Abstract
Aims/hypothesis
Evidence is accumulating that Ca2+-regulated K+ (KCa) channels are important for beta cell function. We used BK channel knockout (BK-KO) mice to examine the role of these KCa channels for glucose homeostasis, beta cell function and viability.
Methods
Glucose and insulin tolerance were tested with male wild-type and BK-KO mice. BK channels were detected by single-cell RT-PCR, cytosolic Ca2+ concentration ([Ca2+]c) by fura-2 fluorescence, and insulin secretion by radioimmunoassay. Electrophysiology was performed with the patch-clamp technique. Apoptosis was detected via caspase 3 or TUNEL assay.
Results
BK channels were expressed in murine pancreatic beta cells. BK-KO mice were normoglycaemic but displayed markedly impaired glucose tolerance. Genetic or pharmacological deletion of the BK channel reduced glucose-induced insulin secretion from isolated islets. BK-KO and BK channel inhibition (with iberiotoxin, 100 nmol/l) broadened action potentials and abolished the after-hyperpolarisation in glucose-stimulated beta cells. However, BK-KO did not affect action potential frequency, the plateau potential at which action potentials start or glucose-induced elevation of [Ca2+]c. BK-KO had no direct influence on exocytosis. Importantly, in BK-KO islet cells the fraction of apoptotic cells and the rate of cell death induced by oxidative stress (H2O2, 10–100 μmol/l) were significantly increased compared with wild-type controls. Similar effects were obtained with iberiotoxin. Determination of H2O2-induced K+ currents revealed that BK channels contribute to the hyperpolarising K+ current activated under conditions of oxidative stress.
Conclusions/interpretation
Ablation or inhibition of BK channels impairs glucose homeostasis and insulin secretion by interfering with beta cell stimulus–secretion coupling. In addition, BK channels are part of a defence mechanism against apoptosis and oxidative stress.
Keywords: Apoptosis, Beta cell, BK channel, Exocytosis, Iberiotoxin, Insulin, Stimulus–secretion coupling
Introduction
Nutrient-induced insulin release critically depends on the activity of ion channels and thus the extent of membrane depolarisation. The key event linking elevated glucose metabolism to alterations of electrical activity and eventually increased exocytosis is the closure of ATP-dependent K+ channels (KATP channels) and subsequent opening of voltage-dependent L-type Ca2+ channels. Besides KATP channels, the beta cells express a variety of other K+ channels that are regulated by voltage and/or by the cytosolic Ca2+ concentration ([Ca2+]c) [1–3]. The primary function of voltage-dependent K+ channels (Kv channels), namely the repolarisation of action potentials, has been established for years [4]; however, the role of Ca2+-activated K+ channels (KCa channels) is less clear. Currents through KCa channels of large conductance (BK channels) were identified electrophysiologically in primary and clonal beta cells more than 20 years ago. Initially, these channels were suggested to play a role in the metabolic potentiation of insulin secretion and/or regulation of the characteristic membrane potential oscillations [5, 6] but subsequent investigations did not confirm these assumptions. Several groups have shown that membrane potential oscillations are not affected by BK channel inhibitors [7, 8] and that the activation of KATP channels, but not of KCa channels, is a key event for induction of the electrically silent interburst phases [9, 10].
However, several recent observations have stimulated renewed interest in KCa channels as regulators of beta cell function: (1) studies with two mouse models lacking sulfonylurea receptor 1 (SUR1)/inward rectifier K+ channel Kir 6 (Kir6.2)-composed KATP channels (Sur1 [also known as Abcc8]- and Kir6.2 [also known as Kcnj11] knockout [KO] mice, respectively) have shown that regulated insulin release is possible via a KATP-channel-independent pathway which involves alterations of plasma membrane potential and [Ca2+]c [11, 12]; (2) the K+ current that is activated during each burst phase of Ca2+ action potentials (Kslow current) is not solely mediated by KATP channels but contains a sulfonylurea-insensitive component that strongly depends on [Ca2+]c [11,13]; (3) KCa channels of small (SK1–3) and intermediate (SK4) conductance have been suggested to contribute to the Kslow current [3, 14]; and (4) knockout of KCa channels of the SK4 type influences in vivo glucose homeostasis [3].
Interestingly, a recent study with beta cells from human non-diabetic donors suggests that the role of BK channels might be underestimated and provides evidence that inhibition of BK channels affects insulin secretion [15].
The generation of BK channel knockout (BK-KO) mice [16] by deletion of the Slo1 (also known as Kcnma1) gene now allows a detailed study of the contribution of these K+ channels to beta cell physiology and regulation of glucose homeostasis. Our data show that BK channels are involved in action potential repolarisation. We demonstrate, for the first time, that loss of BK channels impairs insulin secretion and glycaemic control. In addition, BK-KO increases the sensitivity of beta cells to oxidative stress via a direct effect on cell viability.
Methods
Animals, cell and islet preparation
Experiments were performed with BK-KO and wild-type (WT) mice (in-house breeding). The principles of laboratory animal care were followed (National Institutes of Health publication number 85-23, revised 1985). Experiments were carried out according to German laws (Regierungspräsidium Stuttgart, approval number M 8/03). BK-KO mice were generated as previously described [16]. Mice were killed by CO2 and islets were isolated by collagenase digestion, dispersed in Ca2+-free medium and cultured for up to 4 days (RPMI 1640 medium, 11.1 mmol/l glucose, supplemented with 10% [vol./vol.] fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin).
Solutions and chemicals
Bath solution for [Ca2+]c, membrane potential (Vm) and capacitance (Cm) measurements comprised: 140 mmol/l NaCl, 5 mmol/l KCl, 1.2 mmol/l MgCl2, 2.5 mmol/l CaCl2, 15 mmol/l glucose and 10 mmol/l HEPES, pH 7.4. Pipette solution for perforated-patch recordings comprised: 10 mmol/l KCl, 10 mmol/l NaCl, 70 mmol/l K2SO4, 4 mmol/l MgCl2, 2 mmol/l CaCl2, 10 mmol/l EGTA, 5 mmol/l HEPES, pH 7.15, and amphotericin B, 250 μg/ml. Pipette solution for inside-out recordings comprised: 130 mmol/l KCl, 1.2 mmol/l MgCl2, 2 mmol/l CaCl2, 10 mmol/l EGTA, 20 mmol/l HEPES, pH 7.4. Bath solution comprised: 130 mmol/l KCl, 10 mmol/l EDTA, 20 mmol/l HEPES, pH 7.2, with free Ca2+ adjusted to 10 μmol/l by CaCl2. Pipette solution for Cm determination comprised: 135 mmol/l K-gluconate, 10 mmol/l EGTA, 4 mmol/l MgCl2, 5 mmol/l HEPES, 3 mmol/l Na2ATP, 0.2 mmol/l cAMP, pH 7.2, with free Ca2+ adjusted to 10 μmol/l. Incubation medium for insulin secretion: 122 mmol/l NaCl, 4.8 mmol/l KCl, 2.5 mmol/l CaCl2, 1.1 mmol/l MgCl2, 10 mmol/l HEPES and 0.5%(wt/vol.) bovine serum albumin, pH 7.4.
Fura-2-acetoxymethyl ester (fura-2AM) was from Molecular Probes (Eugene, OR, USA) and iberiotoxin was from Bachem (Bubendorf, Switzerland). RPMI 1640 medium was from PromoCell (Heidelberg, Germany) and penicillin/streptomycin was from GIBCO/BRL (Karlsruhe, Germany). All other chemicals were purchased from Sigma (Deisenhofen, Germany) and Merck (Darmstadt, Germany).
Glucose and insulin tolerance tests
In vivo experiments were performed with male BK-KO mice and WT littermates aged 12–19 weeks. Glucose (2 g/kg body weight) or insulin (1 U/kg body weight) was injected intraperitoneally. Plasma glucose concentration was monitored for 120 (glucose tolerance) or 60 (insulin sensitivity) min. Mice were fasted for 16 h before testing glucose tolerance.
Measurement of intracellular free [Ca2+]c
[Ca2+]c was measured by the fura-2 method using equipment and software from TILL photonics (Gräfelfing, Germany). Cells were identified as beta cells when [Ca2+]c was not decreased by 15 mmol/l glucose as described for alpha cells [17]. Cells were loaded with fura-2AM (5 μmol/l) for 30 min at 37°C. [Ca2+]c was calculated following an in vitro calibration with fura-2 K+-salt [18].
Electrophysiology
Patch pipettes were pulled from borosilicate glass capillaries (Clark, Pangbourne, UK). Vm was recorded at 32°C (EPC-9 patch-clamp amplifier; HEKA, Lambrecht, Germany). Cells were identified as beta cells when they were electrically silent with 0.5 mmol/l glucose but showed Ca2+ action potentials after switching to 15 mmol/l glucose. K+ currents were elicited by 10 mV voltage steps (300 ms) from a holding potential of −70 mV. Cm was determined in the standard whole-cell configuration. To assay exocytosis an 830 Hz sine wave with a peak-to-peak amplitude of 30 mV was applied to the cells (−70 mV holding potential). A train of eight cycles was applied to the cell every 5 s. Cm, membrane conductance and access conductance were derived from analysis of the sinusoidal membrane current at two orthogonal phase angles by the LockIn extension of the Pulse software (‘sine+dc’ protocol, HEKA). Data were analysed with ‘Chart’ software (ADInstruments, Spechbach, Germany).
Insulin secretion
Batches of five islets were incubated for 60 min at 37°C. Insulin was determined by radioimmunoassay using rat insulin (Linco Research, St Charles, MI, USA) as the standard.
Single cell
PCR Cellular contents of single cells were aspirated into RNAse-free borosilicate patch-pipettes containing 7 μl of RNAse-free water and were immediately transferred to an Eppendorf tube for cDNA synthesis using Sensiscript reverse transcriptase (Qiagen, Crawley, UK), RNasin ribonuclease inhibitor (Promega, Southampton, UK) and a mix of random and poly-dT primers in a final volume of 20 μl at 37°C for 1 h. For PCR analysis, 2–5 μl of single-cell cDNA was used in a 20 μl reaction using GoTaq DNA polymerase (Promega). Primers, insulin: forwards 5′-CAGCAAGCAGGTCATTGTTT-3′, reverse 5′-CAGTAGTTCTCCAGCTGGTAGA-3′. Primers for the BK channel alpha subunit spanned the site of splicing C2 to detect BK channel splice variants: forwards 5′-GTTTGTGAGCTGTGTTTTGTG-3′, reverse 5′-CTACGGTTACCAGGTGGTCATGT-3′. Amplicons were run on a 1.5% (wt/vol.) agarose gel and visualised using Sybr Safe.
Determination of apoptotic islet cells
Islet cells were seeded on glass cover slips and cultured in RPMI 1640 medium for 24 h. Apoptosis was determined by active caspase 3 (NucView assay, Biotium, Hayward, CA, USA) or TUNEL staining. In each condition, a minimum of 1,000 cells from three to four different isolations was counted. Growth medium was removed and 40 μl DEVD-NucView 488 caspase 3 substrate was added. Upon enzymatic cleavage of the substrate, the released DNA dye migrates to the cell nucleus where it binds to the DNA resulting in a highly fluorescent complex. For TUNEL labelling, pancreaticislet cells were fixed with 3% (wt/vol.) paraformaldehyde at 20–25°C for 1 h. After rinsing with PBS, beta cells were permeabilised for 2 min on ice [0.1% [wt/vol.] Triton-X and sodium citrate solution). Each sample was covered with 50 μl TUNEL reaction mixture and incubated in a humidified atmosphere for 1 h at 37°C in the dark. TUNEL-positive cells were detected by fluorescein staining (480 nm) and the number of total cells was visualised with Hoechst 3342.
Presentation of results
At least three different cell preparations were used for each series. Means ± SEM are given in the text. Statistical significance of differences was assessed by a one-sample or Student’s t test for paired values; multiple comparisons were made by ANOVA followed by Student–Newman–Keuls test. For action potential characteristics five action potentials of each experiment were averaged. Peak values were set to t=0 ms and data were analysed every 50 ms within the preceding and following 200 ms. A p value of less than 0.05 was considered significant.
Results
Activity and expression of BK channels in pancreatic islet cells
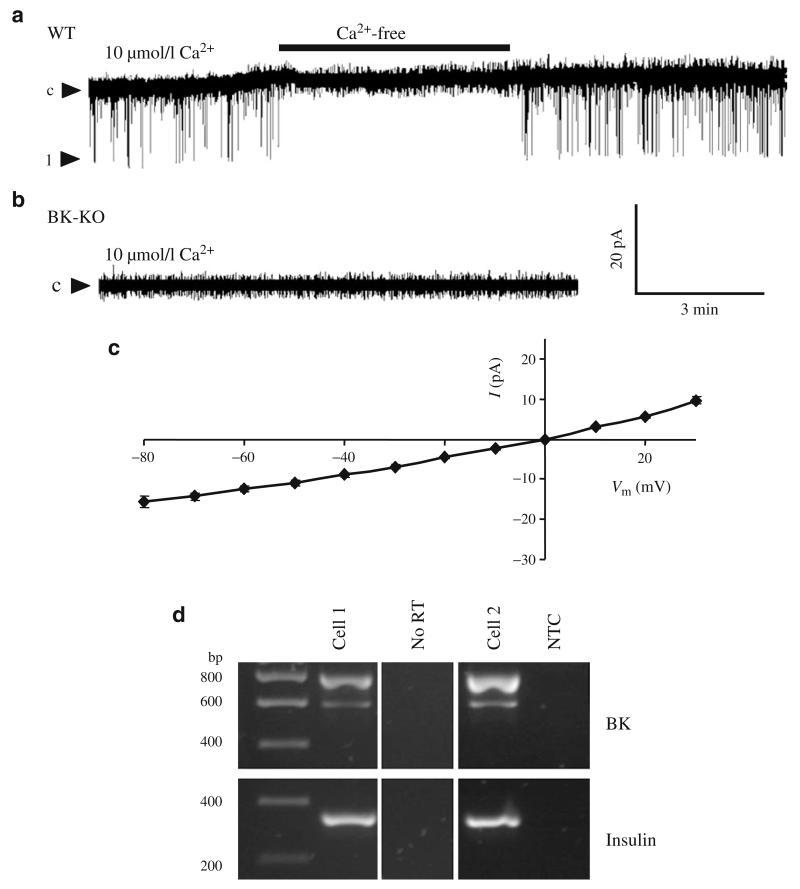
In excised inside-out patches of isolated WT islet cells unitary K+ current amplitudes of 11.0±0.8 pA (holding potential of −50 mV, symmetrical K+ concentration), with an open probability (Po) of 0.012±0.002 (n=5), were detected in 16 out of 43 patches (Fig. 1a). The current was Ca2+ dependent and the cord conductance calculated from the single channel I/V curve was 238±8 pS (n=5, Fig. 1c). As a Ca2+-regulated K+ current with similar properties was absent in excised patches of beta cells obtained from BK-KO mice (n=30, Fig. 1b) the single channel currents were attributed to BK channels. The expression of BK channel pore-forming alpha subunits in pancreatic islet cells was further characterised by single-cell PCR experiments (Fig. 1d). mRNAs of two BK channel alpha subunit splice variants (Zero and Strex) were detected in insulin-positive islet cells, indicating BK channel expression in beta cells.
Fig. 1.
BK channel activity and expression in pancreatic islet cells. a Single channel currents were recorded at a holding potential of −50 mV in inside-out patches of WT islet cells. For the time indicated by the horizontal bar, the BK-channel-positive patches were perifused with Ca2+-free solution. One representative experiment out of 16 single channel recordings is shown. c represents the closed state, 1 represents the open state of one BK channel. b Single channel currents were recorded at a holding potential of −50 mV in inside-out patches of BK-KO islet cells. One representative experiment out of 30 single channel recordings is shown. c I/V curve for single channel currents recorded from inside-out patches of WT beta cells at voltages ranging from −80 to +30 mV. d mRNA of the BK channel splice variants Zero (~600 bp) and Strex (~800 bp) were detected by RT-PCR performed with cytosol derived from single islet cells. Cell 1 and 2 are examples of insulin-positive cells. NTC, non-template control; No RT, control without enzyme
BK-KO mice display reduced insulin secretion and impaired glucose tolerance
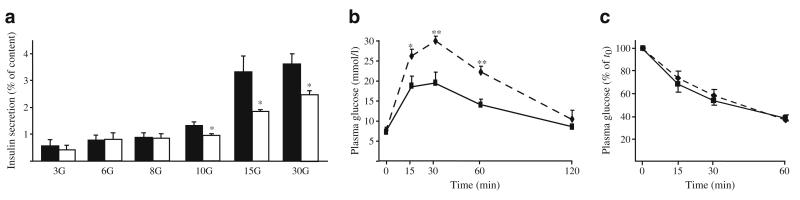
To elucidate whether BK channels are involved in regulation of insulin secretion, the secretory response to glucose was determined in static incubations of isolated WT and BK-KO islets (Fig. 2a). Insulin release was similar in WT and KO islets under resting conditions (3 mmol/l glucose) and at glucose concentrations lower than 10 mmol/l. However, stimulation with 10, 15 and 30 mmol/l glucose was much less effective in BK-KO islets compared with WT controls (p≤0.05, n=4 separate preparations per genotype). WT and BK-KO islets showed no difference in insulin secretion induced by 30 mmol/l K+ in the presence of 250 μmol/l diazoxide (WT 0.9±0.2 ng ml−1 islet−1; BK-KO 0.9±0.5 ng ml−1 islet−1, n=3). The differences in glucose-mediated insulin release were not due to alterations in insulin content (WT 25±1 ng/islet; BK-KO 27±3 ng/islet, n=4 per genotype). The inhibitory effect of BK-KO was mimicked in WT islets treated with the BK channel blockers iberiotoxin (100 nmol/l) or paxillin (10 μmol/l). Insulin secretion of islets stimulated with 15 mmol/l glucose was reduced from 1.9±0.2 to 0.8±0.2 and 0.7±0.1 ng ml−1 islet−1, respectively, when iberiotoxin or paxillin were present during the 60 min incubation period (n=3, p≤0.05).
Fig. 2.
Effect of BK-KO on insulin secretion, glucose tolerance and insulin sensitivity. a Isolated islets were incubated with different glucose concentrations for 60 min. In the presence of 10, 15 and 30 mmol/l glucose, insulin release from BK-KO islets was significantly lower than from WT islets (n=4 different preparations per genotype). b BGC of male WT and BK-KO littermates was monitored before and for 2 h after i.p. injection of glucose (2 g/kg body weight). c For determination of insulin sensitivity, blood glucose was followed before and for 1 h after i.p. injection of insulin (1 U/kg body weight). Glucose tolerance of BK-KO mice was markedly impaired compared with WT mice (n=6 BK-KO and n=8 C57Bl6 WT littermates, respectively), whereas insulin sensitivity was unaffected (n=6 BK-KO and n=5 WT littermates, respectively). *p≤0.05, **p≤0.01; black bars and solid lines, WT mice; white bars and dashed lines, BK-KO mice
To test whether the impaired function of BK-KO islets affects glycaemic control we monitored blood glucose concentration (BGC) in response to glucose and insulin challenge, respectively (Fig. 2b, c).
BGC was similar in WT and BK-KO mice fed ad libitum or after overnight fasting (fed 10.3±0.3 mmol/l for WT vs 10.4±0.5 mmol/l for BK-KO mice; fasted 7.3±0.5 mmol/l for WT and 7.8±0.6 mmol/l for BK-KO mice; n=8 and n=6, respectively). However, an intraperitoneal glucose tolerance test revealed significant differences between the two genotypes. In response to the glucose challenge (2 g/kg body weight) BGC of BK-KO mice was markedly higher compared with WT mice 15, 30 and 60 min after injection. Insulin (1U/kg body weight) injected i.p. reduced the BGC to the same extent in WT and BK-KO mice, respectively.
These data demonstrate that the reduction of insulin secretion caused by BK channel ablation leads to impaired glucose homeostasis in vivo.
Stimulus–secretion coupling in BK-KO beta cells
The observation that glucose-stimulated insulin release was reduced in BK-KO islets whereas K+-induced secretion was unaffected suggests that loss of BK channel function may impair the coupling of glucose metabolism, electrical activity and Ca2+ influx. Consequently, we tested whether the stimulus–secretion cascade was altered in BK channel-deficient beta cells.
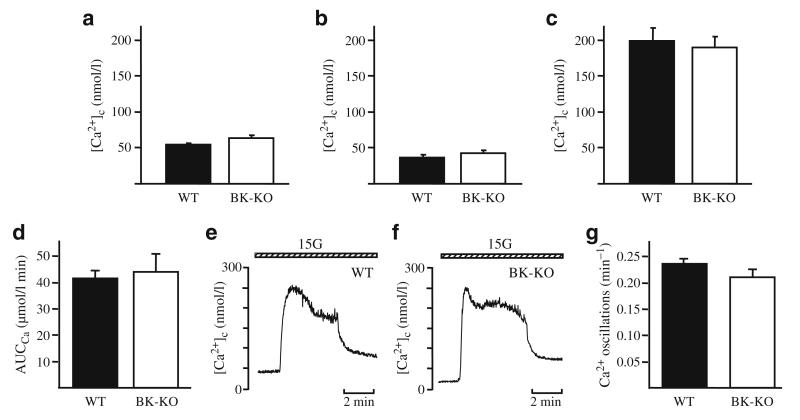
The key event linking glucose metabolism to exocytosis is the increase in [Ca2+]c induced by opening of L-type Ca2+ channels subsequent to membrane depolarisation. Therefore, we investigated whether ablation of BK channels affected [Ca2+]c. In WT beta cells the increase in glucose concentration from 0.5 to 15 mmol/l led to an initial drop of [Ca2+]c due to activation of ATP-dependent Ca2+ pumps. With the opening of L-type Ca2+ channels [Ca2+]c was elevated to a plateau and, finally, characteristic oscillations occurred (n=12). This pattern of activity likewise existed in BK-KO beta cells (n=14, Fig. 3). There was no difference in the area under the curve for the first rise of [Ca2+]c after elevating glucose from 0.5 to 15 mmol/l (Fig. 3d–f) or in the frequency of Ca2+ oscillations (Fig. 3g). The lag time between the elevation of glucose concentration and the rise of [Ca2+]c (WT 140±15 s [n=12] vs BK-KO 146±13 s [n=13]) was also similar for both genotypes.
Fig. 3.
Influence of BK-KO on [Ca2+]c. a BK-KO does not affect basal [Ca2+]c in 0.5 mmol/l glucose (n=14 for both genotypes). b–d After switching to 15 mmol/l glucose there was no difference in the first Ca2+ peak in BK-KO vs WT cells in terms of: (b) the initial decrease (n=14 for both genotypes); (c) the peak [Ca2+]c (WT n=12; BK-KO n=15); or (d) the area under the curve (WT n=12; BK-KO n=13). e, f Representative experiments for (e) WT cells, and (f) BK-KO cells. g A summary of the analysis of the frequency of Ca2+ oscillations in the presence of 15 mmol/l glucose (WT n=51; BK-KO n=23)
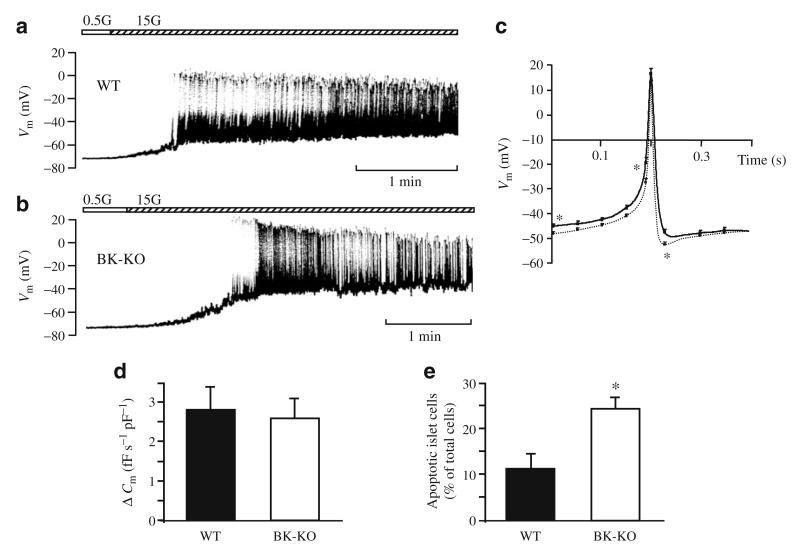
Compatible with the data obtained for [Ca2+]c neither the resting membrane potential in 0.5 mmol/l glucose nor the plateau potential (potential from which Ca2+ action potentials start) with 15 mmol/l glucose were significantly altered by BK-KO (Fig. 4a,b). On average, the resting membrane potential was −69±1 mV (n=10) in BK-KO beta cells and −70±1 mV (n=12) in WT beta cells. The plateau potential was −47±1 mV (n=12) and −48±1 mV (n=12), respectively. Interestingly, BK-KO influenced the shape of Ca2+ action potentials. In BK-KO beta cells, the spike duration of action potentials at half-maximum amplitude was significantly increased from 12±1 ms (WT, n=12) to 18±1 ms (BK-KO, n=12, p≤0.001) and the typical after-hyperpolarisation was completely abolished (Fig. 4c). Identical changes could be induced in WT cells by addition of 100 nmol/l of the specific BK channel blocker iberiotoxin (n=4, not shown), indicating that BK channels are involved in action potential repolarisation. However, BK-KO did not affect action potential frequency (WT 58±10 min−1 [n=12], BK-KO 62±16 min−1 [n=10]) or action potential amplitude (WT 60±2 mV; BK-KO 63±3 mV [n=12]).
Fig. 4.
Electrical activity, exocytosis and cell viability. a,b The effect of glucose stimulation on electrical activity is similar in (a) WT and (b) BK-KO beta cells. Glucose concentrations are indicated by the horizontal bars, with the white section indicating glucose 0.5 mmol/l and the hatched section indicating glucose 15 mmol/l. Experiments were performed in the perforated-patch configuration. The recordings are representative of 12 experiments with each genotype. c Comparison of glucose-induced action potentials recorded from WT (dotted line) and BK-KO (solid line) beta cells. The traces show the average of 12 experiments per genotype. d Exocytosis was determined as the increase in Cm immediately after establishing the standard whole-cell configuration (WT n=14; BK-KO n=13). The cells were dialysed with pipette solution containing 10 μmol/l Ca2+. e BK-KO mice have an increased number of apoptotic islet cells. Apoptotic cells were detected by determination of activated caspase 3. Isolated islet cells were analysed after overnight culture in RPMI medium (11.1 mmol/l glucose). The data were obtained from four different preparations per genotype. *p≤0.05
Furthermore, we examined whether BK channels directly interact with the exocytotic machinery. The standard whole-cell configuration was used to measure Cm and cells were dialysed with pipette solution adjusted to 10 μmol/l free Ca2+. Figure 4d shows that the rate of change in Cm in response to 10 μmol/l Ca2+ was not significantly different between WT (n=14) and BK-KO (n=13) beta cells. To exclude that BK-KO has any effect on cell size, whole-cell capacitance was determined in each experiment (WT 9.7±0.5 pF [n=13]; BK-KO 9.6±0.5 pF [n=14]) and exocytosis was normalised to these values.
BK channels affect beta cell viability
As BK channels have been described to be involved in regulation of cell survival [19] we determined apoptotic cell death in WT and BK-KO cells cultured in 11.1 mmol/l glucose. Importantly, the fraction of apoptotic islet cells was more than doubled in BK-KO vs WT mice (Fig. 4e). In agreement with the increased rate of cell death in BK-KO islets, treatment of WT cells with iberiotoxin (100 nmol/l, 36 h) elevated apoptosis by ~50% (n=3, p≤0.05, not shown).
This suggests that, in addition to the modulation of electrical activity, BK channels are involved in pathways determining apoptotic cell death.
Role of BK channels in response to oxidative stress
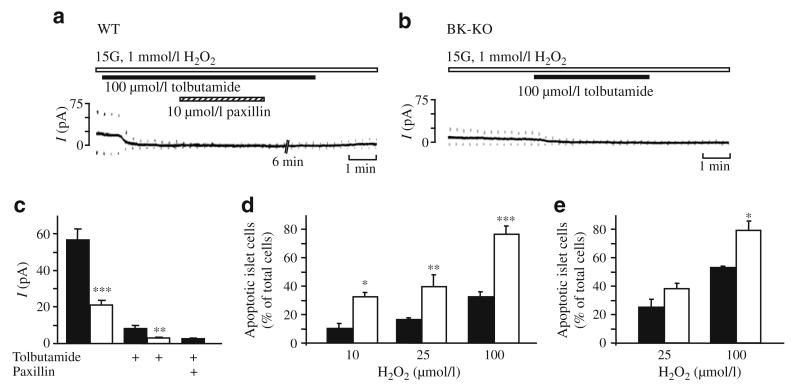
As BK channel activity is linked to cell death we studied the role of these channels in oxidant-induced K+ channel activation and apoptosis. In previous studies we demonstrated that stimulus–secretion coupling of pancreatic beta cells is severely affected by oxidative stress [20–22]. Reactive oxygen species (ROS) such as H2O2 hyperpolarise the plasma membrane via activation of KATP channels, thereby inhibiting insulin secretion. As we could also show that abrogation of electrical activity coincides with a drastic increase in [Ca2+]c [20], activation of KCa channels might also contribute to the hyperpolarising current in addition to KATP channels. To test this hypothesis we measured whole-cell K+ currents (Fig. 5a,b). In WT beta cells stimulated with 15 mmol/l glucose, application of 1 mmol/l H2O2 evoked a marked increase in K+ current that was largely inhibitable by tolbutamide (100 μmol/l, n=8, p≤0.001). However, a small component resistant to the KATP channel blocker was also identified. This component was sensitive to paxillin (10 μmol/l, n=4, p≤0.01), a blocker of BK channels [23]. By contrast, in BK-KO beta cells the current evoked by 1 mmol/l H2O2 was significantly smaller and completely abolished by 100 μmol/l tolbutamide (n=4, p≤0.001). Data are summarised in Fig. 5c. These results demonstrate that current through BK channels contributes to the H2O2-induced hyperpolarisation of pancreatic beta cells. As, for other tissues, activation of BK current has been shown to be an important mechanism to reduce ROS-induced cell damage [24], we tested whether genetic deletion or pharmacological inhibition of BK channels affects the rate of apoptosis in response to H2O2. Figure 5d shows that the increase in the fraction of apoptotic islet cells provoked by 10, 25 and 100 μmol/l H2O2, respectively, was significantly lower in WT compared with BK-KO cells (n=3), suggesting that activation of BK channels is part of the cellular defence mechanism to maintain cell viability under conditions of elevated oxidative stress. To test whether BK channel blockade with iberiotoxin could mimic the effect of BK-KO, WT islet cells were incubated for 36 h with 100 nmol/l iberiotoxin prior to H2O2 application. In this series of experiments the pro-apoptotic action of 100 μmol/l H2O2 was markedly enhanced in iberiotoxin-treated cells vs controls (n=3, Fig. 5e).
Fig. 5.
Influence of H2O2 on ion currents and cell viability in WT and BK-KO islet cells. a, b The K+ current induced by application of H2O2 was composed of KATP and BK current. In the perforated-patch configuration glucose-stimulated beta cells (15 mmol/l glucose) were exposed to 1 mmol/l H2O2 (indicated by the white horizontal bar). In cells from WT islets the current evoked by 10 mV depolarising voltage steps from the holding potential of −70 mV was inhibited by tolbutamide (100 μmol/l; indicated by the black horizontal bar) except for a small component that was sensitive to the BK channel inhibitor paxillin (10 μmol/l; indicated by the hatched horizontal bar). b In cells from BK-KO islets the current was completely blocked by tolbutamide. c The diagram shows the analysis of the experiments described in a, b; black bars, WT; white bars, BK-KO. The number of experiments for H2O2-induced increase in K+ current was 13 for WT and seven for BK-KO beta cells. The effect of tolbutamide was tested in eight WT and four BK-KO cells, respectively. Paxillin was given in addition to the sulfonylurea in four experiments with WT cells. d The fraction of caspase-3-positive islet cells after 1 h incubation with different concentrations of H2O2 (10, 25 and 100 μmol/l, respectively) was significantly higher in BK channel-deficient islet cells. The experiments were performed after overnight culture of dispersed islet cells in RPMI medium (G11.1). The diagram summarises the data obtained from three separate preparations per genotype. Black bars, WT; white bars, BK-KO. e Islets of WT mice were incubated in RPMI medium or in medium supplemented with 100 nmol/l iberiotoxin for 36 h. For an additional 6 h H2O2 (25 or 100 μmol/l) was added and the fraction of apoptotic cells was determined by TUNEL staining (n=3). Black bars, WT; white bars, WT + iberiotoxin. *p≤0.05, **p≤ 0.01, ***p≤0.001 WT vs BK-KO or WT vs iberiotoxin-treated WT cells
Discussion
BK channels are Ca2+- and voltage-regulated K+ channels that occur in most tissues of the body. In excitable cells of endocrine, nervous and vascular systems BK channels link intracellular signalling to electrical activity [16, 25–27].
In 1996 cDNA of the Slo1 gene that encodes the pore-forming alpha subunit of BK channels was identified and characterised in human pancreatic islets [28]. In the present study we detected two splice variants of the alpha subunit, Zero and Strex, in single beta cells (Fig. 1d). In human beta cells, BK current has been reported to account for a significant part of Kv currents and pharmacological inhibition of BK channels influenced by insulin secretion [15]. However, the significance of these observations for glycaemic control of the whole organism remains unclear. The generation of BK-KO mice enabled us to investigate the impact of this channel on regulation of BGC and insulin release. We demonstrate for the first time that loss of BK channels affects glucose homeostasis in vivo. BK-KO did not alter BGC of fasted mice or of animals fed ad libitum but markedly impaired glucose tolerance in response to an intraperitoneal glucose challenge. This effect could be ascribed to a reduction of glucose-stimulated insulin release (Fig. 2).
The fact that BK-KO mice displayed reduced insulin secretion without any change in insulin content pointed to an impairment of beta cell function. Evaluation of glucose-evoked electrical activity revealed that BK-KO did not affect plateau potential or action potential frequency but broadened single action potentials and abolished the after-hyperpolarisation (Fig. 4). Importantly, we obtained similar effects by pharmacological inhibition of BK channels in WT beta cells. As the patch-clamp experiments were performed with single cells or small clusters that do not display the characteristic oscillations recorded from whole islets, the electrophysiological data cannot rule out that BK-KO affects glucose-induced burst frequency. However, this is very unlikely as the frequency of Ca2+ oscillations, which is controlled by Vm, is similar in both genotypes. Our experiments show that inhibition or KO of BK channels does not influence bulk [Ca2+]c (Fig. 3). However, BK channels might participate in regulation of the local Ca2+ concentration which is decisive for control of the exocytotic machinery [29]. The existence of sub-membrane Ca2+ gradients with high Ca2+ concentration directly beneath the plasma membrane has been shown for primary beta cells by Quesada et al. [30]. In addition, the loss of the after-hyperpolarisation might leave more Ca2+ channels in the inactivated state thereby reducing the number of channels that could be recruited by the following action potential. Such subtle changes in Ca2+ influx are most likely too small to change bulk [Ca2+]c, but could alter exocytosis of insulin-containing granules by diminishing [Ca2+] in sub-membrane domains.
BK channels are known to participate in the control of cell mass. For various tumour cell lines it has been shown that BK channel activity modulates proliferation and cell death [19, 31]. Our data provide the first evidence that BK channel ablation affects cell viability in pancreatic islets (Fig. 4e). Compared with WT islet cells, the fraction of apoptotic cells was more than doubled in BK-KO cells. Importantly, similar changes occurred in WT cells after incubation with iberiotoxin. This indicates that alterations in cell viability are not a by-product of the genetic manipulation but are directly linked to loss of BK channel function. The mechanisms by which BK channels modulate signalling pathways determining cell death are not yet resolved. Mitochondrial BK channels have been reported to interfere with Ca2+ sequestration [32] and the mitochondrial permeability transition pore [33, 34]. However, the fact that the non-membrane-permeant peptide iberiotoxin induces similar effects as BK-KO argues against an involvement of mitochondrial BK channels. It is well known that, in several cell types, plasma-membrane-located K+ channels contribute to the regulation of apoptosis. Mostly, inhibition of K+ channels reduces apoptosis but the opposite has also been reported [35, 36]. There are several studies demonstrating that pharmacological or genetic elimination of KATP channels increases apoptotic cell death [22, 37]. Our results suggest that the same holds true for conditions with reduced BK channel activity.
As neither insulin content nor high K+-induced insulin secretion was diminished in BK-KO islets, the proapoptotic effect of BK channel elimination is unlikely to contribute to the impaired secretory response induced by glucose stimulation. However, our study demonstrates that BK channels are important regulators of beta cell viability under conditions of increased oxidative stress (Fig. 5). It is well known that beta cells are extremely vulnerable to ROS due to their poor antioxidant defence mechanisms [38]. Consequently, oxidative stress severely impairs beta cell function and viability [39–42]. BK channels have been reported to be modulated by H2O2 [43]. Our data show that besides KATP channels [44], activation of BK channels contributes to the hyperpolarising current elicited in the presence of H2O2 (Fig. 5a, b) which might serve as a protective mechanism to avoid Ca2+ overload of the cells. At present we cannot rule out that knockout of BK channels induces changes secondary to channel deletion. As the paxillin-sensitive component of the H2O2-induced K+ current is much smaller than the difference between WT and BK-KO beta cells, expression of additional K+ currents might be affected. So far such interactions have not been described for pancreatic beta cells but are reported for the cochlea, where BK-KO leads to disappearance of Kv7.4 channels in outer hair cells [45]. Importantly, compared with WT controls the susceptibility to H2O2-mediated apoptosis was markedly elevated in islet cells derived from BK-KO mice or in iberiotoxin-treated WT cells (Fig. 5d,e). This suggests that activation of BK channels contributes to the defence mechanisms protecting beta cells against oxidative cell damage. In agreement with our results it was demonstrated for hippocampal neurons that pharmacological BK channel inhibition aggravates hypoxia-induced cell death [24]. It is noteworthy that inhibition of BK or KATP channels increases the rate of basal apoptosis but exerts contrary effects on the sensitivity of beta cells to oxidative stress. The protective effect of KATP channel inhibition is caused by an upregulation of antioxidant enzymes that depends on alterations in intracellular Ca2+ sequestration [22]. As limitation of BK channel activity does not coincide with protection against ROS-induced cell death, this indicates that modulation of antioxidative defence mechanisms is particularly related to KATP channels and not generally induced by K+ channel inhibition.
In summary, our investigations show that BK channels play a role in glucose homeostasis and affect the susceptibility of pancreatic beta cells to oxidative stress.
Acknowledgements
We thank I. Breuning for skilful technical assistance. This work was supported by grants from the DFG Dr225/6-3 (G. Drews), DU425/1-2 (M. Düfer) and the Wellcome Trust (M. J. Shipston and P. Ruth). Parts of this study have been published in abstract form (Diabetologia [2004] 47(Suppl 1):208).
Abbreviations
- BGC
Blood glucose concentration
- BK-KO
BK channel knockout
- Cm
Membrane capacitance
- [Ca2+]c
Cytosolic Ca2+ concentration
- KATP channel
ATP-dependent K+ channel
- KCa channel
Ca2+-activated K+ channel
- ROS
Reactive oxygen species
- Vm
Membrane potential
- WT
Wild-type
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contributor Information
M. Düfer, Institute of Pharmacy, Department of Pharmacology and Toxicology, University of Tübingen, Auf der Morgenstelle 8, 72076 Tübingen, Germany
Y. Neye, Institute of Pharmacy, Department of Pharmacology and Toxicology, University of Tübingen, Auf der Morgenstelle 8, 72076 Tübingen, Germany
K. Hörth, Institute of Pharmacy, Department of Pharmacology and Toxicology, University of Tübingen, Auf der Morgenstelle 8, 72076 Tübingen, Germany
P. Krippeit-Drews, Institute of Pharmacy, Department of Pharmacology and Toxicology, University of Tübingen, Auf der Morgenstelle 8, 72076 Tübingen, Germany
A. Hennige, Department of Internal Medicine, Division of Endocrinology, University of Tübingen, Tübingen, Germany
H. Widmer, Division of Biological and Biomedical Science, School of Life Sciences, Glasgow Caledonian University, Glasgow, UK
H. McClafferty, Centre for Integrative Physiology, College of Medicine and Veterinary Medicine, University of Edinburgh, Edinburgh, UK
M. J. Shipston, Centre for Integrative Physiology, College of Medicine and Veterinary Medicine, University of Edinburgh, Edinburgh, UK
H.-U. Häring, Department of Internal Medicine, Division of Endocrinology, University of Tübingen, Tübingen, Germany
P. Ruth, Institute of Pharmacy, Department of Pharmacology and Toxicology, University of Tübingen, Auf der Morgenstelle 8, 72076 Tübingen, Germany
G. Drews, Institute of Pharmacy, Department of Pharmacology and Toxicology, University of Tübingen, Auf der Morgenstelle 8, 72076 Tübingen, Germany
References
- 1.MacDonald PE, Wheeler MB. Voltage-dependent K+ channels in pancreatic beta cells: role, regulation and potential as therapeutic targets. Diabetologia. 2003;46:1046–1062. doi: 10.1007/s00125-003-1159-8. [DOI] [PubMed] [Google Scholar]
- 2.Tamarina NA, Wang Y, Mariotto L, et al. Small-conductance calcium-activated K+ channels are expressed in pancreatic islets and regulate glucose responses. Diabetes. 2003;52:2000–2006. doi: 10.2337/diabetes.52.8.2000. [DOI] [PubMed] [Google Scholar]
- 3.Düfer M, Gier B, Wolpers D, Krippeit-Drews P, Ruth P, Drews G. Enhanced glucose tolerance by SK4 channel inhibition in pancreatic beta-cells. Diabetes. 2009;58:1835–1843. doi: 10.2337/db08-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith PA, Bokvist K, Arkhammar P, Berggren PO, Rorsman P. Delayed rectifying and calcium-activated K+ channels and their significance for action potential repolarization in mouse pancreatic beta-cells. J Gen Physiol. 1990;95:1041–1059. doi: 10.1085/jgp.95.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook DL, Ikeuchi M, Fujimoto WY. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B cells. Nature. 1984;311:269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- 6.Ribalet B, Eddlestone GT, Ciani S. Metabolic regulation of the KATP and a maxi-KV channel in the insulin-secreting RINm5F cell. J Gen Physiol. 1988;92:219–237. doi: 10.1085/jgp.92.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henquin JC. Role of voltage- and Ca2+-dependent K+ channels in the control of glucose-induced electrical activity in pancreatic B cells. Pflügers Arch. 1990;416:568–572. doi: 10.1007/BF00382691. [DOI] [PubMed] [Google Scholar]
- 8.Kukuljan M, Goncalves AA, Atwater I. Charybdotoxin-sensitive KCa channel is not involved in glucose-induced electrical activity in pancreatic beta-cells. J Membr Biol. 1991;119:187–195. doi: 10.1007/BF01871418. [DOI] [PubMed] [Google Scholar]
- 9.Krippeit-Drews P, Düfer M, Drews G. Parallel oscillations of intracellular calcium activity and mitochondrial membrane potential in mouse pancreatic B cells. Biochem Biophys Res Commun. 2000;267:179–183. doi: 10.1006/bbrc.1999.1921. [DOI] [PubMed] [Google Scholar]
- 10.Rolland JF, Henquin JC, Gilon P. Feedback control of the ATP-sensitive K+ current by cytosolic Ca2+ contributes to oscillations of the membrane potential in pancreatic beta-cells. Diabetes. 2002;51:376–384. doi: 10.2337/diabetes.51.2.376. [DOI] [PubMed] [Google Scholar]
- 11.Haspel D, Krippeit-Drews P, Aguilar-Bryan L, Bryan J, Drews G, Düfer M. Crosstalk between membrane potential and cytosolic Ca2+ concentration in beta cells from Sur1−/− mice. Diabetologia. 2005;48:913–921. doi: 10.1007/s00125-005-1720-8. [DOI] [PubMed] [Google Scholar]
- 12.Ravier MA, Nenquin M, Miki T, Seino S, Henquin JC. Glucose controls cytosolic Ca2+ and insulin secretion in mouse islets lacking adenosine triphosphate-sensitive K+ channels owing to a knockout of the pore-forming subunit Kir6.2. Endocrinology. 2009;150:33–45. doi: 10.1210/en.2008-0617. [DOI] [PubMed] [Google Scholar]
- 13.Göpel SO, Kanno T, Barg S, et al. Activation of Ca2+-dependent K+ channels contributes to rhythmic firing of action potentials in mouse pancreatic beta cells. J Gen Physiol. 1999;114:759–770. doi: 10.1085/jgp.114.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Houamed K, Kupershmidt S, Roden D, Satin LS. Pharmacological properties and functional role of Kslow current in mouse pancreatic beta-cells: SK channels contribute to Kslow tail current and modulate insulin secretion. J Gen Physiol. 2005;126:353–363. doi: 10.1085/jgp.200509312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun M, Ramracheya R, Bengtsson M, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 16.Sausbier M, Hu H, Arntz C, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barg S, Galvanovskis J, Göpel SO, Rorsman P, Eliasson L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes. 2000;49:1500–1510. doi: 10.2337/diabetes.49.9.1500. [DOI] [PubMed] [Google Scholar]
- 18.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 19.Weaver AK, Liu X, Sontheimer H. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J Neurosci Res. 2004;78:224–234. doi: 10.1002/jnr.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krippeit-Drews P, Krämer C, Welker S, Lang F, Ammon HP, Drews G. Interference of H2O2 with stimulus-secretion coupling in mouse pancreatic beta-cells. J Physiol. 1999;514:471–481. doi: 10.1111/j.1469-7793.1999.471ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drews G, Krämer C, Krippeit-Drews P. Dual effect of NO on KATP+ current of mouse pancreatic B cells: stimulation by deenergizing mitochondria and inhibition by direct interaction with the channel. Biochim Biophys Acta. 2000;1464:62–68. doi: 10.1016/s0005-2736(99)00242-4. [DOI] [PubMed] [Google Scholar]
- 22.Gier B, Krippeit-Drews P, Aguilar-Bryan L, Bryan J, Düfer M, Drews G. Suppression of KATP channel activity protects murine pancreatic beta cells against oxidative stress. J Clin Invest. 2009;119:3246–3256. doi: 10.1172/JCI38817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knaus HG, McManus OB, Lee SH, et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- 24.Rundén-Pran E, Haug FM, Storm JF, Ottersen OP. BK channel activity determines the extent of cell degeneration after oxygen and glucose deprivation: a study in organotypical hippocampal slice cultures. Neuroscience. 2002;112:277–288. doi: 10.1016/s0306-4522(02)00092-1. [DOI] [PubMed] [Google Scholar]
- 25.Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner R, Peréz GJ, Bonev AD, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 27.Yang MJ, Wang F, Wang JH, et al. PI3-k integrates the effects of insulin and leptin on large-conductance Ca2+-activated K+ channels in neuropeptide Y neurons of the hypothalamic arcuate nucleus. Am J Physiol Endocrinol Metab. 2010;298:E193–E201. doi: 10.1152/ajpendo.00155.2009. [DOI] [PubMed] [Google Scholar]
- 28.Ferrer J, Wasson J, Salkoff L, Permutt MA. Cloning of human pancreatic islet large conductance Ca2+-activated K+ channel (hSlo) cDNAs: evidence for high levels of expression in pancreatic islets and identification of a flanking genetic marker. Diabetologia. 1996;39:891–898. doi: 10.1007/BF00403907. [DOI] [PubMed] [Google Scholar]
- 29.Bokvist K, Eliasson L, Ammälä C, Renström E, Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic B cells. EMBO J. 1995;14:50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quesada I, Martín F, Soria B. Nutrient modulation of polarized and sustained submembrane Ca2+ microgradients in mouse pancreatic islet cells. J Physiol. 2000;525:159–167. doi: 10.1111/j.1469-7793.2000.t01-1-00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheranov SY, Jaggar JH. Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries. J Physiol. 2004;556:755–771. doi: 10.1113/jphysiol.2003.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stowe DF, Aldakkak M, Camara AK, et al. Cardiac mitochondrial preconditioning by big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol. 2006;290:H434–H440. doi: 10.1152/ajpheart.00763.2005. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y, Gu XQ, Bednarczyk P, Wiedemann FR, Haddad GG, Siemen D. Hypoxia increases activity of the BK-channel in the inner mitochondrial membrane and reduces activity of the permeability transition pore. Cell Physiol Biochem. 2008;22:127–136. doi: 10.1159/000149790. [DOI] [PubMed] [Google Scholar]
- 35.Lang F, Huber SM, Szabo I, Gulbins E. Plasma membrane ion channels in suicidal cell death. Arch Biochem Biophys. 2007;462:189–194. doi: 10.1016/j.abb.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 36.Patel AJ, Lazdunski M. The 2P-domain K+ channels: role in apoptosis and tumorigenesis. Pflügers Arch. 2004;448:261–273. doi: 10.1007/s00424-004-1255-8. [DOI] [PubMed] [Google Scholar]
- 37.Miki T, Tashiro F, Iwanaga T, et al. Abnormalities of pancreatic islets by targeted expression of a dominant-negative KATP channel. Proc Natl Acad Sci USA. 1997;22:11969–11973. doi: 10.1073/pnas.94.22.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 39.Tsuura Y, Ishida H, Hayashi S, Sakamoto K, Horie M, Seino Y. Nitric oxide opens ATP-sensitive K+ channels through suppression of phosphofructokinase activity and inhibits glucose-induced insulin release in pancreatic beta cells. J Gen Physiol. 1994;104:1079–1098. doi: 10.1085/jgp.104.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drews G, Krämer C, Düfer M, Krippeit-Drews P. Contrasting effects of alloxan on islets and single mouse pancreatic beta-cells. Biochem J. 2000;352:389–397. [PMC free article] [PubMed] [Google Scholar]
- 41.Di Matteo MA, Loweth AC, Thomas S, et al. Superoxide, nitric oxide, peroxynitrite and cytokine combinations all cause functional impairment and morphological changes in rat islets of Langerhans and insulin secreting cell lines, but dictate cell death by different mechanisms. Apoptosis. 1997;2:164–177. doi: 10.1023/a:1026412414666. [DOI] [PubMed] [Google Scholar]
- 42.Li LX, Skorpen F, Egeberg K, Jørgensen IH, Grill V. Uncoupling protein-2 participates in cellular defense against oxidative stress in clonal beta-cells. Biochem Biophys Res Commun. 2001;282:273–277. doi: 10.1006/bbrc.2001.4577. [DOI] [PubMed] [Google Scholar]
- 43.Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol. 2004;11:171–178. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- 44.Krippeit-Drews P, Lang F, Häussinger D, Drews G. H2O2 induced hyperpolarization of pancreatic B cells. Pflügers Arch. 1994;426:552–554. doi: 10.1007/BF00378534. [DOI] [PubMed] [Google Scholar]
- 45.Rüttiger L, Sausbier M, Zimmermann U, et al. Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc Natl Acad Sci USA. 2004;101:12922–12927. doi: 10.1073/pnas.0402660101. [DOI] [PMC free article] [PubMed] [Google Scholar]