable higher prevalence among women than men (1).
The incidence of obesity in pregnancy has increased over the past two decades concomitant with an increasing prevalence among the general reproductive-aged population (2). Currently nearly 50% of U.S. women aged 15–49 are overweight or obese. In addition to long-term risks of atherosclerotic and cardiovascular disease, diabetes, hypertension, and cancer, obesity in women of reproductive age poses unique risks to perinatal outcomes (3–12). Indeed, multiple studies have demonstrated that obesity independently increases risks for infertility or subfertility as well as gestational diabetes, hypertensive disorders, rate of operative vaginal and cesarean delivery, venous thromboembolism, and postpartum infections and wound complications (3–7). The effects of maternal obesity extend to the fetus, with several large population-based analyses demonstrating independent risks of occurrence of fetal neural tube defects, cardiac malformations, and orofacial clefts (Table I; 8–12). Morbid obesity has additionally been shown to be associated with the development of fetal anomalies and a threefold increased risk of stillbirth (13). Fetal anomalies are a leading cause of infant mortality as 1 in 5 infant deaths in the United States can be attributed to fetal anomalies (14).
Table I.
Obesity exerts an independent increased risk of certain fetal malformations, namely neural tube defects, congenital cardiac anomalies, and orofacial clefts. The consistency of findings and their noted references are as outlined in the Table and discussed further in the text. OR, odds ratio.
Unfortunately, these increased risks are exacerbated by limited the sonographic visualization of fetal and maternal structures due to the maternal body fat layer (8, 15–19). The net effect is that among obese women, a significant independent increased risk for fetal anomalies occurs in conjunction with an inability to view potentially affected anatomic structures. Moreover, there is ongoing evidence to suggest that there exist limitations in the interpretability of many measures of a comprehensive prenatal diagnostic screening program.
The purpose of this summary review is to systematically examine the evidence suggesting an increased risk of fetal malformations in obese mothers, the contributing role of diabetes, and the limitations of prenatal diagnostic and sonographic screening among this increased at-risk population.
DESCRIPTIONAL STUDIES ON FETAL MALFORMATIONS
Prevalence of Obesity and Impact of Nutrition
As demonstrated in Figure 1, obesity complicates pregnancy across broad racial and ethnic groups. According to the National Institute of Health, the Institute of Medicine, and the World Health Organization, obesity is defined as having a body mass index (BMI) of 30 kg/m2 or greater with substratification into Class I (BMI 30–34.9), Class II (BMI 35–39.9) and Class III or morbid obesity (BMI ≥40). Meanwhile, normal weight corresponds to a BMI of 18.5–24.9 kg/m2, and overweight is a BMI of 25–29.9 (20). According to the latest National Health and Nutrition Examination Survey (NHANES) greater than one-third of American women are obese. When broken down by race or ethnicity, 49% of black women are obese compared to 38% of Hispanic women and 31% of Caucasian women (Figure 1, 21).
Figure 1. Prevalence of obesity among racial and ethnic groups among gravidae.
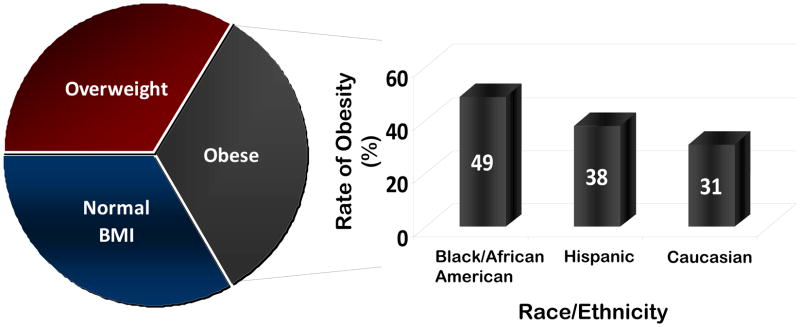
As delineated in the text, the near majority of self-identifying Black or African-American women manifest obesity in pregnancy. A significant and growing minority of both Hispanic-ethnicity and Caucasian women similarly present in pregnancy as obese (20, 21).
Poor nutritional habits—notably high-fat diet consumption--are also on the rise and are likely a contributing factor to the increasing rates of obesity among reproductive-age women and their children (1, 12–14). Could poor nutrition in the obese population possibly be a confounding factor when examining the cause of increased fetal anomalies, such as neural tube defects? Common collective wisdom and multiple lines of evidence have demonstrated that meals lacking or disproportionate in vital nutrients (such as ‘fast food’ or heavily processed food) are cheaper and more readily accessible than meals comprised of fresh and locally-sourced ingredients which are rich in elemental nutrition (1–4). Poor or relatively discordant nutrition becomes particularly concerning when discussing the issue of obesity among pregnant women, and we have published on our findings with a nonhuman primate model of maternal high-fat diet exposure and adverse fetal programming (8, 22–26). Suffice it to say, while there is not a direct kappa value correlation between obesity and poor nutrition, a pregnancy exposed to the triad of increased high-fat dietary intake, relative deficiency in elemental nutrients (such as folic acid), and maternal obesity is at significant further risk for adverse fetal outcomes. Indeed, at least among nonhuman primates, high-fat dietary intake alone is a primary contributor to fetal metabolic dysregulation and risk of future obesity and adult metabolic disease (22–26). The cumulative effect of these fetal programming events is to perpetuate and anticipate the cycle of obesity and diabetes in each successive generation, as presently witnessed across developing and developed nations (1,22,27–29).
Neural Tube Defects
In 1994, Waller et al first demonstrated an increased risk of neural tube defects (spina bifida) in the offspring of obese women (30); multiple other studies have corroborated these findings. A meta-analysis by Stothard et al in 2009 of 39 studies concurred that offspring of obese mothers were more likely to have neural tube defects relative to normal-BMI gravidae (OR 1.87 95% CI 1.62–2.15 P<0.001), with a higher prevalence of both spina bifida and anencephaly. Additionally, offspring of overweight mothers were similarly at increased risk of neural tube defects (OR 1.2, 95% CI 1.04–1.38, P=0.01), but these observations did not extend to anencephaly (14). The significantly increased risk among obese and overweight gravidae may be considered “dose-dependent”, as the relative risk of neural tube defects increases proportionally and linearly with increasing BMI (14, 31).
Multiple theories on biologic plausibility have been proposed to explain the link between maternal obesity and the risk of neural tube defects. First, obese women are at an increased risk for developing diabetes (Figure 2), which in and of itself is a risk factor for many fetal malformations including neural tube defects (12, 32–38). The hyperglycemic state has long been recognized as relatively teratogenic to an embryo, and obese women are at increased risk for hyperglycemia whether via undiagnosed type II diabetes mellitus, a higher likelihood of gestational diabetes, or simply increased insulin resistance (27–29,37, 38). With respect to the higher likelihood of gestational diabetes, it is argued that the increased risk of fetal malformations in obese women with gestational diabetes is suggestive of undiagnosed type II diabetes or increased insulin resistance in many gravidae.
Figure 2. Maternal obesity linearly increases the risk of being diagnosed with (or developing) gestational diabetes.
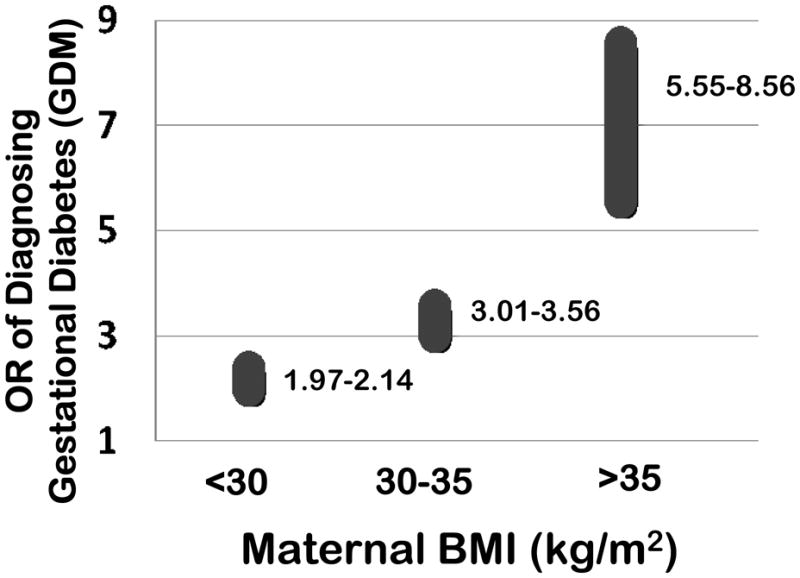
The odds ratios for the diagnosis (or likely development) of gestational diabetes significantly and linearly increases with each IOM obesity classification, with the highest risk among morbidly obese (BMI >35 kg/m2) gravidae. These values are primarily compiled from references 35–37 and 64.
Secondly, it has been hypothesized that the consumption of a relatively nutritionally depleted diet, when paired with a baseline higher nutrient requirement, can lead to the development of neural tube defects. The relationship between folic acid consumption and the risk of neural tube defects is well-established (19,31,39). Longitudinal and cross-sectional population-based data indicate that a higher BMI is correlated with lower serum folate levels, regardless of folate intake (40–43). Moreover, prior and recent data suggest that the risk of neural tube defects in the offspring of obese women has not been decreased by the widespread folic acid fortification of food in the 1990s (40,41). Population based cohort studies have demonstrated that while a diet with more than or equal to 400 micrograms of folate per day is protective against neural tube defects in a woman who weighs less than 70 kg, the same dose effect was not seen in women who weigh more than 70 kg (30,31,40,44). Additionally, obese women have been shown to be deficient in several micronutrients, which is believed to be a result of both a diet relatively low in micronutrients and repetitive attempts at weight loss via rapid diets (31, 40, 44).
Congenital Heart Defects
Children of obese mothers are similarly much more likely to have congenital heart defects, even after controlling for potential confounders. Watkins et al demonstrated a two-fold increase in risk for both overweight and obese mothers to have a child with a cardiac defect by examining the data from the Atlanta Birth Defects Risk Factor Surveillance Study (1993–1997, reference 12). In their analysis, prepregnancy BMI was calculated from self-reported values among three hundred thirty gravidae without diabetes, and anomalies were stratified by BMI criteria. A significant adjusted odds ratio (OR>2) for fetal cardiac malformations was observed for both overweight and obese women (12). Similarly, Cedergren et al conducted a prospective case control nested study comparing 6801 women from the Swedish Medical Health Register that gave birth to a child with a cardiac defect to a total of 812,457 deliveries between 1992 and 2001 (32). Again excluding subjects with diabetes or aneuploid fetuses, increasing BMI was significantly correlated in a linear measure with an increased risk for congenital heart defects (adjusted OR 1.18 at BMI >29 kg/m2; 32). With morbid obesity, the adjusted OR for cardiovascular defects was 1.40 (95% CI 1.22 to 1.64), and for severe cardiovascular defects, the adjusted OR was 1.69 (95% CI, 1.27 to 2.26). There was an increased risk for all specific defects studied among the obese women, but only ventricular and atrial septal defects reached statistical significance after controlling for potential confounders.
It is acknowledged that significant heterogeneity contributes to the risk of congenital heart defects, including familial heritability, undetected type II diabetes in the first trimester, and syndromic variants. However, in a recent meta-analysis of 39 studies (7 of which specifically looked at cardiovascular anomalies with a pooled study size of 9,630 cases in total), it was noted that septal anomalies were specifically and significantly increased in the offspring of the obese population (adjusted OR 1.20) but not amongst the overweight mothers. Moreover, as there did not appear to be a statistically significant increased risk for tetralogy of Fallot, transposition of the great arteries, hypoplastic left heart, or coarctation of the aorta in the obese or overweight population, it raises the notion that there is a unifying potential mechanistic underpinning which is unique to morbidly obese subjects (14). Similarly, Cedergren et al did not observe a statistically significant increased risk of tetralogy of Fallot, transposition, endocardial cushion defects, aortic coarctation, or hypoplastic left heart syndrome among the obese population, despite elevated odds ratios in these patients (32).
While the precise mechanism connecting maternal obesity and fetal cardiac malformations remains to be fully elucidated, one such leading theory raises the notion that impaired glucose metabolism in the absence of overt diabetes may yield milder phenotypic variants in morbidly obese gravidae (32). This is consistent with emerging studies in mice, which suggest that hemodynamic force during the development of the embryo is essential to normal cardiac morphogenesis, and this hemodynamic force is disrupted in metabolically perturbed animals (45–47).
Orofacial Clefts
Comparing a cohort of 1686 women with fetal orofacial clefts against 988,171 controls over a 9 year interval (1992 and 2001; 33), Cedergren and Kallen observed a positive association (adjusted OR 1.3) between obesity and orofacial clefts in offspring (33). Metaanalyses support these observations, as demonstrated by Stothard et al with respect to either cleft palate (adjusted OR 1.23) or cleft lip and palate (adjusted OR 1.2), but not isolated cleft lip. In their analysis, these findings were not significant in the overweight gravidae, again suggesting a dose-dependent relationship (14).
Other Anomalies
Other fetal anomalies have also been linked to maternal obesity. Blomberg et al screened the Swedish Medical Health Registries, consisting of 1,049,582 infants born in Sweden from January 1, 1995, through December 31, 2007, and found that in addition to neural tube defects, cardiac defects, and orofacial clefts, obesity also increased the risk for of hydrocephaly, anal atresia, hypospadias, cystic kidney, pes equinovarus, omphalocele, and diaphragmatic hernia (34). These findings were again largely corroborated by the meta- analysis conducted by Stothard et al, which described that association between maternal obesity and fetal anorectal atresia (OR 1.48, 5% CI 1.12–1.97, P=0.006), hydrocephaly (OR 1.68, 95% CI 1.19–2.36, P=0.003), and limb reduction anomalies (OR 1.34, 95% CI 1.03–1.73, P=0.03; 14). Conversely, fetuses with gastroschisis were significantly more common in the normal BMI population when compared with the obese in both Stothard’s and Blomberg’s studies (14, 34). Interestingly, obesity was found to be protective of one fetal anomaly, gastroschisis, in both Stothard’s and Blomberg’s studies, potentially as a result of confounding covariants including young age and socioeconomic status.
POTENTIAL AND PROBABILITY OF CONFOUNDING: OBESTITY AND DIABETES
Obesity and Diabetes
As introduced above, the fetal risks associated with poor glycemic control have long been established. In utero, the fetus risks hyperinsulinemia (with neonatal hypoglycemia), cardiomyopathy (or ventricular hypertrophy), macrosomia and stillbirth (35, 36). Additionally, there is risk for malformation of major organ systems in the offspring of diabetics, notably among those with pregestational diabetes or gestational diabetes with fasting hyperglycemia or evidence of poor glycemic control (37). Regardless, multiple lines of evidence suggest that obesity accompanies type II and gestational diabetes (Figure 2). In a recent meta-analysis, Torloni et al described the risk of gestational diabetes in association with an increasing prepregnancy BMI. The frequency of gestational diabetes rose by 0.92% for each 1 kg/m2 increase in BMI (95% CI 0.73–1.10). Moreover, the OR for gestational diabetes increased from 1.97 (95% CI 1.77–2.19) to 3.01 (95% CI 2.34–3.87) to 5.55 (95% CI 4.27–7.21) among overweight, moderately obese, and morbidly obese women, respectively (35). Interestingly, pre-gestational BMI has been shown to be an independent predictive variable in the risk analysis for congenital malformations in the offspring of mothers with gestational diabetes. Martinez-Frias et al demonstrated that as BMI increased among mothers with gestational diabetes, so did the risk for fetal malformations (38). Of note, women diagnosed with gestational diabetes in pregnancy have a 20–50% chance of recurrence in subsequent pregnancies (36), an effect partially ameliorated with weight loss (27–29, 36–38).
In sum, the relationship between obesity and diabetes, both gestational and type II, is complex as many diabetic patients are obese and many obese patients are diabetic (27). Moreover, obesity is a known risk factor for insulin resistance which is further amplified by the physiologic insulin resistance of pregnancy, which results in a 60% decrease in insulin sensitivity. It has been suggested that the relative insulin insensitivity of pregnancy superimposed on insulin resistance secondary to obesity predisposes obese pregnant women to gestational diabetes, preeclampsia, and fetal macrosomia (28).
Biologic Plausibility
Unfortunately, the mechanism linking obesity to each of these categories of fetal anomalies is not yet well understood. Many have hypothesized that undiagnosed diabetes is a leading culprit for neural tube defects, congenital cardiac malformations, and facial clefts. However, the specificity in terms of the observed phenotypic variant in each category (i.e., spina bifida but not anencephaly; septal defects but not major endocardial cushion defects; cleft lip and palate, but not isolated cleft lip) is intriguing. Moreover, it is further curious to note the linear relationship between BMI strata and fetal malformation. Such linearity is present but does not uniformly occur for either point prevalence or incidence of diabetes (20,27,28,35–38).
This begs the following question: are effects of obesity and hyperglycemia independent? As has been discussed in the preceding sections, in most of the cited studies diabetic women were excluded with the persistent observations linking obesity to the noted fetal malformations (see above and Table I). Moreover, we find it of interest to note that the association of diabetes and fetal malformations generally holds true for higher glycemic indices. By definition, undetected or undetectable diabetes in obese gravidae implies what is designated as a “normal” glycemic control. To this end, it does deserve comment that the recent findings in the HAPO trial suggest that fetal effects of hyperglycemia are manifested at glucose concentrations below those than what would have met the traditional cut offs for gestational diabetes (29). In the HAPO trial, 23,316 pregnant women, who did not meet traditional diagnostic criteria for gestational diabetes, underwent 75gm glucose tolerance tests between 24 and 32 weeks gestational age. Hyperglycemia (at levels less than traditional criteria correlated with fetal macrosomia) and increased c-peptide levels in cord blood (a surrogate measurement of fetal beta cell function). Additionally, premature delivery, shoulder dystocia, NICU stay, hyperbilirubinemia, and pre eclampsia all increased with increasing maternal glycemia (29).
In summary, the fetal effects of maternal obesity and hyperglycemia appear to be both synergistic and independently valid. Future studies which aim to dissect the mechanistic pathways underlying these observations will be pivotal in ascribing the true risk of fetal malformation with obesity and diabetes—both in isolation and interdependence--may elucidate genomic and epigenomic susceptibility factors which underlie these risks.
PRENATAL DIAGNOSIS: LIMITATIONS IN AN OBESE AND AT RISK POPULATION
Limitations of Maternal Screening Algorithms
Maternal serum screening is recommended for all pregnant women in the first and/or second trimester to evaluate risk for Down syndrome, trisomy 18 and open neural tube defects (48). While obesity does not increase the risk for aneuploidy, as previously noted obese populations are at increased risk for neural tube defects as well as other fetal anomalies. Maternal screening for aneuploidy and open neural tube defects is an integral part of prenatal care, and is influenced both by obesity and diabetes (Table II). For example, the ability to obtain an accurate fetal nuchal translucency in the obese population limits the effectiveness of first-trimester screening (15,49, 50). In the second trimester, screening is based on maternal serum markers (AFP, unconjugated estriol, hCG, and inhibin A) in the absence of NT measurements, but because obese women have increased blood volumes compared to the norm they may appear to have falsely low levels of serum anylates (51). While it is possible to adjust these values to maternal weight (but not BMI per se) and improve detection of trisomy 18 and neural tube defects (52, 53), this adjustment does not improve detection of Down syndrome in the obese population (52). While there are no current guidelines for how to interpret these results in this context, the following discussion may serve as a guide to clinicians informing their obese patients of the limitations in the interpretation of current screening programs.
Table II.
Obesity limits the interpretation of multiple aspects of a comprehensive prenatal diagnostic screening program. Attempts at discerning the contribution of obesity independent of diabetes was made in acknowledgement of the current clinical practice of imputing maternal diabetes into counseling algorithms. At present, no such similar considerations are placed for BMI per se.
| Screening Modality | Effect of Obesity | Effect of Diabetes | References |
|---|---|---|---|
| 1st Trimester Nuchal Translucency (NT) | Decreased visualization, increased failure rate | Uninterruptable due to increased risk of fetal cardiac anomalies, and association with thickened NT | 15, 44, 54, 50 |
| Maternal Serum Screening: 1st Trimester | Dilution effects | Lower free beta-hCG level | 51, 63, 52 |
| Maternal Serum Screening: 2nd Trimester | Dilution effects | Lower AFP, uE3 and DIA levels; increased risk for ONTD (lower MSAFP cutoff) | 51,63,52 |
| Genetic Sonogram | Decreased visualization of anomalies | Increased risk for birth defects | 44,15 |
First Trimester Screening
First trimester screening involves ultrasound evaluation of the nuchal translucency between 11 and 14 weeks of gestation in combination with maternal serum screening using human chorionic gonadotropin (hCG; total or free beta) and pregnancy associated plasma protein-A (PAPP-A). The decreased ability to detect the nuchal translucency in the overweight and obese populations limits effectiveness of first trimester screening for Down syndrome and trisomy 18 (15). Additionally, an increased BMI has been shown to increase the frequency of inadequate nasal bone assessment, increased need for transvaginal ultrasound examination and an increased amount of time to obtain adequate ultrasound images (50, 54). Screening is less effective without a nuchal translucency and studies report up to a 20% failure rate in obtaining a nuchal translucency in morbidly obese populations (49,50). Screening is further limited by the fact that fewer than half of women returned for a repeat attempt when an accurate NT measurement could not be obtained at the first visit (50). This suggests that first trimester screening is not as effective in obese women. Counseling should involve education on decreased ability to detect nuchal translucency measurements and the option of second trimester serum screening.
Second Trimester Screening
Second trimester maternal serum screening is also affected by obesity. Maternal serum levels of alpha-fetoprotein (AFP), unconjugated estriol (uE3), human chorionic gonadotropin (hCG) and inhibin-A (DIA) are measured in the four-analyte screen. These levels are converted to multiples of the medium (MoM) values. Large blood volumes present in overweight and obese women cause dilution effects on these analytes, leading to lower analyte levels as maternal weight increases (51). Detection of open neural tube defects and trisomy 18 improve when maternal serum analytes are adjusted for maternal weight. However weight adjustment of analytes does not increase detection of Down syndrome (51,52,55).
Many laboratories adjust for maternal weight, up to a maximum weight. For example, a common screening laboratory uses a cut-off of 270 lbs (Genzyme Genetics Laboratories). Therefore, women weighing greater than 270 pounds are adjusted using coefficients for 270-pound women. In much heavier women, this adjustment would theoretically decrease the detection of neural tube defects and increase false positives for trisomy 18. While studies have not demonstrated the effect of obesity on integrated and sequential screening, the decreased ability to detect first-trimester ultrasound markers and limitations in maternal weight adjustments for serum analytes can be applied similarly.
Confounding Effect of Diabetes
In addition to the effects of obesity on analyte levels, obese and overweight women are more likely to have diabetes, which requires screening adjustments. It is well known that diabetic women are at increased risk for neural tube defects (30). Studies have also shown that serum markers such as AFP, uE3, DIA and free beta-hCG tend to be lower among women with insulin-dependent diabetes mellitus, even when adjusted for maternal weight (55). Other studies have not demonstrated significantly lower values of these analytes (56). Controversies over these adjustments lead to more uncertainty regarding the accuracy of maternal serum screening in obese and diabetic women.
Limitations of Fetal Ultrasonography in the Obese Population
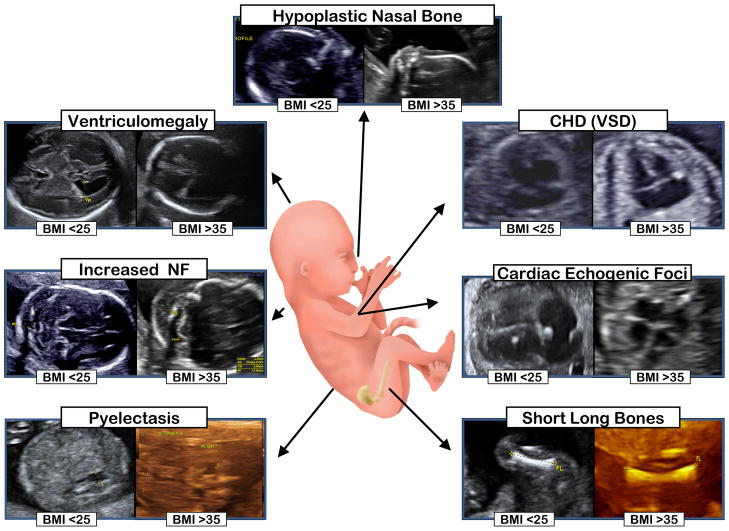
As ultrasonography has become an integral part of obstetrical care, and we and others have shown that it can improve accurate detection of Down syndrome, it has become increasingly apparent that maternal obesity limits optimal visualization or accurate assessment of some (but not all) fetal structures or soft markers (15–19,57,58; Figure 3). Given that among obese women there likely exists an (i) increased prevalence of fetal malformations, with a (ii) concomitant diminished ability to sonographically detect such malformations, it would be clinically advantageous to provide clinicians objective measures of the degree to which visualization might be expected to be limited in an obese population. There is data to suggest that the rate of suboptimal visualization in the obese patient during the genetic ultrasound ranges from 20–50%. The cardiac and craniospinal structures are often the most difficult to visualize (15, 57, 59).
Figure 3. Differential capacity for visualization of fetal structures and markers of aneuploidy in normal weight (BMI <25 kg/m2) and obese women (BMI >35 kg/m2).
As demonstrated in multiple studies (15–19, 54) and visualized herein, maternal obesity decreases the practitioner’s ability to confidently recognize such markers, or accurately assess their dimensions. For example, from the representative images in obese women, please note the poor delineation of the boarders of the lateral ventricles and renal pelvis (which may lead to inaccurate assessment), alongside the decreased echogenicity of the intracardiac echogenic focus (which may lead to missed diagnosis).
It appears to be the common collective experience among clinicians that the size of the body fat layer limits sonographic visualization of fetal and maternal structures (8, 16–19). However, objective analysis of the impact of obesity on the quality of prenatal fetal sonographic surveillance is limited (8, 16–19). In 1990, Wolfe et al described a greater prevalence of suboptimal visualization among women with a BMI above the >90th% (16). Dashe et al performed a retrospective cohort analysis of 10,112 women who had a second trimester anatomy scan over a 5 year period. Ten anatomic structures were chosen for anatomic evaluation (atria of the cerebral ventricles, posterior fossa, midline face, 4-chamber view of the heart, spine, ventral wall, umbilical cord vessels, stomach, kidneys, and bladder). All 10 components were successfully visualized in 57%, 41% and 30% of women with, respectively class 1,2, and 3 obesity, respectively, compared with 72% in women with a normal or underweight BMI (p< 0.001) (57).
Using next-generation ultrasonographic resolution, we and others have replicated their findings in single-institution retrospective cohorts (8), as well as randomized trials aimed at examining the feasibility of second-trimester fetal ultrasound in an unselected population over the 18–22 week interval (18). Thus, while there are prospectively acquired data suggesting that maternal obesity carries a significant independent increased risk for fetal anomalies (8–12,17,18), the limited available data would suggest that our ability to view potentially affected anatomic structures may be suboptimal among obese women (8, 16–18). Focusing on the detection of Down syndrome, the FaSTER trial enrolled participants from 13 centers in order to investigate the role of second trimester genetic ultrasound in modifying maternal serum screening results. Likelihood ratios were calculated for risk modification with presence or absence of structural malformations or ‘soft’ sonographic markers. These markers include a thickened nuchal fold (> 5mm), an echogenic intracardiac focus (papillary muscle that is found to be as bright as bone), echogenic bowel, and renal pyelectasis (a 4 mm or greater anterior posterior diameter of the renal pelvis), shortened long bones, pericardial effusions, choroid plexus cysts, ventriculomegaly, hydrops, liver calcification, two vessel umbilical cord, polydactyly, clinodactyly, sandal gap toe, and club foot (58, 60–62). The presence of these markers may prompt further invasive testing whereas the absence of these markers may be very reassuring.
While the FaSTER trial showed that maternal serum screening in the first and second trimester can be improved with the likelihood ratios from second trimester genetic ultrasound (58). Subsequent analysis of the FaSTER population database investigated the role of maternal body mass index in second trimester genetic sonography. In this secondary analysis, we observed that maternal obesity was associated with elevated false positive results for the category of two or more sonographic markers for Down syndrome and additionally with increased missed diagnosis rate (MDR) of sonographic ‘soft markers’ (15). Additionally, first trimester nuchal translucency measurements were consistently more difficult to obtain with increasing BMI (15). Taken together, these findings and those of others (16–18, 57) illustrate that while the use of likelihood ratios improves aneuploidy screening in the general population, the reported likelihood ratios are not necessarily applicable in the obese subset. Therefore, one might argue that an informed patient discussion will acknowledge the limitations of sonography when used as a tool for aneuploidy risk assessment in obese women.
A great deal of recent discussion has focused on the role of MRI in the obese pregnant patient. MRI is less affected by maternal habitus than ultrasound and may, in some cases, be a preferable imaging modality. There is no evidence of adverse affects of MRI in the pregnant patient (59), but similarly no evidence of any assumed or direct benefit. Since the use of fetal MRI for obese patients is highly limited by cost and availability it is our opinion that it not be used as a routine screening modality in obese subjects but rather be considered as an alternative should serum screening suggest significantly elevated risk of certain fetal malformations. Examples would include an unexplained elevated MSAFP where the fetal spine and abdominal wall cannot be adequately visualized by sonogram.
Summary
The epidemic of obesity has fully washed ashore, and is presently manifesting itself in the reproductive age population with noted fetal sequelae. While the trite solution would be to successfully council obese women to lose weight prior to pregnancy, this is neither feasible nor practical in our current health care environment. That said, preconception counseling should included the risks of obesity from both a maternal and fetal perspective, and enable the patient to be fully informed about the risks of elevated BMI. In an ideal health care system, this counseling would occur in a multidisciplinary manner and involve physician subspecialists including obstetricians, bariatric surgeons, endocrinologists and pulmonologists (due to risk of obstructive sleep apnea), behavioral specialists, as well as genetic counselors and dieticians. In the current health care environment, consideration ought be given to aggressive screening for overt diabetes. We would further encourage patient education about the limitations of genetic ultrasonography and prenatal diagnostic screening among obese gravidae in both the present and absence of overt diabetes, as well as delineating the ramifications that these limitations could pose to early detection of fetal malformations. Finally, future efforts aimed at ascribing optimal care to this at-risk cohort ought to consider the ongoing need for molecular mechanistic studies designed to elucidate the etiology of the described observations herein.
Acknowledgments
The authors wish to acknowledge the contributions of Ms. Monica Murphy, Valerie Jackson, Tamera Wilson, Mary Jo Canale, Stacie Dennison, and Drs. Anthony Johnson and Stacy Strehlow in receipt of sonographic images and critical review of the manuscript. This work was supported by grants to K.A.T. (NIH DP21DP2OD001500-01 and R01DK080558).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dennedy MC, Dunne F. The maternal and fetal impacts of obesity and gestational diabetes on pregnancy outcome. Best Practice & Research Clinical Endocrinology & Metabolism. 2010;24:573–589. doi: 10.1016/j.beem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10 year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 4.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate-A population-based screening study. Am J Obstet Gynecol. 2004;190:1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 5.Cnattingius S, Bergstrom R, Lipworth L, et al. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 6.Robinson HE, O’Connell CM, Joseph KS, et al. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106:1357–64. doi: 10.1097/01.AOG.0000188387.88032.41. [DOI] [PubMed] [Google Scholar]
- 7.UshaKiran TS, Hemmadi S, Bethel J, et al. Outcome of pregnancy in a woman with increased body mass index. BJOG. 2005;112:768–72. doi: 10.1111/j.1471-0528.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- 8.Hendler I, Blackwell SC, Bujold E, et al. The impact of maternal obesity on midtrimester sonographic visualization of fetal cardiac and craniospinal structures. Int J Obesity. 2004;28:1607–1611. doi: 10.1038/sj.ijo.0802759. [DOI] [PubMed] [Google Scholar]
- 9.Watkins ML, Botto LE. Maternal prepregnancy weight and congenital heart defects in the offspring. Epidemiology. 2001;11:439–446. [PubMed] [Google Scholar]
- 10.Shaw GM, Velie EM, Schaffer D. Risk of neural tube defect-affected pregnancies among obese women. JAMA. 1996;275:1093–1096. doi: 10.1001/jama.1996.03530380035028. [DOI] [PubMed] [Google Scholar]
- 11.Wong SF, Chan FY, Cincotta RB, et al. Routine ultrasound screening in diabetic pregnancies. Ultrasound Obstet Gynecol. 2002;19:171–176. doi: 10.1046/j.0960-7692.2001.00560.x. [DOI] [PubMed] [Google Scholar]
- 12.Watkins ML, Rasmussen SA, Nonein MA, et al. Maternal obesity and risk for birth defects. Pediatrics. 2003;111:1152–1158. [PubMed] [Google Scholar]
- 13.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 14.Stothard KJ, Tennant PW, Bell R, et al. Maternal overweight and obesity and the risk of congenital anomalies a systematic review and meta-analysis. JAMA. 2009;301 (6):636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 15.Aagaard-Tillery KM, Porter TF, Malone FD, et al. Influence of maternal body mass index on genetic sonography in the FaSTER trial. Prenat Diag. 2010;30 (1):14–22. doi: 10.1002/pd.2399. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe HM, Sokol RJ, Martier SM, et al. Maternal obesity: a potential source of error in sonographic prenatal diagnosis. Obstet Gynecol. 1990;76:339–342. [PubMed] [Google Scholar]
- 17.DeVore GR, Medearis AL, Bear MB, et al. Fetal echocardiography: factors that influence imaging of the fetal heart during the second trimester of pregnancy. J Ultrasound Med. 1993;12:659–663. doi: 10.7863/jum.1993.12.11.659. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzler P, Senat MV, Holden D, Bernard, et al. Feasibility of the second trimester fetal ultrasound examination in an unselected population at 18, 20, or 22 weeks of pregnancy: a randomized trial. Ultrasound Obstet Gynecol. 1999;14:92–97. doi: 10.1046/j.1469-0705.1999.14020092.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg JC, Guzman ER, Vintzileos AM, et al. Transumbilical placement of the vaginal probe in obese pregnant women. Obstet Gynecol. 1995;85:132–134. doi: 10.1016/0029-7844(94)00342-b. [DOI] [PubMed] [Google Scholar]
- 20.Obesity in pregnancy. ACOG Committee Opinion No. 315. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2005;106:671–675. doi: 10.1097/00006250-200509000-00054. [DOI] [PubMed] [Google Scholar]
- 21.Headley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 22.Aagaard-Tillery KM, Suter MA, Harris RA, et al. Epigenetics and reproduction and the developmental origins of health and disease. Anim Reprod. 2010;7:103–116. [Google Scholar]
- 23.Suter MA, Bocock PN, Showalter L, et al. High fat maternal diet is associated with differential regulation of fetal circadian metabolism. FASEB J. 2010 epub Nov 19. [Google Scholar]
- 24.Aagaard-Tillery KM, Grove K, Bishop J, et al. Developmental origins of disease and determinants of chromatin structure: Maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008;41(2):91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox J, Williams S, Grove K, et al. A maternal high fat diet is accompanied by alterations in the fetal primate metabolome. Am J Obstet Gynecol. 2009;201(3):281.e1–9. doi: 10.1016/j.ajog.2009.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–35. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons D. Diabetes and obesity in pregnancy. Best Practice & Research Clinical Obstetrics and Gynaecology. 2011;25:25–36. doi: 10.1016/j.bpobgyn.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140:365–371. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. NEJM. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 30.Waller DK, Mills JL, Simpson JL, et al. Are obese women at higher risk for producing malformed offspring? Am J Obstet Gynecol. 1994;170:541–548. doi: 10.1016/s0002-9378(94)70224-1. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen SA, Chu SY, Kime SY, et al. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198 (6):611–619. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Cedergren MI, Kallen B. Maternal obesity and infant heart defects. Obes Res. 2003;11:1065–1071. doi: 10.1038/oby.2003.146. [DOI] [PubMed] [Google Scholar]
- 33.Cedergren M, Kallen B. Maternal obesity and the risk for orofacial clefts in offspring. Cleft Palate Craniofac J. 2005;42 (4):367–71. doi: 10.1597/04-012.1. [DOI] [PubMed] [Google Scholar]
- 34.Blomberg MI, Källén B. Maternal obesity and morbid obesity: the risk for birth defects in the offspring. Birth Defects Res A Clin Mol Teratol. 2010;88(1):35–40. doi: 10.1002/bdra.20620. [DOI] [PubMed] [Google Scholar]
- 35.Torloni MR, Betran AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obesity Reviews. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 36.Reece EA. Perspectives on obesity, pregnancy and birth outcomes in the United States: The scope of the problem. Am J Obstet Gynecol. 2008;198 (1):23–27. doi: 10.1016/j.ajog.2007.06.076. [DOI] [PubMed] [Google Scholar]
- 37.Sheffield JS, Butler-Koster EL, Casey BM, et al. Maternal diabetes mellitus and infant malformations. Obstet Gynecol. 2002;100:925–930. doi: 10.1016/s0029-7844(02)02242-1. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Frias ML, Frias JP, Bermejot E, et al. Pre-gestational maternal body mass index predicts an increased risk of congenital malformations in infants of mothers with gestational diabetes. Diabet Med. 2005;33:775–781. doi: 10.1111/j.1464-5491.2005.01492.x. [DOI] [PubMed] [Google Scholar]
- 39.Blencowe H, Cousens S, Modell B, et al. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol. 2010;39(S1):10–20. doi: 10.1093/ije/dyq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray JG, Wyatt PR, Vermeulen MJ, et al. Greater maternal weight and the ongoing risk of neural tube defects after folic acid flour fortification. Obstet Gynecol. 2005;105:261–265. doi: 10.1097/01.AOG.0000151988.84346.3e. [DOI] [PubMed] [Google Scholar]
- 41.Wilson RD, Johnson JA, Wyatt P, et al. Genetics Committee of the Society of Obstetricians and Gynaecologists of Canada and The Motherrisk Program. Preconcepional vitamin/folic acid supplementation 2007: The use of folic acid in combination with a multivitamin supplement for the prevention of neural tude defects and other congenital anomalies. J Obstet Gynaecol Can. 2007;12:1003–26. doi: 10.1016/S1701-2163(16)32685-8. [DOI] [PubMed] [Google Scholar]
- 42.Mahabir S, Ettinger S, Johnson L, et al. Measures of adiposity and body fat distribution in relation to serum folate levels in postmenopausal women in a feeding study. Eur J Clin Nutr. 2008;62(5):644–50. doi: 10.1038/sj.ejcn.1602771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25(11–12):1150–6. doi: 10.1016/j.nut.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Wax JR. Risks and management of obesity in pregnancy: current controversies. Curr Opin Obstet Gynecol. 2009;21:117–123. doi: 10.1097/GCO.0b013e328328d3c7. [DOI] [PubMed] [Google Scholar]
- 45.Culver JC, Dickinson ME. The effects of hemodynamic force on embryonic development. Microcirculation. 2010;17:164–178. doi: 10.1111/j.1549-8719.2010.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poché RA, Larina IV, Scott ML, et al. The Flk1-myr::mCherry mouse as a useful reporter to characterize multiple aspects of ocular blood vessel development and disease. Dev Dyn. 2009;238:2318–2326. doi: 10.1002/dvdy.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucitti JL, Jones EA, Huang C, et al. Vasuclar remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;52:752–60. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Screening for fetal chromosomal abnormalities. ACOG Practice Bulletin No. 77. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2007;109:217–27. doi: 10.1097/00006250-200701000-00054. [DOI] [PubMed] [Google Scholar]
- 49.Wax JR, Pinette MG, Cartin A, et al. The value of repeated evaluation after initial failed nuchal translucency measurement. J Ultrasound Med. 2007;26:825–828. doi: 10.7863/jum.2007.26.6.825. [DOI] [PubMed] [Google Scholar]
- 50.Thornburgh LL, Mulconry M, Post A, et al. Fetal nuchal transluceny thickness evaluation in the overweight and obese gravid. Ultrasound Obsete Gynecol. 2009;33:665–669. doi: 10.1002/uog.6410. [DOI] [PubMed] [Google Scholar]
- 51.Palomaki GE, Panizza DS, Canick JA. Screening for Down syndrome using AFP, uE3, and hCG: effect of maternal weight. Am J Hum Genet. 1990;7:a282. [Google Scholar]
- 52.Wald NJ, Cuckle HS, Densem JW, et al. Maternal serum screening for Down syndrome: the effect of routine ultrasound scan determination of gestational age and adjustment for maternal weight. Bt J Obstet Gynaecol. 1992;99:144–149. doi: 10.1111/j.1471-0528.1992.tb14474.x. [DOI] [PubMed] [Google Scholar]
- 53.Johnson AM, Palomaki GE, Haddow JE. The effect of adjusting maternal serum alpha-feto protein levels for maternal weight in pregnancies with fetal open spina bifida. AM J Obstet Gynecol. 1990;163:9–11. doi: 10.1016/s0002-9378(11)90655-3. [DOI] [PubMed] [Google Scholar]
- 54.Gandhi M, Fox NS, Russo-Stieglitz K, et al. Effect of increased body mass index on first-trimester ultrasound examination for aneuploidy risk assessment. Obstet Gynecol. 2009;114:856–9. doi: 10.1097/AOG.0b013e3181b6bfdc. [DOI] [PubMed] [Google Scholar]
- 55.Wald NJ, Kennard A, Hackshaw A, et al. Prenatal screening for Down syndrome. Prenat Diagn. 1997;4:181–246. [Google Scholar]
- 56.Sancken U, Bartels I. Biochemical screening for chromosomal disorders and neural tube defects (NTDs): is adjustment of maternal alpha-fetoprotein (AFP) still appropriate in insulin-dependent diabetes mellitus (IDDM)? Prenat Diagn. 2001;21:383–386. doi: 10.1002/pd.72. [DOI] [PubMed] [Google Scholar]
- 57.Dashe JS, McIntire DD, Twickler DM. Maternal obesity limits the ultrasound evaluation of fetal anatomy. J Ultrasound Med. 2009;28 :1025–1030. doi: 10.7863/jum.2009.28.8.1025. [DOI] [PubMed] [Google Scholar]
- 58.Aagaard-Tillery KM, Malone FD, Nyberg DA, et al. Role of second trimester genetic sonography following Down syndrome screening. Obstet Gynecol. 2009;114 (6):1189–96. doi: 10.1097/AOG.0b013e3181c15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maxwell C, Glanc P. Imaging and obesity: a perspective in pregnancy. Am J Roentgenol. 2011;196 (2):311–319. doi: 10.2214/AJR.10.5849. [DOI] [PubMed] [Google Scholar]
- 60.Malone FD, Canick JA, Ball RH, et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N Engl J Med. 2005;353:2001–11. doi: 10.1056/NEJMoa043693. [DOI] [PubMed] [Google Scholar]
- 61.Bromley R, Lieberman E, Shipp TD, et al. The genetic sonogram: a method of risk assessment for Down syndrome in the second trimester. J Ultrasound Med. 2002;21:1087–96. doi: 10.7863/jum.2002.21.10.1087. [DOI] [PubMed] [Google Scholar]
- 62.Hobbins JC, Lezotte DC, Persutte WH, et al. An 8-center study to evaluate the utility of midterm genetic sonograms among high-risk pregnancies. J Ultrasound Med. 2003;22:33–38. doi: 10.7863/jum.2003.22.1.33. [DOI] [PubMed] [Google Scholar]
- 63.Neveux LM, Palomaki GE, Larrivee DA, et al. Refinements in managing maternal weight adjustment for interpreting prenatal screening results. Prenat Diag. 1996;16:1115–1119. doi: 10.1002/(SICI)1097-0223(199612)16:12<1115::AID-PD3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 64.Chu SY, Callaghan WM, Kim SY. Maternal obesity and risk of gestational diabetes. Diabetes Care. 2007;30 (8):2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]