Abstract
Streptococcus pneumoniae is an important cause of community-acquired pneumonia, meningitis, and bacteremia. The problem of pneumococcal disease is exacerbated by increasing drug resistance. Furthermore, patients with impaired immunity are at high risk for invasive pneumococcal infections. Thus, there is an urgent need for new approaches to antimicrobial therapy. Antibody therapies take advantage of the specificity and high affinity of the antigen-antibody interaction to deliver antibacterial compounds to a site of infection in the form of naked or conjugated antibodies. We have recently established that radioimmunotherapy (RIT) can be used to treat experimental fungal infections in mice. In the present study, we investigated the feasibility of applying a RIT approach to the treatment of S. pneumoniae infection by evaluating the susceptibility of S. pneumoniae to radiolabeled antibody in vitro and in an animal infection model. For the specific antibody carrier, we used human monoclonal antibody D11, which binds to pneumococcal capsular polysaccharide 8. We have selected the alpha particle emitter 213Bi as the radionuclide for conjugation to the antibody. Incubation of serotype 8 S. pneumoniae with 213Bi-D11 resulted in dose-dependent killing of bacteria. RIT of S. pneumoniae infection in C57BL/6 mice showed that 60% more mice survived in the 213Bi-D11-treated group (80 μCi) than in the untreated group (P < 0.01). The treatment did not cause hematological toxicity, as demonstrated by platelet counts. This feasibility study establishes that RIT can be applied to the treatment of bacterial infections.
Streptococcus pneumoniae is an important cause of community-acquired pneumonia, meningitis, and bacteremia (3). Furthermore, there is an increased prevalence of invasive pneumococcal infections in patients with immune impairment caused by chemotherapy or immune suppression in the setting of organ transplantation or human immunodeficiency virus infection (23). Drug resistance and impaired responses to vaccines (both regular and conjugates) make it difficult to control this disease, particularly in the elderly and immunocompromised (23). Thus, there is an urgent need to develop new approaches to antipneumococcal therapy, which can include new drugs, vaccines, and passive antibody therapy. Antibody-based strategies constitute powerful options for countering the problem of increasing drug resistance and the emergence of new infectious diseases. Antibody therapies take advantage of the specificity and high affinity of the antigen-antibody interaction to deliver antibacterial compounds to a site of infection in the form of naked or conjugated antibodies. It is worth noting that antibody therapy was highly effective against pneumococcal disease in the preantibiotic era (7, 9).
Radioimmunotherapy (RIT) is a therapeutic modality that uses specific antibodies labeled with radioisotopes to deliver lethal doses of particulate radiation to cells, and its development was pioneered for cancer treatment (17). Radiolabeled antibodies provide a valuable alternative to cancer treatment with chemotherapy or external radiation beam by selectively delivering lethal doses of radiation to cancerous cells. We have recently suggested that RIT could be adapted as a novel modality for the treatment of infectious diseases whereby radiolabeled specific antibodies are used to deliver microbicidal radiation to pathogens (13). In contrast to the limitations encountered in the use of RIT against tumors that include few antigenic differences between malignant tissue and normal tissue, RIT of infectious diseases can exploit antigenic differences between microbes and the host, which should translate into specificity for the targeted pathogen and minimal toxicity to the host. A proof of principle for this strategy was recently established by demonstrating that radiolabeled antibodies could be used in the therapy of experimental Cryptococcus neoformans infection in mice. For that study, we developed methods to target ionizing radiation to a fungal cell by labeling a specific monoclonal antibody (MAb) with the radioisotopes 188Re and 213Bi. Radiolabeled antibodies killed C. neoformans cells in vitro, thus converting an antibody with no inherent antimicrobial activity into microbicidal molecules. Administration of radiolabeled antibodies to mice with C. neoformans infections delivered 213Bi or 188Re to the sites of infection, reduced their organ microbial burden, and significantly prolonged their survival without apparent toxicity.
In the present study, we have investigated the feasibility of applying the RIT approach to S. pneumoniae in an animal infection model. As part of the development of this method, we also studied the susceptibility of S. pneumoniae to radiolabeled antibody in vitro. In contrast to fungal infections, which are often chronic, systemic bacterial infections are often characterized by rapid microbial replication and host demise. Furthermore, a comparison of fungal and bacterial cells reveals differences in size, DNA base composition, cell wall composition, and possibly repair mechanisms. Hence, the potential usefulness of RIT for bacterial infections is likely to encounter different challenges than its application to the treatment of fungal infections. For the specific antibody carrier in this study, we used human MAb D11, which binds specifically to pneumococcal capsular polysaccharide 8 (PPS8) and is highly protective against this serotype in several strains of mice and infection models (8, 29). For antibody conjugation, we selected the alpha particle emitter 213Bi. 213Bi (half-life, 45.6 min) emits a high linear energy transfer (LET) alpha particle with E = 5.9 MeV with a path length of 50 to 80 μm that can theoretically kill a cell with one or two hits. We have chosen the alpha emitter 213Bi over the beta emitter 188Re because the much shorter half-life of 213Bi (45.6 min versus 16.9 h) permits delivery of a significant radiation dose in shorter period of time, which may be crucial when quickly dividing cells such as pneumococci are being targeted. 213Bi has been proposed for use in single-cell disorders and some solid cancers (1, 6, 19) and is currently being used in phase I/II clinical trial to treat patients with leukemia (26). The results of this feasibility study confirm that RIT can be applied to the treatment of bacterial infections.
MATERIALS AND METHODS
Bacteria.
S. pneumoniae serotype 8 (ATCC 6308) and serotype 3 (ATCC 6303) were grown in tryptic soy broth (TSB) to mid-log phase at 37% in 5% CO2, frozen in TSB in 10% glycerol, and stored at −80°C. Bacteria were thawed on ice, streaked onto a Trypticase soy agar plate with 5% sheep blood agar, and passaged once in TSB before use.
Antibodies.
Human MAb D11 (immunoglobulin M [IgM]), which binds to PPS8 and was originally generated from the peripheral lymphocytes of a Pneumovax recipient, was produced as described in reference 29. It was purified by affinity chromatography with anti-human IgM agarose beads (Sigma, Minneapolis, Minn.). Commercial human myeloma IgM (Calbiochem, La Jolla, Calif.) was used as an isotype-matching control.
Radioisotope production and radiolabeling of antibodies.
225Ac for 225Ac-213Bi generator construction was obtained from the Institute for Transuranium Elements, Heidelberg, Germany. The 225Ac-213Bi generator was constructed with MP-50 cation exchange resin, and 213Bi eluted with 0.15 M hydroiodic acid in the form of 213BiI3 as described in reference 6. For radiolabeling with 213Bi, antibodies were first conjugated to the bifunctional chelating agent CHXA"{N-[2-amino-3-(p-isothiocyanatophenyl)propyl]-trans-cyclohexane-1,2-diamine-N,N′,N″,N‴,N⁗-pentaacetic acid} as described in references 12 and 21. The average final number of chelates per antibody molecule was determined by the yttrium-arsenazo III spectrophotometric method (22). CHXA"-MAbs were radiolabeled with 213Bi as described in reference 6.
Immunoreactivity of radiolabeled D11.
The immunoreactivity of radiolabeled CHXA"-D11, in comparison with that of unmodified D11, was determined by evaluating binding to solid-phase PPS8 by enzyme-linked immunosorbent assay (ELISA) as described in reference 29. The starting concentration of unmodified D11 and the control IgM was 10 μg/ml, and that of CHXA"-D11 was 2.5 μg/ml.
In vitro activity of labeled antibody against S. pneumoniae.
Approximately 107 S. pneumoniae cells of the serotype 8 strain were placed in microcentrifuge tubes in 50 μl of phosphate-buffered saline (PBS). The 213Bi-CHXA"-D11 MAb in 0.5 ml of PBS was added to obtain the desired concentrations of radioactivity per sample (0 to 4 μCi), with the total amount of MAb per sample kept constant at 20 μg. After 30 min of incubation at 37°C, the cells were collected by centrifugation. The pellets were washed with PBS and diluted, and approximately 1,000 cells were plated on blood agar plates to determine CFU. To account for nonspecific cell killing, S. pneumoniae cells of the serotype 3 strain were incubated with 213Bi-CHXA"-D11. This experiment served as a control because MAb D11 does not bind to cells of S. pneumoniae serotype 3.
In another series of experiments with serotype 8 S. pneumoniae, radiolabeled irrelevant control MAb 213Bi-CHXA"-IgM was also included to account for nonspecific cell killing in comparison with 213Bi-CHXA"-D11 treatment.
RIT of experimental S. pneumoniae infection in mice.
The efficacy of RIT against experimental S. pneumoniae infection was evaluated in mouse strain C57BL/6. Mice were infected intraperitoneally (i.p.) with 1,000 bacteria, as measured by CFU counting. Preliminary experiments were carried out to determine the amount of the MAb that conferred protection against S. pneumoniae infection in this animal model by giving the mice 1 or 10 μg of unlabeled D11 i.p. at 1 h postinfection. A D11 dose of 1 μg/mouse did not confer protection against S. pneumoniae, but increasing the dose to 10 μg/mouse increased the percentage of mice surviving infection relative to that of nontreated controls from 30 to 70%. Thus, for RIT we selected the intermediate amount of 5 μg of MAb D11 per mouse. At 1 h postinfection, groups of 8 to 10 animals were treated i.p. with 80 μCi of 213Bi-CHXA"-D11 or 213Bi-CHXA"-IgM (5 μg), or 5 μg of unlabeled D11 or left untreated. The animals were observed for survival and vital signs for 14 days.
Determination of bacterial loads of RIT-treated mice.
As untreated mice die from bacteremia in the animal model of systemic infection used in this study, we determined whether RIT had an effect on the serum bacterial burden. Ten mice were infected with 1,470 CFU of S. pneumoniae. Five infected animals were treated i.p. with 80 μCi of 213Bi-CHXA"-D11 while five others served as controls. Blood was obtained from the tail veins of 213Bi-CHXA"-D11-treated mice and control at 3, 6, and 10 h posttherapy and from survivors on days 3 and 14 posttherapy, dilutions of the blood in TSB were plated in duplicate on blood agar plates and incubated for 18 h, and the number of CFU per milliliter of blood was determined. The limit of detection was 13 CFU.
Determination of platelet counts.
Platelet counts were used as a marker of RIT toxicity. For measurement of platelet counts, the blood of C57BL/6 mice used in the therapeutic studies was collected from the tail vein into heparinized capillary tubes on day 3, 7, and 14 days posttherapy. The background measurement of the platelet counts was obtained for healthy C57BL/6 mice. Four microliters of blood was mixed with 2.5 μl of 10% EDTA. One microliter of the anticoagulated blood was then mixed with 100 μl of 1% ammonium citrate. Platelets were counted in a hemocytometer by phase-contrast microscopy at ×400 magnification as described in reference 20.
Statistical analysis.
Student's t test for unpaired data was used to analyze differences in the number of CFU between differently treated groups during in vitro therapy studies. The log-rank test was used to assess the course of animal survival. Differences were considered statistically significant when P values were <0.05. Prism statistical software (GraphPad Software, San Diego, Calif.) was used for statistical calculations.
RESULTS
Radiolabeling of antibodies with 213Bi.
Both MAb D11 and the control IgM proved to be robust immunoglobulins suitable for conjugation with CHXA" ligand. The average number of chelates per antibody molecule ranged from 1.5 to 3.0, as determined by the yttrium-arsenazo III spectrophotometric method. The radiolabeling of CHXA"-MAb conjugates with 213Bi resulted in 95% ± 3% yields.
Immunoreactivity of radiolabeled 213Bi-CHXA"-D11 MAb.
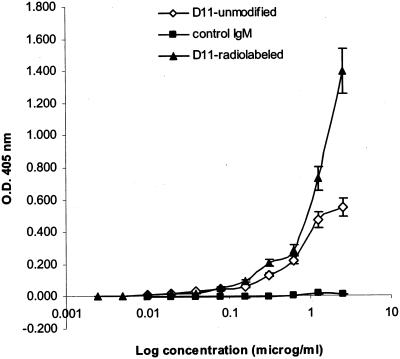
The results of 213Bi-CHXA"-D11 (referred to further in the text as 213Bi-D11) binding to solid PPS8 are presented in Fig. 1. Attachment of CHXA" ligand, followed by radiolabeling with 213Bi, did not compromise the ability of MAb D11 to bind PPS8. Some differences in binding between unmodified and radiolabeled D11 were observed at concentrations of >1 μg/ml, which might be explained by the contribution of the electrostatic interaction between the antigen in the wells and the negatively charged CHXA" group on the antibody. These results established the feasibility of radiolabeling MAb D11 while retaining its ability to bind to PPS8.
FIG. 1.
Immunoreactivity of radiolabeled MAb D11 by ELISA. Binding to solid-phase PPS8 was evaluated by ELISA as described in reference 29. The starting concentration of unmodified D11 and control IgM was 10 μg/ml, and that of CHXA"-D11 was 2.5 μg/ml. O.D., optical density.
Activity of radiolabeled MAb against S. pneumoniae in vitro.
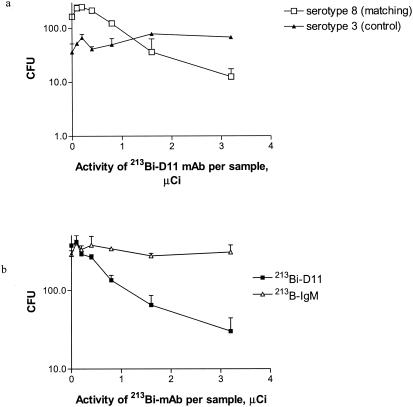
We evaluated the susceptibility of S. pneumoniae to 213Bi alpha particles delivered by S. pneumoniae-specific antibody in vitro. Incubation of serotype 8 S. pneumoniae with 213Bi-D11 resulted in dose-dependent killing of bacteria (Fig. 2a). The slight increase in CFU of serotype 8 S. pneumoniae when low activities of 213Bi-D11 were applied (Fig. 2a) may be a result of negatively charged CHXA" groups on the antibody molecules preventing the organisms from clumping. In contrast, incubation of S. pneumoniae serotype 3 with 213Bi-D11 with the same specific activity produced only minimal killing within the investigated range of activities (P = 0.001). In another series of experiments, the ability of 213Bi-D11 to eradicate S. pneumoniae serotype 8 was compared to the effect of irrelevant control MAb 213Bi-IgM used in the same range of activities (Fig. 2b). No killing of S. pneumoniae with 213Bi-IgM was observed. The significantly higher killing associated with the specific antibody almost certainly reflects higher exposure of S. pneumoniae to radiation as a consequence of antibody binding to PPS8. Hence, radiolabeled anti-S. pneumoniae MAb was bactericidal in vitro.
FIG. 2.
RIT of S. pneumoniae with PPS8-specific 213Bi-D11 MAb in vitro. (a) S. pneumoniae serotypes 8 and 3 (control) were incubated with 20 μg of MAb D11 labeled with increasing activities of 213Bi. (b) Serotype 8 S. pneumoniae cells were incubated with 20 μg of either MAb D11 or IgM (control) labeled with increasing activities of 213Bi. Each experiment was performed in duplicate. Error bars represent standard deviations.
In vivo RIT of S. pneumoniae infection and bacterial loads in treated mice.
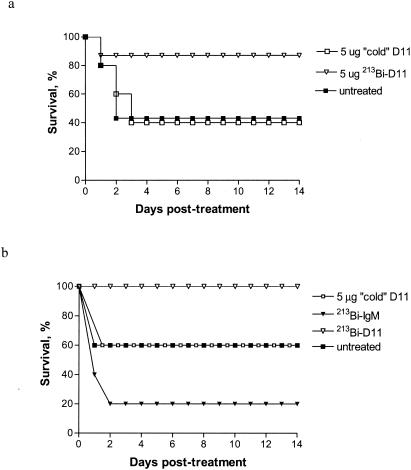
Figure 3 shows the results of RIT for S. pneumoniae infection in C57BL/6 mice (data from two different treatment experiments are shown). A greater percentage of mice survived in the 213Bi-D11-treated group than in the untreated group (P < 0.01). In contrast, administration of unlabeled D11 (5 μg) did not prolong survival in comparison to that of untreated mice (P > 0.05). Radiolabeled control IgM also did not have any therapeutic effect (P > 0.05). Mice in control groups succumbed to bacteremia on days 1 to 3, while mice treated with 80 μCi of 213Bi-D11 demonstrated 87 to 100% survival. Furthermore, mice treated with 213Bi-D11 were not bacteremic at 3, 6, and 10 h posttreatment, as determined by counting the CFU in their blood (Table 1), as well as on days 3 and 14 (data not shown). Treatment with radiolabeled D11 was very well tolerated—no weight loss or changes in eating and drinking habits or grooming routines were observed in treated animals.
FIG. 3.
RIT of S. pneumoniae infection with 213Bi-labeled MAbs in C57BL/6 mice. Eight to 10 mice per group were used. Mice were infected i.p. with 1,000 organisms 1 h before treatment with MAbs. Results of two experiments are presented. Cold = unlabeled.
TABLE 1.
Bacterial loads in C57BL/6 mice infected i.p. with S. pneumoniae and treated with 213Bi-D11a
| Animal no. | Bacterial load (CFU/ml of blood)
|
||
|---|---|---|---|
| 3 h | 6 h | 10 h | |
| Nontreated | |||
| 1 | 2.3 × 103 | 7.8 × 105 | 4.9 × 108 |
| 2 | 1.5 × 103 | 1.5 × 105 | 5.6 × 108 |
| 3 | 250 | 250 | 250 |
| 4 | 250 | 250 | 250 |
| 5 | 2.1 × 103 | 7.8 × 105 | 3.8 × 108 |
| Treated | |||
| 1 | 250 | 250 | 250 |
| 2 | 250 | 250 | 250 |
| 3 | 250 | 1.5 × 103 | 3.0 × 107 |
| 4 | 250 | 250 | 250 |
| 5 | 250 | 250 | 250 |
Ten mice were infected i.p. with 1,470 CFU of S. pneumoniae, and 5 of the mice were treated 1 h later with 80 μCi of 213Bi-D11. The lower limit of detection was 13 CFU. In untreated infected mice, the survival rate was 40%; in mice infected and treated with 213Bi-D11, it was 80%.
Toxicity studies.
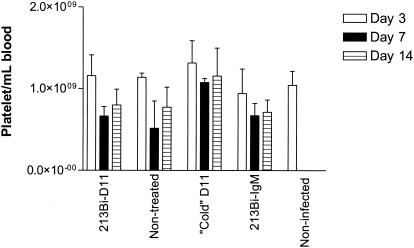
The number of platelets in peripheral blood was determined as a measure of radiation toxicity. Platelet counts in S. pneumoniae-infected C57BL/6 mice treated with 213Bi-D11 and 213Bi-IgM MAbs were compared to those of mice treated with unlabeled D11 and with those of nontreated infected animals (Fig. 4). It should be noted that platelet counts in 213Bi-IgM-treated, unlabeled-D11-treated, and infected nontreated mice were acquired in few surviving animals while the platelet counts in 213Bi-D11-treated animals were obtained from a significantly larger number of animals. There was no statistically significant difference in platelet counts on day 3 postinfection in treated or nontreated infected mice in comparison with those of healthy animals. The drop in platelet counts was observed in all groups on day 7 postinfection, including the 213Bi-D11-treated group, where the counts were significantly different (P = 0.01) in comparison with those on day 3. This can be explained by the effect of prior S. pneumoniae infection on platelets, as well as by the fact that the nadirs in platelet counts are usually reached at 1 week after radiolabeled antibody administration in RIT of cancer patients (4). On day 14 posttherapy, the platelet count in the 213Bi-D11-treated group increased and there was no difference from the count on day 3 (P = 0.06), which reflects the lack of toxicity of 213Bi-D11 treatment in C57BL/6 mice.
FIG. 4.
Platelet counts in C57BL/6 mice infected i.p. with S. pneumoniae and treated with 213Bi-labeled MAbs at 1 h postinfection. Cold = unlabeled.
DISCUSSION
Our earlier encouraging results with RIT for C. neoformans infection provided the impetus for applying RIT to an experimental bacterial infection. In the present study, we have investigated the feasibility of using RIT for systemic S. pneumoniae disease. RIT with an S. pneumoniae-specific MAb radiolabeled with the alpha-emitting radioisotope 213Bi proved to be an efficient and safe treatment modality in the C57BL/6 mouse strain. In contrast to fungal infections, which are usually chronic, disseminated bacterial infections can progress rapidly and bacteria replicate at a considerably faster rate than fungi. For example, the replication rates of S. pneumoniae and C. neoformans are 20 min and 3 h, respectively. Cryptococci have average diameters that are approximately 20 times larger than those of pneumococci (24) and consequently present significantly larger targets for RIT than do bacterial cells. It remains to be seen if the sensitivity of a microorganism to particulate radiation depends on the amount of DNA per volume of matter. In addition, these two microbes elicit different inflammatory responses, with neutrophils predominating in pneumococcal infections and macrophages predominating in cryptococcal infections. Consequently, the success of RIT against experimental cryptococcal infection could not be extrapolated to bacteria from the prior fungal study.
There are several factors that make S. pneumoniae potentially more amenable to RIT in animal models than fungal infections. First, bacteria are known to be at least 1 order of magnitude more radiosensitive to external radiation in vitro than fungi (10). We have recently shown that, despite their extreme radioresistance to external radiation, it is possible to achieve significant killing of the pathogenic fungi C. neoformans and Histoplasma capsulatum with specific radiolabeled antibodies both in vitro (13, 14) and in vivo (13). Thus, the increased susceptibility of bacteria to external radiation relative to that of fungi may translate into better RIT outcomes.
In our studies, we used an i.p. infection animal model that has been used to establish the efficacy of using human MAbs to target pneumococci in mice (11, 29). This model has been shown to be predictive of antibody efficacy in the treatment of human pneumococcal infection and was used to develop serum therapy for pneumococcal pneumonia (7, 9). In this historical serum potency model, the administration of a specific antibody before infection was predictive of its efficacy in patients when given after the onset of pneumococcal disease (7). In our study, we modified this model by administering the radiolabeled antibody after infection. The advantage of using an i.p. infection animal model to assess the potential of RIT of S. pneumoniae is that the targeted cells and radiolabeled MAb molecules are initially confined to the peritoneal cavity, thus increasing the probability of a MAb molecule binding to an S. pneumoniae cell. In fact, i.p. infection models are widely used in cancer research when applicability of RIT with alpha-emitting radionuclides to the treatment of micrometastatic spread from gastric, ovarian, and other cancers is evaluated (2, 25). It might be possible to increase the survival of mice to 100% by increasing the amount of unlabeled D11 in the 213Bi-D11 preparation.
The RIT protocol used in our experiments is theoretically capable of delivering a 213Bi atom to every S. pneumoniae cell in this animal model. At 1 h after i.p. infection with 103 S. pneumoniae cells, there can be expected to be a maximum of 8 × 103 S. pneumoniae cells in the host, assuming a 20-min doubling time with no cell killing by host defenses. The therapeutic dose of 80 μCi of 213Bi-CHXA"-D11 contains 1.2 × 1010 213Bi atoms. The alpha particles emitted by 213Bi have a path length of 50 to 80 μm and a high LET of ∼100 keV/μm and are very efficient at killing a cell by a direct hit. As the range of alpha particles in tissue is more than one cell diameter, even if a particular cell has not been targeted with a radiolabeled antibody molecule, it may be killed by radiation from a distant cell by the so-called crossfire effect. Another mechanism contributing to the killing of bacterial cells in RIT is the bystander effect, which is the biological response of surrounding cells not directly targeted by radiation. The bystander effect has been recently considered to play an important role in radionuclide therapy (5, 28). Also, there could be some synergy between the effect of the radioactivity and the antibody itself on the microbial cells, since antibody molecules have been recently reported to catalyze ozone formation, which can promote bacterial killing and inflammation (27). Finally, since D11 has been shown to downregulate chemokine production from human effector cells (8), its immunomodulatory properties may also be beneficial in the setting of RIT. More studies are needed to establish the specific mechanism(s) of RIT efficacy against pneumococci.
The fact that administration of 213Bi-D11 (80-μCi dose) proved to be therapeutic and safe in the systemic model of S. pneumoniae infection is consistent with our previous finding that the 213Bi-18B7 MAb (100-μCi dose) had a therapeutic effect and was relatively nontoxic in a murine C. neoformans infection (13). In mice treated with irrelevant control 213Bi-IgM, a significant dose is delivered to the organs in the intraperitoneal cavity owing to the retention of IgM molecules in the cavity for several hours. During this period, practically all of the 213Bi decays, depositing its alpha particles nonspecifically. The combination of this radiation dose with the effects of the S. pneumoniae infection itself may explain the poorer survival of 213Bi-IgM-treated animals in comparison with that of the untreated group (Fig. 3b). In fact, we have observed the same effect in C. neoformans-infected mice when high doses of 213Bi-labeled MAb were nontoxic to uninfected animals but toxic to infected ones (15).
As alpha particles can kill a host cell with just one or two hits (18), cells struck by alpha radiation are, as a rule, not able to survive, and hence there should not be any pronounced late cytogenic effects of RIT. In other words, alpha particles kill cells by causing catastrophic damage and this effect is different from the DNA damage associated with other forms of radiation, which is mutagenic. The gamma component of 213Bi emission should not cause late cytogenic effects either. The evidence for this comes from the treatment of patients with metastatic thyroid cancer with large cumulative doses of 131I (which emits more energetic gamma rays than 213Bi) where there is a very low risk of secondary leukemia—0.4 death/104 patient year-Gy (16). The 80-μCi dose administered to a mouse in our study translates into ∼200 mCi for a human, which is comparable to the cumulative 131I doses. We have also recently demonstrated that RIT of C. neoformans infection is safe in regard to long-term pulmonary toxicity in mice (15).
For future clinical trials, we envision that the antibody-chelator conjugate can be produced at a cGMP facility while radiolabeling with 213Bi can be performed on site in the radiopharmacy laboratory by using a 213Bi-225Ac generator. It is noteworthy that such an approach has been successfully used in clinical trials with 213Bi-labeled MAb for the treatment of leukemia (26).
We have recently witnessed a global outbreak of SARS and the introduction of monkeypox virus into the United States. Hence, there is an urgent need for new approaches to the treatment of emerging and drug-resistant infectious diseases that can translate into the rapid development of new antimicrobial agents. Current strategies for the development of antimicrobial drugs and vaccines take many years to yield clinically useful products. However, antibodies can be made very rapidly and the linkage of radionuclides to specific antibodies provides the means to generate microbicidal antibodies. We believe that the combination of immune and radionuclide therapies provides an exciting new strategy that may be potentially useful against a variety of bacterial infections.
Acknowledgments
We thank K. Datta and R. Maitta for help with animal experiments and T. Zhang for help with platelet counts.
This research was in part supported by NIH grants AI52042 (E.D.), AI52733 (J.N.), AI035370 (L.P.), and AI033774 (A.C.).
REFERENCES
- 1.Adams, G. P., C. C. Shaller, L. L. Chappell, C. Wu, E. M. Horak, H. H. Simmons, S. Litwin, J. D. Marks, L. M. Weiner, and M. W. Brechbiel. 2000. Delivery of the alpha-emitting radioisotope bismuth-213 to solid tumors via single-chain Fv and diabody molecules. Nucl. Med. Biol. 27:339-346. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, H., J. Elgqvist, G. Horvath, R. Hultborn, L. Jacobsson, H. Jensen, B. Karlsson, S. Lindegren, and S. Palm. 2003. Astatine-211-labeled antibodies for treatment of disseminated ovarian cancer: an overview of results in an ovarian tumor model. Clin. Cancer Res. 9:3914S-3921S. [PubMed] [Google Scholar]
- 3.Appelbaum, P. C. 2002. Resistance among Streptococcus pneumoniae: implications for drug selection. Clin. Infect. 34:1613-1620. [DOI] [PubMed] [Google Scholar]
- 4.Behr, T. M., M. Behe, M. G. Stabin, E. Wehrmann, C. Apostolidis, R. Molinet, F. Strutz, A. Fayyazi, E. Wieland, S. Gratz, L. Koch, D. M. Goldenberg, and W. Becker. 1999. High-linear energy transfer (LET) alpha versus low-LET beta emitters in radioimmunotherapy of solid tumors: therapeutic efficacy and dose-limiting toxicity of 213Bi- versus 90Y-labeled CO17-1A Fab′ fragments in a human colonic cancer model. Cancer Res. 59:2635-2643. [PubMed] [Google Scholar]
- 5.Bishayee, A., D. V. Rao, and R. W. Howell. 1999. Evidence for pronounced bystander effects caused by nonuniform distributions of radioactivity using a novel three-dimensional tissue culture model. Radiat. Res. 152:88-97. [PMC free article] [PubMed] [Google Scholar]
- 6.Boll, R. A., S. Mirzadeh, and S. J. Kennel. 1997. Optimization of radiolabeling of immunoproteins with 213Bi. Radiochim. Acta 79:145-149. [Google Scholar]
- 7.Buchwald, U. K., and L. Pirofski. 2003. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future role of antibody therapy, therapeutic vaccination and biological response modifiers. Curr. Pharm. Des. 9:945-968. [DOI] [PubMed] [Google Scholar]
- 8.Burns, T., Z. Zhong, M. Steinitz, and L. Pirofski. 2003. Modulation of polymorphonuclear cell interleukin-8 secretion by human monoclonal antibodies to type 8 pneumococcal capsular polysaccharide. Infect. Immun. 71:6775-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadevall, A., and M. D. Scharff. 1994. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob. Agents Chemother. 38:1695-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casarett, A. P. 1968. Radiation biology. Prentice-Hall, Englewood Cliffs, N.J.
- 11.Chang, Q., Z. Zhong, A. Lees, M. Pekna, and L. Pirofski. 2002. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin loci. Infect. Immun. 70:4977-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chappell, L. L., E. Dadachova, D. E. Milenic, K. Garmestani, and M. W. Brechbiel. 2000. Synthesis and characterization of a novel bifunctional chelating agent for lead(II). Conjugation to a monoclonal antibody, radiolabeling with Lead-203 and serum stability determination. Nucl. Med. Biol. 27:93-100. [DOI] [PubMed] [Google Scholar]
- 13.Dadachova, E., A. Nakouzi, R. Bryan, and A. Casadevall. 2003. Ionizing radiation delivered by specific antibody is therapeutic against a fungal infection. Proc. Natl. Acad. Sci. USA 100:10942-10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dadachova, E., R. W. Howell, R. A. Bryan, A. Frenkel, J. D. Nosanchuk, and A. Casadevall. 2004. Susceptibility of human pathogens Cryptococcus neoformans and Histoplasma capsulatum to gamma radiation versus radioimmunotherapy with alpha- and beta-emitting radioisotopes. J. Nucl. Med. 45:313-320. [PubMed]
- 15.Dadachova, E., R. A. Bryan, A. Frenkel, T. Zhang, C. Apostolidis, J. S. Nosanchuk, J. D. Nosanchuk, and A. Casadevall. 2004. Acute hematological and long-term pulmonary toxicity of radioimmunotherapy of Cryptococcus neoformans infection in murine models. Antimicrob. Agents Chemother. 48:1004-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edmonds, C. J., and T. Smith. 1986. The long-term hazards of the treatment of thyroid cancer with radioiodine. Br. J. Radiol. 59:45-51. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg, D. M (ed.). 1995. Cancer therapy with radiolabeled antibodies. CRC Press, Inc., Boca Raton, Fla.
- 18.Kozak, R. W., R. W. Atcher, O. A. Gansow, A. M. Friedman, J. J. Hines, and T. A. Waldmann. 1986. Bismuth-212-labeled anti-Tac monoclonal antibody: alpha particle-emitting radionuclides as modalities for radioimmunotherapy. Proc. Natl. Acad. Sci. USA 83:474-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDevitt, M. R., E. Barendswaard, D. Ma, L. Lai, M. J. Curcio, G. Sgouros, A. M. Ballangrud, W. H. Yang, R. D. Finn, V. Pellegrini, M. W. Geerlings, M. Lee, M. W. Brechbiel, N. H. Bander, C. Cordon-Cardo, and D. A. Scheinberg. 2000. An alpha particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res. 60:6095-6100. [PubMed] [Google Scholar]
- 20.Miale, J. B. 1982. Laboratory medicine hematology, p. 864. The CV Mosby Company, St. Louis, Mo.
- 21.Mirzadeh, S., M. W. Brechbiel, R. W. Atcher, and O. A. Gansow. 1990. Radiometal labeling of immunoproteins: covalent linkage of 2-(4-isothiocyanatobenzyl)diethylenetriamine-pentaacetic acid ligands to immunoglobulin. Bioconjug. Chem. 1:59-65. [DOI] [PubMed] [Google Scholar]
- 22.Pippin, C. G., T. A. Parker, T. J. McMurry, and M. W. Brechbiel. 1992. Spectrophotometric method for the determination of a bifunctional DTPA ligand in DTPA-monoclonal antibody conjugates. Bioconjug. Chem. 3:342-345. [DOI] [PubMed] [Google Scholar]
- 23.Pirofski, L.-A., and A. Casadevall. 1998. Use of licensed vaccines for active immunization of the immunocompromised host. Clin. Microbiol. Rev. 11:1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan, K. J. (ed.). 1994. Sherris medical microbiology. An introduction to infectious diseases. Appleton & Lange, Norwalk, Conn.
- 25.Senekowitsch-Schmidtke, R., C. Schuhmacher, K. F. Becker, T. K. Nikula, C. Seidl, I. Becker, M. Miederer, C. Apostolidis, C. Adam, R. Huber, E. Kremmer, K. Fischer, and M. Schwaiger. 2001. Highly specific tumor binding of a 213Bi-labeled monoclonal antibody against mutant E-cadherin suggests its usefulness for locoregional alpha-radioimmunotherapy of diffuse-type gastric cancer. Cancer Res. 61:2804-2808. [PubMed] [Google Scholar]
- 26.Sgouros, G., A. M. Ballangrud, J. G. Jurcic, M. R. McDevitt, J. L. Humm, Y. E. Erdi, B. M. Mehta, R. D. Finn, S. M. Larson, and D. A. Scheinberg. 1999. Pharmacokinetics and dosimetry of an alpha particle emitter labeled antibody: 213Bi-HuM195 (anti-CD33) in patients with leukemia. J. Nucl. Med. 40:1935-1946. [PubMed] [Google Scholar]
- 27.Wentworth, P., Jr., J. E. McDunn, A. D. Wentworth, C. Takeuchi, J. Nieva, T. Jones, C. Bautista, J. M. Ruedi, A. Gutierrez, K. D. Janda, B. M. Babior, A. Eschenmoser, and R. A. Lerner. 2002. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science 298:2195-2199. [DOI] [PubMed] [Google Scholar]
- 28.Xue, L. Y., N. J. Butler, G. M. Makrigiorgos, S. J. Adelstein, and A. I. Kassis. 2002. Bystander effect produced by radiolabeled tumor cells in vivo. Proc. Natl. Acad. Sci. USA 99:13765-13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]